Abstract
The members of the claudin family are major integral transmembrane protein constituents of tight junctions. Normal and neoplastic tissues can be characterized by unique qualitative and quantitative distribution of claudin subtypes, which may be related to clinicopathological features. Differential diagnosis and prognosis of nonmuscle invasive tumor entities of urinary bladder epithelium are often challenging. The aim was to investigate the expression profile of claudins in inverted urothelial papillomas (IUPs), urothelial papillomas (UPs), papillary urothelial neoplasms of low malignant potential (PUNLMPs), and intraepithelial (Ta), low-grade urothelial cell carcinomas (LG-UCCs) in order to reveal potential prognostic and differential diagnostic values of certain claudins. Claudin-1, -2, -4, and -7 protein expressions detected by immunohistochemistry and clinical data were analyzed in 15 IUPs, 20 UPs, 20 PUNLMPs, and 20 LG-UCCs. UPs, PUNLMPs, and LG-UCCs showed significantly decreased claudin-1 expression in comparison to IUPs. LG-UCCs expressing claudin-4 over the median were associated with significantly shorter recurrence-free survival. PUNLMPs expressing claudin-1 over the median revealed significantly longer recurrence-free survival. High claudin-1 protein expression might help to differentiate IUP from UPs, PUNLMPs, and LG-UCCs. High claudin-4 expression may determine an unfavorable clinical course of LG-UCCs, while high claudin-1 expression in PUNLMP was associated with markedly better clinical outcome.
Keywords: inverted urothelial papilloma, urothelial cell cancer, low grade, PUNLMP, urothelial papilloma, claudin
The appropriate pathology report of noninvasive urothelial neoplasms of the human urinary bladder epithelium is challenging. Differential diagnosis of noninvasive tumor entities such as urothelial papilloma (UP), inverted urothelial papilloma (IUP), urothelial carcinoma with inverted growth pattern (UCIGP), papillary neoplasm of low malignant potential (PUNLMP), and low-grade urothelial carcinoma (LG-UCC) is based mainly on H&E morphology and often presents serious differential diagnostic problems for pathologists. Moreover, sampling techniques and haphazard orientation of tissue fragments may lead to difficulties in correct interpretation concerning histological subtype, grading, and staging. Distinction between UP and PUNLMP or PUNLMP and LG-UCC, and further, IUP and LG-UCC in certain cases, might be extremely challenging. On the other hand, patients with identical clinicopathological tumor parameters (grade and stage) may show basically different clinical courses of the disease (Montironi et al. 2008). Patients with intraepithelial, noninvasive Ta-stage UCCs can also suffer from a relapse of the disease. Many pathology surveys report that in a relatively high percentage of cases, interobserver or intraobserver variabilities exist in regard to diagnosis (Samaratunga et al. 2002; Boireau et al. 2007; Eiber et al. 2007). The pathology report, however, basically determines postoperative clinical management including treatment and surveillance strategies. This urges the search for new markers to increase the specificity and reliability of pathological diagnosis. So far, there is not a single marker at hand that could overcome the predictive value of routine histological analysis. Therefore, new prognostic and predictive markers are needed to identify the group of those patients who would need more aggressive treatment to provide longer recurrence-free and/or overall survival.
The importance of tight junction morphology in the maintenance of integrity of uroepithelium and further in the prevention of recurrence of UCC was recognized quite a long time ago (Stravoravdi et al. 1996). However, the molecular composition of tight junction, including the expression of claudins, has been the focus of studies since previous years. Claudins are integral transmembrane proteins of tight junctions with 24 known members in human tissues. The expression profile of various claudin types is organ and tissue specific and has been investigated by many working groups. The possible role of claudins in human tumorigenesis is strongly suspected. Multiple pieces of evidence proved that the molecular composition of tight junctions changes in several pathological conditions, especially during tumorigenesis. Aberrant expression correlates with tissue of origin, type of cancer, and even with invasiveness and survival (Awsare et al. 2007; Forster 2008; Martin and Jiang 2009; Ouban and Ahmed 2010). The claudin profile characterizes different tumor entities; therefore, it might be utilized for differential diagnostic purposes (Lodi et al. 2006; Sobel et al. 2006; Paschoud et al. 2007; Sheehan et al. 2007; Nemeth et al. 2009). Moreover, data on claudin expression profile in different premalignant and malignant alterations suggest that claudins might serve not only as diagnostic but also as prognostic markers (Lechpammer et al. 2008). Claudins might also dock therapeutic molecules and at the same time be primary targets of future therapeutic modalities (Saeki et al. 2010). There are only few data available on the claudin expression profiles of various urothelial neoplasms of the human urinary bladder (Boireau et al. 2007).
IUP of the human urinary bladder is a relatively rare occurrence. IUPs are almost invariably characterized by benign biological behavior, supposing that the pathological diagnosis was achieved by means of strict morphological criteria (Sung et al. 2006b). However, this statement has been challenged because in rare cases, IUPs coexist with malignant urothelial neoplasms (Cheville et al. 2000). Molecular analyses also confirmed that IUPs arise on the basis of genetic changes, which are strikingly different from those of high-grade/high-stage urothelial neoplasms (Sung et al. 2006a; Lott et al. 2009). Therefore, distinction between IUP and inverted growth pattern of LG-UCC is essential. Moreover, diagnosing IUP might present difficulties because it may share morphological characteristics with UCIGP and might mimic LG-UCC as well. Morphological separation of UP, PUNLMP, and LG-UCC might also pose severe problems for the pathologist because neither morphological descriptions nor microphotographs in specialized handbooks provide substantial help in difficult cases.
It is well documented that noninvasive urinary bladder tumors have markedly better prognosis compared with patients with invasive cancers. Nonetheless, within this more favorable patient group—low-grade, noninvasive carcinomas—certain patients still have much shorter recurrence-free survival period often associated with decreased overall survival.
Proliferation marker Ki-67, cytokeratin-5/6 (CK-5/6), and cytokeratin-20 (CK-20) are expressed both in the normal and tumorous urothelium. CK-5/6 is localized in the basal and CK-20 in the superficial layers of normal urothelium, while Ki-67 nuclear positivity might be found in every layer (Cheville et al. 2000; Castillo-Martin et al. 2010). Abnormal CK-20 expression (negative or more than 10% positivity) and increased Ki-67 expression are associated with higher grade and stage of UCCs (Jones et al. 2007; van Oers et al. 2007). Dysregulation of these proteins might support the differential diagnosis, but the data are insufficient regarding well-differentiated, noninvasive urothelial neoplasms (van Oers et al. 2007; Bryan et al. 2010).
In the present work, our aim was to characterize the claudin expression profile and localization of claudins in UP, IUP, PUNLMP, and noninvasive LG-UCC cases as well as in independent normal urothelium. Moreover, besides studying the potential differential diagnostic value of claudins, we investigated whether differences in claudin expression bear with prognostic value regarding nonmuscle invasive urothelial neoplasms.
Materials and Methods
The claudin expression profile and clinical data of 80 transurethral resection specimens were analyzed: 15 IUPs, 20 UPs, 20 PUNLMPs, 20 LG-UCCs, and 5 independent normal samples. None of the control patients had any neoplastic urinary bladder lesions earlier. All investigations were performed under the permission of the Regional Ethical Committee of Semmelweis University.
A total of 30 IUPs were diagnosed in our institute between April 1994 and December 2010. Three cases were associated with simultaneous UCC. The clinical data of these samples were analyzed and published previously by our group (Riesz et al. 2010). After the IUP cases were reviewed by a board of pathologists (ES,TS,JT,ZS,AKiss) according to the updated classification of WHO (2004), 15 formalin-fixed, paraffin-embedded (FFPE) blocks proved to be suitable for immunohistochemical analysis. The mean follow-up period was 59.79 months (range = 3–126). The mean age of patients was 60.8 years (range = 9–91). The male/female ratio was 1.5:1 (IUPs = 2.75/1). Recurrence-free survival (RFS) was determined from initial diagnosis to the time of recurrence.
Methods
Tissue microarrays (TMAs) were created from 15 IUP samples, 20 UP samples, 20 PUNLMPs, and 20 LG-UCC cases. From each case, two core pieces 2 mm in diameter were taken for TMA blocks. Three randomly selected cases of each tumor group and all of the normal samples were analyzed in full-mount sections of FFPE tissue blocks. Immunohistochemical reactions were scored and measured by morphometry as well on both TMA and full-mount sections. There were no relevant differences between the TMA and full-mount immunohistochemistry results.
Histology and Immunohistochemistry
Tissues were fixed in 4% neutral buffered formalin for 24 hours. Diagnosis was established using H&E-stained slides cut from paraffin-embedded blocks. Paraffin-embedded, 3- to 4-µm-thick sections were used for immunohistochemistry. Reactions were carried out in BenchMark XT automatic immunostainer using multimer technology and diaminobenzidine as chromogen, with reagents registered according to the manufacturer’s protocol (Ultra View Universal DAB detection kit; Ventana, Tucson, AZ). Antibodies, dilutions, and positive controls recommended by the manufacturers are listed in Table 1. The appropriate antibody was omitted for negative controls, and only the antibody diluent was applied. The reactions revealed no signals.
Table 1.
Antibody Dilutions and Positive Controls
| Antibody | Dilution | Positive Control | Manufacturer | Antibody Type | Lot No. |
|---|---|---|---|---|---|
| CLDN-1 | 1:100 | Skin | Invitrogen | Rabbit polyclonal | 624568A |
| CLDN-2 | 1:50 | Colon | Invitrogen | Mouse monoclonal | 787052A |
| CLDN-3 | 1:80 | Colon | Invitrogen | Rabbit polyclonal | 760684A |
| CLDN-4 | 1:200 | Colon | Invitrogen | Mouse monoclonal | 716863A |
| CLDN-7 | 1:100 | Breast | Invitrogen | Rabbit polyclonal | 673366A |
| Ki-67 | 1:100 | Tonsil | DAKO | mouse monoclonal | 00070375 |
| CK-5/6 | 1:2000 | Tonsil | DAKO | Mouse monoclonal | 00033034 |
| CK-20 | 1:600 | Colon adenocarcinoma | DAKO | Mouse monoclonal | 00032659 |
Note: Ki-67 = kinase inhibitor 67; CK-5/6 = cytokeratin-5/6; CK-20 = cytokeratin-20; Invitrogen (Carlsbad, CA); DAKO (Glostrup, Denmark).
Evaluation of Immunohistochemistry
Evaluation was performed by quantitative and semiquantitative methods as well. Four independent examiners (ES,PT,TS,AKiss) evaluated the reactions to register proper quality and tissue localization of the immunohistochemical reaction. Score values for semiquantitative analysis were calculated for each reaction: intensity multiplied with area positivity. Intensity scores were 0 for negative, 1 for weak, 2 for moderate, and 3 for strong immunohistochemical reaction. Area positivity scores were as follows: 1 (0%–5%), 2 (6%-25%), 3 (26%-50%), 4 (51%-75%), and 5 (76%-100%). For quantitative analysis, slides were digitalized using Mirax Midi slide-scanner system (3D Histech, Budapest, Hungary). Fifteen nonoverlapping, independent microphotographs (400x) were taken from each case, and then area positivity was evaluated quantitatively using Leica QWin V3 morphometrical software (Leica Microsystems Imaging Solutions Ltd., Cambridge, UK) for claudins, CK-5/6, and CK-20. The nonepithelial areas were excluded from the morphometrical analysis. Positive cells/total cells were calculated for Ki-67 evaluation from each slide. The mean values of each case were used for statistical analysis.
Statistics
All statistical analyses were performed using Statistica V8.0 software (StatSoft Inc., Tulsa, OK). After normality testing, nonparametric ANOVA test (Kruskal-Wallis) was used to examine the differences between normal, IUP, UP, PUNLMP, and LG-UCC cases because samples did not completely follow normal distribution. Kaplan-Meier method was used to analyze RFS. For Kaplan-Meier analysis, tumors of each entity were divided into two groups according to claudin expression: one group contained cases expressing claudin, CK-5/6, CK-20, or Ki-67 over the median (high expression), while the other group expressed claudin, CK-5/6, CK-20, or Ki-67 under the median (low expression). Comparison between survival parameters for different strata was assessed with log-rank statistics. To test the correlation between the result of scoring analysis and morphometry, Spearman rank correlation was used. p < 0.05 values were considered as significant.
Results
Immunohistochemistry
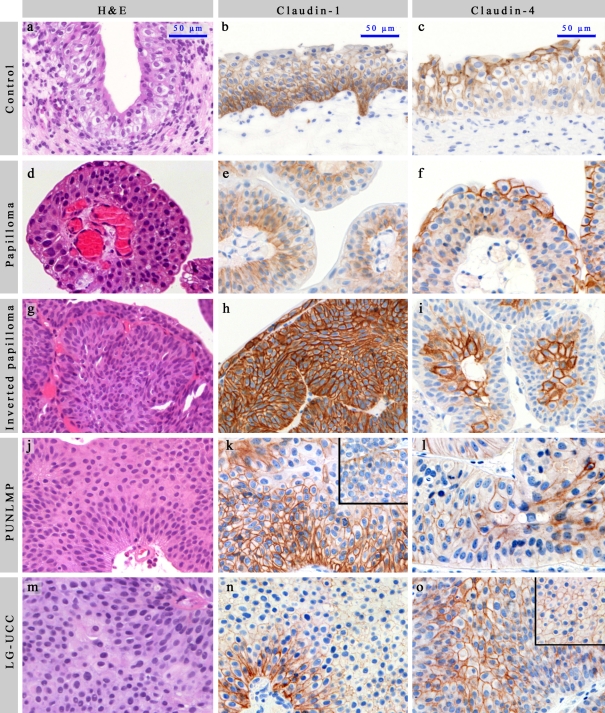
The epithelial expression pattern and intraepithelial distribution of individual claudins in normal, UP, IUP, PUNLMP, and LG-UCC cases were similar to previously published data of normal urothelium (Varley et al. 2006; Boireau et al. 2007; Nakanishi et al. 2008). In certain IUP samples, it was rather difficult to assess the basal or superficial orientation in H&E sections (Figure 1G). It is noteworthy, therefore, that the standard intraepithelial differences in claudin distribution aided the orientation of the small-sized tissue fragments. Claudin-1 showed membranous reaction in the basal layers, mainly at the basal surface of cells having connection with the connective tissue, while upper layers showed no staining (Figure 1B, 1E, 1K, 1N). Claudin-2 revealed perimembranous and cytoplasmic granular reaction, with the reaction being stronger in the basal/parabasal layers. In some cases, the umbrella cells also showed positivity. Claudin-3 revealed only weak scattered expression, mainly at the membrane of the umbrella cells. Claudin-4 positivity was detected in the upper layers, diminishing towards the basal layers (Figure 1C, 1F, 1I, 1L, 1O). Claudin-7 positivity was weak and membranous, detectable in a similar localization as claudin-4.
Figure 1.
Each photograph (including inserts) was taken with standard adjustments. Scale bar: 50 µm. (A, D, G, J, M) H&E in normal, UP, IUP, PUNLMP, and LG-UCC, respectively. (B, E, H, K, N) Claudin-1 in normal, UP, IUP, PUNLMP, and LG-UCC, respectively. (C, F, I, L, O) Claudin-4 in normal, UP, IUP, PUNLMP, and LG-UCC, respectively. LG-UCC expressing claudin-4 over the median (O) and under the median (insert of O). PUNLMP expressing claudin-1 over the median (K) and under the median (insert of K). UP = urothelial papilloma; IUP = inverted urothelial papilloma; PUNLMP = papillary urothelial neoplasm of low malignant potential; LG-UCC = low-grade urothelial cell carcinoma. Only linear adjustments of brightness/contrast and color balance were applied for whole images in order to create uniform-looking pictures for the composite image.
In some cases of PUNLMPs and LG-UCCs, only claudin-1 and -4 were observed in the whole extent of the epithelium. On the other hand, however, in 73% of IUP cases (11/15), claudin-1 positivity appeared in the whole depth of the tumor tissue (Figure 1H). Claudin-1 and -2 expressions were lower in LG-UCCs in comparison to UPs, IUPs, and PUNLMPs. However, only IUPs revealed significantly higher claudin-1 expression when compared with UPs, PUNLMPs, and LG-UCCs upon both morphometrical and scoring analyses (Table 2; Figure 2A and 2B). Claudin-3, -4, and -7 expressions did not show major differences between analyzed groups. None of the cases showed marked claudin-3 expression (data not shown).
Table 2.
Results of morphometry and scoring analysis
| Stain | Group | Mean | Standard Error | Kruskal-Wallis Testp Values | Versus UPp Values | Versus IUPp Values | Versus PUNLMPp Values | Versus LG-UCCp Values |
|---|---|---|---|---|---|---|---|---|
| Results of morphometry | ||||||||
| Claudin-1 | Normal | 13.45 | 1.82 | 0.001* | 1.000 | 0.040* | 1.000 | 1.000 |
| UP | 20.21 | 2.09 | 0.006* | 1.000 | 1.000 | |||
| IUP | 34.95 | 3.74 | 0.047* | 0.002* | ||||
| PUNLMP | 22.74 | 3.14 | 1.000 | |||||
| LG-UCC | 18.65 | 3.46 | ||||||
| Claudin-2 | Normal | 0.44 | 0.15 | 0.104 | 1.000 | 1.000 | 1.000 | 1.000 |
| UP | 1.27 | 0.29 | 1.000 | 1.000 | 0.057 | |||
| IUP | 1.21 | 0.56 | 1.000 | 1.000 | ||||
| PUNLMP | 0.81 | 0.29 | 1.000 | |||||
| LG-UCC | 0.33 | 0.08 | ||||||
| Claudin-4 | Normal | 2.56 | 1.20 | 0.631 | 1.000 | 1.000 | 1.000 | 1.000 |
| UP | 4.77 | 0.77 | 1.000 | 1.000 | 1.000 | |||
| IUP | 3.92 | 1.02 | 1.000 | 1.000 | ||||
| PUNLMP | 4.11 | 0.51 | 1.000 | |||||
| LG-UCC | 4.03 | 0.66 | ||||||
| Claudin-7 | Normal | 0.284 | 0.094 | 0.162 | 1.000 | 1.000 | 1.000 | 0.620 |
| UP | 0.15 | 0.06 | 1.000 | 1.000 | 1.000 | |||
| IUP | 0.24 | 0.10 | 1.000 | 1.000 | ||||
| PUNLMP | 0.21 | 0.09 | 0.720 | |||||
| LG-UCC | 0.07 | 0.05 | ||||||
| Results of scoring analysis | ||||||||
| Claudin-1 | Normal | 6.00 | 1.155 | 0.002* | 1.000 | 0.047* | 1.000 | 1.000 |
| UP | 8.68 | 0.75 | 0.039* | 1.000 | 1.000 | |||
| IUP | 11.63 | 0.97 | 0.027* | 0.006* | ||||
| PUNLMP | 8.82 | 0.85 | 1.000 | |||||
| LG-UCC | 7.50 | 0.98 | ||||||
| Claudin-2 | Normal | 2.4 | 0.65 | 0.567 | 1.000 | 1.000 | 1.000 | 1.000 |
| UP | 3.52 | 0.59 | 1.000 | 1.000 | 1.000 | |||
| IUP | 3.36 | 0.76 | 1.000 | 1.000 | ||||
| PUNLMP | 3.83 | 0.73 | 1.000 | |||||
| LG-UCC | 2.79 | 0.52 | ||||||
| Claudin-4 | Normal | 3.00 | 0.58 | 0.459 | 1.000 | 1.000 | 1.000 | 1.000 |
| UP | 3.75 | 0.49 | 1.000 | 1.000 | 1.000 | |||
| IUP | 4.27 | 0.73 | 1.000 | 1.000 | ||||
| PUNLMP | 3.62 | 0.47 | 1.000 | |||||
| LG-UCC | 4.89 | 0.62 | ||||||
| Claudin-7 | Normal | 1.67 | 0.33 | 0.096 | 1.000 | 1.000 | 1.000 | 0.164 |
| UP | 0.83 | 0.26 | 1.000 | 1.000 | 1.000 | |||
| IUP | 1.33 | 0.66 | 1.000 | 1.000 | ||||
| PUNLMP | 0.59 | 0.17 | 0.651 | |||||
| LG-UCC | 0.37 | 0.15 | ||||||
| Results of Ki-67 analysis | ||||||||
| Ki-67 | Normal | 2.10 | 1.05 | <0.001* | 1.000 | 1.000 | 0.323 | <0.001* |
| UP | 3.27 | 0.41 | 1.000 | 0.038* | <0.001* | |||
| IUP | 5.37 | 1.55 | 0.223 | <0.001* | ||||
| PUNLMP | 9.27 | 1.87 | 0.621 | |||||
| LG-UCC | 17.88 | 2.87 | ||||||
Note: Mean and standard error values are listed together with comparisons of the protein expression between normals, UPs, IUPs, PUNLMPs, and LG-UCCs. Horizontal groups were compared with vertical groups using the Kruskal-Wallis test. Significant changes (p < 0.05) are marked with an asterisk (*). UP = urothelial papilloma; IUP = inverted urothelial papilloma; PUNLMP = papillary urothelial neoplasm of low malignant potential; LG-UCC = low-grade urothelial cell carcinoma.
Figure 2.
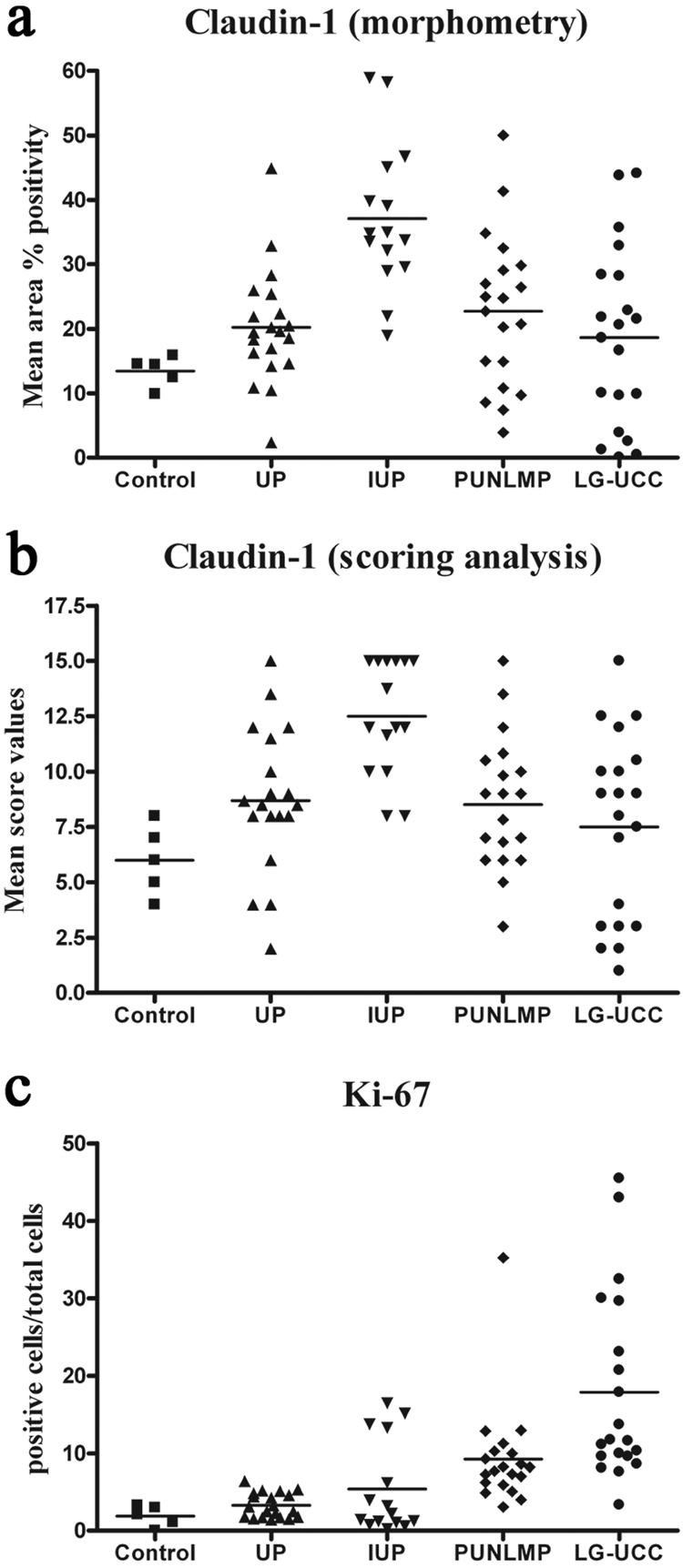
Dot plot of claudin-1 morphometry (A), scoring analysis (B), and Ki-67 (C). IUPs revealed significantly higher claudin-1 expression in comparison to the other investigated groups. LG-UCCs revealed significantly higher Ki-67 expression when compared with normals, UPs, and IUPs. PUNLMPs showed significantly higher Ki-67 expression in comparison to UPs. UP = urothelial papilloma; IUP = inverted urothelial papilloma; PUNLMP = papillary urothelial neoplasm of low malignant potential; LG-UCC = low-grade urothelial cell carcinoma.
CK-5/6 expression was detected in the basal and CK-20 expression in the superficial layers of the investigated entities in concordance with the literature (Eiber et al. 2007; Castillo-Martin et al. 2010). Dysregulated CK-20 expression was generally manifested in increased number of positive cells situated not only in the superficial layers but also in the intermediate layers of LG-UCC. PUNLMPs were characterized by significantly lower CK-20 expression in comparison to normal urothelium and LG-UCCs. Otherwise, there were no measured differences between the groups (data not shown).
LG-UCCs revealed significantly higher Ki-67 expression when compared with normals, UPs, and IUPs (Table 2; Figure 2C). Morphometry altogether correlated significantly with the results of scoring (Spearman rank correlation, r = 0.75).
Follow-up Analysis
The mean follow-up period was 59.79 months (range = 3–126). One patient died of nonurological disease. All samples, except 2 PUNLMPs, were primary tumors. A total of 20 LG-UCC cases and 20 PUNLMPs, 18 UPs, and 15 IUPs were suitable for follow-up analysis. Recurrence appeared in 16 cases (10/20 LG-UCCs, 6/20 PUNLMPs); the mean RFS was 21.43 months (range = 4–60) in these cases. IUPs and UPs did not recur.
Two LG-UCC patients received one local mitomycin C (MMC) instillation within 6 hours after surgery. Thirteen patients received 6 MMC (2 PUNLMPs, 11 LG-UCCs), while 5 patients received 6 farmorubicin (2 PUNLMPs, 3 LG-UCCs) therapy treatments after the first operation. In case of UPs and IUPs, chemotherapy was not administered. There was no significant association between recurrence and chemotherapy. However, patients who received postoperative local instillation had longer RFS in comparison to patients who did not (22 months vs 16 months).
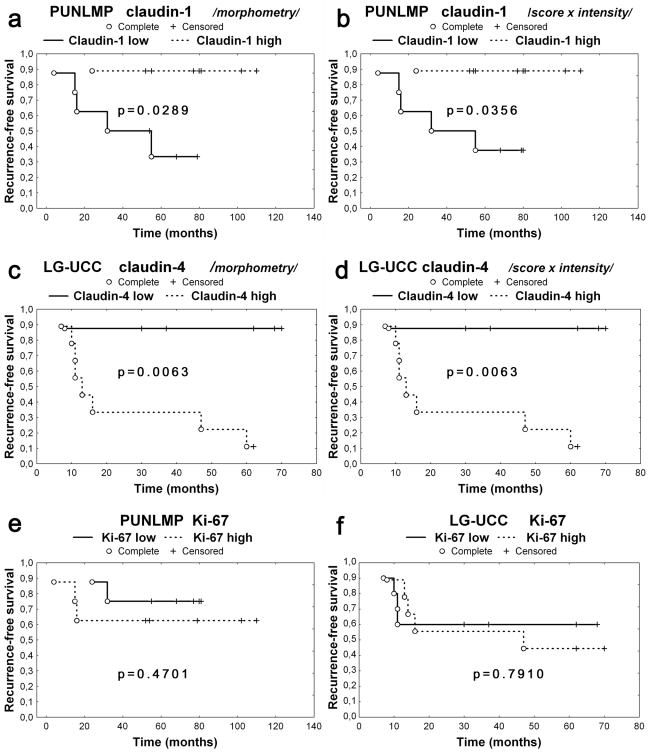
PUNLMPs showing decreased claudin-1 expression (under the median) revealed significantly shorter RFS in comparison to PUNLMPs highly expressing claudin-1 (over the median) in Kaplan-Meier analysis (log-rank test, p = 0.0289) (Figure 3A and 3B). Cases of LG-UCCs highly expressing claudin-4 (over the median) showed significantly shorter RFS as compared with other noninvasive UCCs expressing claudin-4 under the median (log-rank test, Kaplan-Meier analysis, p = 0.0063) (Figure 3C and 3D). RFS showed no significant association with expressions of claudin-2 and -7, CK-5/6, CK-20, or Ki-67 (Figure 3E and 3F).
Figure 3.
LG-UCCs and PUNLMPs were divided into two groups for RFS analysis according to claudin expression over the median and under the median. PUNLMPs expressing claudin-1 under the median showed significantly shorter RFS compared with PUNLMPs expressing claudin-1 over the median (Kaplan-Meier analysis; [A] morphometry, p = 0.0289; [B] scoring analysis, p = 0.0356). LG-UCCs expressing claudin-4 over the median revealed significantly shorter RFS compared with LG-UCCs expressing claudin-4 under the median (Kaplan-Meier analysis, p = 0.0063; [C] morphometry; [D] scoring analysis). RFS did not show association with Ki-67 expression being under or above the median (Kaplan-Meier analysis; [E] PUNLMP, p = 0.4701; [F] LG-UCC, p = 0.7910). Complete event = recurrence occurred; censored event = follow-up was terminated either because patient died or follow-up period ended; RFS = recurrence-free survival; UP = urothelial papilloma; IUP = inverted urothelial papilloma; PUNLMP = papillary urothelial neoplasm of low malignant potential; LG-UCC = low-grade urothelial cell carcinoma.
Discussion
Similarly to earlier observations (Nakanishi et al. 2008), the distribution of claudins showed urothelium-specific topographical distribution. Claudin-1 expression was typically found in the basal and intermediate layers, while claudins-3, -4, and -7 were detected mainly in the upper layers of the urothelium. This expression profile in case of claudin-4 and -7 was found to be especially marked in the UP, PUNLMP, and LG-UCC cases. Claudin-3 expression, on the other hand, was scattered and weak in our set of samples. Nakanishi et al. (2008) found weak claudin-3 expression in superficial UCCs of the upper urinary tract: 16 cases were negative (33%, 16/49), and 31 cases showed weak reaction (<10% positivity; 63%, 31/49), whereas only two superficial UCCs (4%, 2/49) revealed more than 10% positivity of claudin-3. The topographical difference between parabasal and superficial layers regarding claudin-1, -4, and -7 expressions can help the orientation of tumorous cell nests in crowded tumor tissues. Further, claudins could support definition of whether haphazard orientation of crowded stalks gives the impression of increased epithelial thickness or rather that the lesion is really composed of thickened, multilayered neoplastic epithelium.
Despite the fact that all investigated groups of urothelial neoplasias were noninvasive, the LG-UCCs revealed significantly decreased claudin-1 and significantly increased Ki-67 expressions when compared with the IUPs. These features could be possible aids for pathologists in establishing differential diagnosis of difficult cases. Such a decision has clinical relevance because primary IUPs are not treated after surgical resection, whereas LG-UCC patients receive postoperative treatment. Moreover, it would be crucial to determine which patient would need more aggressive treatment, resulting in more effective medical intervention and increased survival. Eiber et al. (2007) described significantly decreased Ki-67 expression in IUPs in comparison to urothelial carcinoma with inverted growth pattern, but in the expression of CK-20, there was no difference between the two groups. Ki-67 nuclear positivity was higher in LG-UCCs than in PUNLMPs; however, the difference was not significant.
Analysis of CK-5/6 did not reveal significant differences between the groups. CK-5/6 was found to be expressed in the basal layers, while CK-20 was detected in the upper layers of normal and tumorous urothelium. The literature also acknowledges the loss or gain of expression of CK-20 and CK-5/6 in UCCs (van Oers et al. 2007; Kaufmann et al. 2001). However, lack of expression of CK-20 and Ki-67 was also reported in IUP (Jones et al. 2007). In fact, in our data sets as well, very high and very low expression was found in all investigated tumor groups. Higher CK-20 expression might help to separate LG-UCCs from PUNLMPs. Otherwise, it would be very difficult to draw a general conclusion, which could help establish the diagnosis in questionable cases.
Our findings of decreased claudin-1 and -2 expressions in LG-UCCs in comparison to UPs, IUPs, and PUNLMPs strengthen the notion that altered tight junction composition characterizes the process of carcinogenesis and tumor progression. However, only lower claudin-1 expression of LG-UCCs compared to IUPs proved to be statistically significant. Therefore, an unequivocal and general conclusion cannot be made on decreased expression of claudins in LG-UCCs. Decreased claudin-1 expression found in carcinomas in comparison to normal tissues characterizes several malignant tumor entities, including breast cancer (Tokes et al. 2005), colon cancer (Resnick et al. 2005), and prostate cancer (Krajewska et al. 2007). Others, however, found that claudin-1 is strongly associated with colon carcinogenesis and is overexpressed in colorectal carcinoma (Kinugasa et al. 2007; Huo et al. 2009; Mees et al. 2009). On the other hand, Nakanishi et al. (2008) observed increased claudin-1, -3, and -4 expressions in advanced stages of UCC of the upper urinary tract associated with poor survival. Our finding of high claudin-4 expression in low-grade, noninvasive UCCs with worse clinical course and shorter RFS altogether supports the association between high claudin-4 expression and more advanced stage of urinary carcinogenesis and progression. This finding suggests that the claudin expression profile might be used to predict the clinical behavior of UCCs. Further, novel in vitro data suggest that the claudin-4 expressing tumor cells might be a therapeutic target for genetically engineered cytotoxic fusion proteins recognizing claudin-4 as a docker molecule (Saeki et al. 2009). An in vivo animal model study demonstrated that this fusion protein can even antagonize tumor metastasis formation of claudin-4 presenting tumor cells (Saeki et al. 2010). We could not find any significant association between the expressions of claudin-1, claudin-7, CK-5/6, CK-20, and Ki-67 in urothelial tumors (whether benign or malignant) and RFS. Interestingly, inverse correlation was detected between moderate/strong claudin-3 and -4 expressions and overall survival in clear-cell renal cell carcinomas (Lechpammer et al. 2008). Similarly, expression of claudin-4 correlated with advanced-stage prostate adenocarcinomas, while decreased claudin-1 expression independently predicted disease recurrence in multivariate analysis (Sheehan et al. 2007). On the contrary, Boireau et al. (2007) found decreased claudin-4 expression in invasive and high-grade UCCs by immunohistochemical and mRNA analyses, whereas superficial, low-grade UCCs did not show this phenomenon. The observation regarding altered claudin-4 expression, however, was made by comparing UCCs with their surrounding nontumorous uroepithelium. This evaluation setup might be influenced by field carcinogenesis and the lack of comparison with independent normal urinary bladder urothelium samples (Jones et al. 2005).
Conclusion
High claudin-1 protein expression might help to differentiate IUP from UPs, PUNLMPs, and LG-UCCs. High claudin-4 expression in case of low-grade papillary urothelial cancer, whereas low claudin-1 expression in case of PUNLMPs, might determine poor clinical outcome, while these expressional changes are associated with shorter RFS. On the contrary, low claudin-1 expression of PUNLMPs and high claudin-4 expression of LG-UCCs are associated with a markedly better clinical outcome. These features might assist the selection of patients who need more detailed patient follow-up and more aggressive treatment modalities.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the authorship and publication of this article.
This work was supported by the grant of the Hungarian Scientific Research Fund (OTKA)# 75468, the grant of Jedlik Anyos National R&D Fund (NKFT) # 07-A1/2007 and the grant of the Department of Health Scientific Council (ETT) # 252/2009.
References
- Awsare N, Martin T, Haynes M, Watkins G, Matthews P, Jiang W. 2007. The expression of tight junction proteins claudins 11 and 15 in normal human urothelium and transitional cell carcinoma. BJU Int. 99:46–4717092289 [Google Scholar]
- Boireau S, Buchert M, Samuel MS, Pannequin J, Ryan JL, Choquet A, Chapuis H, Rebillard X, Avances C, Ernst M, et al. 2007. DNA-methylation-dependent alterations of claudin-4 expression in human bladder carcinoma. Carcinogenesis. 28:246–258 [DOI] [PubMed] [Google Scholar]
- Bryan RT, Zeegers MP, James ND, Wallace DM, Cheng KK. 2010. Biomarkers in bladder cancer. BJU Int. 105:608–613 [DOI] [PubMed] [Google Scholar]
- Castillo-Martin M, Domingo-Domenech J, Karni-Schmidt O, Matos T, Cordon-Cardo C. 2010. Molecular pathways of urothelial development and bladder tumorigenesis. Urol Oncol. 28:401–408 [DOI] [PubMed] [Google Scholar]
- Cheville JC, Wu K, Sebo TJ, Cheng L, Riehle D, Lohse CM, Shane V. 2000. Inverted urothelial papilloma: is ploidy, MIB-1 proliferative activity, or p53 protein accumulation predictive of urothelial carcinoma? Cancer. 88:632–636 [DOI] [PubMed] [Google Scholar]
- Eiber M, van Oers JM, Zwarthoff EC, van der Kwast TH, Ulrich O, Helpap B, Stoerkel S, Blaszyk H, Cheville J, Sauter G, et al. 2007. Low frequency of molecular changes and tumor recurrence in inverted papillomas of the urinary tract. Am J Surg Pathol. 31:938–946 [DOI] [PubMed] [Google Scholar]
- Forster C. 2008. Tight junctions and the modulation of barrier function in disease. Histochem Cell Biol. 130:55–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huo Q, Kinugasa T, Wang L, Huang J, Zhao J, Shibaguchi H, Kuroki M, Tanaka T, Yamashita Y, Nabeshima K, Iwasaki H. 2009. Claudin-1 protein is a major factor involved in the tumorigenesis of colorectal cancer. Anticancer Res. 29:851–857 [PubMed] [Google Scholar]
- Jones TD, Wang M, Eble JN, MacLennan GT, Lopez-Beltran A, Zhang S, Cocco A, Cheng L. 2005. Molecular evidence supporting field effect in urothelial carcinogenesis. Clin Cancer Res. 11:6512–6519 [DOI] [PubMed] [Google Scholar]
- Jones TD, Zhang S, Lopez-Beltran A, Eble JN, Sung MT, MacLennan GT, Montironi R, Tan PH, Zheng S, Baldridge LA, Cheng L. 2007. Urothelial carcinoma with an inverted growth pattern can be distinguished from inverted papilloma by fluorescence in situ hybridization, immunohistochemistry, and morphologic analysis. Am J Surg Pathol. 31:1861–1867 [DOI] [PubMed] [Google Scholar]
- Kaufmann O, Fietze E, Mengs J, Dietel M. 2001. Value of p63 and cytokeratin 5/6 as immunohistochemical markers for the differential diagnosis of poorly differentiated and undifferentiated carcinomas. Am J Clin Pathol. 116:823–830 [DOI] [PubMed] [Google Scholar]
- Kinugasa T, Huo Q, Higashi D, Shibaguchi H, Kuroki M, Tanaka T, Futami K, Yamashita Y, Hachimine K, Maekawa S, et al. 2007. Selective up-regulation of claudin-1 and claudin-2 in colorectal cancer. Anticancer Res. 27:3729–3734 [PubMed] [Google Scholar]
- Krajewska M, Olson AH, Mercola D, Reed JC, Krajewski S. 2007. Claudin-1 immunohistochemistry for distinguishing malignant from benign epithelial lesions of prostate. Prostate. 67:907–910 [DOI] [PubMed] [Google Scholar]
- Lechpammer M, Resnick MB, Sabo E, Yakirevich E, Greaves WO, Sciandra KT, Tavares R, Noble LC, DeLellis RA, Wang LJ. 2008. The diagnostic and prognostic utility of claudin expression in renal cell neoplasms. Mod Pathol. 21:1320–1329 [DOI] [PubMed] [Google Scholar]
- Lodi C, Szabo E, Holczbauer A, Batmunkh E, Szijarto A, Kupcsulik P, Kovalszky I, Paku S, Illyes G, Kiss A, Schaff Z. 2006. Claudin-4 differentiates biliary tract cancers from hepatocellular carcinomas. Mod Pathol. 19:460–469 [DOI] [PubMed] [Google Scholar]
- Lott S, Wang M, Zhang S, MacLennan GT, Lopez-Beltran A, Montironi R, Sung MT, Tan PH, Cheng L. 2009. FGFR3 and TP53 mutation analysis in inverted urothelial papilloma: incidence and etiological considerations. Mod Pathol. 22:627–632 [DOI] [PubMed] [Google Scholar]
- Martin TA, Jiang WG. 2009. Loss of tight junction barrier function and its role in cancer metastasis. Biochim Biophys Acta. 1788:872–891 [DOI] [PubMed] [Google Scholar]
- Mees ST, Mennigen R, Spieker T, Rijcken E, Senninger N, Haier J, Bruewer M. 2009. Expression of tight and adherens junction proteins in ulcerative colitis associated colorectal carcinoma: upregulation of claudin-1, claudin-3, claudin-4, and beta-catenin. Int J Colorectal Dis. 24:361–368 [DOI] [PubMed] [Google Scholar]
- Montironi R, Mazzucchelli R, Scarpelli M, Lopez-Beltran A, Cheng L. 2008. Morphological diagnosis of urothelial neoplasms. J Clin Pathol. 61:3–10 [DOI] [PubMed] [Google Scholar]
- Nakanishi K, Ogata S, Hiroi S, Tominaga S, Aida S, Kawai T. 2008. Expression of occludin and claudins 1, 3, 4, and 7 in urothelial carcinoma of the upper urinary tract. Am J Clin Pathol. 130:43–49 [DOI] [PubMed] [Google Scholar]
- Nemeth Z, Szasz AM, Tatrai P, Nemeth J, Gyorffy H, Somoracz A, Szijarto A, Kupcsulik P, Kiss A, Schaff Z. 2009. Claudin-1, -2, -3, -4, -7, -8, and -10 protein expression in biliary tract cancers. J Histochem Cytochem. 57:113–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouban A, Ahmed AA. 2010. Claudins in human cancer: a review. Histol Histopathol. 25:83–90 [DOI] [PubMed] [Google Scholar]
- Paschoud S, Bongiovanni M, Pache JC, Citi S. 2007. Claudin-1 and claudin-5 expression patterns differentiate lung squamous cell carcinomas from adenocarcinomas. Mod Pathol. 20:947–954 [DOI] [PubMed] [Google Scholar]
- Resnick MB, Konkin T, Routhier J, Sabo E, Pricolo VE. 2005. Claudin-1 is a strong prognostic indicator in stage II colonic cancer: a tissue microarray study. Mod Pathol. 18:511–518 [DOI] [PubMed] [Google Scholar]
- Riesz P, Szekely E, Torzsok P, Majoros A, Szendroi A, Dombovari P, Romics I. 2010. [Can inverted papilloma in urinary bladder be considered as a benign tumor]. Orv Hetil. 151:92–95 [DOI] [PubMed] [Google Scholar]
- Saeki R, Kondoh M, Kakutani H, Matsuhisa K, Takahashi A, Suzuki H, Kakamu Y, Watari A, Yagi K. 2010. A claudin-targeting molecule as an inhibitor of tumor metastasis. J Pharmacol Exp Ther. 334:576–582 [DOI] [PubMed] [Google Scholar]
- Saeki R, Kondoh M, Kakutani H, Tsunoda S, Mochizuki Y, Hamakubo T, Tsutsumi Y, Horiguchi Y, Yagi K. 2009. A novel tumor-targeted therapy using a claudin-4-targeting molecule. Mol Pharmacol. 76:918–926 [DOI] [PubMed] [Google Scholar]
- Samaratunga H, Makarov DV, Epstein JI. 2002. Comparison of WHO/ISUP and WHO classification of noninvasive papillary urothelial neoplasms for risk of progression. Urology. 60:315–319 [DOI] [PubMed] [Google Scholar]
- Sheehan GM, Kallakury BV, Sheehan CE, Fisher HA, Kaufman RP, Jr., Ross JS. 2007. Loss of claudins-1 and -7 and expression of claudins-3 and -4 correlate with prognostic variables in prostatic adenocarcinomas. Hum Pathol. 38:564–569 [DOI] [PubMed] [Google Scholar]
- Sobel G, Nemeth J, Kiss A, Lotz G, Szabo I, Udvarhelyi N, Schaff Z, Paska C. 2006. Claudin 1 differentiates endometrioid and serous papillary endometrial adenocarcinoma. Gynecol Oncol. 103:591–598 [DOI] [PubMed] [Google Scholar]
- Stravoravdi P, Natsis K, Kirtsis P, Retalis G, Konstandinidis E, Polyzonis M. 1996. Ultrastructural study of the noninvolved urothelium of tumor-bearing patients after two years of intravesical interferon therapy. Eur Urol. 29:477–482 [DOI] [PubMed] [Google Scholar]
- Sung MT, Eble JN, Wang M, Tan PH, Lopez-Beltran A, Cheng L. 2006a. Inverted papilloma of the urinary bladder: a molecular genetic appraisal. Mod Pathol. 19:1289–1294 [DOI] [PubMed] [Google Scholar]
- Sung MT, Maclennan GT, Lopez-Beltran A, Montironi R, Cheng L. 2006b. Natural history of urothelial inverted papilloma. Cancer. 107:2622–2627 [DOI] [PubMed] [Google Scholar]
- Tokes AM, Kulka J, Paku S, Szik A, Paska C, Novak PK, Szilak L, Kiss A, Bogi K, Schaff Z. 2005. Claudin-1, -3 and -4 proteins and mRNA expression in benign and malignant breast lesions: a research study. Breast Cancer Res. 7:R296–R305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Oers JM, Wild PJ, Burger M, Denzinger S, Stoehr R, Rosskopf E, Hofstaedter F, Steyerberg EW, Klinkhammer-Schalke M, Zwarthoff EC, et al. 2007. FGFR3 mutations and a normal CK20 staining pattern define low-grade noninvasive urothelial bladder tumours. Eur Urol. 52:760–768 [DOI] [PubMed] [Google Scholar]
- Varley CL, Garthwaite MA, Cross W, Hinley J, Trejdosiewicz LK, Southgate J. 2006. PPARgamma-regulated tight junction development during human urothelial cytodifferentiation. J Cell Physiol. 208:407–417 [DOI] [PMC free article] [PubMed] [Google Scholar]