Abstract
Chronic intense UV radiation is the main cause of epidermal tumors. Because hyaluronan (HA), a large extracellular polysaccharide, is known to promote malignant growth, hyaluronan expression was studied in a model in which long-term UV radiation (UVR) induces epidermal tumors. Mouse back skin was exposed three times a week for 10.5 months to UVR corresponding to one minimal erythema dose, processed for histology, and stained for hyaluronan and the hyaluronan receptor CD44. This exposure protocol caused epidermal hyperplasia in most of the animals; tumors, mainly squamous cell carcinomas (SCCs), were found in ~20% of the animals. Specimens exposed to UVR showed increased hyaluronan and CD44 staining throughout the epidermal tissue. In hyperplastic areas, hyaluronan and CD44 stainings correlated positively with the degree of hyperplasia. Well-differentiated SCCs showed increased hyaluronan and CD44 staining intensities, whereas poorly differentiated tumors and dysplastic epidermis showed areas where HA and CD44 were locally reduced. The findings indicate that HA and CD44 increase in epidermal keratinocytes in the premalignant hyperplasia induced by UV irradiation and stay elevated in dysplasia and SCC, suggesting that the accumulation of hyaluronan and CD44 is an early marker for malignant transformation and may be a prerequisite for tumor formation.
Keywords: epidermis, hyaluronan, CD44, squamous cell carcinoma, UV radiation
Ultraviolet radiation (UVR) is widely known for its role in carcinogenesis (Ley et al. 1989; Devary et al. 1992; Buckman et al. 1998), and it is considered the main cause of skin squamous cell carcinomas (SCCs) and basal cell carcinomas (BCCs) (Armstrong and Kricker 2001). The relative contribution of UVB and UVA to SCC development in humans is not known, but because UVA causes less direct DNA damage than UVB, it has been regarded less carcinogenic. However, both can cause SCC in mice (Sterenborg and van der Leun 1990), and UVA-induced fingerprint mutations are common in the basal cell layer of precarcinogenic skin (Agar et al. 2004).
Expression of hyaluronan, a large linear polysaccharide composed of repeating units of N-acetyl-glucosamine and glucuronic acid (Tammi et al. 2002), has been linked to tumor development because it can enhance cell proliferation and migration (Toole 2004; Tammi et al. 2008) by producing a large hydrated matrix around the cells (Knudson and Knudson 1990; Evanko et al. 1999) and by activating intracellular signaling routes through binding to its receptors (Heldin et al. 2008; Maxwell et al. 2008). Increased hyaluronan content is found either on the cancer cells or in the tumor stroma of many adenocarcinomas, like breast, ovarian, colon, or gastric cancers, and it often is associated with aggressive tumor type and poor patient prognosis (reviewed in Tammi et al. 2008). Modulation of the amount of hyaluronan in experimental tumor models has provided direct evidence for the importance of hyaluronan in cancer growth and spreading (reviewed in Itano et al. 2008). Overexpression of hyaluronan synthases increases migration, for example, in rat fibroblasts (Itano et al. 2002) and mesothelioma cells (Li and Heldin 2001), whereas their downmodulation with antisense or siRNA techniques has been associated with decreased migration, as shown in osteosarcoma cells (Nishida et al. 2005), breast cancer cells (Li et al. 2007) and zebrafish (Bakkers et al. 2004). Over-expression of Has2 or Has3 also promotes anchorage- independent growth (Kosaki et al. 1999; Zoltan-Jones et al. 2003), whereas transfection with antisense Has2 or Has3 diminishes invasion (Udabage et al. 2005), anchorage- independent growth (Bullard et al. 2003), and attachment to bone marrow endothelial cells (Simpson et al. 2002).
Carcinomas derived from stratified squamous epithelium show a biphasic hyaluronan expression pattern. In early squamous epithelial lesions, hyaluronan content is increased whereas in poorly differentiated squamous tumors, hyaluronan content is decreased. This pattern has been found in squamous cell malignancies arising from skin, mouth, larynx, and lung (Pirinen et al. 1998; Hirvikoski et al. 1999; Karvinen et al. 2003; Kosunen et al. 2004). The reduction of hyaluronan in the poorly differentiated tumors correlates with poor patient prognosis in oral SCC (Kosunen et al. 2004).
UV exposure affects skin hyaluronan both in cell culture experiments and in human and mouse skin in vivo. In a mouse model, chronic UVB and UVA exposures induce accumulation of hyaluronan in the dermal compartment (Schwartz. 1988; Margelin et al. 1996; Koshiishi et al. 1999). More recently, however, Dai and coworkers reported that long term UVB-irradiation of mice induces loss of dermal hyaluronan and downregulates the expression of the hyaluronan synthases (HAS1-3) (Dai et al. 2007). Although there is no information about the effects of long-term UVR on epidermal hyaluronan metabolism, it has been shown that single, low-dose UVB and UVA exposures caused a rapid, transient downregulation of epidermal hyaluronan content in a mouse model (Calikoglu et al. 2006). In line with this mouse model, cultured keratinocytes when exposed to UVB show reduced secretion of hyaluronan 3 hr after the exposure, whereas stimulation occurs afterward (Averbeck et al. 2007). Hyaluronan synthesis in human keratinocytes shows a biphasic dose response: low doses of UVB stimulate the secretion of HA and the expression of HAS2 and 3, whereas higher doses inhibit those (Kakizaki et al. 2008). In the latter experimental setup, however, UVA shows minor effects on hyaluronan synthesis, whereas high UVA doses slightly inhibit hyaluronan content and HAS expression (Kakizaki et al. 2008).
Because there is currently no information whether long-term, high-dose UVR influences epidermal hyaluronan and CD44 expressions, we studied them in a mouse model using histochemical stainings. Our results show that the epidermal hyperplasia caused by UVR is associated with increased signals for hyaluronan and CD44 in the epidermis and that the degree of the hyperplasia positively correlates with the hyaluronan and CD44 staining intensities. Dysplastic lesions and squamous cell carcinomas generally showed increased hyaluronan and CD44 stainings. However, occasionally an irregular staining pattern was observed, with sites of decreased staining in these lesions.
Materials and Methods
The study protocols including the UVR exposures have been described in detail previously (Kumlin et al. 1998). The mice (total 42) were female non-transgenic littermates of the line K2, developed at the University of Kuopio, and were 6–9 months old at the beginning of the experiment. Twenty-one animals were exposed to UV light and 21 control animals were exposed to sham treatment only. The animals were exposed to UVR using lamps simulating the solar spectrum (Philips HP3136). Wavelengths from 280 to 400 nm were used. During the UVR treatments, the mice were kept in cages 50 cm below the lamp, and the back skin of each mouse was shaved once a week with an electronic clipper (Aesculap Favorita II, Tuttlingen, Germany). Three exposures a week, each 35 min corresponding to one human minimum erythema dose (MED), were performed during the whole period of 10.5 months. Sham treatment for control animals was performed using similar exposure system with no light applied.
Histological Specimens
Skin samples from the exposed animals were fixed in 10% buffered formalin, embedded in paraffin, and sectioned 5 µm thick. The sections were stained with hematoxylin and eosin for the evaluation of tissue morphology and for hyaluronan and CD44, as described below. One representative biopsy was analyzed from each mouse.
Hyaluronan Staining
The sections were rehydrated in descending xylene–ethanol series and incubated with 3% H2O2 for 5 min to block endogenous peroxidases; then they were incubated with 1% BSA in 0.1 M Na-phosphate buffer (PB), pH 7.0, for 30 min at 37C to block unspecific binding of the probe, followed by overnight incubation at 4C with 3 µg/ml biotinylated hyaluronan binding complex (bHABC), isolated from bovine articular cartilage and containing the biotinylated complex of link protein and G1 domain of aggrecan (Tammi et al. 1994).
After washes with PB, the sections were incubated with avidin–biotin peroxidase (Vector Laboratories, Irvine, CA, 1:200) for 1 hr. The color was developed with 0.05% 3,3′-diaminobenzidine (DAB; Sigma, St. Louis, MO) containing 0.03% H2O2. The sections were counterstained with Mayer’s hematoxylin for 2 min, washed, dehydrated, and mounted in DePex (BDH Laboratory Supplies, Poole, UK). To control the specificity of the staining, hyaluronan was removed by preincubating the sections with Streptomyces hyaluronidase (Seikagaku, Kogyo, Tokyo, Japan), or the bHABC probe was blocked with HA oligosaccharides (Tammi et al. 1994).
CD44 Staining
Specimens were fixed and processed as described above. After rehydration, the sections were incubated for 30 min at 95C in antigen retrieval solution (Dako, Carpentaria, CA). After blocking the endogenous peroxidase activity and unspecific binding as described above, the sections were incubated with an anti-CD44 antibody recognizing all CD44 splice variants (1:100 IM7, a generous gift from Dr. Jayne Lesley, San Diego, CA) overnight at 4C and then with biotinylated anti-rat secondary antibody (Vector Laboratories, 1:100) for 1 hr at room temperature. Avidin–biotin peroxidase and DAB treatments were carried out as described above. Control sections were stained similarly, but omitting the primary antibody.
HAS Stainings
The deparaffinized sections were incubated in 10 mM citrate buffer, pH 6.0, for 5 min in a pressure cooker at 120C, washed with PB, and treated for 5 min with 1% H2O2 to block endogenous peroxidase activity. Thereafter the sections were incubated in 1% bovine serum albumin (BSA) and 0.1% gelatin (Sigma G-2500, Sigma, MO) in PB for 30 min to block nonspecific binding. The sections were incubated overnight at 4C with polyclonal antibodies (2 µg/ml dilution in 1% BSA, Santa Cruz Biotechnology, Santa Cruz, CA) for HAS1 (sc-34021), HAS2 (sc-34067), and HAS3 (sc-34204) followed by 1 hr incubation with biotinylated anti-goat antibody (1:1000, Vector Laboratories). The bound antibodies were visualized with the avidin–biotin peroxidase method, as described above. After washes, the sections were counterstained with Mayer’s hematoxylin for 1 min, washed, dehydrated, and mounted in DPX. Treatment of primary antibodies with corresponding peptides (sc-34021 P for HAS1, sc-34067 P for HAS2, and sc-34204 P for HAS3; Santa Cruz Biotechnology) was used as control.
Evaluation of the Hyaluronan and CD44 Stainings
The stainings were analyzed blind regarding the experimental group. General morphological features of the sections were analyzed and scored for epidermal hyperplasia (no hyperplasia, mild, moderate, strong hyperplasia). In addition, the sections were scored for the presence of tumors, dysplasia, and squamous cell carcinomas. The area of HA and CD44 stainings in the epidermis was estimated with a four-level scoring from 0 to 3. Score 0 was given when no staining or only minimal staining around 1–2 hair follicles was detected. Score 1 was given when less than 33% of the interfollicular area was stained, score 2 when 33–66% of the interfollicular area was stained, and score 3 when more than 66% of the interfollicular area was stained. The intensity of epidermal staining was estimated with a four-level scoring from 0 to 3. The homogeneity of the staining was recorded as well as the intensity of dermal staining and the staining of skin areas showing abnormal morphology.
Statistical Analysis
The statistical calculations were performed using the SPSS program for MacIntosh (version 11.0, SPSS, Chicago, IL). The Kruskal-Wallis and Mann-Whitney U-tests were used to compare the extent and intensity of the stainings between treatments. The amount of epidermal hyperplasia was correlated to the HA and CD44 parameters using Kendall’s test. Probability values less than 0.05 were considered significant.
Results
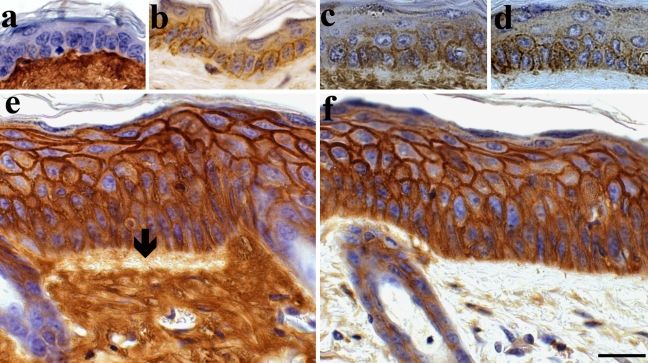
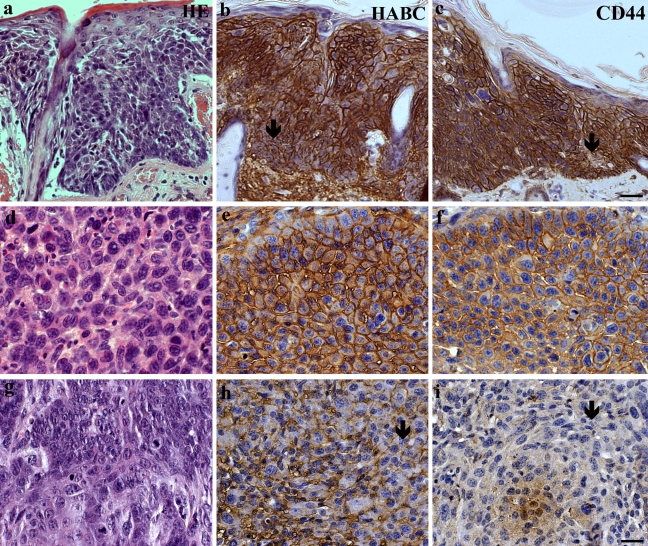
In the present work, mouse back skin was exposed to 1 MED of UVR three times a week for 10.5 months. Histological evaluation indicated that in most of the treated animals the epidermal tissue showed marked, approximately 3–4-fold thickening (Fig. 1, e and f) compared with control skin (Fig. 1, a and b, Table 1). One fifth of the UVR-exposed animals developed squamous cell carcinomas (SCC, Fig. 2, d–i, and Table 1), and one third showed dysplastic changes (Fig. 2, a–c). Only one of the control animals showed mild hyperplasia, whereas dysplasia or SCC was not detected in any of the control animals (Table 1).
Figure 1.
Hyaluronan and CD44 in skin following chronic UV-irradiation. Specimens from shaved mouse back were stained for hyaluronan (a, c, e) and CD44 (b, d, f). (a, b) Control mouse. (c–f) Mouse exposed to UVR for 10.5 months, three times per week. Dermal connective tissue is positive for hyaluronan in all specimens, but there is a loss of dermal hyaluronan staining below the basement membrane in samples showing epidermal hyperplasia (arrow in e). Epidermal tissue in control skin is negative for hyaluronan (a) and shows faint CD44-positive staining (b). Both mildly (c and d) and strongly (e and f) hyperplastic epidermal areas of UVR epidermis show strong bHABR and CD44-positive staining that covers most of the interfollicular epidermis and is localized in all living cell layers from basal up to the granular cell layer. Magnification bar 20 µm.
Table 1.
Hyperplasia, Dysplasia, and Squamous Cell Carcinoma Following Long-Term UVR
| Hyperplasia, n (%) |
|||||
|---|---|---|---|---|---|
| Group | n | Mild | Moderate/Strong | Dysplasia, n (%) | SCC, n (%) |
| Control | 21 | 1 (5%) | 0 | 0 | 0 |
| UVR | 21 | 4 (19%) | 12 (57%) | 6 (29%) | 4 (19%) |
SCC, squamous cell carcinoma; UVR, UVR treated.
Figure 2.
Hyaluronan and CD44 in dysplastic skin and SCC induced by UVR. (a-c) Dysplastic skin. (d-i) SCC. Dermal connective tissue in all specimens contains cells with a strong positive staining. Hyaluronan and CD44 stainings form a net-like pattern around the epithelial cells. The intensities of the stainings are generally moderate or strong, but in dysplastic (a–c) and some of the SCC (g–i) the staining is inhomogeneous with local loss of hyaluronan and CD44 stainings (arrows in b, c, h, and i). Magnification bar 40 µm in c for a–c and 20 µm in i for d–i.
Hyaluronan and CD44 Stainings
In normal, untreated skin, dermal connective tissue was intensely stained for hyaluronan, whereas most of the epidermis either was negative (Fig. 1a) or showed a weak positive signal around the orifices of hair follicles (Table 1), as previously described in mouse ear and tail (Tammi et al. 2005). Although CD44 immunostaining in control skin was generally weak, some CD44-positive cells were found throughout the whole interfollicular epidermis, in both the basal and suprabasal layers (Fig. 1b, Table 2).
Table 2.
Scoring of Epidermal and Dermal Coverage for Hyaluronan and CD44 and Their Staining Intensities
| Coverage |
Intensity |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Group | 0 | 1 | 2 | 3 | p | Weak | Moderate | Strong | p |
| Epidermis | |||||||||
| Hyaluronan | |||||||||
| Control | 19 | 0 | 1 | 1 | 2 | 0 | 0 | ||
| UVR | 3 | 0 | 0 | 18 | a | 7 | 11 | 1 | a |
| CD44 | |||||||||
| Control | 1 | 1 | 3 | 16 | 14 | 5 | 1 | ||
| UVR | 0 | 0 | 0 | 20 | b | 3 | 13 | 4 | a |
| Dermis | |||||||||
| Hyaluronan | |||||||||
| Control | 0 | 20 | 0 | ||||||
| UVR | 1 | 18 | 2 | NS | |||||
| CD44 | |||||||||
| Control | 3 | 18 | 0 | ||||||
| UVR | 0 | 18 | 3 | NS | |||||
NS, not significant; UVR, UVR treated.
p<0.001, b p<0.05, when compared with control group (Mann-Whitney U-test).
Coverage: 0 = no staining or only local staining; 1 = <33% of the interfollicular area positive; 2 = 33–66% of the interfollicular area positive; 3 = >66% of the interfollicular area positive. Staining intensity was scored into 3 grades: weak, moderate, or strong. The values represent numbers of samples scored into each class.
In UVR-treated animals, hyaluronan-positive cells covered more than two thirds of the epidermal area in most of the specimens (Table 2), and the intensity of epidermal hyaluronan staining was significantly increased compared with untreated epidermis (Table 2, Fig. 1 and 2). CD44 immunostaining also showed significantly increased epidermal staining intensity and coverage in UVR-treated animals (Figs. 1 and 2, Table 2). Epidermal areas showing benign hyperplasia showed moderate or strong hyaluronan and CD44 stainings, even in the case of moderate hyperplasia (Fig. 1, c and d); however, the number of positive layers was increased when the degree of hyperplasia increased (Fig. 1, e and f), with all vital cell layers except granular cells being positive for hyaluronan and CD44. Epidermal hyperplasia showed a significant positive correlation with both intensity and coverage of hyaluronan staining (Kendall’s τ-b test, p<0.01). Similar correlation was also observed between epidermal hyperplasia and CD44 staining intensity and coverage (Kendall’s τ-b test, p<0.01 and 0.05, respectively).
Epidermal areas containing dysplastic cells were generally moderately or intensely positive for hyaluronan and CD44; however, they often contained patches with reduced staining or even no staining at all (Fig. 2, b and c). Similarly, invasive squamous cell carcinomas showed generally moderate or strong hyaluronan and CD44 stainings (Fig. 2, e and f), but patches of lower staining intensity made the appearance inhomogeneous in some of the tumors (Fig. 2, h and i).
Dermal connective tissue stained for hyaluronan in all samples. In semiquantitative scoring of dermal hyaluronan staining intensity, all samples in control group were graded as moderately stained, whereas in the UVR group 1 of 21 was graded as weakly stained, 2 were graded as strongly stained, and the remaining were graded as moderate (NS). However, in UVR-treated animals showing epidermal hyperplasia, there was a loss of subepidermal hyaluronan staining in 69% of the hyperplastic samples (Fig. 1e).
HAS Immunostainings
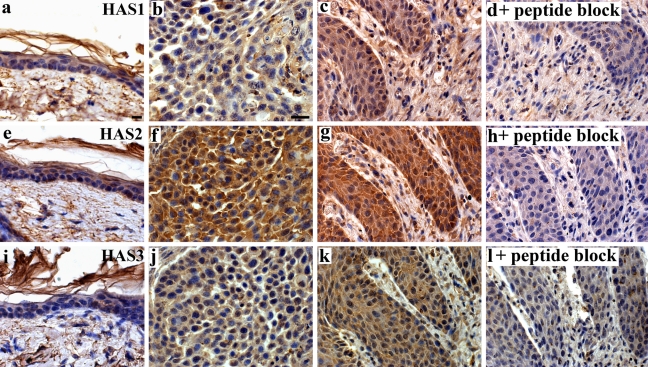
We performed immunostainings with antibodies raised against hyaluronan synthase enzymes 1, 2, and 3 (Fig. 3). In the control skin that was not exposed to UVB (Fig. 3, a, e and i) just a few epidermal cells showed HAS-immunoreactivity above the level of diffuse, low-intensity background signal. The dermal cells stained weakly or with moderate intensity for HAS1, HAS2, and HAS3 (Fig. 3, a, e and i). The staining intensity in specimens exposed to the UVR was clearly higher with all HAS antibodies, in both epidermis and dermis (Fig. 3, b, c, f, g, j and k). Strongest induction of staining intensity was found with the HAS2 antibody (Fig. 3, f and g). The increased HAS immunostainings were seen both in hyperplastic areas (Fig. 3, c, g and k) and in the squamous cell carcinomas (Fig. 3, b, f and j). Fig. 3, d, h and l shows specimens stained with HAS-antibodies preincubated with peptides used for immunization. Most of the staining was lost, indicating the specificity of the signal. The retrieval at high temperature required for the exposure of the epitopes (Rilla et al, unpublished) tended to tear the fragile mouse skin sections and precluded HAS immunostainings of sufficient quality for comprehensive scoring of all specimens.
Figure 3.
Hyaluronan synthases (HAS1-3) in UV-treated epidermis. Specimens from mouse back skin were stained with antibodies against HAS1 (a–d), HAS2 (e–h), and HAS3 (i–l). In d, h, and l, the antibodies were preincubated with the peptides used in the immunization. All HAS antibodies gave a low-level signal in control, unirradiated epidermis, whereas dermal cells showed a stronger immunostaining (a, e, i). In UV-treated skin, hyperplastic areas (c, g, k) and SCCs (b, f, j) keratinocytes and fibroblasts showed increased staining intensity for all HASes compared with control skin. Magnification bar 20 µm (in a for a, e, i and in b for b–d, f–h, and j–l).
Discussion
The present study shows for the first time that chronic UV irradiation of skin leads to the accumulation of hyaluronan and its receptor CD44 in the epidermis and that this accumulation correlates with the development of epidermal hyperplasia.
Although the effect of chronic UVR on GAG and hyaluronan metabolism in the dermal compartment has been studied previously (Schwartz 1988; Margelin et al. 1996; Koshiishi et al. 1999; Dai et al. 2007), none of the previous publications has reported changes in the epidermal compartment. The reports concerning the response of epidermal cells to acute UVR have been contradictory (Calikoglu et al. 2006; Averbeck et al. 2007; Kakizaki et al. 2008), probably because of differences in the dose and UV source.
The single dose used in the present work (20 mJ/cm2 corresponding one minimum erythema dose) is comparable to the doses that have been reported to stimulate hyaluronan synthesis in in vitro experiments (Averbeck et al. 2007; Kakizaki et al. 2008). However, the doses cannot be directly compared, because under in vitro conditions epidermal cells lack the natural protective mechanisms, e.g., melanosomes and stratum corneum, and are therefore more sensitive to the UVR. The UV source used in the present work simulated solar irradiation, containing a broad spectrum of wavelengths from 310 to 400 nm (Kumlin et al. 1998). The main part (98%) of the physical dose is comprised of UVA; however, about 70% of the biologically effective (erythema-inducing) irradiation is expected to come from the UVB wavelength. UVA may either be ineffective in stimulating hyaluronan synthesis (Kakizaki et al. 2008) or even inhibit it when used at high doses (Calikoglu et al. 2006). Although in the present experiment we cannot differentiate between the effects of UVB and UVA on hyaluronan metabolism, our findings indicate that in a chronic setting, UVR resembling solar irradiation at intensities causing skin erythema (1 MED) causes a dramatic change in epidermal hyaluronan metabolism.
In the present material, the increase in HAS-immunostaining intensities in the UVR exposed epidermis suggests that the increased hyaluronan content in UVR-treated epidermis is at least partly explained by increased HAS activity. All HAS isoforms showed increased immunostaining in UVR exposed skin. In line with the previous cell culture experiments (Averbeck et al. 2007), HAS2 showed highest increase due to UVR. However, possible differences in the sensitivity of the antibodies preclude direct conclusions concerning HAS2 as the major target. UVR is known to activate several signaling routes, but there are no comprehensive data showing which of the signals are involved in the UV-induced upregulation of hyaluronan synthesis and HAS expression (Averbeck et al. 2007; Kakizaki et al. 2008). The acute phase upregulation of HAS2 and HAS3 was inhibited by blocking IL-1β, suggesting that an inflammatory pathway is involved (Kakizaki et al. 2008). Chronic UVB has been reported to cause enhanced activation of pathways associated with EGFR and ErbB2, PI3K and AKT, JNK, NFkB, and Stat3 (Sano et al. 2005; Katiyar and Meeran. 2007; Wunderlich et al. 2008), all known to influence HAS2 or HAS3 expression (Pienimäki et al. 2001; Saavalainen et al. 2005; Madson and Hansen. 2007; Han et al. 2008).
The strongly elevated level of CD44 is likely to contribute to the increased epidermal content of hyaluronan by binding and immobilizing hyaluronan on the plasma membranes of keratinocytes. The close correlation between CD44 and hyaluronan staining patterns observed in the present work and in previous publications (Pirinen et al. 1998; Hirvikoski et al. 1999; Karvinen et al. 2003; Kosunen et al. 2004) supports this conclusion. However, acute high-dose UVB and UVA irradiation was reported to cause depletion of CD44 (Calikoglu et al. 2006), and our own data in cultured keratinocytes support this finding (Rauhala et al. unpublished), indicating that the CD44 response to UVR is biphasic. Mutations of p53 are typically found in epidermal keratinocytes exposed to solar radiation very early, before the actual tumor (de Gruijl and Rebel. 2008; Klein et al. 2010), and cause upregulation of CD44 expression (Godar et al. 2008), suggesting a plausible mechanism for the increased CD44 levels in our model. The p53-induced effect of CD44 on cell growth and survival was mediated by EGFR (Godar et al. 2008). In our material the epidermal hyperplasia strongly correlated with the levels of epidermal CD44 and hyaluronan. Likewise, epidermal hyperplasia during wound healing (Tammi et al. 2005) and in psoriasis (Tammi et al. 1994) shows increased CD44 and hyaluronan content. Furthermore, lack of CD44 inhibits the capacity of mouse epidermis to respond to various growth-promoting signals (Kaya et al. 1997). Hyaluronan binding to CD44 has been shown to modulate and enhance signaling of a number of cell surface growth factor receptors including EGFR (Kim et al. 2008) and ErbB2 (Bourguignon et al. 2007; Misra et al. 2008). Therefore, both CD44 and hyaluronan may be required for epidermal activation and hyperplasia, whether it occurs in injury, inflammation, or development of SCC. Hyaluronan accumulation in the epidermis may also be a protective response against UVR because it can bind and inactivate reactive oxygen species. High molecular mass hyaluronan has been shown to decrease apoptosis and inflammation of corneal keratinocytes exposed to UVR (Pauloin et al. 2009).
Earlier reports concerning the effect of chronic UVR on dermal hyaluronan have been contradictory, ranging from hyaluronan accumulation (Schwartz. 1988; Margelin et al. 1996; Koshiishi et al. 1999) to its depletion (Dai et al. 2007). In our material, based on the histochemical analysis of hyaluronan, we observed a loss of subepidermal hyaluronan staining in most of the hyperplastic samples. The reason for these contradictory results may come from differences in the exposure regimens or wavelengths used, perhaps having differential effects on reactive oxygen species and enzymes that control hyaluronan synthesis and catabolism, all influenced by UVR (Rees et al. 2004; Averbeck et al. 2007).
In conclusion, long-term chronic UVR treatment of mouse skin, simulating excessive sunlight, causes accumulation of hyaluronan around keratinocytes in the hyperplastic and SCC lesions probably by activated expression of both hyaluronan synthesizing enzymes and the cell surface receptor CD44, reproducing the pattern in the premalignant and malignant human SCCs (Wang et al. 1996; Pirinen et al. 1998; Karvinen et al. 2003). This indicates that hyaluronan and CD44 augmentations are inherent features of the early phases of SCC, suggesting that they contribute to the development of these malignancies.
Acknowledgments
The authors appreciate Kari Kotikumpu and Eija Rahunen for expert technical assistance and preparation of the histological samples.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the authorship and publication of this article.
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants from The Finnish Medical Foundation (HS), Finnish Cultural Foundation (HS), Paavo Koistinen’s Foundation (KT), Finnish Cancer Foundation (RT), Juselius Foundation (RT, MT) and EVO funds of Kuopio University Hospital (MT).
References
- Agar NS, Halliday GM, Barnetson RS, Ananthaswamy HN, Wheeler M, Jones AM. 2004. The basal layer in human squamous tumors harbors more UVA than UVB fingerprint mutations: a role for UVA in human skin carcinogenesis. Proc Natl Acad Sci U S A. 101:4954–4959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong BK, Kricker A. 2001. The epidemiology of UV induced skin cancer. J Photochem Photobiol B. 63:8–18 [DOI] [PubMed] [Google Scholar]
- Averbeck M, Gebhardt CA, Voigt S, Beilharz S, Anderegg U, Termeer CC, Sleeman JP, Simon JC. 2007. Differential regulation of hyaluronan metabolism in the epidermal and dermal compartments of human skin by UVB irradiation. J Invest Dermatol. 127:687–697 [DOI] [PubMed] [Google Scholar]
- Bakkers J, Kramer C, Pothof J, Quaedvlieg NE, Spaink HP, Hammerschmidt M. 2004. Has2 is required upstream of Rac1 to govern dorsal migration of lateral cells during zebrafish gastrulation. Development. 131:525–537 [DOI] [PubMed] [Google Scholar]
- Bourguignon LY, Gilad E, Peyrollier K. 2007. Heregulin-mediated ErbB2-ERK signaling activates hyaluronan synthases leading to CD44-dependent ovarian tumor cell growth and migration. J Biol Chem. 282:19426–19441 [DOI] [PubMed] [Google Scholar]
- Buckman SY, Gresham A, Hale P, Hruza G, Anast J, Masferrer J, Pentland AP. 1998. COX-2 expression is induced by UVB exposure in human skin: implications for the development of skin cancer. Carcinogenesis. 19:723–729 [DOI] [PubMed] [Google Scholar]
- Bullard KM, Kim HR, Wheeler MA, Wilson CM, Neudauer CL, Simpson MA, McCarthy JB. 2003. Hyaluronan synthase-3 is upregulated in metastatic colon carcinoma cells and manipulation of expression alters matrix retention and cellular growth. Int J Cancer. 107:739–746 [DOI] [PubMed] [Google Scholar]
- Calikoglu E, Sorg O, Tran C, Grand D, Carraux P, Saurat JH, Kaya G. 2006. UVA and UVB decrease the expression of CD44 and hyaluronate in mouse epidermis, which is counteracted by topical retinoids. Photochem Photobiol. 82:1342–1347 [DOI] [PubMed] [Google Scholar]
- Dai G, Freudenberger T, Zipper P, Melchior A, Grether-Beck S, Rabausch B, de Groot J, Twarock S, Hanenberg H, Homey B, Krutmann J, et al. 2007. Chronic ultraviolet B irradiation causes loss of hyaluronic acid from mouse dermis because of down-regulation of hyaluronic acid synthases. Am J Pathol. 171:1451–1461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Gruijl FR, Rebel H. 2008. Early events in UV carcinogenesis—DNA damage, target cells and mutant p53 foci. Photochem Photobiol. 84:382–387 [DOI] [PubMed] [Google Scholar]
- Devary Y, Gottlieb RA, Smeal T, Karin M. 1992. The mammalian ultraviolet response is triggered by activation of Src tyrosine kinases. Cell. 71:1081–1091 [DOI] [PubMed] [Google Scholar]
- Evanko SP, Angello JC, Wight TN. 1999. Formation of hyaluronan- and versican-rich pericellular matrix is required for proliferation and migration of vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 19:1004–1013 [DOI] [PubMed] [Google Scholar]
- Godar S, Ince TA, Bell GW, Feldser D, Donaher JL, Bergh J, Liu A, Miu K, Watnick RS, Reinhardt F, et al. 2008. Growth-inhibitory and tumor- suppressive functions of p53 depend on its repression of CD44 expression. Cell. 134:62–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han CY, Lim SC, Choi HS, Kang KW. 2008. Induction of ErbB2 by ultraviolet A irradiation: potential role in malignant transformation of keratinocytes. Cancer Sci. 99:502–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldin P, Karousou E, Bernert B, Porsch H, Nishitsuka K, Skandalis SS. 2008. Importance of hyaluronan-CD44 inter-actions in inflammation and tumorigenesis. Connect Tissue Res. 49:215–218 [DOI] [PubMed] [Google Scholar]
- Hirvikoski P, Tammi R, Kumpulainen E, Virtaniemi J, Parkkinen JJ, Tammi M, Johansson R, Ågren U, Karhunen J, et al. 1999. Irregular expression of hyaluronan and its CD44 receptor is associated with metastatic phenotype in laryngeal squamous cell carcinoma. Virchows Arch. 434:37–44 [DOI] [PubMed] [Google Scholar]
- Itano N, Atsumi F, Sawai T, Yamada Y, Miyaishi O, Senga T, Hamaguchi M, Kimata K. 2002. Abnormal accumulation of hyaluronan matrix diminishes contact inhibition of cell growth and promotes cell migration. Proc Natl Acad Sci U S A. 99:3609–3614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itano N, Zhuo L, Kimata K. 2008. Impact of the hyaluronan-rich tumor microenvironment on cancer initiation and progression. Cancer Sci. 99:1720–1725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakizaki I, Itano N, Kimata K, Hanada K, Kon A, Yamaguchi M, Takahashi T, Takagaki K. 2008. Up-regulation of hyaluronan synthase genes in cultured human epidermal keratinocytes by UVB irradiation. Arch Biochem Biophys. 471:85–93 [DOI] [PubMed] [Google Scholar]
- Karvinen S, Kosma VM, Tammi MI, Tammi R. 2003. Hyaluronan, CD44 and versican in epidermal keratinocyte tumours. Br J Dermatol. 148:86–94 [DOI] [PubMed] [Google Scholar]
- Katiyar SK, Meeran SM. 2007. Obesity increases the risk of UV radiation-induced oxidative stress and activation of MAPK and NF-kappaB signaling. Free Radic Biol Med. 42:299–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaya G, Rodriguez I, Jorcano JL, Vassalli P, Stamenkovic I. 1997. Selective suppression of CD44 in keratinocytes of mice bearing an antisense CD44 transgene driven by a tissue-specific promoter disrupts hyaluronate metabolism in the skin and impairs keratinocyte proliferation. Genes Dev. 11:996–1007 [DOI] [PubMed] [Google Scholar]
- Kim Y, Lee YS, Choe J, Lee H, Kim YM, Jeoung D. 2008. CD44-epidermal growth factor receptor interaction mediates hyaluronic acid-promoted cell motility by activating protein kinase C signaling involving Akt, Rac1, Phox, reactive oxygen species, focal adhesion kinase, and MMP-2. J Biol Chem. 283:22513–22528 [DOI] [PubMed] [Google Scholar]
- Klein AM, Brash DE, Jones PH, Simons BD. 2010. Stochastic fate of p53-mutant epidermal progenitor cells is tilted toward proliferation by UV B during preneoplasia. Proc Natl Acad Sci U S A. 107:270–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudson CB, Knudson W. 1990. Similar epithelial-stromal interactions in the regulation of hyaluronate production during limb morphogenesis and tumor invasion. Cancer Lett. 52:113–122 [DOI] [PubMed] [Google Scholar]
- Kosaki R, Watanabe K, Yamaguchi Y. 1999. Overproduction of hyaluronan by expression of the hyaluronan synthase Has2 enhances anchorage-independent growth and tumorigenicity. Cancer Res. 59:1141–1145 [PubMed] [Google Scholar]
- Koshiishi I, Horikoshi E, Mitani H, Imanari T. 1999. Quantitative alterations of hyaluronan and dermatan sulfate in the hairless mouse dorsal skin exposed to chronic UV irradiation. Biochim Biophys Acta. 1428:327–333 [DOI] [PubMed] [Google Scholar]
- Kosunen A, Ropponen K, Kellokoski J, Pukkila M, Virtaniemi J, Valtonen H, Kumpulainen E, Johansson R, Tammi R, Tammi M, et al. 2004. Reduced expression of hyaluronan is a strong indicator of poor survival in oral squamous cell carcinoma. Oral Oncol. 40:257–263 [DOI] [PubMed] [Google Scholar]
- Kumlin T, Kosma VM, Alhonen L, Janne J, Komulainen H, Lang S, Rytomaa T, Servomaa K, Juutilainen J. 1998. Effects of 50 Hz magnetic fields on UV-induced skin tumourigenesis in ODC-transgenic and non-transgenic mice. Int J Radiat Biol. 73:113–121 [DOI] [PubMed] [Google Scholar]
- Ley RD, Applegate LA, Padilla RS, Stuart TD. 1989. Ultraviolet radiation–induced malignant melanoma in Monodelphis domestica. Photochem Photobiol 50:1–5 [DOI] [PubMed] [Google Scholar]
- Li Y, Heldin P. 2001. Hyaluronan production increases the malignant properties of mesothelioma cells. Br J Cancer. 85:600–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Li L, Brown TJ, Heldin P. 2007. Silencing of hyaluronan synthase 2 suppresses the malignant phenotype of invasive breast cancer cells. Int J Cancer. 120:2557–2567 [DOI] [PubMed] [Google Scholar]
- Madson JG, Hansen LA. 2007. Multiple mechanisms of Erbb2 action after ultraviolet irradiation of the skin. Mol Carcinog. 46:624–628 [DOI] [PubMed] [Google Scholar]
- Margelin D, Medaisko C, Lombard D, Picard J, Fourtanier A. 1996. Hyaluronic acid and dermatan sulfate are selectively stimulated by retinoic acid in irradiated and nonirradiated hairless mouse skin. J Invest Dermatol. 106:505–509 [DOI] [PubMed] [Google Scholar]
- Maxwell CA, McCarthy J, Turley E. 2008. Cell-surface and mitotic-spindle RHAMM: moonlighting or dual oncogenic functions? J Cell Sci. 121:925–932 [DOI] [PubMed] [Google Scholar]
- Misra S, Obeid LM, Hannun YA, Minamisawa S, Berger FG, Markwald RR, Toole BP, Ghatak S. 2008. Hyaluronan constitutively regulates activation of COX-2-mediated cell survival activity in intestinal epithelial and colon carcinoma cells. J Biol Chem. 283:14335–14344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida Y, Knudson W, Knudson CB, Ishiguro N. 2005. Antisense inhibition of hyaluronan synthase-2 in human osteosarcoma cells inhibits hyaluronan retention and tumorigenicity. Exp Cell Res. 307:194–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauloin T, Dutot M, Joly F, Warnet JM, Rat P. 2009. High molecular weight hyaluronan decreases UVB-induced apoptosis and inflammation in human epithelial corneal cells. Mol Vis. 15:577–583 [PMC free article] [PubMed] [Google Scholar]
- Pienimäki JP, Rilla K, Fulop C, Sironen RK, Karvinen S, Pasonen S, Lammi MJ, Tammi R, Hascall VC, Tammi MI. 2001. Epidermal growth factor activates hyaluronan synthase 2 in epidermal keratinocytes and increases pericellular and intracellular hyaluronan. J Biol Chem. 276:20428–20435 [DOI] [PubMed] [Google Scholar]
- Pirinen RT, Tammi RH, Tammi MI, Pääkko PK, Parkkinen JJ, Ågren UM, Johansson RT, Viren MM, Törmänen U, Soini YM, et al. 1998. Expression of hyaluronan in normal and dysplastic bronchial epithelium and in squamous cell carcinoma of the lung. Int J Cancer. 79:251–255 [DOI] [PubMed] [Google Scholar]
- Rees MD, Hawkins CL, Davies MJ. 2004. Hypochlorite and superoxide radicals can act synergistically to induce fragmentation of hyaluronan and chondroitin sulphates. Biochem J. 381:175–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saavalainen K, Pasonen-Seppänen S, Dunlop TW, Tammi R, Tammi MI, Carlberg C. 2005. The human hyaluronan synthase 2 gene is a primary retinoic acid and epidermal growth factor responding gene. J Biol Chem. 280:14636–14644 [DOI] [PubMed] [Google Scholar]
- Sano S, Chan KS, Kira M, Kataoka K, Takagi S, Tarutani M, Itami S, Kiguchi K, Yokoi M, Sugasawa K, et al. 2005. Signal transducer and activator of transcription 3 is a key regulator of keratinocyte survival and proliferation following UV irradiation. Cancer Res. 65:5720–5729 [DOI] [PubMed] [Google Scholar]
- Schwartz E. 1988. Connective tissue alterations in the skin of ultraviolet irradiated hairless mice. J Invest Dermatol 91:158–161 [DOI] [PubMed] [Google Scholar]
- Simpson MA, Wilson CM, Furcht LT, Spicer AP, Oegema TR, McCarthy JB. 2002. Manipulation of hyaluronan synthase expression in prostate adenocarcinoma cells alters pericellular matrix retention and adhesion to bone marrow endothelial cells. J Biol Chem. 277:10050–10057 [DOI] [PubMed] [Google Scholar]
- Sterenborg HJ, van der Leun JC. 1990. Tumorigenesis by a long wavelength UV-A source. Photochem Photobiol. 51:325–330 [DOI] [PubMed] [Google Scholar]
- Tammi MI, Day AJ, Turley EA. 2002. Hyaluronan and homeostasis: a balancing act. J Biol Chem. 277:4581–4584 [DOI] [PubMed] [Google Scholar]
- Tammi R, Ågren UM, Tuhkanen AL, Tammi M. 1994. Hyaluronan metabolism in skin. Prog Histochem Cytochem. 29:1–81 [DOI] [PubMed] [Google Scholar]
- Tammi R, Pasonen-Seppänen S, Kolehmainen E, Tammi M. 2005. Hyaluronan synthase induction and hyaluronan accumulation in mouse epidermis following skin injury. J Invest Dermatol. 124:898–905 [DOI] [PubMed] [Google Scholar]
- Tammi RH, Kultti A, Kosma VM, Pirinen R, Auvinen P, Tammi MI. 2008. Hyaluronan in human tumors: pathobiological and prognostic messages from cell-associated and stromal hyaluronan. Semin Cancer Biol. 18:288–295 [DOI] [PubMed] [Google Scholar]
- Toole BP. 2004. Hyaluronan: from extracellular glue to pericellular cue. Nat Rev Cancer. 4:528–539 [DOI] [PubMed] [Google Scholar]
- Udabage L, Brownlee GR, Waltham M, Blick T, Walker EC, Heldin P, Nilsson SK, Thompson EW, Brown TJ. 2005. Antisense-mediated suppression of hyaluronan synthase 2 inhibits the tumorigenesis and progression of breast cancer. Cancer Res. 65:6139–6150 [DOI] [PubMed] [Google Scholar]
- Wang C, Tammi M, Guo H, Tammi R. 1996. Hyaluronan distribution in the normal epithelium of esophagus, stomach, and colon and their cancers. Am J Pathol. 148:1861–1869 [PMC free article] [PubMed] [Google Scholar]
- Wunderlich L, Paragh G, Wikonkal NM, Banhegyi G, Karpati S, Mandl J. 2008. UVB induces a biphasic response of HIF-1alpha in cultured human keratinocytes. Exp Dermatol. 17:335–342 [DOI] [PubMed] [Google Scholar]
- Zoltan-Jones A, Huang L, Ghatak S, Toole BP. 2003. Elevated hyaluronan production induces mesenchymal and transformed properties in epithelial cells. J Biol Chem. 278:45801–45810 [DOI] [PubMed] [Google Scholar]