Abstract
Pancreatic ductal neoplasms exhibit gastric epithelium–like characteristics. In this study, we evaluated the expression of claudin-18 (CLDN18), a gastric epithelium–associated claudin, in pancreatic intraepithelial neoplasias (PanINs), intraductal papillary mucinous neoplasms (IPMNs), mucinous cystic neoplasms (MCNs), and pancreatic ductal adenocarcinomas (PDACs) using immunohistochemistry. We observed a high level of expression of CLDN18 in PanINs (31/32, 97%), IPMNs (61/65, 95%), and MCNs (4/5, 80%) using ordinary tissue section analysis. Furthermore, we observed a high level of CLDN18 expression in PDACs (109/156, 70%) using tissue microarray analysis. However, the normal pancreatic duct or the ductal metaplasia of the acinar cells was not immunoreactive. Comparative analysis of CLDN18 and phenotypic markers in IPMNs revealed that simultaneous expression of CLDN18 and intestinal markers frequently occurred, even in intestinal-type IPMNs. CLDN18 variant 2 mRNA was expressed and was similarly upregulated by phorbol 12–myristate 13–acetate (PMA) treatment in pancreatic cancer cell lines and in a gastric cancer cell line. An inhibitor of pan-PKC (GF109203X) completely suppressed this upregulation in pancreatic cancer cells. These results indicate that CLDN18, a marker for the early carcinogenetic process, is commonly expressed in precursor lesions of PDAC. Activation of the PKC pathway might be involved in CLDN18 expression associated with pancreatic carcinogenesis.
Keywords: claudin-18, pancreatic ductal neoplasm, PanIN, IPMN, MCN, gastric phenotype
Pancreatic ductal adenocarcinoma (PDAC) is the most common pancreatic malignancy. This cancer’s poor prognosis and late presentation emphasize the importance of early detection; therefore, accumulating knowledge about the pathological details and the molecular alterations of early or precursor lesions of PDAC is essential. Three distinct epithelial lesions—pancreatic intraepithelial neoplasias (PanINs), intraductal papillary mucinous neoplasms (IPMNs), and mucinous cystic neoplasms (MCNs)—are recognized as the precursors of PDAC (Maitra et al. 2005). The multistep progression models of these precursor lesions have been clarified using morphological and molecular examinations (Maitra et al. 2005; Maitra et al. 2003).
Several lines of evidence indicate that phenotypic changes of the pancreatic epithelium, especially the acquisition of gastric epithelium–like characteristics, constitute a crucial event in the early stage of pancreatic carcinogenesis (Ban et al. 2006; Prasad et al. 2005; Kim et al. 2002; Basturk et al. 2010). IPMNs with gastric foveolar epithelium–like morphology are a major subset of IPMNs (gastric-type IPMNs) (Ban et al. 2006). The foregut markers are characteristically upregulated in PanINs, including the ectopic appearance of the gastric epithelial phenotype (Prasad et al. 2005). Accordingly, several gastric markers, such as MUC5AC and MUC6, have been shown to label the pancreatic precursor lesions (Kim et al. 2002); however, these markers might fail to detect early neoplastic changes. Those molecules that are expressed more ubiquitously in the precursor lesions demand further analysis to gain a better understanding of the early carcinogenetic process of PDACs.
Claudins (CLDNs) are a family of proteins that form tight junctions and maintain the polarity of epithelial and endothelial cells (Tsukita and Furuse 2000). CLDN18 is specifically expressed in the stomach and lung. Of the two CLDN18 isoform transcripts produced by alternative splicing, CLDN18.2 is a highly selective gastric lineage marker that determines the gastric phenotype in a neoplastic condition, whereas CLDN18.1 is lung specific (Sahin et al. 2008; Niimi et al. 2001; Sentani et al. 2008; Sanada et al. 2006; Yasui et al. 2009). Interestingly, recent articles have reported the frequent expression of CLDN18 in PDACs and PanINs (Sahin et al. 2008; Karanjawala et al. 2008).
In the present study, we conducted a detailed analysis of CLDN18 expression in PDACs and in their precursor lesions (PanINs, IPMNs, and MCNs) using immunohistochemistry. We sought to determine whether CLDN18 was an early-stage marker of pancreatic ductal neoplasms. We also compared the expression levels of CLDN18 and known phenotypic (gastric and intestinal) markers in IPMNs to verify that CLDN18 is a gastric marker. Furthermore, we determined the variant of CLDN18 expressed in pancreatic cancer cell lines and investigated its regulatory mechanism to gain insight into the early-stage abnormalities of pancreatic carcinogenesis.
Materials and Methods
Cases
We reviewed 224 cases of pancreatic ductal neoplasms, including 156 PDACs, 64 IPMNs, and 5 MCNs, and two cases of chronic pancreatitis from The University of Tokyo Hospital pathology archives from the period 1986 to 2009. Thirty-two PanINs were also selected from these specimens for immunohistochemical analysis. All aspects of the present study were approved by The University of Tokyo Ethics Committee.
Histopathological Examination
For each case, all the tissue slides were reviewed. The entire tumor of the surgically resected specimen was fixed in 10% formalin at room temperature and was sectioned at intervals of 0.5 to 1.0 cm, with all tumor-containing sections routinely processed and embedded in paraffin. The serial sections of each tumor were cut and stained with hematoxylin and eosin (H&E).
The histological diagnosis of each lesion was based on the World Health Organization classification (Hamilton and Aaltonen 2000) and the textbook from the Armed Forces Institute of Pathology (Hruban et al. 2007). The PanINs and IPMNs were subclassified according to a recent consensus (Hruban et al. 2004; Furukawa et al. 2005). Because of the heterogeneity of the dysplastic grades within the lesions, we recorded the histological grades of the noninvasive components of the IPMNs by assigning them to two broad categories: low-grade lesions (mild and moderate dysplasia) and high-grade lesions (high-grade dysplasia, including carcinoma in situ). All the MCNs in the present study corresponded to adenoma. The PDACs were also graded as well differentiated, moderately differentiated, or poorly differentiated adenocarcinoma.
Tissue Microarrays
Tissue microarrays were constructed for the immunohistochemical analysis of the PDACs. The archived paraffin-embedded tissues of the PDAC cases were placed as 2-mm cores into the recipient block in duplicate to create tissue microarrays (TMAs). Only the microarray cores that comprised at least 30% invasive carcinoma were evaluated.
Immunohistochemical Evaluation
The levels of protein expression were measured using immunohistochemical labeling of the TMAs of the PDACs and the whole tissue sections of the precursor lesions. Four-micrometer-thick sections of formalin-fixed and paraffin-embedded tissue were subjected to immunohistochemical analyses using the streptavidin–biotin peroxidase method with an automated immunostainer (Ventana Medical Systems Inc., Tucson, AZ). The following primary antibodies were used: CLDN18 (ZMD395, 1:1000; Zymed, San Francisco, CA), MUC2 (Ccp59, 1:200; Novocastra, Newcastle Upon Tyne, UK), MUC5AC (CLH2, 1:500; Novocastra), MUC6 (CLH5, 1:100; Novocastra), and CDX-2 (CDX2-88, 1:50; BioGenex, Fremont, CA). The antigen-antibody reaction was visualized using chromogen 3,30-diaminobenzidine. The sections were lightly counterstained with hematoxylin.
The immunostaining of CLDN18 was evaluated as being either negative, weakly positive, moderately positive, or strongly positive. The labeling of CLDN18 was considered to be strong (3+) if more than 80% of the neoplastic cells were labeled at a strong intensity; moderate (2+) if 25% to 80% of the neoplastic cells were labeled at any intensity or if more than 80% of these cells were labeled at a moderate or weak intensity; weak (1+) if 1% to 25% of the neoplastic cells were labeled at any intensity; and negative (0) if less than 1% of the neoplastic cells were labeled. The respective immunostainings of MUC5AC, MUC6, MUC2, and CDX2 were evaluated following the evaluation of the CLDN18 expression. At the same time, the immunostaining of CDX2 was evaluated as positive or negative. We defined 2+ or 3+ as positive and 0 or 1+ as negative. Normal gastric epithelial cells were used as a control for MUC5AC, MUC6, and CLDN18, and normal small intestinal epithelial cells were used as a control for MUC2 and CDX2. Two or three pathologists (MT,JS,NF) reviewed each slide using a multiheaded microscope with no knowledge of the clinical features of each case. A consensus among these pathologists was reached in all cases.
Cell Lines and Culture Conditions
The pancreatic carcinoma cell lines used in the study were Panc-1, PK-1, PK-8, PK-9, PK-45H, PK-45P, KLM-1, and MiaPaca-2. The cell lines were cultured in RPMI 1640 (Nacalai Tesque Inc., Kyoto, Japan) or DMEM (Nacalai Tesque Inc.) supplemented with 10% FCS (MP Biomedicals, Santa Ana, CA), penicillin (40 U/mL), and streptomycin (50 µg/mL) at 37C in a 5% CO2 incubator.
RNA Extraction and RT-PCR
The reverse-transcription PCR (RT-PCR) analysis of CLDN18 was performed in eight pancreatic carcinoma cell lines. The total RNA was extracted from the pancreatic carcinoma cell lines using an RNeasy Mini Kit (Qiagen Inc., Hilden, Germany). The reverse transcription of mRNA was conducted using a SuperScript III RT system (Invitrogen Corp., Carlsbad, CA) and random primers. The following primers were used for the PCR: CLDN18 variant 1 primer: (forward) 5′-CGGGCGGCCAGGATCATGTC-3′, (reverse) 5′-ACTGCCTGCAGCATGGCTGG-3′, or (forward) 5′-TC-CACCACCACATGCCAAGTG-3′, (reverse) 5′-GTGTACA-TGTTAGCTGTGGAC-3′; CLDN18 variant 2: (forward) 5′-TGTGCGCCACCATGGCCGTG-3′, (reverse) 5′-ACT-CGGTGAAGCCAGAGCTC-3′; and glyceraldehyde-3-phosphate dehydrogenase (GAPDH): (forward) 5′-GA-AGGTGAAGGTCGGAGTC-3′, (reverse) 5′-GAAGAT-GGTGATGGGATTC-3′. The PCR reactions were performed using a GeneAmp PCR 9700 system (Applied Biosystems, Foster City, CA). On a 2% agarose gel, 10 µL of each PCR product was separated, stained with ethidium bromide, and photographed under an ultraviolet transilluminator using a compact gel documentation system (Gel Doc EZ; Bio-Rad Laboratories Inc., Hercules, CA). Images were saved in tagged information file format (tiff) files for quantification. The optical density was determined for both CLDN18 and GAPDH using the Photoshop Extended System (Adobe Systems Inc., Mountain View, CA). Then, the ratio of CLDN18 band intensity to GAPDH band intensity was calculated. Normal human lung tissue and gastric epithelium were used as positive controls for the RT-PCR analysis of CLDN18 variant 1 and variant 2.
Reagents and Treatments
Phorbol 12–myristate 13–acetate (PMA) was obtained from Sigma (St. Louis, MO). It was dissolved in dimethylsulfoxide (DMSO; Sigma) at a stock concentration of 2.0 mM and was stored at −20C. Three cell lines—Panc-1, PK-1, and MiaPaca-2—were treated with DMSO or 50 nM, 100 nM, or 200 nM PMA. The cells were harvested at 0 hours, 6 hours, or 12 hours after the PMA treatment. A pan-PKC inhibitor (GF109203X) was purchased from Wako Pure Chemical Industries Ltd. (Osaka, Japan).
Statistical Analysis
All the calculations in the present study were conducted using StatView 5.0J (Abacus Concepts Inc., Berkeley, CA). An exact contingency table test tool (χ2 test) was used for correlation analysis of CLDN18 staining intensity and other gastric or intestinal marker staining intensity or the pathological features of each patient. p values of ≤0.05 were considered to be statistically significant.
Results
CLDN18 Expression in Nonneoplastic and Neoplastic Pancreases
The results of the CLDN18 expression analysis in nonneoplastic and various ductal neoplasms of the pancreas are presented in Table 1. All three types of the precursor lesions (PanIN, IPMN, and MCN) exhibited frequent immunoreactivity for CLDN18. Staining of CLDN18 at the basolateral membrane without staining of the apical cell surface and the cytoplasm reflects its role as a component of a tight junction. The immunoreactivity was scored 0 to 3+ according to the criteria presented above. Among cases with the expression score 1+, no case showed strongly positive cells even in a scattered distribution for CLDN18, MUC5AC, MUC6, MUC2, or CDX2.
Table 1.
CLDN18 Expression in Normal, Metaplastic, and Neoplastic Pancreatic Ductal Lesions
| CLDN18 |
||
|---|---|---|
| Histology | Positive Rate | Expression Score (3+/ 2+/1+/0) |
| Normal pancreatic duct | Negative | — |
| Ductal metaplasia of acinar cells | Negative | — |
| PanIN | 31/32 (96.8%) | 25/6/0/1 |
| PanIN-1 | 18/19 (94.7%) | 15/3/0/1 |
| PanIN-2 | 9/9 (100%) | 9/0/0/0 |
| PanIN-3 | 4/4 (100%) | 1/3/0/0 |
| IPMN | 61/64 (95.3%) | 37/21/3/3 |
| Low grade | 38/39 (97.4%) | 25/12/1/1 |
| High grade | 23/25 (92.0%) | 12/9/2/2 |
| Gastric type | 44/45 (97.8%) | 31/13/0/1a |
| Intestinal type | 13/15 (86.7%) | 3/8/2/2a |
| Onocytic type | 4/4 (100%) | 3/0/1/0 |
| MCN | 4/5 (80.0%) | 2/2/0/1 |
| PDAC | 109/156 (69.9%) | 45/33/31/47 |
| Well differentiated | 28/31 (90.3%) | 19/7/2/3b |
| Moderately differentiated | 71/98 (72.4%) | 26/23/22/27b |
| Poorly differentiated | 10/27 (37.0%) | 0/1/9/17b |
The significant difference was found between gastric-type IPMN and intestinal-type IPMN (p = 0.001).
The significant differences were found between well and poorly differentiated carcinomas (p < 0.0001) and between moderately and poorly differentiated carcinomas (p = 0.0002).
CLDN18 expression in nonneoplastic pancreases
In nonneoplastic pancreases, the pancreatic duct epithelia (Figure 1A and 1B) and the ductal metaplasia of the acinar cells (Figure 1C and 1D) were not immunoreactive for CLDN18. The acinar cells, neuroendocrine cells, and mesenchymal fibroblasts that surrounded the neoplastic lesions were negative for CLDN18.
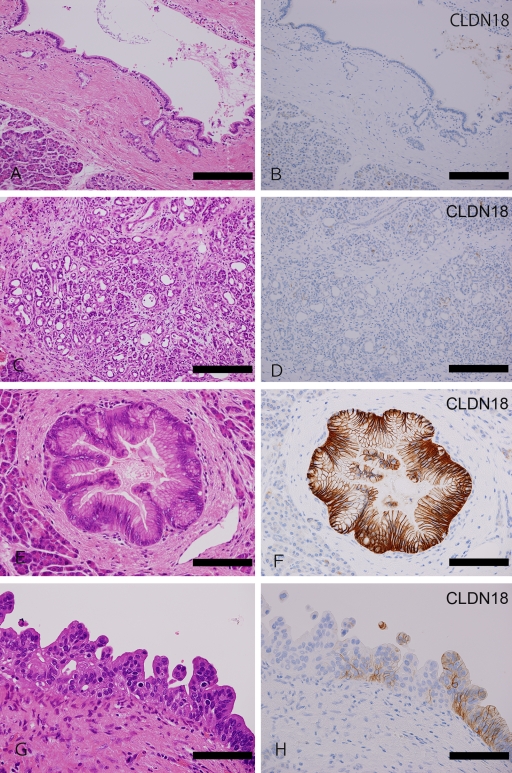
Figure 1.
CLDN18 expression in nonneoplastic pancreas and PanINs. (A-D) Nonneoplastic pancreas. Normal pancreatic ducts ([A] H&E staining; [B] CLDN18 immunostaining) and ductal metaplasia of acinar cells ([C] H&E; [D] CLDN18) do not express claudin-18. (E, F) PanINs. In the PanIN-1 lesion, CLDN18 is strongly expressed in the basolateral membrane ([E] H&E; [F] CLDN18). In the PanIN-3 lesion, CLDN18 is still expressed, but its intensity is weak ([G] H&E; [H] CLDN18). Bar = 2.0 mm.
CLDN18 expression in PanINs
CLDN18 expression was observed in almost all the PanINs, irrespective of their histological grade (31 of 32 cases, 96.9%). Although the expression of CLDN18 in PanIN-3 was slightly weaker, the PanINs exhibited strong expression overall (Table 1, Figure 1E-1H).
CLDN18 expression in IPMNs
CLDN18 was expressed with a high frequency in the intraductal components of all grades and subtypes of the IPMNs (61 of 64 cases, 95.3%). However, the expression scores depended on the grade and the subtype of the tumor. The low-grade lesions tended to exhibit higher expression scores than the high-grade lesions, although this difference was not statistically significant. In terms of the subtypes, the expression scores in the gastric-type IPMNs were significantly higher than in the intestinal-type IPMNs (p = 0.001) (Table 1, Figure 2). Twelve IPMNs had an invasive component, and the CLDN18 expression was noted to have a somewhat lower staining intensity in an invasive component than in an intraductal component.
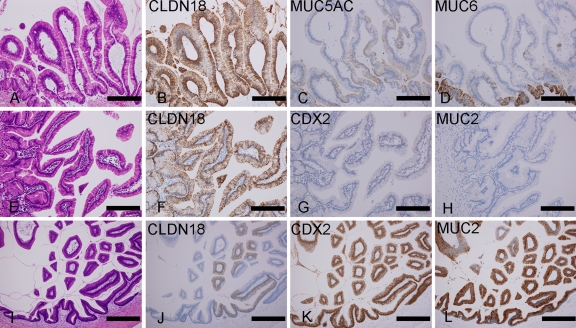
Figure 2.
CLDN18 expression in IPMN. (A-D) Gastric-type IPMN. Gastric-type IPMN shows strong immunoreactivity to CLDN18 ([A] H&E; [B] CLDN18). The expression is broader than MUC5AC (C) and MUC6 (D). (E-L) Intestinal-type IPMNs. In an intestinal-type IPMN, CLDN18 is diffusely expressed, whereas intestinal markers (CDX2 and MUC2) are only weakly expressed ([E] H&E; [F] CLDN18; [G] CDX2; [H] MUC2). Another intestinal IPMN showed diffuse expression of intestinal markers with decreased CLDN18 expression ([I] H&E; [J] CLDN18; [K] CDX2; [L] MUC2). Some fractions of the tumor cells showed simultaneous expression of CLDN18 and intestinal markers. Bar = 2.0 mm.
CLDN18 expression in MCNs
Four of the five MCNs were immunoreactive for CLDN18 (80%), with relatively high expression scores.
CLDN18 expression in PDACs
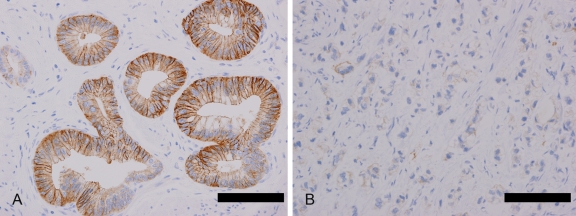
PDACs also exhibited positive immunoreactivity to CLDN18. The expression in these cases was observed with lower frequency than in the precursor lesions described above (109 of 156 cases, 69.9%), and the expression scores were lower (Table 1). However, the materials examined in these instances (TMA vs whole tissue section) may have affected the results. The expression states in the PDACs seemed to depend on the histological grade; well-differentiated and moderately differentiated tumors were immunoreactive with a higher frequency and a higher intensity than the poorly differentiated tumors (p < 0.0001) (Table 1, Figure 3).
Figure 3.
CLDN18 expression in PDAC. (A) Well-differentiated carcinomas exhibit strong CLDN18 expression. (B) Poorly differentiated carcinomas exhibit weak and focal expression. Bar = 2.0 mm.
CLDN18 Expression Versus MUC5AC and MUC6 Expression
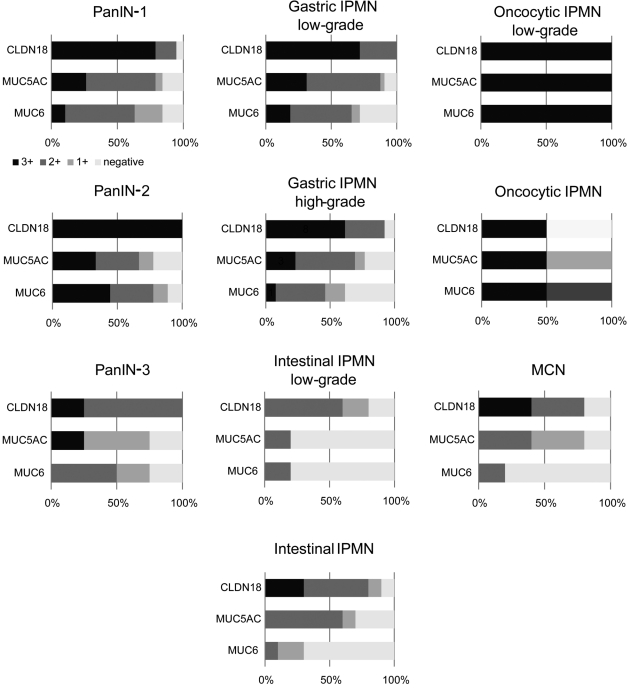
Because CLDN18 was expressed broadly in the precursor lesions, we compared the expression of CLDN18 to that of the widely used early-stage markers for pancreatic precursor lesions MUC5AC and MUC6 (Table 2, Figure 4). All three of these markers were expressed in most of the PanIN cases; however, CLDN18 expression was somewhat more frequently observed than that of MUC5AC or MUC6 (96.9%, 81.3%, and 84.4%, respectively). Moreover, CLDN18 tended to label a much wider range of tumor area than the other two markers. Similarly, more frequent and broader labeling of CLDN18, compared to MUC5AC and MUC6, was observed in the MCNs and IPMNs, especially in the gastric-type IPMNs (Figure 2A-2D). Within the examined precursor lesions, the percentages of lesions that expressed none of the CLDN18, MUC5AC, and MUC6 markers were as follows: 0% in PanIN-1, -2, and -3 and low/high-grade lesions of gastric IPMN and oncocytic IPMN, 10% in the high-grade lesions of intestinal IPMN, and 20% in the low-grade lesions of intestinal IPMN and MCN.
Table 2.
Expression of CLDN18, MUC5AC, and MUC6 in PanINs, IPMNs, and MCNs
| No. of Cases |
||||||
|---|---|---|---|---|---|---|
| Total | 3+ | 2+ to 1+ | Negative | |||
| PanIN | PanIN-1 | CLDN18 | 19 | 15 | 3 | 1 |
| MUC5AC | 19 | 5 | 11 | 3 | ||
| MUC6 | 19 | 2 | 14 | 3 | ||
| PanIN-2 | CLDN18 | 9 | 9 | 0 | 0 | |
| MUC5AC | 9 | 3 | 4 | 2 | ||
| MUC6 | 9 | 4 | 4 | 1 | ||
| PanIN-3 | CLDN18 | 4 | 1 | 3 | 0 | |
| MUC5AC | 4 | 1 | 2 | 1 | ||
| MUC6 | 4 | 0 | 3 | 1 | ||
| Gastric IPMN | Low grade | CLDN18 | 32 | 23 | 9 | 0 |
| MUC5AC | 32 | 10 | 19 | 3 | ||
| MUC6 | 32 | 6 | 17 | 9 | ||
| High grade | CLDN18 | 13 | 8 | 4 | 1 | |
| MUC5AC | 13 | 3 | 7 | 3 | ||
| MUC6 | 13 | 1 | 7 | 5 | ||
| Intestinal IPMN | Low grade | CLDN18 | 5 | 0 | 4 | 1 |
| MUC5AC | 5 | 0 | 1 | 4 | ||
| MUC6 | 5 | 0 | 1 | 4 | ||
| High grade | CLDN18 | 10 | 3 | 6 | 1 | |
| MUC5AC | 10 | 0 | 7 | 3 | ||
| MUC6 | 10 | 0 | 3 | 7 | ||
| Oncocytic IPMN | Low grade | CLDN18 | 2 | 2 | 0 | 0 |
| MUC5AC | 2 | 2 | 0 | 0 | ||
| MUC6 | 2 | 2 | 0 | 0 | ||
| High grade | CLDN18 | 2 | 1 | 1 | 0 | |
| MUC5AC | 2 | 1 | 1 | 0 | ||
| MUC6 | 2 | 1 | 0 | 1 | ||
| MCN | CLDN18 | 5 | 2 | 2 | 1 | |
| MUC5AC | 5 | 0 | 4 | 1 | ||
| MUC6 | 5 | 0 | 1 | 4 | ||
Figure 4.
Stacked bar graphs (100%) of CLDN18, MUC5AC, and MUC6 expression percentage in precancerous lesions. The horizontal axis indicates percentage. The black bar indicates strong labeling for CLDN18, MUC5AC, or MUC6. The dark gray bar shows moderate labeling, the light gray bar shows mild labeling, and the white bar shows negative labeling.
CLDN18 and Known Phenotypic Markers in IPMNs
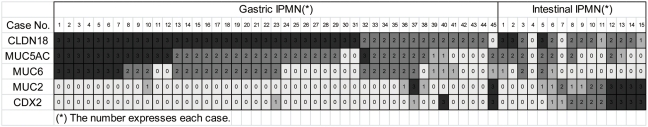
The results presented above indicate clearly that CLDN18 is a highly sensitive marker of all three types of pancreatic precursor lesion. To confirm that CLDN18 expression is associated with the gastric phenotype, we further examined the relationship between CLDN18 and the known phenotypic (gastric and intestinal) markers expressed in IPMNs (Figure 5, Table 3).
Figure 5.
Expression patterns of CLDN18 and known phenotypic markers in gastric and intestinal IPMNs. Each square represents an individual case. The number within each square is an expression score of that case. Each box is colored according to the expression score: black is 3+, dark gray is 2+, light gray is 1+, and off white is negative. Clusters with high expression scores of CLDN18, MUC5AC, and MUC6 are formed in gastric IPMNs. Clusters with high expression scores of CDX2 and MUC2 are detected in intestinal IPMNs.
Table 3.
Relationship between CLDN18 Expression and Mucin or CDX2 Expression in IPMN
| CLDN18 |
||||||
|---|---|---|---|---|---|---|
| 3+ | 2+ | 1+ | Negative | p Valuea | ||
| MUC5AC | 3+ | 15 | 1 | 0 | 0 | 0.0013 |
| 2+ | 20 | 10 | 1 | 1 | ||
| 1+ | 0 | 3 | 0 | 0 | ||
| Negative | 2 | 7 | 2 | 2 | ||
| MUC6 | 3+ | 10 | 0 | 0 | 0 | 0.01 |
| 2+ | 15 | 7 | 0 | 0 | ||
| 1+ | 1 | 4 | 0 | 1 | ||
| Negative | 11 | 10 | 3 | 2 | ||
| MUC2 | 3+ | 0 | 4 | 1 | 1 | 0.0006 |
| 2+ | 0 | 1 | 1 | 1 | ||
| 1+ | 3 | 5 | 0 | 0 | ||
| Negative | 34 | 11 | 1 | 1 | ||
| CDX2 | Positive | 0 | 7 | 2 | 1 | <0.0001 |
| Negative | 37 | 14 | 1 | 2 | ||
Correlation of expression scores of CLDN18 is calculated with those of MUC5AC, MUC6, MUC2, or CDX2 using a χ2 test.
As expected, the expression scores of CLDN18 in the IPMNs were significantly correlated with those of the gastric markers (MUC5AC and MUC6) and were inversely correlated with those of the intestinal markers (MUC2 and CDX-2). Interestingly, some of the intestinal-type IPMNs, despite the characteristic dark tumor cells and villous architecture, exhibited intense CLDN18 expression, and in these cases, intestinal markers were rarely expressed (Figures 2E-2H and 5). Intestinal markers appeared in the cases with decreased CLDN18 expression (Figures 2I-2L and 5). For the tissue sections, the tumor areas that exhibited positive immunoreactivity for the intestinal markers tended to lack CLDN18 expression, but tumor cells with simultaneous expression of CLDN18 and intestinal markers were observed frequently.
CLDN18 Expression in Pancreatic Cancer Cell Lines
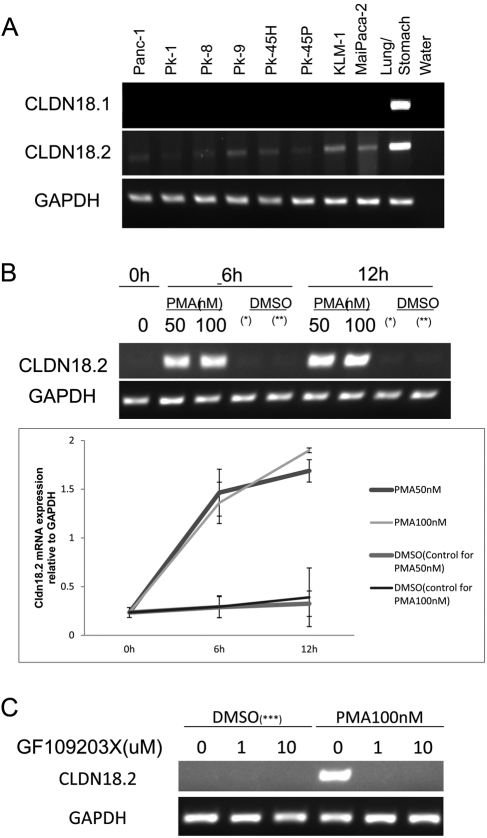
The expression of CLDN18.1 and CLDN18.2 was examined in eight pancreatic cancer cell lines: Panc-1, PK-1, PK-8, PK-9, PK-45H, PK-45P, KLM-1, and MiaPaca-2. According to RT-PCR analyses, all the cell lines expressed low to moderate levels of CLDN18.2 but were negative for CLDN18.1 (Figure 6A).
Figure 6.
(A) Analysis of CLDN18 isoforms in eight pancreatic cancer cell lines using RT-PCR. (B) Pancreatic cancer cell line PK-1 was treated with 50 nM and 100 nM PMA for different time periods. The CLDN18.2 mRNA levels were evaluated using RT-PCR. The average volumes of triplicate experiments were plotted with the respective standard deviations. GAPDH was used as the internal control. (*) The control for the sample with 50 nM PMA treatment. (**) The control for the sample with 100 nM PMA treatment. (C) Pancreatic cancer cell line PK-1 was treated with 100 nM with or without the pan-PKC inhibitor, GF109203X, for 12 hours. Each experiment was performed at least three times. (***) The control for the sample with 100 nM PMA treatment.
Subsequently, we investigated the effects of PMA treatment on CLDN18.2 expression in Panc-1 and PK-1. Our results indicate that CLDN18.2 was upregulated by PMA treatment in these cell lines, and 50 nM PMA induced robust expression of CLDN18.2 mRNA within 6 hours of administration (Figure 6B).
Subsequently, we examined whether the PKC pathway is involved in upregulation of CLDN18.2 by PMA. PK-1 was pretreated with a pan-PKC inhibitor, GF109203X (10 µM), 30 minutes before treatment of 100 nM PMA for 12 hours. Results of RT-PCR showed that even 1 µM GF109203X completely suppressed the upregulation of CLDN18.2 by PMA (Figure 6C).
Discussion
In the present study, we observed that CLDN18 was expressed ubiquitously in pancreatic ductal neoplasms, including PDACs and all three types of precursor lesions (PanINs, IPMNs, and MCNs). The expression of CLDN18 was particularly prevalent in the precursor lesions. Furthermore, CLDN18 was expressed more frequently and broadly than either MUC5AC or MUC6, which are widely used early-stage markers for pancreatic ductal neoplasms (Kim et al. 2002). Therefore, CLDN18 can serve as an exceptionally useful early-stage marker of pancreatic ductal neoplasms.
Of the two CLDN18 isoform transcripts, CLDN18.2 is a more highly selective gastric lineage marker and determines the gastric phenotype in neoplastic conditions (Sahin et al. 2008; Niimi et al. 2001; Sentani et al. 2008; Sanada et al. 2006; Yasui et al. 2009). Although the antibody used in the present immunohistochemical study does not discriminate between these two isoforms, the CLDN18 immunoreactivity in the IPMNs was correlated with the expression of known gastric markers (MUC5AC and MUC6) and was inversely correlated with the expression of the intestinal markers (MUC2 and CDX2). In addition, we confirmed the expression of CLDN18.2, but not CLDN18.1, in all of the pancreatic cancer cell lines examined using RT-PCR. These results agree with those described in several recent articles, which demonstrated an association between the gastric phenotype and early pancreatic ductal lesions (Prasad et al. 2005; Kim et al. 2002; Basturk et al. 2010).
The staining results in the IPMNs were intriguing, especially in terms of the relationship between the gastric and intestinal IPMN subtypes. Although the gastric-type and intestinal-type IPMNs have different histological and biological characteristics (Ban et al. 2006), the precise pathways of their evolution have yet to be elucidated. Some intestinal-type IPMNs in the present study, despite their characteristic dark cell morphology and villous architecture, showed immunoreactivity to CLDN18 in a diffuse manner and to MUC5AC and MUC6, albeit with lesser intensity. By contrast, immunoreactivity to the intestinal markers (MUC2 and CDX2) was rarely observed. Therefore, the intestinal markers appeared in cases with decreased CLDN18 expression, and some fractions of the tumor cells showed the simultaneous expression of CLDN18 and intestinal markers. These findings support the hypothesis that gastric-type IPMNs acquire an intestinal phenotype and progress to intestinal-type IPMNs (Ban et al. 2006).
The protein kinase C (PKC)/mitogen-activated protein kinase (MAPK)/activator protein-1 (AP-1)–dependent pathway regulates the expression of CLDN18 in gastric neoplasms (Yano et al. 2008). PMA is the most commonly used phorbol ester; it binds to and activates PKC, causing a wide range of effects in cells and tissues. In the present study, CLDN18.2 was upregulated by PMA treatment. This upregulation was repressed by pan-PKC inhibitor in pancreatic cancer cell lines. Tight junction proteins are transcriptionally controlled via a PKC signal pathway in human telomerase reverse transcriptase–transfected human pancreatic duct epithelial cells (Sanada et al. 2010). Activation of the PKC pathway is apparently involved in CLDN18.2 expression in pancreatic carcinogenesis.
The role of CLDN18 in the gastric or gastric-type epithelia is still poorly understood. In a specialized columnar epithelium of the Barrett esophagus, in which CLDN18 is reported to be the dominant claudin, CLDN18 contributes to greater acid resistance (Jovov et al. 2007). It remains unknown whether aberrant CLDN18 expression in the pancreatic ductal epithelium exerts a similar functional role under unknown stimuli toward carcinogenesis.
In conclusion, the immunohistochemical study described herein revealed the ubiquitous expression of CLDN18 in pancreatic ductal neoplasms, including PDACs and all three types of their precursor lesions (PanIN, IPMN, and MCN). CLDN18 may be used as an early-stage marker of pancreatic ductal carcinogenesis. In addition, the regulatory mechanisms of CLDN18 may highlight key pathways in pancreatic carcinogenesis for use in additional studies.
Acknowledgments
This work was supported by the Industrial Technology Research Grant Program (2008) from the New Energy and Industrial Technology Development Organization (NEDO) of Japan (SI) and a Grant-in-Aid for Scientific Research on Innovative Areas from the Ministry of Education, Culture, Sports, Science and Technology of Japan (SI).
Footnotes
The author(s) declared no potential conflicts of interest with respect to the authorship and publication of this article.
The author(s) received no financial support for the research and authorship of this article.
References
- Ban S, Naitoh Y, Mino-Kenudson M, et al. 2006. Intraductal papillary mucinous neoplasm (IPMN) of the pancreas: its histopathologic difference between 2 major types. Am J Surg Pathol. 30:1561–1569 [DOI] [PubMed] [Google Scholar]
- Basturk O, Khayyata S, Klimstra D, et al. 2010. Preferential expression of MUC6 in oncocytic and pancreatobiliary types of intraductal papillary neoplasms highlights a pylopancreatic pathway, distinct from the intestinal pathway, in pancreatic carcinogenesis. Am J Surg Pathol. 34:364–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa T, Klöppel G, Adsay VN, et al. 2005. Classification of types of intraductal papillary-mucinous neoplasm of the pancreas: a consensus study. Virchows Arch. 447:794–799 [DOI] [PubMed] [Google Scholar]
- Hamilton R, Aaltonen LA, eds. Tumors of the Digestive System. Lyon: IARC; 2000 [Google Scholar]
- Hruban RH, Pitman MB, Klimstra DS, et al. Intraductal neoplasms. In: Tumors of the Pancreas. AFIP Atlas of Tumor Pathology Series 4 Washington DC: American Registry of Pathology; 2007. p 75–110 [Google Scholar]
- Hruban RH, Takaori K, Klimstra DS, et al. 2004. An illustrated consensus on the classification of pancreatic intraepithelial neoplasia and intraductal papillary mucinous neoplasms. Am J Surg Pathol. 28:977–987 [DOI] [PubMed] [Google Scholar]
- Jovov B, Van Itallie CM, Shaheen NJ, et al. 2007. Claudin-18: a dominant tight junction protein in Barrett’s esophagus and likely contributor to its acid resistance. Am J Physiol Gastrointest Liver Physiol. 293:G1106–G1113 [DOI] [PubMed] [Google Scholar]
- Karanjawala ZE, Illei PB, Ashfaq R, et al. 2008. New markers of pancreatic cancer identified through differential gene expression analyses: claudin 18 and annexin A8. Am J Surg Pathol. 32:188–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim GE, Bae HI, Park HU, et al. 2002. Aberrant expression of MUC5AC and MUC6 gastric mucins and sialyl Tn antigen in intraepithelial neoplasms of the pancreas. Gastroenterology. 123:1052–1060 [DOI] [PubMed] [Google Scholar]
- Maitra A, Adsay NV, Argani P, et al. 2003. Multicomponent analysis of the pancreatic adenocarcinoma progression model using a pancreatic intraepithelial neoplasia tissue microarray. Mod Pathol. 16:902–912 [DOI] [PubMed] [Google Scholar]
- Maitra A, Fukushima N, Takaori K, Hruban RH. 2005. Precursors to invasive pancreatic cancer. Adv Anat Pathol. 12:81–91 [DOI] [PubMed] [Google Scholar]
- Niimi T, Nagashima K, Ward JM, et al. 2001. Claudin-18, a novel downstream target gene for the T/EBP/NK-X2.1 homeodomain transcription factor, encodes lung- and stomach-specific isoforms through alternative splicing. Mol Cell Biol. 21:7380–7390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad NB, Biankin AV, Fukushima N, et al. 2005. Gene expression profiles in pancreatic intraepithelial neoplasia reflect the effects of Hedgehog signaling on pancreatic ductal epithelial cells. Cancer Res. 65:1619–1626 [DOI] [PubMed] [Google Scholar]
- Sahin U, Koslowski M, Dhaene K, et al. 2008. Claudin-18 splice variant 2 is a pan-cancer target suitable for therapeutic antibody development. Clin Cancer Res. 14:7624–7634 [DOI] [PubMed] [Google Scholar]
- Sanada Y, Hirose Y, Osada S, et al. 2010. Immunohistochemical study of claudin 18 involvement in intestinal differentiation during the progression of intraductal papillary mucinous neoplasm. Anticancer Res. 30:2995–3003 [PubMed] [Google Scholar]
- Sanada Y, Oue N, Mitani Y, Yoshida K, Nakayama H, Yasui W. 2006. Down-regulation of the claudin-18 gene, identified through serial analysis of gene expression data analysis, in gastric cancer with an intestinal phenotype. J Pathol. 208:633–642 [DOI] [PubMed] [Google Scholar]
- Sentani K, Oue N, Tashiro T, et al. 2008. Immunohistochemical staining of Reg IV and claudin-18 is useful in the diagnosis of gastrointestinal signet ring cell carcinoma. Am J Surg Pathol. 32:1182–1189 [DOI] [PubMed] [Google Scholar]
- Tsukita S, Furuse M. 2000. Pores in the wall: claudins constitute tight junction strands containing aqueous pores. J Cell Biol. 149:13–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano K, Imaeda T, Niimi T. 2008. Transcriptional activation of the human claudin-18 gene promoter through two AP-1 motifs in PMA-stimulated MKN45 gastric cancer cells. Am J Physiol Gastrointest Liver Physiol. 294:G336–G343 [DOI] [PubMed] [Google Scholar]
- Yasui W, Oue N, Sentani K, Sakamoto N, Motoshita J. 2009. Transcriptome dissection of gastric cancer: identification of novel diagnostic and therapeutic targets from pathology specimens. Pathol Int. 59:121–136 [DOI] [PubMed] [Google Scholar]