Abstract
The sorting nexin (SNX) family proteins, which contain a Phox homology (PX) domain, play crucial roles in regulating the intracellular membrane trafficking of the endocytic pathway. The proper coordination of this pathway is important for axonal elongation; however, little is known about the expression and intracellular dynamics of the SNX members during the formation of the nervous system. Here the authors found that SNX18, which belongs to the Src-homology-3-PX-Bin/Amphiphysin/Rvs domain-containing SNX subfamily, was specifically expressed in differentiating motor neurons in the chick and mouse embryonic spinal cord. The expression of SNX18 in embryonic spinal motor neurons was transient and was downregulated as the neurons matured. The authors further demonstrated that the localization of EGFP-SNX18 in growth cones was dynamically regulated and accumulated especially at areas in contact with permissive substrates. These findings collectively suggest that SNX18 may play an active role in axonal elongation.
Keywords: sorting nexin, SNX18, SNX9-family, motor neuron, DRG neuron, growth cone
Axonal growth is precisely regulated by the concerted actions of multiple factors, ranging from extracellular guidance cues and the factors that recognize them to the intracellular molecules that process such information and alter growth cone behavior (Chilton 2006; Geraldo and Gordon-Weeks 2009). To facilitate the progression of this complex process, the participating molecules need to be actively distributed and sorted. Given that the expression of axon guidance factors is tightly controlled throughout the process of neural circuit formation, it is plausible that the expression of some of their regulatory molecules is also developmentally regulated. However, little has been reported about the differential expression of molecules involved in intracellular protein sorting during nervous system development.
The sorting nexin (SNX) family proteins are cytoplasmic/membrane-associated proteins containing a phosphoinositide-binding module called the Phox homology (PX) domain, which targets these proteins to phosphoinositide-enriched membranes (Ellson et al. 2002). SNXs play crucial roles in the endosomal sorting system that regulates the intracellular trafficking of plasma membrane receptors (Worby and Dixon 2002; Carlton et al. 2005; Seet and Hong 2006; Cullen 2008). The SNX family has more than 30 members, which are categorized into subgroups according to their structural organization and function. This diversity of SNX family members may reflect the complexity and variety of the endocytic system, of which parts must be regulated differently in different neurons during neural circuit formation.
The SNX9 family is a subgroup of SNXs consisting of SNX9, SNX18, and SNX33 (Lundmark and Carlsson 2009). These three proteins share the same structural architecture: Src-homology-3 (SH3) at the N-terminus followed by PX and Bin/Amphiphysin/Rvs (BAR) domains; these features are not shared by other SNX molecules (Cullen 2008). SNX9 is a major binding partner of dynamin, and it is required for efficient clathrin-mediated endocytosis (Lundmark and Carlsson 2004; Soulet et al. 2005). SNX9 also interacts with N-WASP through its SH3 domain, to couple actin dynamics with membrane remodeling during endocytosis (Yarar et al. 2007; Shin et al. 2007), further indicating its important role in regulating the endocytic pathway. All the SNX9 family members interact and co-localize with dynamin 2 (Håberg et al. 2008), suggesting that they may function in part through a common molecular mechanism. Interestingly, the SNX9 family proteins are differentially localized in HeLa cells; SNX18 interacts and partly co-localizes with AP-1 but not AP-2 in these cells (Håberg et al. 2008), whereas SNX9 interacts with AP-2 (Lundmark and Carlsson 2003). These observations collectively suggest that the SNX9 family members may play different roles in the complex endosomal sorting machinery, with some members being selectively used during nervous system development.
Here we report that the expression of SNX18 is regulated in embryonic spinal neurons. SNX18 was specifically expressed by newly generated motor neurons in the chick and mouse embryonic spinal cord, and its expression was later downregulated as the motor neurons matured. We also observed that the localization of EGFP-SNX18 was dynamically regulated in growth cones. Collectively, our findings suggest that this member of the SNX9 family could play a specific role in neural circuit formation.
Materials and Methods
Isolation of Chick and Mouse TY35/SNX18 cDNAs
The cDNA fragment of the 3′-UTR, obtained by screening genes selectively expressed by chick embryonic motor neurons (Tanabe et al. 1998; Fujimura et al. 2006), was used to isolate longer cDNAs to identify their coding sequences. Of these, one named TY35 was later identified as the chick ortholog of SNX18. Mouse TY35/SNX18 cDNAs were isolated from a cDNA library made from the brain of a neonatal mouse under low-stringency hybridization and washing conditions, using the coding regions of chick TY35/SNX18 as a probe. The cDNAs for mouse and chick SNX9 and a fragment of SNX33 were purchased from Invitrogen (Carlsbad, CA) or Delaware Biotechnology Institute (Newark, DE). The cRNA probes were prepared from these plasmids (3.6 kb, 2.2 kb, 3 kb, and 3.1 kb for chick SNX9, SNX18, and SNX33 and mouse SNX18, respectively).
In Situ Hybridization
In situ hybridization was performed as described (Schaeren-Wiemers and Gerfin-Moser 1993; Tsuchida et al. 1994). Briefly, embryos were fixed in 4% paraformaldehyde (PFA)/PBS overnight at 4C. The fixed embryos were successively placed in 10%, 20%, and 30% sucrose solutions overnight. The fixed and cryoprotected embryos were embedded in OCT compound (Sakura Finetech; Tokyo, Japan) and sectioned into serial 12-µm sections on a CM3000 cryostat (Leica Microsystems; Wetzler, Germany). The resultant sections were postfixed in 4% PFA/PBS at room temperature (RT) for 5 min, washed three times with PBS, and incubated in 1 µg/ml Proteinase K (Roche Applied Science; Penzburg, Germany) in 6.25 mM EDTA pH 8.0 (Dojindo Laboratories; Kumamoto, Japan) and 50 mM Tris pH 7.5 (Wako Pure Chemical Industries; Osaka, Japan) at RT for 5 min. The sections were refixed in 4% PFA/PBS at RT for 5 min, washed three times with PBS, and acetylated in 1.33% triethanol amine (Sigma-Aldrich; St. Louis, MO) and 0.75% acetic anhydride solution (Wako Pure Chemical Industries) at RT for 10 min. The acetylated sections were washed three times with PBS and incubated in hybridization buffer (50% formamide [Sigma-Aldrich], 0.25 mg/ml yeast RNA [Sigma-Aldrich], 0.5 mg/ml herring sperm DNA [Roche Applied Science], 5× Denhard’s [Sigma-Aldrich], 5× SSC [0.75 M NaCl, 75 mM sodium citrate, pH 7.0]) at RT for 2 hr, then with digoxigenin-labeled cRNA probes in hybridization buffer (~1 ng/µl) at 72C for 16 hr. The hybridized sections were washed in 5× SSC at 72C for 10 min and then in 0.2× SSC for 1 hr. The washed sections were incubated with 10% heat-inactivated goat serum (Roche Applied Science) in 100 mM Tris pH 7.5 and 0.15 M NaCl solution at RT for 1 hr, followed by incubation with alkaline phosphatase-conjugated anti-digoxigenin antibody (Roche Applied Science; 1:5000) in the same solution at 4C overnight. The sections were washed three times with 100 mM Tris pH 7.5 and 0.15 M NaCl solution and twice with 100 mM Tris pH 9.5, 0.1 M NaCl, and 50 mM MgCl2 solution, followed by incubation with NBT/BCIP (Roche Applied Science) in the same solution containing 0.24 mg/ml levamisole (Sigma-Aldrich) at RT in the dark. The reaction was stopped by immersing the sections in PBS–5 mM EDTA. Whole-mount in situ hybridization was performed as described (Streit and Stern 2001) using the same probe. Briefly, the fixed embryos were washed with PBS three times and immersed in absolute methanol. The embryos were rehydrated in PBT (PBS–0.1% Tween 20 [Sigma-Aldrich]), bleached for 1 hr in 6% H2O2, and digested for 10 min in 10 µg/ml Proteinase K/PBT at RT. The embryos were washed with PBT three times and postfixed for 20 min at RT in 0.2% glutalaldehyde (Sigma-Aldrich)–4% PFA/PBT. After washing with PBT, the treated embryos were prehybridized in hybridization solution (50% formamide, 5× SSC, 50 µg/ml yeast RNA, 50 µg/ml heparin [Sigma-Aldrich]) for 2 hr at 65C followed by hybridization with the indicated cRNA probes (~0.2 ng/µl) overnight at 65C. The embryos were washed twice with 50% formamide, 5× SSC, and 1% SDS solution at 65C for 30 min and three times with TBS-T (20 mM Tris pH 7.5, 0.13 M NaCl, 0.1% Tween 20). The washed embryos were incubated with 20% heat-inactivated goat serum in TBS-T for 2 hr at RT, followed by incubation with alkaline phosphatase-conjugated anti-digoxigenin antibody (1:5000) in the same solution at 4C overnight. The embryos were washed with TBS-T three times for 5 min and four times for 1 hr at RT and overnight at 4C. The washed embryos were incubated with 100 mM Tris pH 9.5, 0.1 M NaCl, 50 mM MgCl2, and 0.1% Tween 20 solution followed by incubation with NBT/BCIP in the same solution containing 0.24 mg/ml levamisole at RT in the dark. The reaction was stopped by immersing the sections in PBS–5 mM EDTA. The developmental stages of chick embryos were determined according to the table of Hamburger and Hamilton (1951).
Plasmids
Mouse N-WASP cDNA was purchased from Invitrogen. For the construction of pACT-N-WASP, a cDNA fragment of N-WASP containing the whole open reading frame was inserted into the EcoRI site of pACT2 (Clontech; Mountain View, CA). pAS-SNX18, pAS-SNX18N, pAS-SNX18C, and pAS-SNX18SH3 were generated by inserting the NcoI, NcoI-StuI, NaeI-ScaI, and NcoI-SmaI fragments of mouse SNX18 cDNA, respectively, into the NcoI or NcoI-SmaI site of pAS2 (Clontech). pEGFP-SNX18 was prepared by inserting the BamHI-EcoRI fragment of mouse SNX18 cDNA into the BglII-EcoRI site of pEGFP-C1 (Clontech). pDsRed-NWASP was generated by inserting the corresponding coding sequences into the HindIII-EcoRI site of pDsRed-Monomer (Clontech).
Generation of an Anti-SNX18 Antibody
An expression construct for producing a glutathione S-transferase (GST) fusion protein of mouse SNX18 (1-232) in Escherichia coli was generated by inserting the BamHI-StuI fragment of the mouse SNX18 cDNA fragment into the BamHI-SmaI site of pGEX-5X-3 (Pharmacia; Stockholm, Sweden). The purified recombinant proteins were used to immunize guinea pigs. Immunohistochemistry was performed as described (Fujimura et al. 2006). The mouse anti-Islet1/2 antibody was purchased from Developmental Studies Hybridoma Bank (Iowa City, IA). Donkey secondary antibodies (which show minimal cross-reaction to the serum proteins of other species) were purchased from Jackson Immuno Research Laboratories (West Grove, PA).
Ectopic Generation of Motor Neurons
Ectopic motor neurons were generated as described (Briscoe et al. 2000) by introducing RCAS-Nkx6.1 (gift from Dr. Jessell, Columbia University) into HH12 chick embryonic spinal cord using a CUY21 electroporator (NEPA GENE; Chiba, Japan). After electroporation, the eggs were incubated for 2 days at 39C, and the embryos were fixed in 4% PFA/PBS for 2 hr at 4C. The fixed embryos were cryoprotected in 30% sucrose, embedded in OCT compound, and sectioned into 12-µm slices on a CM3000 cryostat for immunohistochemistry as described above.
Yeast Two-Hybrid Assay
Mouse N-WASP cDNA was purchased from Invitrogen. For the construction of pACT-N-WASP, the cDNA fragment of N-WASP containing the whole open reading frame was inserted into the EcoRI site of pACT2. pAS-SNX18, pAS-SNX18 (1-232), pAS-SNX18 (182-615), and pAS-SNX18SH3 were generated by inserting the NcoI, NcoI-StuI, NaeI-ScaI, and NcoI-SmaI fragments of mouse SNX18 cDNA, respectively, into the NcoI, NcoI-SmaI, or SmaI site of pAS2 (Clontech). The yeast two-hybrid assay was performed by following the manufacturer’s instructions (Clontech). Briefly, the indicated pAS2-derived plasmids and pACT2 or pACT-N-WASP were co-transfected into Y190 cells by the lithium acetate-mediated method. The resultant transformants were grown on SD-Leu-Trp-His plates containing 50 mM 3-amino-1,2,4-triazol (Sigma-Aldrich).
GST Pull-Down Assay
The N-WASP cDNA was inserted into the EcoRI site of pcDNA3.1 (Invitrogen) to produce 35S-labeled N-WASP protein, using a TNT-coupled reticulocyte lysate system, following the manufacturer’s instructions (Promega; Madison, WI). GST- or GST-SNX18 (1-232)–bound beads were prepared by incubating 50 µg of the purified proteins with 10 µl of Glutathione-Sepharose 4B beads (GE Healthcare; Piscataway, NJ) for 10 min at room temperature, followed by washing twice with PBS–0.1% Triton-X100 (Sigma-Aldrich). The resultant beads were preincubated at 4C for 30 min with the reticulocyte lysate to minimize nonspecific interactions. Then, 25 µl of the resultant lysate containing the 35S-labeled N-WASP was incubated with 5 µl of GST- or GST-SNX18 (1-232)–bound beads at 4C for 30 min with occasional mixing. The beads were extensively washed with PBS–0.1% Triton X100 and subjected to SDS-PAGE. The resultant gel was dried and autoradiographed.
Phospholipid Filter Binding Assay
The phospholipid filter binding assay was performed using PIP MicroStrips (Invitrogen), following the manufacturer’s instructions. Briefly, each PIP MicroStrip filter was incubated with 1 ng/µl purified GST-SNX18 (1-232) or GST-SNX18 (182-411) in TBS (10 mM Tris-HCl pH 8.0, 150 mM NaCl)–0.1% Tween 20 (Sigma-Aldrich) containing 30 mg/ml fatty acid–free BSA (Sigma-Aldrich) for 30 min at room temperature. After extensively washing the filters with TBS–0.1% Tween 20, the bound GST fusion proteins were detected using an anti-GST antibody (Medical and Biological Laboratories; Nagoya, Japan) and visualized by DAB staining (Vector; Burlingame, CA). For the preparation of GST-SNX18 (182-411), the fragment of mouse SNX18 PCR-amplified using the following oligonucleotides as a primer (CGCACTAGTCGCTACCGCCTGTCCACCCGCT and CCGCTCGAGTCAGTCCAGGGCGGCGGCT) was digested with SpeI and XhoI and inserted into the SpeI-XhoI site of pET42b (Merck; Darmstadt, Germany). The resultant expression plasmid was transfected into BL21(DE3);pLysS cells (Merck). The cells were cultured and treated with 0.5 mM isopropyl-β-D-thiogalactopyranoside (IPTG) for 5 min at 30C to minimize aggregation of the protein product. The produced GST fusion protein was immediately purified from the cell lysate, and the purified protein (~20 ng/µl) was quickly frozen in small aliquots.
Live-Cell Imaging
CAD mouse neuroblastoma cells were plated on a chambered coverglass (Nalge Nunc; Rochester, NY) and cultured in DMEM/F12 (1:1) containing 8% calf serum. pEGFP-SNX18 and pDcRed-N-WASP were co-transfected with Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. Twenty-four hours after transfection, the culture medium was changed to DMEM/F12 (1:1; without phenol red) containing 8% calf serum and 25 mM HEPES (pH 7.4), and the cells were observed using an LSM510 confocal microscope (Carl Zeiss; Jena, Germany).
Observation of EGFP-SNX18 in the Growth Cones of Elongating DRG Neurons
pEGFP-SNX18 was electroporated into the neural tube of HH 12 chick embryos as described previously (Arakawa et al. 2008). At this stage, the expression plasmids could be introduced into the neural crest cells that become DRG neurons. After electroporation, the eggs were incubated for 5 days at 39C, and the embryos were dissected in L-15 medium (Sigma-Aldrich) under a fluorescence microscope MZ10F (Leica Microsystems). The DRGs showing faint green fluorescence were collected and plated on a polyethyleneimine-coated glass-bottom dish (Matsunami Glass; Kishiwada, Japan). The dissected DRG explants were cultured in DMEM/F12 containing 1xITS3, 10 ng/ml NGF, and 10 ng/ml NT3 (Sigma-Aldrich) at 37C for 16 hr. The fluorescent signals in the growth cones of elongated axons were observed, and time-lapse images were collected at 37C with a BZ-9000 microscope (Keyence; Osaka, Japan).
Results
SNX18 is specifically and transiently expressed by motor neurons in the embryonic chick and mouse spinal cord
In the course of searching for genes selectively expressed in chick embryonic motor neurons, using a single-cell PCR technique (Fujimura et al. 2006), we isolated a cDNA fragment of chick SNX18. SNX18 encodes a protein containing one PX domain in its middle region (Fig. 1A) and is categorized as a member of the SNX family proteins. To verify that SNX18 was expressed in motor neurons of the developing spinal cord, we performed in situ hybridization. In the developing chick brachial spinal cord, SNX18 expression was first observed in some ventral neurons around Hamburger and Hamilton stage (HH) 15, when the motor neurons begin to be generated (Hollyday and Hamburger 1977), as confirmed by the expression of Isl1, a marker for motor neurons at this stage, in the same regions (Fig. 1B-a and b). At HH20, SNX18 was robustly expressed by ventral spinal neurons, whose position was confirmed by the expression of Isl1 (Fig. 1B). The whole-mount in situ hybridization of HH20 embryos showed that SNX18 was expressed in the ventral spinal cord along the entire A-P axis where motor neurons reside (Fig. 2B). These observations collectively suggested that SNX18 was selectively expressed by embryonic motor neurons in the spinal cord.
Figure 1.
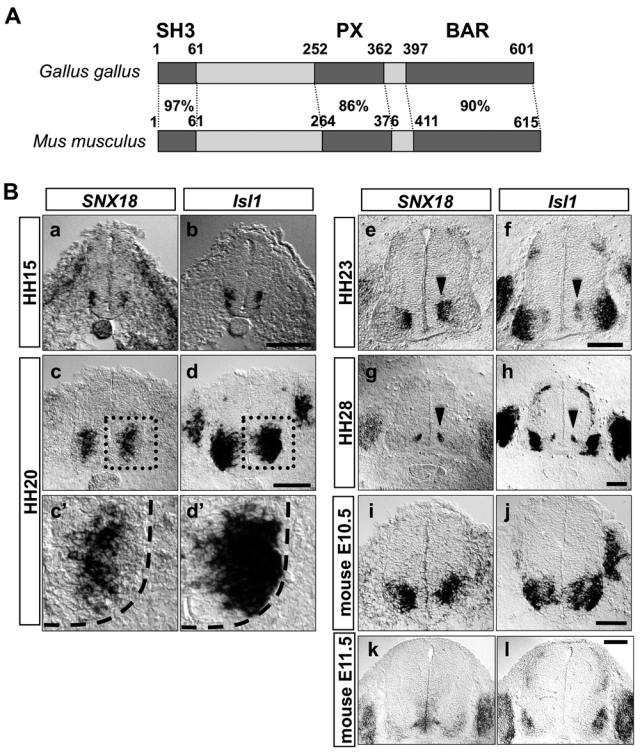
SNX18 is specifically and transiently expressed by motor neurons in the embryonic spinal cord. (A) Primary structures of chick and mouse SNX18. The percentage of identical amino acid residues between the structural domains of the chick and mouse orthologs is indicated. (B) SNX18 expression in the embryonic spinal cord. The expression of SNX18 in the brachial-level embryonic spinal cord was observed by in situ hybridization in chick embryos at HH15 (a), HH20 (c), HH23 (e), and HH28 (g) and in mouse at E10.5 (i), E11.5 (k). The expression of Isl1 in the adjacent sections (b, d, f, h, j, l) is shown to indicate the areas where motor neurons reside. The magnified view of the dotted boxes in c and d is indicated in c′ and d′. The outline of the spinal cord is shown by a dashed line in c′ and d′. The expression of SNX18 was downregulated in the chick and mouse motor neurons that moved laterally and became mature. Highest expression of SNX18 at HH23 and HH28 is observed in the region where newly generated motor neurons reside, shown by the expression of Isl1 (e-h, arrowheads). DRG neurons started expressing SNX18 around HH23. Scale bar: 100 µm.
Figure 2.
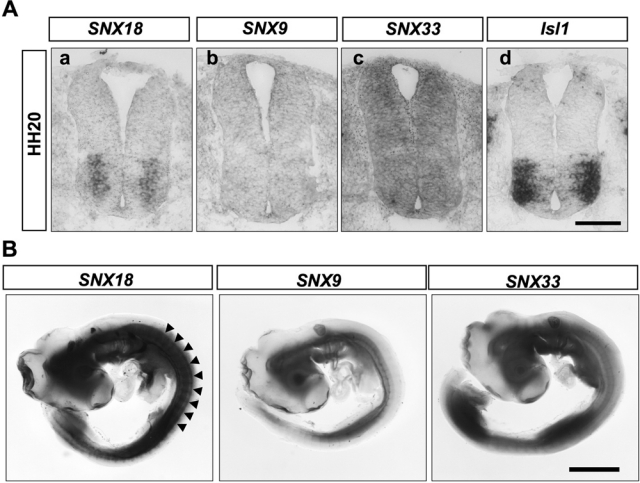
SNX9 family members are differently expressed in chick HH20 embryo. Expression of the indicated SNX9 family members in HH20 chick spinal cord (A) and whole embryo (B). The column of spinal motor neurons expressing SNX18 is partly highlighted by the line of arrowheads. Scale bar: 100 µm (A) and 2 mm (B).
Interestingly, the signal strength of the SNX18 expression in motor neurons decreased as the neurons moved laterally, which was clearly seen at HH20 to HH23 (Fig. 1B and Suppl. Fig. S1), when the spinal motor neurons are most rapidly generated (Ericson et al. 1992). At HH20, all embryonic motor neurons generated by this stage express Isl1. Of the Isl1-expressing motor neurons, SNX18 mRNA was barely detected in the laterally located motor neurons that were generated earlier. At HH23, Isl1 was expressed by newly born motor neurons residing near the ventricular zone (Fig. 1B-f, pointed by the arrowhead) and LMCm motor neurons located in the ventral horn (Fig. 1B-f). SNX18 mRNA was detected around the region where newly generated Isl1-positive neurons reside (Fig. 1B-e, pointed by the arrowhead) but rarely detected in mature LMCm motor neurons. At HH28, SNX18 mRNA was detected near the ventricular zone at the area where Isl1-expressing neurons were still observed (Fig. 1B-g and h, pointed by arrowheads). These observations collectively indicated that expression of SNX18 was observed around the area where newly generated motor neurons reside, further suggesting that the expression of SNX18 is transient in motor neurons, peaking soon after their birth and then becoming downregulated as these neurons mature. SNX18 expression was almost undetectable in the spinal cord at HH28 (Fig. 1B-g), confirming that little SNX18 was expressed by mature motor neurons. The expression profiles of two closely related family members, SNX9 and SNX33, were dissimilar to that of SNX18 (Fig. 2 and Suppl. Fig. S1).
To examine whether this characteristic expression takes place in other animals, we isolated the mouse ortholog of chick SNX18. The deduced amino acid sequence of mouse SNX18 shared high identity with the chick ortholog (82% identity; Fig. 1A), suggesting that SNX18 plays an evolutionally conserved role. As anticipated, the mouse SNX18 was also specifically and transiently expressed in the ventral spinal cord, where motor neurons reside at E10.5 (Fig. 1B). Its expression became faint at E11.5 (Fig. 1B), indicating that SNX18 functions at relatively early stages of motor neuron differentiation and maturation. Later in development, the dorsal root ganglia neurons began to express SNX18 (Fig. 1B).
To confirm whether SNX18 protein was selectively expressed by motor neurons, we generated an anti-SNX18 antibody. The antibody specifically recognized exogenous chick and mouse SNX18 expressed in Neuro2a cells but did not react with mouse SNX9 (Suppl. Fig. S2). Immuno-histochemistry using the prepared anti-SNX18 antibody clearly showed the SNX18 protein around all and only the ventral Isl1/2-positive nuclei. Because Isl2 is also expressed by LMCl motor neurons that cease expressing Isl1 soon after their births (Tsuchida et al. 1994), this anti-Isl1/2 antibody can label all motor neurons in the ventral spinal cord. This observation confirmed that the SNX18 protein was specifically expressed by motor neurons in both the mouse and chick developing spinal cord (Fig. 3A). Next, to test whether SNX18 was expressed by any type of motor neurons, we verified its expression in artificially and ectopically induced motor neurons. The misexpression of a homeobox-containing transcription factor Nkx6.1 induces motor neurons ectopically (Briscoe et al. 2000; Fig. 3C). SNX18 was also expressed in these ectopically induced motor neurons (Fig. 3C, in the area surrounded by the dotted line). This observation, together with the fact that we failed to find any spinal motor neurons that did not express SNX18 (data not shown), strongly suggests that SNX18 is expressed by all types of spinal motor neurons.
Figure 3.
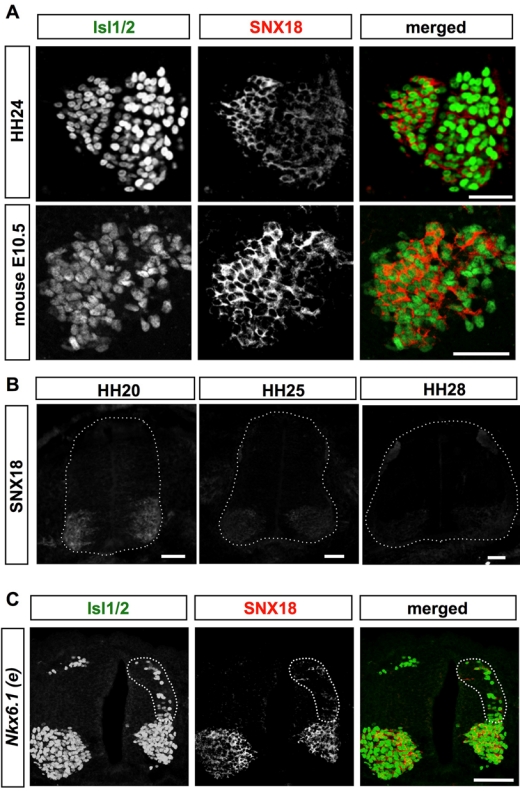
SNX18 protein is expressed in embryonic motor neurons and in ectopically induced chick embryonic spinal motor neurons. (A) Localization of SNX18 protein in the ventral spinal cord of chick HH24 and mouse E10.5 embryos. The indicated proteins were visualized by FITC–anti-mouse (Isl1/2) and Cy3–anti–guinea pig (SNX18) antibodies. Scale bar: 50 µm. (B) Distribution of SNX18 at HH20, 25, and 28 chick spinal cord. The chick embryos were simultaneously fixed, embedded, cryosectioned, and immunostained with anti-SNX18 antibody. Images were taken from the same slide in the same conditions. Scale bar: 50 µm. (C) SNX18 is expressed by ectopically generated motor neurons. An expression plasmid for rat Nkx6.1 was introduced into the right side of spinal neuron progenitors by in ovo electroporation at HH12. The ectopically generated motor neurons expressing Isl1/2 at the dorsal region of the electroporated side (surrounded by dotted line) expressed SNX18. Scale bar: 100 µm.
SNX18 proteins were detected in virtually all motor neurons at HH24 (Fig. 3A), supporting the conclusion that SNX18 is once expressed by all motor neurons. Considering that SNX18 mRNA was barely detected in earlier generated LMCm neurons by HH23 (Fig. 1B), this observation may also suggest that SNX18 protein is relatively stable in the cell. The relative amounts of SNX18 protein in the motor neuron cell bodies were estimated by immunohistochemistry using a simultaneously prepared specimen. SNX18 proteins in the motor neuron cell bodies decreased over time but were still slightly detectable at HH28 (Fig. 3B), confirming that SNX18 is transiently expressed by embryonic motor neurons.
SNX18 interacts with N-WASP through its SH3 domain, and its PX domain binds to a broad range of phosphoinositides
It has been shown that SNX9 family members share the ability to bind dynamin through their SH3 domain (Håberg et al. 2008; Schöbel et al. 2008). N-WASP is another molecule that interacts with SNX9 and SNX33 through their SH3 domain (Yarar et al. 2007; Zhang et al. 2009); however, it is not reported whether SNX18 also binds to N-WASP. To test this, we performed a yeast two-hybrid assay. In this assay, SNX18 showed an interaction with N-WASP, and its SH3 domain was essential and sufficient for the binding (Fig. 4A). This interaction was further confirmed by a pull-down assay using in vitro–translated N-WASP, in which 35S-methione-labeled N-WASP bound GST-SNX18 (1-232) (Fig. 3B). These findings together indicated that SNX18 interacts with N-WASP. Moreover, the coexpression of SNX18 and N-WASP in CAD mouse neuroblastoma cells showed they were co-localized in live cells as previously reported for SNX9 (Yarar et al. 2007), further supporting the idea that SNX18 interacts with N-WASP.
Figure 4.
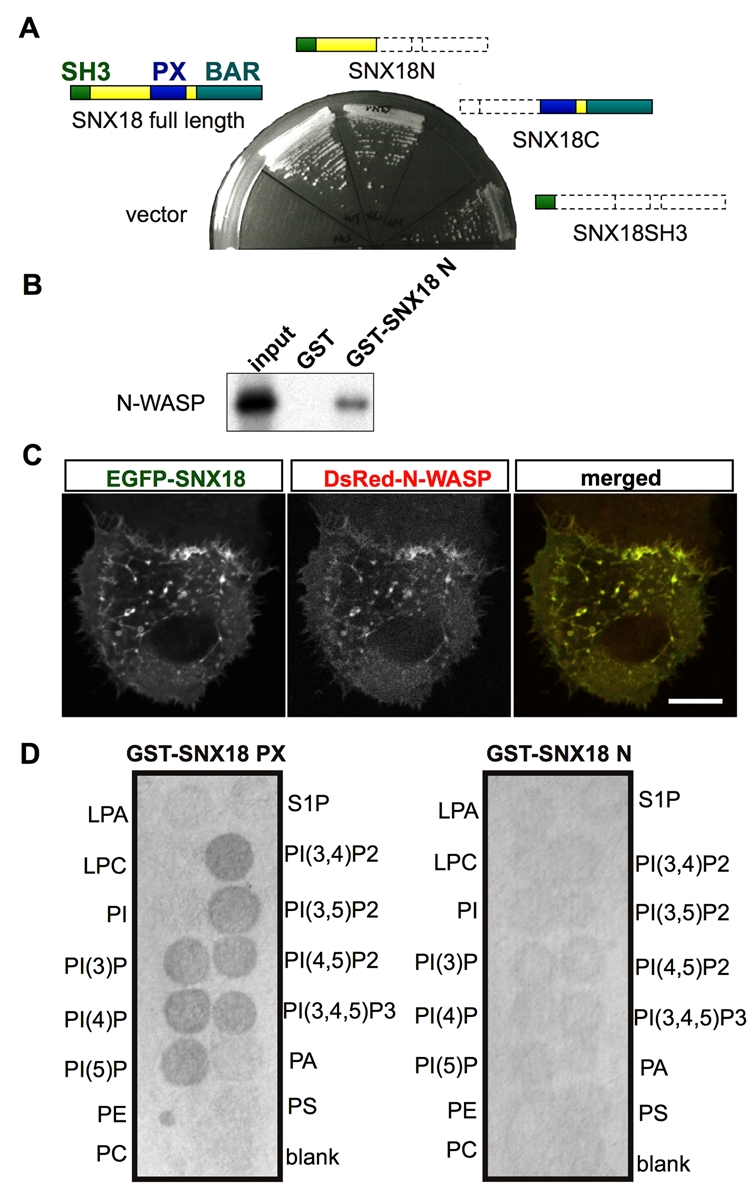
SNX18 interacts with N-WASP and phosphatidylinositol phosphates. (A) Yeast two-hybrid analysis. The constructs expressing the indicated SNX18 deletion mutants were co-introduced with N-WASP-expressing constructs. (B) Glutathione S-transferase (GST) pull-down assay. 35S-labeled N-WASP was incubated with GST- or GST-SNX18 (1-232)–bound beads. The beads were extensively washed and subjected to SDS-PAGE. The resultant gel was dried and autoradiographed. (C) Confocal image of live CAD cells expressing EGFP-SNX18 and DsRed-N-WASP. Scale bar: 10 µm. (D) PIP filter-binding assay. The GST fusion proteins of the SNX18 PX domain (GST-SNX18PX) or the N-terminal region before the PX domain (GST-SNX18N) were incubated with the filter, and the bound proteins were detected by an anti-GST antibody. LPA, lysophosphatidyl acid; LPC, lysophosphatidylcholine; PI, phosphatidylinositol; PI(3)P, phosphatidylinositol 3-phosphate; PI(4)P, phosphatidylinositol 4-phosphate; PI(5)P, phosphatidylinositol 5-phosphate; PE, phosphatidylethanolamine; PC, phosphatidylcholine; S1P, sphingosine 1-phosphate; PI(3,4)P2, phosphatidylinositol 3,4-diphosphate; PI(3,5)P2, phosphatidylinositol 3,5-diphosphate; PI(4,5)P2, phosphatidylinositol 4,5-diphosphate; PI(3,4,5)P3, phosphatidylinositol 3,4,5-triphosphate; PA, phosphatidic acid; PS, phosphatidylserine.
The PX domain is a phosphoinositide-binding domain (Ellson et al. 2002), and each PX domain of the different SNX family members shows a different PIP-binding activity (Seet and Hong 2006; Cullen 2008). Full-length SNX9 binds liposomes containing a relatively broad range of PIPs (Lundmark and Carlsson 2003; Yarar et al. 2008), whereas the SNX9 PX domain alone preferentially binds to PIP3 spotted on a filter (Badour et al. 2007). The PX and BAR lipid-binding domains of SNX9 also act cooperatively to promote PIP-containing lipid targeting and tubulation (Pylypenko et al. 2007), and the broad specificity of SNX9 seems to be conferred by structures outside of the PX domain. Full-length SNX18 was previously shown to preferentially bind liposomes containing PIP2s (Håberg et al. 2008). We therefore performed a filter-binding assay to examine whether the lipid-binding preference of the SNX18 PX domain was narrow or broad. We found that GST-SNX18 (182-411), which consisted of SNX18’s PX domain, bound all of the PIPs spotted on a filter (Fig. 4D), suggesting that the broad lipid-binding potential of the PX domain of SNX18 may be modulated by the BAR domain to ensure a stricter PIP-binding preference.
The localization of SNX18 is dynamically regulated in the growth cones of DRG neurons
Transient expression of SNX18 in motor neurons at their births, when motor neurons start extending their axons out from the spinal cord, suggested that SNX18 might play a role in axonal growth regulation. Because axonal growth is closely correlated with the behavior of growth cones, we used chick embryonic DRG neurons, which express SNX18 after HH23, because their growth cones are readily visible.
Because SNX9’s expression is known to change dynamically in live cells (Yarar et al. 2007), we investigated the distribution of EGFP-SNX18 proteins in the growth cones of living DRG neurons during axon elongation. Plasmids expressing EGFP-SNX18 were electroporated into the neural tubes of HH10 to HH12 chick embryos to introduce the expression plasmids into neural crest cells. To minimize the possible side effects caused by the overexpression of EGFP-SNX18, we selected and cultured DRGs that only faintly expressed the EGFP-SNX18 protein. As shown in Figure 5A, the distribution of the EGFP-SNX18 protein in the growth cones was very dynamic. It appeared transiently as a radial line, and the fluorescence then disappeared within a minute (Fig. 5A, arrowheads). In the course of axonal growth, the EGFP-SNX18 protein also appeared to be actively redistributed where growth cones formed contacts with permissive substrates (Fig. 5B, arrowheads). These observations collectively suggest that SNX18 may function in the growth cones to affect the elongation of peripheral nerves.
Figure 5.
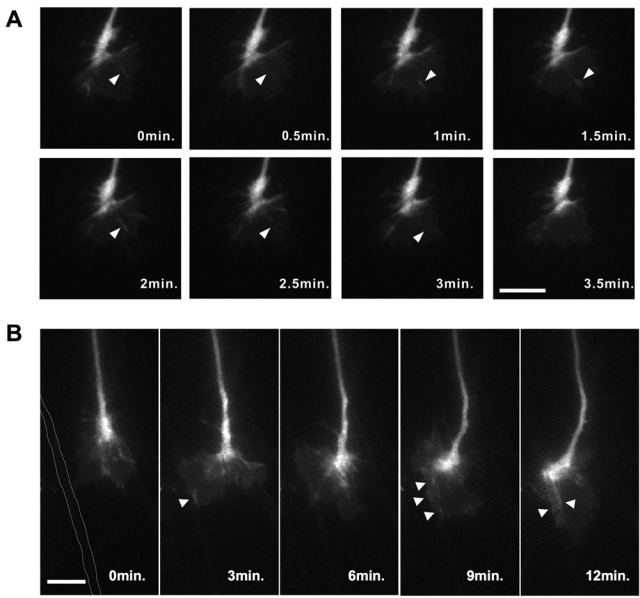
SNX18 is dynamically distributed in the growth cones of elongating DRG neurons. (A) Time-lapse images of EGFP-SNX18 in the growth cone of an elongating chick DRG axon. Arrowheads highlight the transient radial accumulation of EGFP-SNX18. Scale bar: 20 µm. (B) Time-lapse images of an elongating EGFP-SNX18-expressing DRG axon. The axon shaft that attracted the growth cone is highlighted by white dotted lines at 0 min. EGFP-SNX18 proteins were actively and dynamically distributed along the contact area (arrowheads). Scale bar: 20 µm.
Discussion
Here we provide evidence that the expression of SNX18 is differentially regulated during neural development. In the embryonic spinal cord, SNX18 expression was obvious in newly generated motor neurons. Our observations suggest that the endosomal system could be dynamically controlled by the expression of the appropriate components in accordance with a cell’s particular needs. The relative amounts of SNX9 family proteins are known to vary among tissues in mice (Håberg et al. 2008), collectively suggesting that they function in cell- and tissue-specific manners. It is still unknown which, if any, aspect of motor neuron differentiation and/or axonal elongation requires SNX18; however, the specificity and transience of its expression suggest that it might play a role in responding to situations that are specifically experienced just after these neurons are born. In the central nervous system, motor neurons are the only ones that project their axons into the periphery. Soon after motor neurons are generated, they begin to elaborate axons, and their growth cones must exit the ordered neuroepithelial tissue of their birth to enter the relatively unstructured region of the outer mesenchymal cells, which are surrounded by a different extracellular matrix. That is, the growth cones immediately encounter a completely novel environment. SNX18 might help motor neurons prepare for and/or deal with these drastic changes by facilitating the intracellular trafficking of plasma membrane receptors. Further investigations are obviously required to examine these possibilities.
A characteristic transience of the SNX18 expression in embryonic spinal motor neurons was observed in both the chick and mouse, suggesting it might be functionally relevant. It is possible that SNX18 later disrupts the proper regulation of the endosomal pathway in motor neurons if its expression is maintained. In addition, although the SNX18 mRNA of laterally located earlier-born motor neurons at HH20 was below the detection level of our ISH, the SNX18 protein was still observed in the motor neuron cell bodies even at HH24, suggesting that once produced, the SNX18 protein is relatively stable. Our observations collectively suggest that the expression of SNX18 in motor neurons may have to be transient because the level of SNX18 protein would soon become too high to support normal axonal elongation. That prolonged expression of exogenous SNX18 by in ovo electroporation slightly but significantly attenuated the growth of motor axons (SN, unpublished observation) may support our speculation. Future studies aimed at determining the specific functional role of SNX18 in the endosomal pathway should help explain the functional significance of this characteristic transience of the SNX18 expression.
We have also showed evidence suggesting that SNX18 may function with N-WASP in the cells. During preparation of this manuscript, Park et al. (2010) reported that SNX18 interacts with N-WASP. Our observation confirms their conclusion, and SNX18 appears to share the same binding partners. However, the PX domain of SNX18 binds a broad range of phopholipids spotted on a filter, whereas the PX domain of SNX9 preferentially binds to PIP3 on a filter (Badour et al. 2007). As a whole molecule, it has been shown that SNX18 preferentially binds to liposome-containing PIP2s, whereas SNX9 binds a broad range of lipids (Håberg et al. 2008; Lundmark and Carlsson 2003; Yarar et al. 2008). These observations collectively suggest that the different lipid binding preferences of SNX9 family members might be partly attained by a combination of the different nature of each lipid-binding domain, which could be used differently in the cells.
Endosomal sorting is a highly dynamic process, and the localization of EGFP-SNX18 proteins in growth cones was also actively regulated, as we anticipated. Interestingly, EGFP-SNX18 tended to accumulate at sites of contact between growth cones and an attractive substrate. In Drosophila, SNX9 is found in a complex containing DSCAM, an axon guidance molecule, and AP50, a clathrin adaptor protein (Worby et al. 2001). SNX18 may play a similar role in sorting molecules that have interacted with the outer surrounding environment in growth cones. We cannot exclude the possibility that its observed accumulation might represent stacked overexpressed SNX18 proteins. However, we sought to minimize this possibility by examining growth cones that showed a dim fluorescence that was just sufficient to acquire images. EGFP-SNX18 was electroporated into neural crest cells just before they moved out from the spinal cord, and the resultant observed DRG neurons were descendants of the neural crest cells that migrated, differentiated, and elongated axons apparently normally, suggesting that the amount of introduced EGFP-SNX18 should be below the level affecting such complex biological events. Even if too much SNX18 protein was still present, our observations at least indicate that SNX18 is actively recruited to such contact sites. Future studies on the function of SNX18 together with its family members in growth cones could help elucidate the significance of the endosomal pathway in regulating axonal behavior.
Acknowledgments
We thank Dr. Thomas M. Jessell (Columbia University) and Fumio Matsuzaki (RIKEN) for encouraging us to pursue the project.
Footnotes
Supplementary material for this article is available on the Journal of Histochemistry & Cytochemistry Web site at http://jhc.sagepub.com/supplemental.
The author(s) declared no potential conflicts of interest with respect to the authorship and/or publication of this article.
The author(s) disclosed receipt of the following financial support for the research and/or authorship of this article: This work was partly supported by a Grant-in-Aid for Scientific Research 22590055 (TY) and a Grant-in-Aid for Scientific Research on Innovative Areas 20200011 (TY) from the Ministry of Education, Science, Culture, Sports, and Technology, Japan.
References
- Arakawa T, Iwashita M, Matsuzaki F, Suzuki T, Yamamoto T. 2008. Paths, elongation and projections of ascending chick embryonic spinal commissural neurons after crossing the floor plate. Brain Res. 1233:25-33 [DOI] [PubMed] [Google Scholar]
- Badour K, McGavin MK, Zhang J, Freeman S, Vieira C, Filipp D, Julius M, Mills GB, Siminovitch KA. 2007. Interaction of the Wiskott-Aldrich syndrome protein with sorting nexin 9 is required for CD28 endocytosis and cosignaling in T cells. Proc Natl Acad Sci U S A. 104:1593-1598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briscoe J, Pierani A, Jessell TM, Ericson J. 2000. A homeodomain protein code specifies progenitor cell identity and neuronal fate in the ventral neural tube. Cell. 101:435-445 [DOI] [PubMed] [Google Scholar]
- Carlton J, Bujny M, Rutherford A, Cullen P. 2005. Sorting nexins: unifying trends and new perspectives. Traffic. 6:75-82 [DOI] [PubMed] [Google Scholar]
- Chilton JK. 2006. Molecular mechanisms of axon guidance. Dev Biol. 292:13-24 [DOI] [PubMed] [Google Scholar]
- Cullen P. 2008. Endosomal sorting and signalling: an emerging role for sorting nexins. Nat Rev Mol Cell Biol. 9:574-582 [DOI] [PubMed] [Google Scholar]
- Ellson CD, Andrews S, Stephens LR, Hawkins PT. 2002. The PX domain: a new phosphoinositide-binding module. J Cell Sci. 115:1099-1105 [DOI] [PubMed] [Google Scholar]
- Ericson J, Thor S, Edlund T, Jessell TM, Yamada T. 1992. Early stages of motor neuron differentiation revealed by expression of homeobox gene Islet-1. Science. 256:1555-1560 [DOI] [PubMed] [Google Scholar]
- Fujimura Y, Iwashita M, Matsuzaki F, Yamamoto T. 2006. MDGA1, an IgSF molecule containing a MAM domain, heterophilically associates with axon- and muscle-associated binding partners through distinct structural domains. Brain Res. 1101:12-19 [DOI] [PubMed] [Google Scholar]
- Geraldo S, Gordon-Weeks PR. 2009. Cytoskeletal dynamics in growth-cone steering. J Cell Sci. 122:3595-3604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Håberg K, Lundmark R, Carlsson SR. 2008. SNX18 is an SNX9 paralog that acts as a membrane tubulator in AP-1-positive endosomal trafficking. J Cell Sci. 121:1495-1505 [DOI] [PubMed] [Google Scholar]
- Hamburger H, Hamilton H. 1951. A series of normal stages in the development of the chick embryo. J Morphol. 88:49-92 [PubMed] [Google Scholar]
- Hollyday M, Hamburger V. 1977. An autoradiographic study of the formation of the lateral motor column in the chick embryo. Brain Res. 132:197-208 [DOI] [PubMed] [Google Scholar]
- Lundmark R, Carlsson SR. 2003. Sorting nexin 9 participates in clathrin-mediated endocytosis through interactions with the core components. J Biol Chem. 278:46772-46781 [DOI] [PubMed] [Google Scholar]
- Lundmark R, Carlsson SR. 2004. Regulated membrane recruitment of dynamin-2 mediated by sorting nexin 9. J Biol Chem. 279:42694-42702 [DOI] [PubMed] [Google Scholar]
- Lundmark R, Carlsson SR. 2009. SNX9—a prelude to vesicle release. J Cell Sci. 122:5-11 [DOI] [PubMed] [Google Scholar]
- Park J, Kim Y, Lee S, Park JJ, Park ZY, Sun W, Kim H, Chang S. 2010. SNX18 shares a redundant role with SNX9 and modulates endocytic trafficking at the plasma membrane. J Cell Sci. 123:1742-1750 [DOI] [PubMed] [Google Scholar]
- Pylypenko O, Lundmark R, Rasmuson E, Carlsson SR, Rak A. 2007. The PX-BAR membrane-remodeling unit of sorting nexin 9. EMBO J. 26:4788-4800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeren-Wiemers N, Gerfin-Moser A. 1993. A single protocol to detect transcripts of various types and expression levels in neural tissue and cultured cells: in situ hybridization using digoxigenin-labelled cRNA probes. Histochemistry. 100:431-440 [DOI] [PubMed] [Google Scholar]
- Schöbel S, Neumann S, Hertweck M, Dislich B, Kuhn PH, Kremmer E, Seed B, Baumeister R, Haass C, Lichtenthaler SF. 2008. A novel sorting nexin modulates endocytic trafficking and alpha-secretase cleavage of the amyloid precursor protein. J Biol Chem. 283:14257-14268 [DOI] [PubMed] [Google Scholar]
- Seet LF, Hong W. 2006. The Phox (PX) domain proteins and membrane traffic. Biochim Biophys Acta. 1761:878-896 [DOI] [PubMed] [Google Scholar]
- Shin N, Lee S, Ahn N, Kim SA, Ahn SG, Yong-Park Z, Chang S. 2007. Sorting nexin 9 interacts with dynamin 1 and N-WASP and coordinates synaptic vesicle endocytosis. J Biol Chem. 282:28939-28950 [DOI] [PubMed] [Google Scholar]
- Soulet F, Yarar D, Leonard M, Schmid SL. 2005. SNX9 regulates dynamin assembly and is required for efficient clathrin-mediated endocytosis. Mol Biol Cell. 16:2058-2067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit A, Stern CD. 2001. Combined whole-mount in situ hybridization and immunohistochemistry in avian embryos. Methods. 23:339-344 [DOI] [PubMed] [Google Scholar]
- Tanabe Y, William C, Jessell TM. 1998. Specification of motor neuron identity by the MNR2 homeodomain protein. Cell. 95:67-80 [DOI] [PubMed] [Google Scholar]
- Tsuchida T, Ensini M, Morton SB, Baldassare M, Edlund T, Jessell TM, Pfaff SL. 1994. Topographic organization of embryonic motor neurons defined by expression of LIM homeobox genes. Cell. 79:957-970 [DOI] [PubMed] [Google Scholar]
- Worby CA, Dixon JE. 2002. Sorting out the cellular functions of sorting nexins. Nat Rev Mol Cell Biol. 3:919-931 [DOI] [PubMed] [Google Scholar]
- Worby CA, Simonson-Leff N, Clemens JC, Kruger RP, Muda M, Dixon JE. 2001. The sorting nexin, DSH3PX1, connects the axonal guidance receptor, Dscam, to the actin cytoskeleton. J Biol Chem. 276:41782-41789 [DOI] [PubMed] [Google Scholar]
- Yarar D, Surka MC, Leonard MC, Schmid SL. 2008. SNX9 activities are regulated by multiple phosphoinositides through both PX and BAR domains. Traffic. 9:133-146 [DOI] [PubMed] [Google Scholar]
- Yarar D, Waterman-Storer CM, Schmid SL. 2007. SNX9 couples actin assembly to phosphoinositide signals and is required for membrane remodeling during endocytosis. Dev Cell. 13:43-56 [DOI] [PubMed] [Google Scholar]
- Zhang J, Zhang X, Guo Y, Xu L, Pei D. 2009. Sorting nexin 33 induces mammalian cell micronucleated phenotype and actin polymerization by interacting with Wiskott-Aldrich syndrome protein. J Biol Chem. 284:21659-21669 [DOI] [PMC free article] [PubMed] [Google Scholar]


