Abstract
The purpose of this study was to evaluate the neuroprotective effects of intranasally delivered recombinant human neuronal erythropoietin (Neuro-EPO) on brain injury induced by unilateral permanent ischemia in the Mongolian gerbil. Expression of EPO receptor (EPOR) and neuroglobin (Ngb) over 5 weeks after intranasal treatment with Neuro-EPO was determined using immunohistochemistry. Mortality of Neuro-EPO-treated gerbils decreased after surgery, and the sensory and motor function was significantly improved. Histopathological mapping showed that Neuro-EPO significantly reduced delayed neuronal death in the brain. Expression of Ngb was upregulated in the cerebral cortex at most time points (expect for 10 min and 48 hr) and in the hippocampus at 10 min and from 48 hr to 5 weeks, whereas EPOR was almost downregulated or unchanged in the brain (expect for 48 hr). The 10 min and 48 hr seemed to be two time points for the brain to switch the expression of both Ngb and EPOR to early and late recovery phase, respectively. In addition, there were two phases, 10 min to 1 hr and 24 hr to 72 hr, respectively, closing to the “golden hour” of about 60 min and the “silver day” of 1 to 3 days, for the brain to recover from stroke onset with intranasal Neuro-EPO treatment. Therefore, the results suggest that the intranasal administration of Neuro-EPO is effective in the treatment of acute brain ischemia. The different expression patterns of Ngb and EPOR is probably due to ischemic tolerance in the cerebral cortex and ischemic sensitivity in the hippocampus.
Keywords: erythropoietin, erythropoietin receptor, neuroglobin, ischemia, intranasal drug delivery, Neuro-EPO, neuroprotection, biphasic expression pattern
Stroke is one of the most critical diseases of the nervous system. It is believed that neurotrophic factors, including erythropoietin (EPO), may be potential neuroprotective agents. Considerable evidence has demonstrated that exogenously administrated recombinant human EPO (rhEPO) provides neuroprotection in different paradigms of brain damage (Buemi et al. 2000; Erbayraktar et al. 2003; Sirén, Knerlich, et al. 2001). However, many clinical situations may likely require multiple doses of rhEPO, which may lead to potentially harmful increases in the red cell mass. Studies in animal models clearly show that EPO-induced increases in hematocrit can cause and worsen brain injury (Wiessner et al. 2001); however, Neuro-EPO with a truncated oligosaccharide chain has no erythropoietic properties and does not increase erythrocyte mass but remains fully neuroprotective in animal models of stroke, spinal cord injury, and peripheral neuropathy (Erbayraktar et al. 2003; Hasselblatt et al. 2006). However, a way of targeting the drug to the ischemic regions is still lacking because the blood-brain barrier selectively regulates the input and output of various molecules into and out of the brain. Therefore, it is important to develop novel ways for drug delivery, such as intranasal administration to the brain (Yu et al. 2005). In our previous studies, we have shown that intranasal delivery of Neuro-EPO significantly reduces infarct size in the gerbil brain even when administered a very short time (less than 5-10 min) after ischemic injury (Iliana et al. 2008; Sosa Testé et al. 2006); these results suggest that Neuro-EPO is therapeutically effective against stroke, and its intranasal administration is a valuable drug delivery method. In addition, this approach could be safer, less invasive, and 10 times more rapid than the intravenous route, although it may be associated with some nasal cavity discomfort (Iliana et al. 2008; Sosa Testé et al. 2006).
It has been shown that a number of mechanisms are involved in the neuroprotective effects of EPO, such as activation of specific protein kinases, inhibition of reactive oxygen species and glutamate overproduction, modulation of neurotransmission, attenuation of apoptosis, and stimulation of angiogenesis (Acker T and Acker H 2004; Flynn et al. 2008; Garcia-Rodriguez and Sosa-Teste 2009; Hossmann 2008; Kulik et al. 2008; Lakhan et al. 2009); however, the neuroprotective effects of intranasal administration of Neuro-EPO are poorly understood. Considering that the EPO receptor (EPOR) is important for EPO’s effect, and neuroglobin (Ngb) is an endogenous neuroprotector for the brain, we proposed that the expressions of EPOR and Ngb are probably related, and these proteins are involved in the neuroprotection of intranasal delivery of Neuro-EPO. Ngb, a novel heme-containing, respiratory globin protein, is widely expressed in the brain, including the cerebral cortex, hippocampus, thalamus, cerebellum, and brainstem (Burmester et al. 2004; Burmester et al. 2000; Schmidt-Kastner et al. 2006; Zhang et al. 2002). Ngb is upregulated under hypoxic stress and is considered a neuroprotective protein both in vitro and in vivo (Hang et al. 2009; Li W et al. 2010; Sun et al. 2001; Sun et al. 2003). Accordingly, the purpose of the current study was to examine the dynamic changes in EPOR and Ngb expression in the brain with intranasal delivery of Neuro-EPO over an extended recovery period, from 10 min to up to 5 weeks, and to characterize the underlying neuroprotective events in response to ischemic insults.
Materials and Methods
Animals and Preparation of Ischemic Insults
One hundred male Mongolian gerbils (12-15 weeks; 70-90 g) were provided by the National Center for Laboratory Animal Breeding (CENPALAB, La Havana, Cuba) and adapted to experimental conditions for 7 days. The animals were maintained in controlled environmental rooms with the following conditions: temperature of 22 ± 2C, relative humidity of 55% to 60%, 12-hr/12-hr light-dark cycle, and 15% to 20% room air changes per hour. All materials used to maintain the animals were autoclaved at 121C for 20 min. Food and water were provided ad libitum. Animal use was approved by a local animal care and use committee to minimize the overall use of animals. Each protocol was discussed and approved by the Institutional Ethics Committee of CENPALAB, considering the international regulations established by the International Council for Laboratory Animal Science (ICLAS).
The EPO used in the study was Neuro-EPO with sialic acid content at 4 to 7 mol/mol protein. During the biotechnological production of Neuro-EPO, various isoforms with different contents of sialic acid were obtained. EPO with sialic acid content at 4 to 7 mol/mol protein is considered low sialic acid containing, which is very similar to the one that occurs in the mammalian brain. Low sialic acid–containing EPO will be rapidly degraded by the liver.
Animals were divided into two groups: unilateral permanent ischemia coupled with intranasal treatment of Neuro-EPO (Nasal-Neuro-EPO) and unilateral permanent ischemia without Neuro-EPO treatment as the control group (Nasal-Vehicle). To examine the time course of expression of Ngb and EPOR in the brain, the following postischemia time points were used: 10 min, 1 hr, 12 hr, 24 hr, 48 hr, 72 hr, 1 week, and 5 weeks. Neuro-EPO was provided by the Center of Molecular Immunology (CIM; Havana, Cuba) and diluted in phosphate-buffered saline (PBS; pH 7.0) at 0.15 mM. The dosage of Neuro-EPO via nasal administration was 10 µl, 3 times daily for 4 days, beginning immediately after surgery.
Surgical Procedure and Measurement of Spontaneous Exploratory Activity
Gerbils were anesthetized with ketamine-atropine-diazepam (47 mg/kg, 0.02 mg/kg, and 5 mg/kg, respectively). Lesions were performed according to the Butterfield and McGraw (1978) method. Briefly, the right common carotid artery (CCA) was isolated, double ligated using silk suture, and transected. In sham-operated animals, the artery was only isolated. Twenty-four hours after unilateral permanent ischemia, the appearance of some clinical signs of infarction was assessed: palpebral ptosis, bristling, loss of tone and reflexes in the four limbs, postural asymmetry, rolling or circling, and death. The animals were checked twice daily, morning and afternoon, to record the mortality. Neurological evaluation was made 24 hr after surgery using the scale. The method of the neuronal scoring was elaborated to give items tested and score definitions according to the existing table as reported by Lawner et al. (1979). Each sign was scored separately, and then the sum of the scores created a general neurological score for each animal. The percentage of protection was calculated by comparing the neuronal scoring of each group with the control group.
Gerbils were placed in the center of a round 30-cm-diameter open field surrounded by a 25-cm high wall. A rearing event was defined as the animal assuming an upright posture on its hind limbs and tail and then returning any forelimb to the floor or touching the open field wall with any forelimb. It was not considered a rearing event if the animal subsequently straightened after a being in an upright position but did not touch the floor with a forelimb or if the animal assumed a kangaroo-like posture. Exploratory activity was determined by counting the rearing events during 3 min or 9 min in the open field. In the 9-min trials, the time was subdivided into three periods of 3 min each, and the number of events was recorded, producing three values per trial. Each value was plotted, and the line obtained by the least squares method was considered the habituation curve. The slope of the habituation curve was used to characterize the state of the animal.
Tissue Preparation and Immunohistochemical Mapping
At different time points after surgery, the gerbils were transcardially perfused with saline solution followed by buffered 4% formaldehyde solution (pH 7.0). Brains were carefully removed, maintained for several days in the fixative, and then processed for paraffin sectioning.
Three- to 5-µm-thick tissue sections were obtained and de-waxed in xylene and rehydrated through graded alcohols to PBS. The slides were placed in 3% hydrogen peroxide for 10 min to eliminate endogenous peroxidase activity. Microwave antigen retrieval for 20 min was then performed. After several rinses in PBS (pH 7.4), the tissue was incubated for 30 min in solution A (containing 5% normal goat serum) to block nonspecific binding. Tissue sections were then incubated in primary antibody overnight at 4C in PBS containing 1% goat normal serum and 0.25% Triton X-100. After several rinses, the tissue was incubated for 1 hr at 37C in solution B (biotinylated goat anti-rabbit/mouse immunoglobulin). Sections were rinsed and incubated in solution C (avidin-biotin-peroxidase complex) for 30 min. The avidin-biotin-peroxidase complex reaction was visualized by incubation in PBS containing 0.05% diaminobenzidine (DAB; Sigma, St Louis, MO) and 0.005% hydrogen peroxide for 5 min and then halted by several washings in PBS.
All antibodies were diluted in PBS (pH 7.0) containing 0.1% Triton X-100 and 0.05% BSA. The primary antibodies used were mouse-derived anti-Ngb (1:200; homemade) (Shang et al. 2006; Zhang et al. 2002) and rabbit-derived anti-EPOR (1:100; Boster, Wuhan, China). Corresponding biotinylated secondary antibodies and avidin-biotin-peroxidase complex solution were produced by Beijing Zhongshan Inc. (Beijing, China).
Stereological Analysis
Usually, there are two parameters for positive signal analysis in immunohistochemistry micrographs: intensity and area, represented by mean optical density (MOD) and total per area (TPA; area of positive signal per unit area of stained tissue) in the image analysis software Image Pro Plus (version 5.1; Media Cybernetics, Bethesda, MD). By combining the two parameters together as MOD × TPA, we can easily determine the positive signals within the manually selected area of interest (AOI) in different brain regions of the micrographs (Gao et al. 2009). Therefore, the artificial unit of MOD × TPA was employed for measurement of the stereological analysis, which indicated the integrated optical density (IOD) of positive signal per unit area of stained tissue.
In detail, images of the stained sections (five sections per animal) were captured with a microscope connected to a CCD camera and a desktop computer. Seven representative brain regions were analyzed, including the cingulate cortex, striate cortex, auditory cortex, piriform cortex, and the CA1, CA2 to CA3, and CA4 areas of the hippocampus. To have an overall measurement of protein levels in the brain, we used the summation of all four cortex regions to represent the protein expression in the cerebral cortex. Similarly, we used the summation of CA1 to CA4 areas to represent protein expression in the hippocampus.
For the analysis of the expression pattern of Ngb and EPOR, we used the ratio of IOD to represent the expression changes of the Nasal-Neuro-EPO group versus the Nasal-Vehicle group, which is displayed as IODNeuro-EPO/IODvehicle. In addition, the expression level of the two proteins at each time point was compared with the non-ischemia control group, which is displayed as IODNeuro-EPO/IODcontrol.
Data Analysis
All data are expressed as mean ± SD. Statistical analysis of quantification of Ngb and EPOR immunoreactive cells was performed by means of the one-way ANOVA test. Student’s t-test was used to compare the two means of different groups. p<0.05 was considered significant, whereas p<0.01 was considered highly significant.
Results
Mortality of Gerbils Treated with Nasal-Neuro-EPO Decreased after Surgery
The protective effect of Neuro-EPO was not evident at the 24-hr postischemia time point, when the number of deaths in both groups was similar (Table 1). However, the mortality at 48 hr after brain ischemia was greater in the Nasal-Vehicle group than that in the Nasal-Neuro-EPO group (p=0.02). Seventeen animals in the Nasal-Vehicle group were dead at 48 hr after ischemia, whereas no animals from the Nasal-Neuro-EPO group died before 48 hr postischemia.
Table 1.
Mortality of Gerbils with Intranasal Neuro-EPO Treatment
| Time Point | Nasal-Neuro-EPO, n (%) | Nasal-Vehicle, n (%) | Total, n (%) |
|---|---|---|---|
| 24 hr | 9 (26.4) | 8 (23.6) | 17 (50.0) |
| 48 hr | 0 (0)* | 17 (50.0) | 17 (50.0) |
| Total | 9 (26.4)* | 25 (73.6) | 34 (100.0) |
Neuro-EPO = neuronal erythropoietin.
p<0.05, compared with Nasal-Vehicle group.
Changes in the Neurological State of Gerbils Treated with Nasal-Neuro-EPO after Surgery
The animals treated with Nasal-Neuro-EPO showed better general states. The gerbils with higher neurological scores not only showed deterioration in the extremities corresponding to the damaged hemisphere but also showed defects on the opposite side, including bilateral palpebral ptosis. The neurological scores of the Nasal-Neuro-EPO and Nasal-Vehicle groups are shown in Figure 1A.
Figure 1.
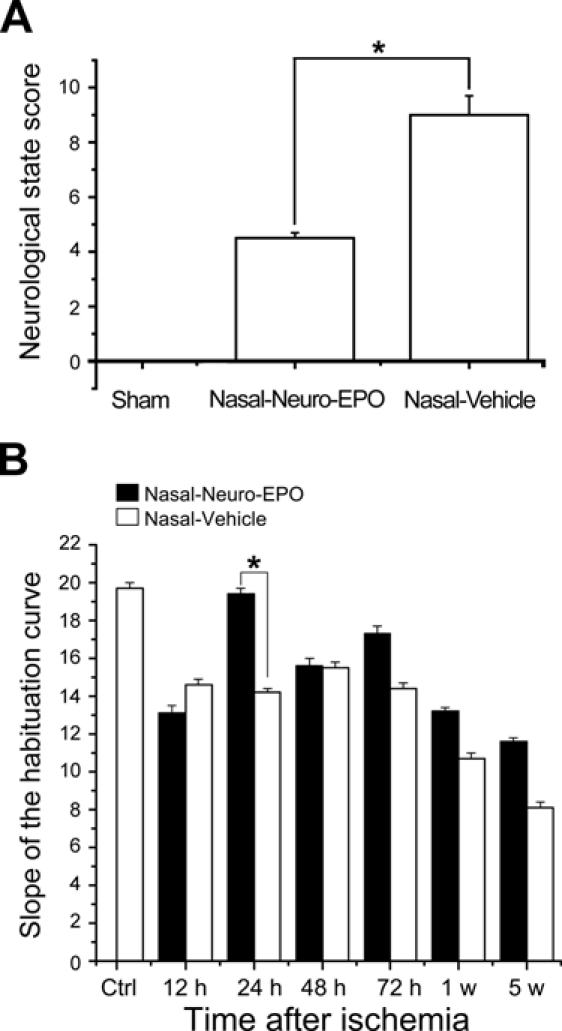
Diagram showing the neurological state score of the animals at 24 hr after the surgery (A) and values of the slope for spontaneous exploratory activity (B) in the Nasal-Neuro-EPO group and the Nasal-Vehicle group at different time points after surgery. N=100; *p<0.05.
The open field testing to evaluate the exploratory activity of the animals showed significant differences (p<0.05) when comparing activity before surgery to after surgery in all groups of ischemic animals, except in the groups treated with Nasal-Neuro-EPO, which showed no difference. A marked behavioral difference existed at 24 hr between the ischemic animals in both treatment groups. The Nasal-Neuro-EPO group presented a slope of the habituation curve further from zero than the Nasal-Vehicle group (p=0.03; Fig. 1B).
Morphometric Evaluation of Ischemic Neuronal Damage with Intranasal Neuro-EPO Treatment
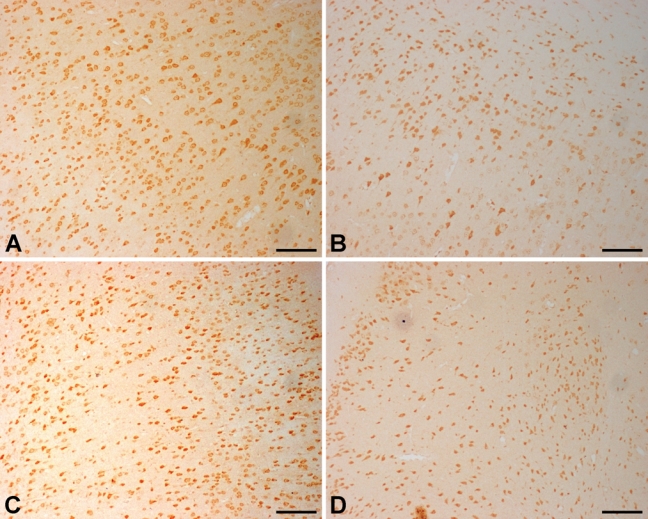
The distribution of neuronal damage was evaluated by hematoxylin and eosin (HE) staining and Nissl staining. Brains of the Nasal-Neuro-EPO group showed less ischemic cell changes than the Nasal-Vehicle group. Ischemic damage after permanent unilateral common carotid occlusion increased over time. Morphometric analysis ascertained that the damaged neurons in the auditory cortex assessed 24 hr after ischemia were significantly decreased in the Nasal-Neuro-EPO group (Fig. 2A and Fig. 3A). In the hippocampus, morphological changes of the neurons were also significant. Five weeks after ischemia, extensive and severe morphological changes in neuronal cells were detected in the CA2 to CA3 regions of the hippocampus from the Nasal-Vehicle group (Fig. 2D and Fig. 3D). However, no obvious neuronal damage was observed in the Nasal-Neuro-EPO group (Fig. 2C and Fig. 3C).
Figure 2.
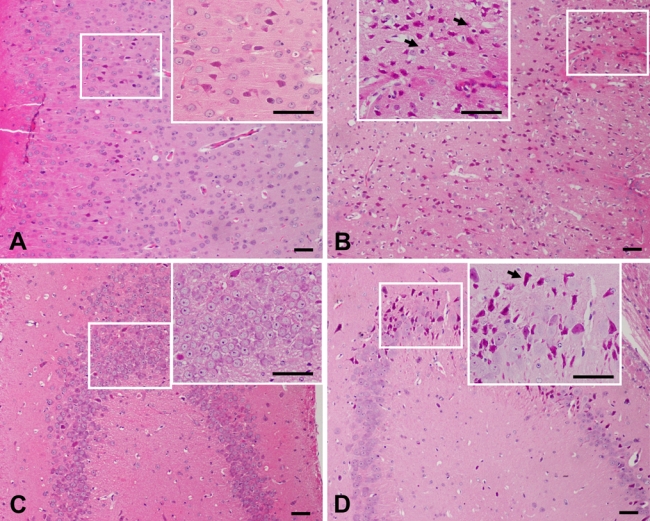
Hematoxylin and eosin–stained coronal brain sections indicate the ischemic injured region of the Nasal-Neuro-EPO and Nasal-Vehicle groups. At 24 hr after ischemia, the number of pyknotic, edematous, and necrotic cells in the auditory cortex of the Nasal-Neuro-EPO-treated group (A) is less than that in the Nasal-Vehicle group (B). At 5 weeks after ischemia, the number of pyknotic, edematous, and necrotic cells in the hippocampus of the Nasal-Neuro-EPO group (C) is less than that in the Nasal-Vehicle group (D). Arrowheads indicate cells with edema and necrosis. Scale bar = 50 µm.
Figure 3.
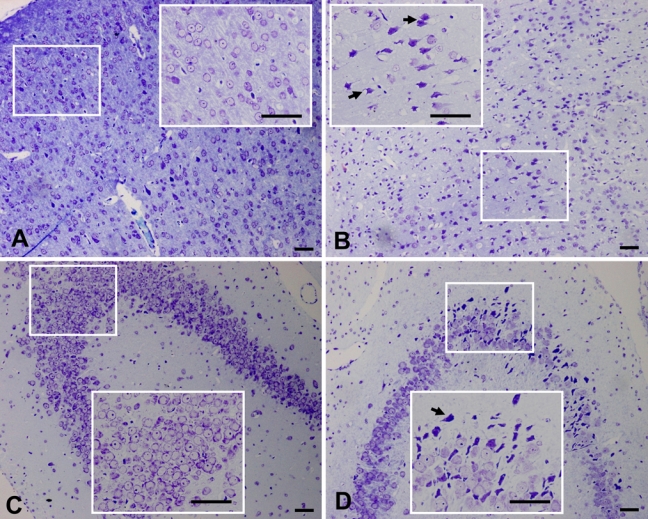
Nissl-stained coronal brain sections indicate the ischemic-injured region of Nasal-Neuro-EPO and Nasal-Vehicle. At 24 hr after ischemia, the number of pyknotic, edematous, and necrotic cells in the auditory cortex of the Nasal-Neuro-EPO group (A) is less than that in the Nasal-Vehicle group (B). At 5 weeks after ischemia, the number of pyknotic, edematous, and necrotic cells in the hippocampus of the Nasal-Vehicle group (C) is less than that in the Nasal-Vehicle group (D). Arrowheads indicate cells with edema and necrosis. Scale bar = 50 µm.
Dynamic Changes of Ngb Expression in the Gerbil Brain with Intranasal Neuro-EPO Treatment
The level of Ngb expression after ischemia was upregulated in both the Nasal-Neuro-EPO and Nasal-Vehicle groups compared with the control, as partially shown for the auditory cortex at 12 hr (Fig. 4A,B) or 5 weeks (Fig. 4C,D). However, the IODNeuro-EPO/IODvehicle changes in Ngb in the cerebral cortex decreased at two time points, 10 min and 48 hr, but upregulated at 1 hr to 24 hr and 72 hr to 5 weeks (Fig. 5). At 48 hr after ischemia, Ngb expression in the Nasal-Neuro-EPO group was lower than that in the Nasal-Vehicle group and decreased until 72 hr (Table 2). However, the expression pattern of Ngb in the hippocampus was completely different from that in the cerebral cortex (Fig. 6); hippocampal expression showed downregulated Ngb in the Nasal-Neuro-EPO group compared to the Nasal-Vehicle group at 1 hr to 24 hr (Table 2) but upregulated expression at all other time points compared to its preischemia expression level (data not shown).
Figure 4.
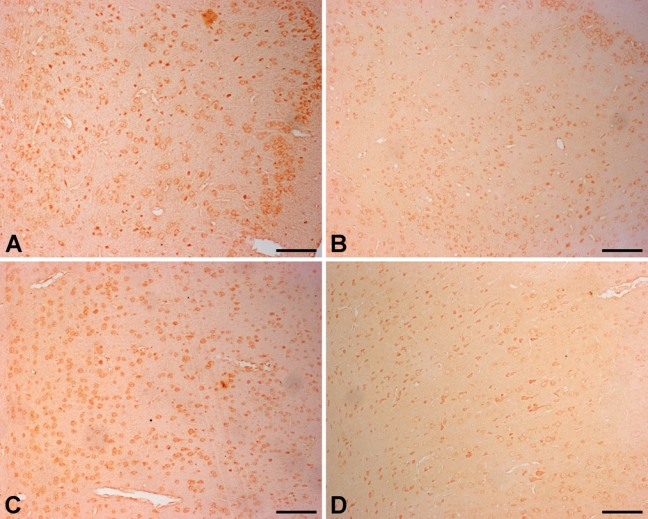
Dynamic expression of neuroglobin (Ngb) in the gerbil brain with intranasal Neuro-EPO treatment. At 12 hr after ischemia, Ngb expression in the auditory cortex of the Nasal-Neuro-EPO group (A) is higher than that in the Nasal-Vehicle group (B). At 5 weeks after ischemia, Ngb expression in the auditory cortex of the Nasal-Neuro-EPO group (C) is still higher than that in the Nasal-Vehicle group (D). Scale bar = 100 µm.
Figure 5.
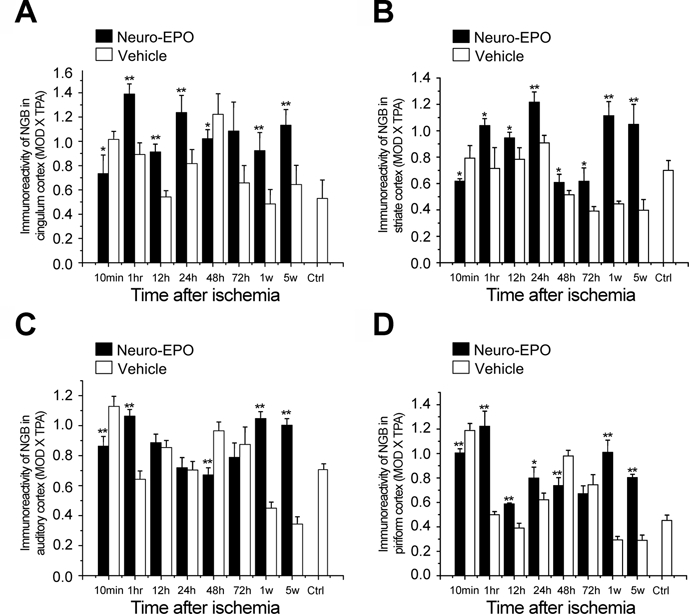
Expression of neuroglobin (Ngb) in the cerebral cortex of the gerbil brain in the Nasal-Neuro-EPO group compared with the Nasal-Vehicle group from 10 min to 5 weeks after permanent unilateral common carotid occlusion. (A) Cingulum cortex, (B) striate cortex, (C) auditory cortex, and (D) piriform cortex. *p<0.05. **p<0.01.
Table 2.
Expression Pattern of Ngb and EPOR in the Cerebral Cortex and Hippocampus of Gerbils in the Nasal-Neuro-EPO Group Compared to Nasal-Vehicle group
| Time Postischemic Insults of the Gerbil Brain | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 10 min | 1 hr | 12 hr | 24 hr | 48 hr | 72 hr | 1 Week | 5 Weeks | ||
| Golden Hour (~ 1 hr) | Silver Day (1-3 days) | ||||||||
| Protein | Brain Region | Early Recovery Phase (1-24 hr) | Late Recovery Phase (72 h to 5 weeks) | ||||||
| Ngb | Cerebral cortex | ↓↓ | ↑↑ | ↑↑ | ↑↑ | ↓↓ | ↑↑ | ↑↑ | ↑↑ |
| Hippocampus | ↑↑ | ↓ | ↓↓ | ↓↓ | ↑↑ | ↑↑ | ↑↑ | ||
| EPOR | Cerebral cortex | ↓↓ | ↓↓ | ↓↓ | ↑↑ | ||||
| Hippocampus | ↑ | ↓ | ↓↓ | ↓↓ | ↑↑ | ↓↓ | ↓↓ | ↑ | |
Note: Data represented as IODNeuro-EPO/IODvehicle. ↑ = upregulation; ↓ = downregulation, compared with control group. ↑ and ↓: p<0.05; ↑↑ and ↓↓: p<0.01. Neuro-EPO = neuronal erythropoietin; EPOR = erythropoietin receptor; Ngb = neuroglobin.
Figure 6.
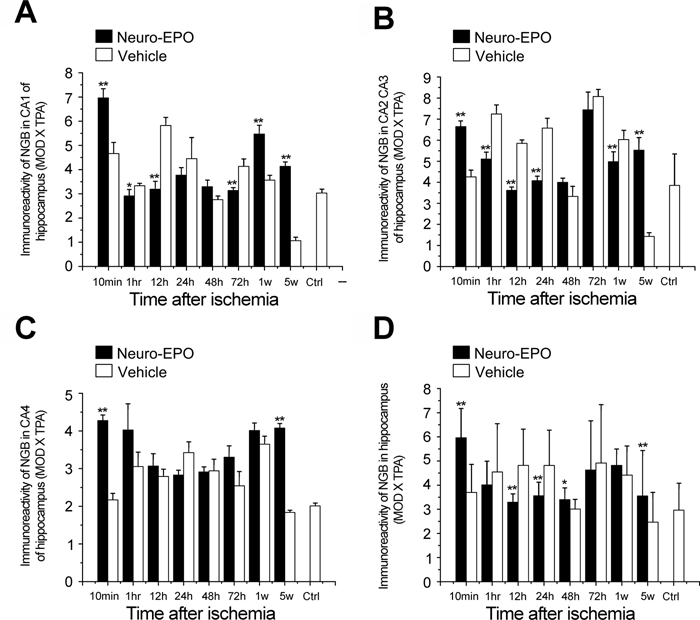
Expression of neuroglobin (Ngb) in the hippocampus of the gerbil brain in the Nasal-Neuro-EPO group compared with the Nasal-Vehicle group from 10 min to 5 weeks after permanent unilateral common carotid occlusion. (A) CA1 of the hippocampus, (B) CA2 to CA3 of the hippocampus, (C) CA4 of the hippocampus, and (D) whole hippocampus. *p<0.05. **p<0.01.
Dynamic Changes of EPOR Expression in the Gerbil Brain with Intranasal Neuro-EPO Treatment
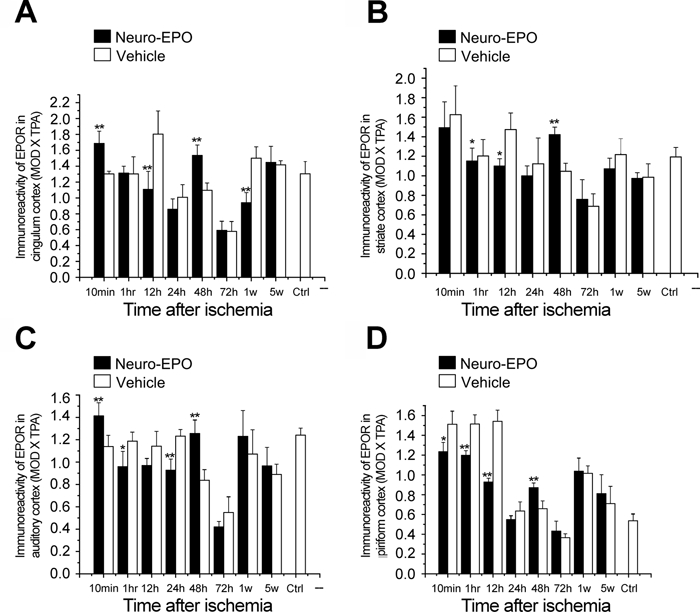
We further examined the expression pattern of EPOR in gerbil brains treated with intranasal Neuro-EPO. EPOR was observed to be extensively expressed in the gerbil brain not only in the cerebral cortex but also in the hippocampus. EPOR expression in the Nasal-Neuro-EPO group was higher than that in the Nasal-Vehicle group in three cerebral cortex regions at 10 min and in all cortex regions at 48 hr after ischemia (Fig. 7 and Fig. 8). After a global morphometric evaluation, we surprisingly found that the IODNeuro-EPO/IODvehicle changes in EPOR in the cerebral cortex showed a continuous downregulation from 1 hr to 24 hr (Fig. 8 and Table 2). However, in the hippocampus, IODNeuro-EPO/IODvehicle changes in EPOR showed a biphasic pattern similar to that of Ngb: a quick upregulation at 10 min, downregulation from 1 hr to 24 hr, upregulation again at 48 hr, downregulation again at 72 hr and 1 week, and a slight upregulation at 5 weeks (Fig. 9 and Table 2). These patterns were also similar in the hippocampus between IODNeuro-EPO/IODvehicle and IODNeuro-EPO/IODnormal (its expression level before ischemia; data not shown).
Figure 7.
Dynamic expression of erythropoietin receptor (EPOR) in the gerbil brain with intranasal Neuro-EPO treatment. At 48 hr after ischemia, EPOR expression in the striate cortex (A) of the Nasal-Neuro-EPO group is higher than that of the Nasal-Vehicle group (B). At 48 hr after ischemia, EPOR expression in the auditory cortex of the Nasal-Neuro-EPO group (C) is higher than that of the Nasal-Vehicle group (D). Scale bar = 100 µm.
Figure 8.
Expression of erythropoietin receptor (EPOR) in the cerebral cortex of the gerbil brain of the Nasal-Neuro-EPO group compared with the Nasal-Vehicle group from 10 min to 5 weeks after permanent unilateral common carotid occlusion. (A) Cingulum cortex, (B) striate cortex, (C) auditory cortex, and (D) piriform cortex. *p<0.05. **p<0.01.
Figure 9.
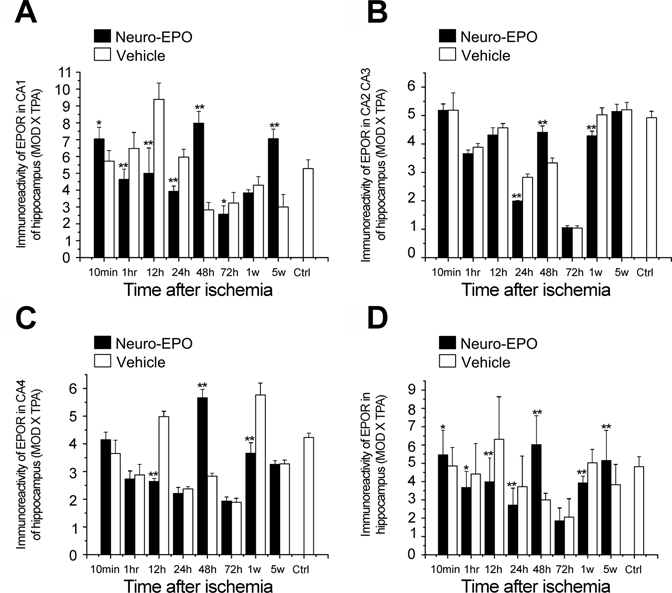
Expression of erythropoietin receptor (EPOR) in the hippocampus of the gerbil brain of the Nasal-Neuro-EPO group compared with the Nasal-Vehicle group from 10 min to 5 weeks after permanent unilateral common carotid occlusion. (A) CA1 of the hippocampus, (B) CA2 to CA3 of the hippocampus, (C) CA4 of the hippocampus, and (D) whole hippocampus. *p<0.05. **p<0.01.
Discussion
Clearly, the mortality rate and behavioral differences of the animals observed between the Nasal-Neuro-EPO and the Nasal-Vehicle groups demonstrate the effectiveness of Neuro-EPO to counteract the acute effect of brain ischemia. The mortality rate of the Nasal-Neuro-EPO group was 26.4%, which was lower than the Nasal-Vehicle group, which showed a mortality rate of 73.6%. Moreover, a greater functional integrity at day 7 in the Nasal-Neuro-EPO group was observed compared with the Nasal-Vehicle group (data not shown). Motor and exploratory activity expressed by rearing counts was preserved in the Nasal-Neuro-EPO group. Results demonstrate the effectiveness of intranasal Neuro-EPO to counteract the deleterious effects of acute brain ischemia.
EPO is a cytokine synthesized by the kidney to regulate hematopoiesis. There are receptors for EPO throughout the central nervous system (CNS). EPO has a direct protective role against a variety of neurotoxic insults, such as hypoxia (Marti et al. 1996), glutamate toxicity (Morishita et al. 1997), free-radical injury, and exposure to neurotoxicants (Villa et al. 2003). EPO has also shown neuroprotective effects in rodent stroke models, reducing infarct size and improving behavioral and cognitive outcomes (Sirén et al. 2006). It was reported that intranasally administrated I125-labeled Neuro-EPO in Mongolian gerbils was detected in the olfactory bulb and cerebellum at 5 min (Sosa Testé et al. 2006). EPO has now been safely administered to stroke patients (Ehrenreich et al. 2002; Hasselblatt et al. 2006), but little was known about the mechanisms mediating the neuroprotective activity of EPO in vivo. As EPO and Ngb are both important proteins in the therapeutic responses to ischemia (Milano and Collomp 2005), our current studies revealed the potential therapeutic effect of Nasal-Neuro-EPO treatment.
As an oxygen carrier and potential neuroprotective factor in neuronal cells, Ngb may enhance the tolerance to ischemic insults (Wang et al. 2008) and be a potential biomarker for brain insults after ischemia (Shang et al. 2006). In the acute phase 10 min after ischemia, expression of Ngb in the Nasal-Neuro-EPO treatment group decreased immediately in the cerebral cortex but increased in the hippocampus, supporting the hypothesis that the hippocampus is more sensitive to ischemia than the cerebral cortex. This expression pattern was continuously higher in the cerebral cortex and lower in the hippocampus from 1 hr to 24 hr, the early recovery phase, which is consistent with the cerebral cortex having superior ischemic tolerance. After the adjustment phase of 48 hr and during the late recovery phase from 72 hr to 5 weeks, the Nasal-Neuro-EPO/Nasal-Vehicle ratio for Ngb in the cerebral cortex continuously increased, whereas in the hippocampus, it increased from 48 hr to 5 weeks, except for the 72-hr time point. Therefore, within the first 48-hr postischemic insults, the expression patterns of Ngb in the cerebral cortex and the hippocampus are quite contradictory (Table 2), indicating a completely different mechanism of Nasal-Neuro-EPO neuroprotection against ischemic insults in the cerebral cortex versus the hippocampus. This is the typical biphasic pattern of Ngb expression in the cerebral cortex and the hippocampus. Therefore, the upregulation of Ngb seemed to be a neuroprotective marker for ischemic insults at different time points (early recovery phase of 1 hr to 24 hr and late recovery phase of 3 days to 5 weeks) in the cerebral cortex. On the other hand, Ngb was downregulated at the early recovery phase of 1 hr to 24 hr in the hippocampus, which is consistent with the fact that the hippocampus is sensitive to ischemic insults.
The EPO/EPOR system is neuroprotective against ischemic insults in the CNS (Ehrenreich et al. 2009; Minnerup et al. 2009). Alcalá-Barraza et al. (2010) recently demonstrated that intranasal delivery of EPO in adult rats resulted in substantial concentrations of this neurotrophic factor widely throughout the brain and cervical spinal cord. The EPOR is expressed in many neurons throughout the CNS, particularly enriched in the cortex and hippocampus (Brines et al. 2000). The upregulation of EPOR in the cortex and hippocampus mediated the neuroprotection of Nasal-Neuro-EPO treatment (Sanchez et al. 2009). Exogenous EPO added postischemia resulted in EPOR upregulation in the brain, suggesting the therapeutic potential for brain injury (Sirén, Fratelli, et al. 2001). In the ischemic insults model in this study, the expression of EPOR in the brain also showed a postischemia dynamic change. From our data, the expression of EPOR in the cerebral cortex was downregulated from 1 hr to 24 hr but upregulated at 48 hr, which was complementary with Ngb within the first 48-hr postischemic insults. It is interesting to point out that the 48-hr postischemic insults seemed to be a switching time point for the expression of both EPOR and Ngb in the brain. During the late recovery phase 72 hr to 5 weeks postischemic insults, the expression of Ngb was upregulated in the cerebral cortex, whereas the expression of EPOR was not significantly changed (Table 2). Thus, Ngb and EPOR may act as important intercoordination neuroprotectors in the cerebral cortex during early recovery phase (1-24 hr) postischemia, but the mechanism of the complementary expression between these two important proteins needs to be further investigated. It is worth mentioning that in the acute phase of 10 min, EPOR showed a slight upregulation in the hippocampus. Although it has been reported that after intranasal administration of Neuro-EPO in this model, it reaches the brain in 5 min (Sosa Testé et al. 2006), the significant changes of EPOR protein expression at 10 min after intranasal Neuro-EPO administration may be attributable not only to Neuro-EPO treatment but also probably because of stroke surgery or some unknown mechanisms. For example, Sirén et al. reported that 25 min after intranasal EPO administration, EPO was detectable in many regions of CNS in sufficient concentrations to activate the prosurvival PI3-Kinase/Akt pathway (Sirén, Knerlich, et al. 2001). However, the expression of EPOR in the hippocampus was accordant with Ngb within the first 48-hr postischemic insults. During the late recovery phase, EPOR still remained downregulated in the hippocampus from 72 hr to 1 week but upregulated at 5 weeks (Table 2). The similar expression pattern between Ngb and EPOR in the hippocampus and the overall downregulation of endogenous EPOR after the ischemic insults were probably related to the lower ischemic tolerance of the hippocampus with respect to the higher ischemic tolerance of the cerebral cortex under intranasal treatment of Neuro-EPO. This will be further studied by using quantitative approaches such as reverse transcription–polymerase chain reaction (RT-PCR) and Western blot in future studies, together with the immunohistochemical methods.
Otherwise, the protective effect of intranasal delivery of Neuro-EPO may be ascribed to the therapeutics in time. It is well known that the term golden hour is now a general concept in emergency medicine that is applied to conditions in which hyperacute therapy is more effective than later intervention, including trauma, myocardial ischemia, septic shock, cardiopulmonary resuscitation, and stroke (Hildebrand et al. 2005; Safar 2000; Saver et al. 2010). A pilot retrospective study showed that the first 60 min is the important “golden hour,” which means that patients who receive thrombolytic therapy more immediately will get eusemia (Saver et al. 2010), and there would be the lowest death percentage for patients with occult hypoperfusion (OH) within the first 24 hr, including stroke insult (Blow et al. 1999). Therefore, the 10-min to 1-h postischemia period was the most important “golden hour” in our study, as also shown by switching time of the expression of Ngb and EPOR, which was also the transition time from acute insults to the early recovery phase. A pilot retrospective study also showed that ischemic insults can be mitigated when patients receive proper treatment on the “silver day,” which is 1 to 3 days after ischemia (Safar 2000). Accordingly, our data showed that during the silver day of the 24- to 72-h postischemic insults, the gerbils had transited from the early recovery phase to the late recovery phase of the brain.
In summary, our current results not only showed that the intranasal delivery of Neuro-EPO is a valuable neuroprotective approach for the treatment of ischemia but also suggested that Ngb and the EPO/EPOR system have a close relationship with ischemic insults. Ngb may act as a positive neuroprotective biomarker for brain ischemic insults or brain protection in vertebrates, including humans (Jin et al. 2010). Thus, the overall expression patterns of Ngb at different time points after ischemic insults suggested that it might be an indicator of the neuroprotective action of Neuro-EPO in the brain. Actually, recent studies have also reported that treatment with EPO after brain ischemia could upregulate Ngb expression in the brain (Li P et al. 2009; Li Y and Tang 2010; Ye et al. 2009), concurring with this study. Furthermore, the neuroprotective effects of EPO have various complementary actions, including antagonism of the effects of glutamate, increased expression of antioxidant enzymes, changes in the production of neurotransmitters, and induction of Ngb (Milano and Collomp 2005). In addition, we have recently demonstrated that Ngb has antioxidant and free-radical scavenging activities, providing fundamental evidence for the neuroprotective function of this protein (Li W et al. 2010). Therefore, the neuroprotective functions of Ngb and EPO/EPOR in the brain are probably closely related. The dramatic expression pattern of endogenous Ngb and EPOR is possibly important for improving gerbil survival with intranasal Neuro-EPO treatment. EPO played a neuroprotective role mediated by EPOR accompanied with Ngb upregulation. In addition, the distinctive biphasic expression patterns of Ngb in the gerbil brain are largely associated with ischemic tolerance in the cerebral cortex and ischemic sensitivity in the hippocampus. These data are important for understanding the different reaction properties of different brain regions, which are subjected to ischemic insults at specific time points and also important for improving the therapeutic effects of intranasal Neuro-EPO administration.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the authorship and/or publication of this article.
The author(s) disclosed receipt of the following financial support for the research and/or authorship of this article: This work is supported by the General Program (81070741, 30772293, 30700246) of the National Natural Science Foundation of China, the Special Key Programs for Science and Technology of China (2009ZX09103-616, 2009ZX09503-002, 2009ZX09301-002), the National Basic Research Project (973 program) (2006CB504100), the Major Program for Science and Technology Research of the Beijing Municipal Bureau (7061004), and the Major Project Plan on Science and Technology Research of the Beijing Municipal Bureau (Z07000200540702).
References
- Acker T, Acker H. 2004. Cellular oxygen sensing need in CNS function: physiological and pathological implications. J Exp Biol. 207:3171-3188 [DOI] [PubMed] [Google Scholar]
- Alcalá-Barraza SR, Lee MS, Hanson LR, McDonald AA, Frey WH, II, McLoon LK. 2010. Intranasal delivery of neurotrophic factors BDNF, CNTF, EPO, and NT-4 to the CNS. J Drug Target. 18:179-190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blow O, Magliore L, Claridge JA, Butler K, Young JS. 1999. The golden hour and the silver day: detection and correction of occult hypoperfusion within 24 hours improves outcome from major trauma. J Trauma. 47:964-969 [DOI] [PubMed] [Google Scholar]
- Brines ML, Ghezzi P, Keenan S, Agnello D, de Lanerolle NC, Cerami C, Itri LM, Cerami A. 2000. Erythropoietin crosses the blood-brain barrier to protect against experimental brain injury. Proc Natl Acad Sci U S A. 97:10526-10531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buemi M, Grasso G, Corica F, Calapai G, Salpietro FM, Casuscelli T, Sfacteria A, Aloisi C, Alafaci C, Sturiale A, et al. 2000. In vivo evidence that erythropoietin has a neuroprotective effect during subarachnoid hemorrhage. Eur J Pharmacol. 392:31-34 [DOI] [PubMed] [Google Scholar]
- Burmester T, Haberkamp M, Mitz S, Roesner A, Schmidt M, Ebner B, Gerlach F, Fuchs C, Hankeln T. 2004. Neuroglobin and cytoglobin: genes, proteins and evolution. IUBMB Life. 56:703-707 [DOI] [PubMed] [Google Scholar]
- Burmester T, Weich B, Reinhardt S, Hankeln T. 2000. A vertebrate globin expressed in the brain. Nature. 407:520-523 [DOI] [PubMed] [Google Scholar]
- Butterfield JD, McGraw CP. 1978. Effect of DPPD (diphenyl-para-phenylenediamine) on stroke and cerebral edema in gerbils. Stroke. 9:480-483 [DOI] [PubMed] [Google Scholar]
- Ehrenreich H, Hasselblatt M, Dembowski C, Cepek L, Lewczuk P, Stiefel M, Rustenbeck HH, Breiter N, Jacob S, Knerlich F, et al. 2002. Erythropoietin therapy for acute stroke is both safe and beneficial. Mol Med. 8:495-505 [PMC free article] [PubMed] [Google Scholar]
- Ehrenreich H, Weissenborn K, Prange H, Schneider D, Weimar C, Wartenberg K, Schellinger PD, Bohn M, Becker H, Wegrzyn M, et al. 2009. Recombinant human erythropoietin in the treatment of acute ischemic stroke. Stroke. 40:e647-e656 [DOI] [PubMed] [Google Scholar]
- Erbayraktar S, Grasso G, Sfacteria A, Xie QW, Coleman T, Kreilgaard M, Torup L, Sager T, Erbayraktar Z, Gokmen N, et al. 2003. Asialoerythropoietin is a nonerythropoietic cytokine with broad neuroprotective activity in vivo. Proc Natl Acad Sci U S A. 100:6741-6746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn RW, MacWalter RS, Doney AS. 2008. The cost of cerebral ischaemia. Neuropharmacology. 55:250-256 [DOI] [PubMed] [Google Scholar]
- Gao Y, Wu YH, Zhang CG. 2009. Quantitative analysis of micrographs using integration of mean optical density and total per area [in Chinese]. Bull Acad Military Med Sci. 33:405-408 [Google Scholar]
- Garcia-Rodriguez JC, Sosa-Teste I. 2009. The nasal route as a potential pathway for delivery of erythropoietin in the treatment of acute ischemic stroke in humans. ScientificWorldJournal. 9:970-981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hang X, Li P, Li Z, Qu W, Yu Y, Li H, Shen Z, Zheng H, Gao Y, Wu Y. 2009. Transcription and splicing regulation in human umbilical vein endothelial cells under hypoxic stress conditions by exon array. BMC Genomics. 10:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselblatt M, Ehrenreich H, Siren AL. 2006. The brain erythropoietin system and its potential for therapeutic exploitation in brain disease. J Neurosurg Anesthesiol. 18:132-138 [DOI] [PubMed] [Google Scholar]
- Hildebrand F, van Griensven M, Giannoudis P, Schreiber T, Frink M, Probst C, Grotz M, Krettek C, Pape HC. 2005. Impact of hypothermia on the immunologic response after trauma and elective surgery. Surg Technol Int. 14:41-50 [PubMed] [Google Scholar]
- Hossmann KA. 2008. Cerebral ischemia: models, methods and outcomes. Neuropharmacology. 55:257-270 [DOI] [PubMed] [Google Scholar]
- Iliana S, Yuneidys M, Jorge DG, Nelvis S, Janette C, Adriana M, Yanier N, Julio CG. 2008. Recombinant human erythropoietin as a neuroprotective therapy in brain ischemia. Pharmacol Online. 25:223-229 [Google Scholar]
- Jin K, Mao Y, Mao X, Xie L, Greenberg DA. 2010. Neuroglobin expression in ischemic stroke. Stroke. 41:557-559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulik T, Kusano Y, Aronhime S, Sandler AL, Winn HR. 2008. Regulation of cerebral vasculature in normal and ischemic brain. Neuropharmacology. 55:281-288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakhan SE, Kirchgessner A, Hofer M. 2009. Inflammatory mechanisms in ischemic stroke: therapeutic approaches. J Transl Med. 7:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawner P, Laurent J, Simeone F, Fink E, Rubin E. 1979. Attenuation of ischemic brain edema by pentobarbital after carotid ligation in the gerbil. Stroke. 10:644-647 [DOI] [PubMed] [Google Scholar]
- Li P, He J, Zhang W. 2009. Study of rh-EPO on NGB expression in cerebral cortex after traumatic brain injury in rats [in Chinese]. Chin J Neurosurg Dis Res. 8:101-104 [Google Scholar]
- Li W, Wu Y, Ren C, Lu Y, Gao Y, Zheng X, Zhang C. 2010. The activity of recombinant human neuroglobin as an antioxidant and free radical scavenger. Proteins. September 7 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Li Y, Tang Y. 2010. The effect of erythropoietin on the expression of neuroglobin after cerebral ischemia-reperfusion injury in rats [in Chinese]. China Trop Med. 10:75-76 [Google Scholar]
- Marti HH, Wenger RH, Rivas LA, Straumann U, Digicaylioglu M, Henn V, Yonekawa Y, Bauer C, Gassmann M. 1996. Erythropoietin gene expression in human, monkey and murine brain. Eur J Neurosci. 8:666-676 [DOI] [PubMed] [Google Scholar]
- Milano M, Collomp R. 2005. Erythropoietin and neuroprotection: a therapeutic perspective. J Oncol Pharm Pract. 11:145. [DOI] [PubMed] [Google Scholar]
- Minnerup J, Heidrich J, Rogalewski A, Schabitz WR, Wellmann J. 2009. The efficacy of erythropoietin and its analogues in animal stroke models: a meta-analysis. Stroke. 40:3113-3120 [DOI] [PubMed] [Google Scholar]
- Morishita E, Masuda S, Nagao M, Yasuda Y, Sasaki R. 1997. Erythropoietin receptor is expressed in rat hippocampal and cerebral cortical neurons, and erythropoietin prevents in vitro glutamate-induced neuronal death. Neuroscience. 76:105-116 [DOI] [PubMed] [Google Scholar]
- Safar P. 2000. On the future of reanimatology. Acad Emerg Med. 7:75-89 [DOI] [PubMed] [Google Scholar]
- Sanchez PE, Fares RP, Risso JJ, Bonnet C, Bouvard S, Le-Cavorsin M, Georges B, Moulin C, Belmeguenai A, Bodennec J, et al. 2009. Optimal neuroprotection by erythropoietin requires elevated expression of its receptor in neurons. Proc Natl Acad Sci U S A. 106:9848-9853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saver JL, Smith EE, Fonarow GC, Reeves MJ, Zhao X, Olson DM, Schwamm LH. 2010. The “golden hour” and acute brain ischemia: presenting features and lytic therapy in >30,000 patients arriving within 60 minutes of stroke onset. Stroke. 41:1431-1439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Kastner R, Haberkamp M, Schmitz C, Hankeln T, Burmester T. 2006. Neuroglobin mRNA expression after transient global brain ischemia and prolonged hypoxia in cell culture. Brain Res. 1103:173-180 [DOI] [PubMed] [Google Scholar]
- Shang A, Zhou D, Wang L, Gao Y, Fan M, Wang X, Zhou R, Zhang C. 2006. Increased neuroglobin levels in the cerebral cortex and serum after ischemia-reperfusion insults. Brain Res. 1078:219-226 [DOI] [PubMed] [Google Scholar]
- Sirén AL, Fratelli M, Brines M, Goemans C, Casagrande S, Lewczuk P, Keenan S, Gleiter C, Pasquali C, Capobianco A, et al. 2001. Erythropoietin prevents neuronal apoptosis after cerebral ischemia and metabolic stress. Proc Natl Acad Sci U S A. 98:4044-4049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirén AL, Knerlich F, Poser W, Gleiter CH, Bruck W, Ehrenreich H. 2001. Erythropoietin and erythropoietin receptor in human ischemic/hypoxic brain. Acta Neuropathol. 101:271-276 [DOI] [PubMed] [Google Scholar]
- Sirén AL, Radyushkin K, Boretius S, Kammer D, Riechers CC, Natt O, Sargin D, Watanabe T, Sperling S, Michaelis T, et al. 2006. Global brain atrophy after unilateral parietal lesion and its prevention by erythropoietin. Brain. 129:480-489 [DOI] [PubMed] [Google Scholar]
- Sosa Testé I, García Rodríguez J, García Salman JD, Santana J, Subirós Martínez N, González Triana C, Rodríguez Cruz Y, Cruz Rodríguez J. 2006. Intranasal administration of recombinant human erythropoietin exerts neuroprotective effects on post-ischemic brain injury in Mongolian Gerbils. Pharmacol Online. 1:100-112 [Google Scholar]
- Sun Y, Jin K, Mao XO, Zhu Y, Greenberg DA. 2001. Neuroglobin is up-regulated by and protects neurons from hypoxicischemic injury. Proc Natl Acad Sci U S A. 98:15306-15311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Jin K, Peel A, Mao XO, Xie L, Greenberg DA. 2003. Neuroglobin protects the brain from experimental stroke in vivo. Proc Natl Acad Sci U S A. 100:3497-3500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa P, Bigini P, Mennini T, Agnello D, Laragione T, Cagnotto A, Viviani B, Marinovich M, Cerami A, Coleman TR, et al. 2003. Erythropoietin selectively attenuates cytokine production and inflammation in cerebral ischemia by targeting neuronal apoptosis. J Exp Med. 198:971-975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Liu J, Zhu H, Tejima E, Tsuji K, Murata Y, Atochin DN, Huang PL, Zhang C, Lo EH. 2008. Effects of neuroglobin overexpression on acute brain injury and long-term outcomes after focal cerebral ischemia. Stroke. 39:1869-1874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiessner C, Allegrini PR, Ekatodramis D, Jewell UR, Stallmach T, Gassmann M. 2001. Increased cerebral infarct volumes in polyglobulic mice overexpressing erythropoietin. J Cereb Blood Flow Metab. 21:857-864 [DOI] [PubMed] [Google Scholar]
- Ye S, Tang Y, Li Y. 2009. Protective effects of erythropoietin against cerebral ischemia-reperfusion injury [in Chinese]. J Int Neurol Neurosurg. 36:391-394 [Google Scholar]
- Yu YP, Xu QQ, Zhang Q, Zhang WP, Zhang LH, Wei EQ. 2005. Intranasal recombinant human erythropoietin protects rats against focal cerebral ischemia. Neurosci Lett. 387:5-10 [DOI] [PubMed] [Google Scholar]
- Zhang C, Wang C, Deng M, Li L, Wang H, Fan M, Xu W, Meng F, Qian L, He F. 2002. Full-length cDNA cloning of human neuroglobin and tissue expression of rat neuroglobin. Biochem Biophys Res Commun. 290:1411-1419 [DOI] [PubMed] [Google Scholar]


