Abstract
Tumor-initiating cells of pancreatic ductal adenocarcinoma (PDAC) have been isolated based on expression of either CD133 or CD44. The authors aimed to visualize pancreatic cells simultaneously expressing both these cell surface markers by employing the same antibodies commonly used in cell-sorting studies. Normal and diseased pancreatic tissue, including 51 PDAC cases, were analyzed. CD44 and CD133 expression was determined by immunohistochemical double staining on formalin-fixed material and subcellular protein distribution evaluated by immunofluorescence/confocal microscopy. In the normal pancreas, CD44 and CD133 were coexpressed in the centroacinar regions but in non-overlapping subcellular compartments. As expected, CD44 was found mainly basolaterally, whereas CD133 was present on the apical/endoluminal membrane. This was also the case in chronically inflamed/atrophic pancreatic tissue and in PDAC. In some malignant ducts, CD44 was found at the apical cell membrane adjacent to but never overlapping with CD133 expression. CD44 level was significantly associated with the patient’s lymph node status. In conclusion, a CD44+/CD133+ cell population does exist in the normal and neoplastic pancreas. The preferentially centroacinar localization of the doubly positive cells in the normal parenchyma suggests that this population could be of particular interest in attempts to identify tumor-initiating cells in PDAC. This article contains online supplemental material at http://www.jhc.org. Please visit this article online to view these materials.
Keywords: CD44, CD133, immunohistochemistry, pancreas, pancreatic cancer
Subpopulations of cells with malignant potential have recently been isolated from a variety of tumors by fluorescence- or magnetic-activated cell sorting (FACS, MACS) based on the expression of certain cell surface markers (Rosen and Jordan 2009). These subpopulations are denoted tumor-initiating cells or, when a unique self-renewal potential is assumed, cancer stem cells. If proven, the concept of cancer stem cells is important because it could change the therapeutic target from the bulk of tumor cells to a specific cell population initiating and/or maintaining tumor growth (Reya et al. 2001).
The standard assay to validate if tumor-initiating cells can be isolated from solid human cancers is xenografting cell subpopulations into mice. However, the results must be interpreted with caution as the model systems cross species borders, pay little attention to the tumor stroma (mainly cancer cells are transplanted), and attenuate the immune response (severely immunocompromised animals serve as recipients). It is also likely that the harsh mechanical and enzymatic treatment of tumor specimens prior to sorting may lead to molecular (and morphological) changes of the cells. This could result in an altered expression pattern of some surface molecules. Accordingly, the single-cell suspensions obtained prior to sorting are not necessarily representative of the tumor tissue as a whole. Nevertheless, the tissue distribution of the surface markers used in cell sorting is an issue that seldom is addressed in studies focusing on cancer stem cell identification.
Positivity for the surface proteins CD133 and CD44 has been employed in many studies to isolate cells with stem cell–like and cancer-initiating properties from disintegrated solid tumors (e.g., breast [Al-Hajj et al. 2003]; brain [Singh et al. 2003]; prostate [Patrawala et al. 2006]; exocrine pancreas [Hermann et al. 2007; Li et al. 2007]; liver [Ma et al. 2007]; skin melanoma [Monzani et al. 2007]; colon [O’Brien et al. 2007; Ricci-Vitiani et al. 2007; Dalerba et al. 2007]; head and neck [Prince et al. 2007]). CD133, also known as prominin-1, is a five-transmembrane glycoprotein that is expressed in the plasma membrane (reviewed in Mizrak et al. 2008). It has been recognized for several years as a surface marker of hematopoietic and neural stem cells (Yin et al. 1997; Uchida et al. 2000), but its specific function and ligand(s) are still a matter of speculation. However, CD133 is present on apical cytoplasm protrusions of different epithelial and non-epithelial cells and may have a role in organizing plasma membrane topology (Weigmann et al. 1997; Corbeil et al. 2001; Corbeil et al. 2010). We and others have previously reported the distribution of CD133 in a variety of normal and neoplastic epithelial tissues (Weigmann et al. 1997; Corbeil et al. 2000; Bussolati et al. 2005; Florek et al. 2005; Immervoll et al. 2008; Karbanová et al. 2008). CD133 expression has also been evaluated as a prognostic marker in different cancer types. A high level, for example, has been related to adverse prognosis in colon and brain tumors (Zeppernick et al. 2008; Li et al. 2009) and to chemotherapy resistance in colon and lung tumors (Salnikov et al. 2010; Ong et al. 2010).
CD44 is a type I transmembrane cell surface protein acting as a major receptor for hyaluronic acid and other extracellular matrix components (Aruffo et al. 1990). It takes part in physiological and pathologic processes such as lymphocyte homing, T cell activation, wound healing, angiogenesis, and malignant disease (reviewed in Ponta et al. 2003; Marhaba and Zoller 2004). The complexity of the CD44 molecule, with an array of isoforms due to alternative RNA splicing and posttranslational modifications, suggests a variety of functions (Ponta et al. 2003). CD44 is widely expressed on hematopoietic cells, inflammatory cells, fibroblasts, and epithelial cells (e.g., Stamenkovic et al. 1991; Mackay et al. 1994; Fox et al. 1994; Sneath and Mangham 1998). There are two major versions of CD44: the standard isoform (CD44s) characteristic for hematopoietic cells and variant isoforms (CD44v) produced by alternative mRNA splicing and found in epithelial cells (Stamenkovic et al. 1991; Marhaba and Zoller 2004). The expression of CD44s and the CD44v variants has been investigated in many solid tumors (reviewed in Sneath and Mangham 1998; Goodison et al. 1999). The variant isoforms of the protein are generally overexpressed in tumors and have been connected to matrix degradation, invasiveness, and metastasis (Günthert et al. 1995; Marhaba and Zoller 2004; Naor et al. 2008; Klingbeil et al. 2009). In studies on cancer cell lines, expression of CD44s has been linked to reduced tumorigenicity (Tanabe et al. 1995) and suppression of the metastatic process (Gao et al. 1998).
We are focusing on pancreatic ductal adenocarcinoma (PDAC), a highly malignant disease with a dismal prognosis (reviewed in Hidalgo 2010). CD133 or CD44, in combination with other cell surface markers, have been used in identification of postulated pancreatic cancer stem cells (Hermann et al. 2007; Li et al. 2007). A direct identification in pancreatic tissues of cells simultaneously expressing both proteins would therefore be of interest. In the normal pancreas parenchyma, CD133 is expressed apically/endoluminally in the ductal epithelium and also in centroacinar cells (Immervoll et al. 2008; Karbanová et al. 2008; Lardon et al. 2008). Most PDACs are positive for CD133 expression, showing a varying degree of apical cell surface expression (Immervoll et al. 2008). The CD44 isoforms are known to be differently expressed in normal pancreas, PDAC, and pancreatic cancer cell lines (Günthert et al. 1991; Rall and Rustgi 1995; Gansauge et al. 1995; Ringel et al. 2001). Gotoda et al. (1998) found that the expression of the variant isoforms CD44v6 and CD44v2 correlated with decreased overall survival of pancreatic cancer patients. Recently, expression of CD44 was linked to chemotherapy (gemcitabine) resistance in two pancreatic cancer cell lines (Hong et al. 2009).
Here we present an immunohistochemical study of the expression pattern of CD44 in combination with CD133 in formalin-fixed human pancreatic tissues. Our characterization is based on the use of identical antibody clones to those commonly employed in FACS- or MACS-based experiments claiming to sort and enrich cancer cells with stem cell–like properties. We find that a pancreatic population of CD44+/CD133+ cells does exist and that the subcellular distribution of these two surface markers does not overlap. Our findings may have implications not only for studies of stem cells in the normal and neoplastic pancreas but could also indicate that the role of plasma membrane subdomains in cancer needs further exploration.
Materials and Methods
Tissue Specimens
The pancreatic tumor samples were an extended series of that described by Immervoll et al. (2006). Normal pancreatic tissue was taken from 10 pancreas fragments surgically removed due to non-pancreatic tumors and from 7 sudden-death cases. Atrophic and inflammatory pancreatic tissue was investigated in 5 surgically removed specimens from chronic pancreatitis patients or in specimens taken from regions adjacent to pancreatic tumors. Further details about the patient material, reviewing of tissue sections/patient records, and construction of tissue microarrays (TMAs) are given in Immervoll et al. (2008). The study was approved by the Regional Ethics Committee and performed according to the Helsinki declaration.
Immunohistochemistry
Immunohistochemical staining for CD44 and CD133 was done on 2- to 5-µm sections of formalin-fixed paraffin-embedded tissues, placed on chrome-alum gelatin-coated glass slides, and dried 30 min at 70°C. Antigen retrieval was performed by incubation in a pressurized heating chamber (Pascal; Dako, Glostrup, Denmark) at 120°C for 1 min in Tris-EDTA buffer (pH 9). The slides were then cooled in running tap water. CD44 was detected by the monoclonal antibody G44-26 (BD Biosciences, San Jose, CA), which recognizes the standard and variant CD44 isoforms. The antibody was diluted 1:50 in TBS antibody diluent (50 mM Tris, 150 mM NaCl, 1% BSA, 0.1% NaAzid, 0.05% Tween 20 [pH 7.4]). CD133 was detected by the monoclonal antibody AC133 (Miltenyi, Bergisch Gladbach, Germany) diluted 1:25 in an antibody diluent with reduced salt concentration (25 mM Tris, 75 mM NaCl, 1% BSA, 0.1% NaAzid, 0.05% Tween 20 [pH 7.4]). The incubation time for the primary antibodies was 60 min. This and all subsequent steps were carried out at room temperature. Primary antibody detection was performed for CD44 with EnVision+ System–HRP for mouse primary antibodies (Dako) and for CD133 with MACH3 (Biocare Medical, Concord, CA), in accordance with the suppliers’ instructions. The antigens were visualized by developing with diaminobenzidine DAB+ (Dako) for 5 and 10 min for CD44 and CD133, respectively. Between each step, there were two washing steps of 1 min each on a rocking platform in washing buffer (50 mM Tris, 150 mM NaCl, 0.05% Tween 20 [pH 7.5]). Blocking for unspecific peroxidase activity was done by exposing the sections to an aqueous solution of 3% hydrogen peroxide for 5 min. The sections were counterstained with Harris’s hematoxylin (Histolab Products, Gothenburg, Sweden) for 30 sec and then dehydrated in alcohol, cleared in xylene, and coverslipped using a Mountex permanent mounting medium (Histolab Products).
Double-staining for CD133 and CD44 was done in two steps. CD133 (1:25 or 1:30) was applied first, using the protocol described above. After color development in DAB, the slides were rinsed in running tap water and then placed in preheated (100°C) Tris EDTA buffer (pH 9) for 2 min (modified antigen retrieval). Next, the sections were incubated with anti-CD44, using different antibody dilution factors (1:25, 1:35, 1:50, and 1:100), at room temperature for 60 min. Primary antibody detection and visualization of CD44 were achieved using the MACH2 kit and the Vulcan Fast Red Chromogen kit (both from Biocare Medical). Sections were counterstained, dehydrated, cleared, and coverslipped as described above.
Controls and Assessment of Immunohistochemical Staining
Specificity of the antibody AC133 was checked as described previously (Immervoll et al. 2008). Specificity of the antibody G44-26 was tested on a Western blot with extracts from two pancreatic cancer cell lines (not shown). Moreover, frozen sections containing normal pancreas were stained and compared with formalin-fixed sections, and CD44 detection was routinely performed with the primary antibody omitted to control for background staining (not shown). The CD44 pattern observed when double-staining for CD133 was compared to parallel slides stained for CD44 alone, either with the same antibody/incubations used for the double-staining (clone G44-26) or with the CD44 antibody clone DF1485 (M7082; Dako) using a protocol established in routine diagnostics (available on request).
The quality of staining was further judged by examining sections from different organs/tumors and sections from TMA blocks containing an array of control tissues, followed by a comparison to data on CD133 and CD44 expression in the literature. Tumor cell CD44 expression was scored independently by two of the authors (H.I. and A.M.) in TMA sections from the series of PDACs as negative (0), weakly positive (1), or strongly positive (2). Similarly, stromal CD44 expression was scored as weakly (1), medium (2), or strongly positive (3). As validation for TMA interpretation, whole sections from the border between the adenocarcinoma and nearby normal pancreatic tissue were made from 10 of the cases included in the TMA block and treated and evaluated in the same way as the TMA slides. For CD133 expression alone, data and slides from the previous study were evaluated (Immervoll et al. 2008).
Immunofluorescence Staining and Confocal Microscopy
Antigen retrieval and antibody diluents were the same as described above for G44-26 and AC133. The AC133 antibody (1:20) was applied first and incubated at 4°C for 18 to 20 hr. All subsequent steps were carried out at room temperature. Primary antibody detection was performed for 30 min by the MACH-2 AP-polymer (Biocare Medical) for mouse primary antibodies. The antigens were visualized as red fluorescence by developing with the Vector Red Alkaline Phosphatase substrate kit (SK-5100; Vector Laboratories, Burlingame, CA) for 15 min. Next, the sections were incubated with the CD44 antibody G44-26 (1:200) for 60 min. Primary antibody detection and visualization of CD44 as green fluorescence was achieved by using Alexa Fluor 488 goat anti-mouse IgG2b (Invitrogen Molecular Probes, Eugene, OR), diluted (1:200) and incubated for 30 min. Between each step, there were two washing steps of 1 min each on a rocking platform in washing buffer (50 mM Tris, 150 mM NaCl, 0.05% Tween 20 [pH 7.5]). Finally, the sections were coverslipped using an anti-fading mounting medium containing DAPI (Vectashield H-1500; Vector Laboratories). The stained sections were analyzed by confocal scanning laser microscopy, using an LSM 510 Meta laser scanning microscope (Carl Zeiss, Jena, Germany).
Statistics
The statistical analyses were performed using the software package Statistica 6.0 (StatSoft, Tulsa, OK). A p value <0.05 was chosen for statistical significance. Categorical data with comparison of two proportions were analyzed by the χ2 test. The Product–Limit (Kaplan–Meier) Analysis Module was used for comparing survival between multiple groups. Survival times versus cumulative proportion surviving, according to breakdown by different CD44 staining intensity groups, were plotted.
Results
CD44 and CD133 Expression in the Normal Pancreas
We have previously described the distribution of CD133+ cells in the normal and pathological pancreas (Immervoll et al. 2008). CD133 was visualized with AC133, an antibody commonly used to enrich cells with a postulated cancer stem cell function (Table 1). Here we extend the analysis by adding the antibody G44-26, which in several reports has been employed to isolate tumor-initiating cells based on surface expression of the CD44 protein (Table 1). We first examined the distribution of the two markers by double-staining a variety of surgically removed tissues with normal or near-normal morphology. Representative images (retina, adrenal gland, lymphoid tissue, prostate, salivary gland, gall bladder, colon) and a description are given as online Supplemental Figure S1. In general, when CD44 and CD133 were expressed simultaneously in epithelial tissues, the markers were present in the same cells, displaying an apparently non-overlapping pattern at the subcellular level. As expected, CD44 positivity was observed on the membrane facing the extracellular matrix (basally) and the neighboring epithelial cells (laterally). Additional strong cytoplasmic CD44 staining was present in some cells and tissues (e.g., nerves, prostate basal cells, lymphocytes; Suppl. Fig. S1). CD133, on the other hand, was expressed on the membrane part facing a free surface (apically/endoluminally).
Table 1.
Studies Where Positivity for CD44 and/or CD133 Has Been Used in the Isolation of Tumor-Initiating Human Cells
| Organ | CD44 | CD133 | Additional Marker(s) | Reference |
|---|---|---|---|---|
| Pancreas | X | CXCR4 | Hermann et al. (2007)a | |
| X | CD24, ESA | Li et al. (2007) | ||
| Breast | X | CD24, ESA | Al-Hajj et al. (2003) | |
| Brain | X | — | Bao et al. (2006)a | |
| X | — | Singh et al. (2003); Singh et al. (2004)a | ||
| Colon | X | X | ESA, CD166 | Dalerba et al. (2007)a |
| X | — | Ricci-Vitiani et al. (2007)a | ||
| X | — | O’Brien et al. (2007)a | ||
| X | X | — | Haraguchi et al. (2008)a | |
| X | X | — | Du et al. (2008)a | |
| Head and neck | X | — | Prince et al. (2007)a | |
| Prostate | X | — | Patrawala et al. (2006) | |
| X | X | α2β1 integrin | Collins et al. (2005) | |
| X | CD24 | Hurt et al. (2008) | ||
| Liver | X | — | Ma et al. (2007)a | |
| Skin (melanoma) | X | ABCG2 | Monzani et al. (2007)a |
Studies that specify that the AC133 and/or the G44-26 antibodies have been employed.
We then investigated normal pancreatic tissue. When staining for CD44 alone (Fig. 1A,B) and together with CD133 (Fig. 1C,D), a similar pattern of CD44 positivity was seen in consecutive sections. CD44 was expressed in varying intensity. We observed CD44-negative areas located close to areas without overt morphological signs of disease but with relatively strong CD44 positivity within a lobule (Fig. 1E). CD44 expression was most abundant in centroacinar cells and in the smallest ducts (intercalating ducts) seen as both cytoplasmic and membrane positivity (Fig. 1F). In the ductal epithelium, staining intensity decreased toward the larger ducts, and intralobular ducts were partially CD44 negative (Fig. 1B,D). In the endocrine compartment (i.e., the islets of Langerhans), a few cells showed faint CD44 positivity (Fig. 1E and not shown).
Figure 1.
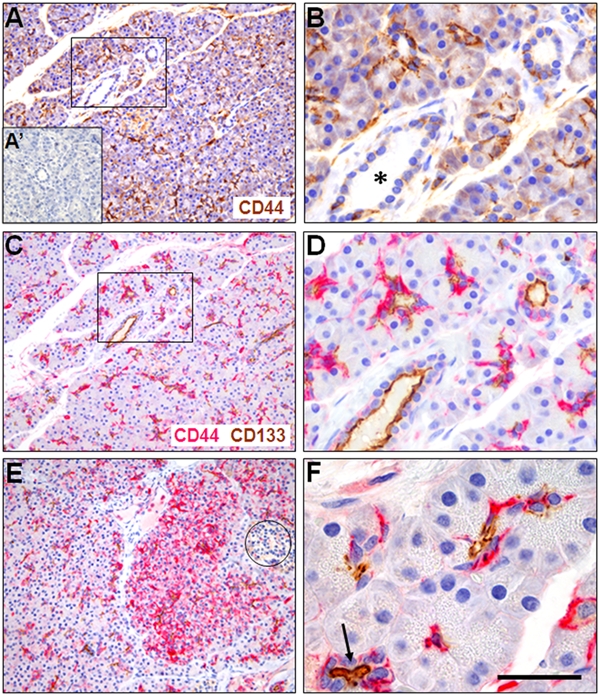
CD44 expression in normal human pancreas and its relation to CD133. (A) CD44 expression (brown) in exocrine tissue is most abundant in centroacinar regions and intercalated ducts. A negative control staining without primary antibody is shown as A′. (B) Higher magnification of the marked area in A. CD44 staining appears weaker in larger ducts (asterisk). (C) Double-staining of CD44 (red) and CD133 (brown) in a consecutive section of A. CD44 is expressed in similar localization and amount as in the single staining. (D) Higher magnification of the marked area in C. (E) Variation of CD44 positivity between two pancreatic lobes. An islet of Langerhans is encircled. (F) Example of acini with moderate centroacinar CD44 positivity and CD133 expression at the apical cell membrane of cells bordering a lumen. The arrow points to an intralobular duct. Scale bar in F corresponds to 250 µm (A, C, E), 80 µm (B, D), and 50 µm (F).
In CD44–CD133 double-stainings (Fig. 1C–F), the centroacinar regions were highlighted by both markers. The basolateral membrane/cytoplasm and the apical/endoluminal cell surface were the preferential sites of CD44 and CD133 expression, respectively (Fig. 1D). In intralobular ducts, CD133 staining intensity was kept at a high level while CD44 staining was reduced (Fig. 1B,D).
CD44 and CD133 Expression in Non-malignant Pancreatic Disease
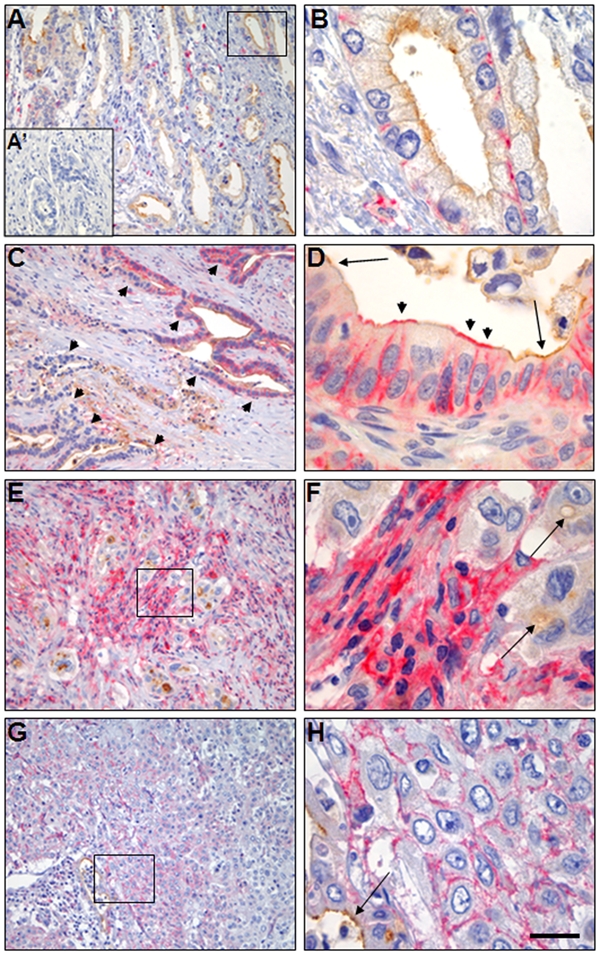
Next, we investigated pancreatic CD44/CD133 expression in areas of pancreatic tissue characterized by inflammation and atrophy. CD44 expression was pronounced in the vicinity of inflammatory infiltrates and in areas showing atrophy (Fig. 2A–D). The epithelial cells showed a subcellular distribution similar to that of normal pancreas, with CD133 expressed apically and CD44 in the basolateral membrane and cytoplasm. Moreover, the epithelia of tubular complexes in areas of advanced atrophy showed particularly strong CD44 expression in the cytoplasm and membrane. The majority of epithelial cells remaining in the atrophic areas expressed both CD44 and CD133 (Fig. 2C,D). Cells and tissues not specific for the pancreas (inflammatory cells, nerves, and, less constantly, fibroblasts and vessel walls) also showed CD44 positivity.
Figure 2.
CD44 and CD133 expression in inflamed and atrophic pancreas. (A, B) Double-staining of the two markers in a specimen with early stage of chronic inflammation. (C, D) Double-staining in a later stage of chronic inflammation with atrophy of the acinar tissue. CD44 is visualized in red and CD133 in brown. B and D represent higher magnifications of the marked areas in A and C. (E, F) Immunofluorescence double-staining visualized by confocal microscopy. CD44 is shown in green and CD133 in red. F is a higher digital magnification of the marked area on the left side. Note that CD44 positivity is seen on basolateral membrane surfaces, whereas CD133 is expressed apically/endoluminally. In general, there is strong expression of CD44 in tubular complexes and concomitant expression of CD133 at the apical membrane as the amount of connective tissue increases and the exocrine tissue undergoes atrophy. Compare with panels C and D. Scale bar in D corresponds to 125 µm (A, C) and 50 µm (B, D). Scale bars in E and F correspond to 20 µm.
CD44 and CD133 Expression in Pancreatic Ductal Adenocarcinomas
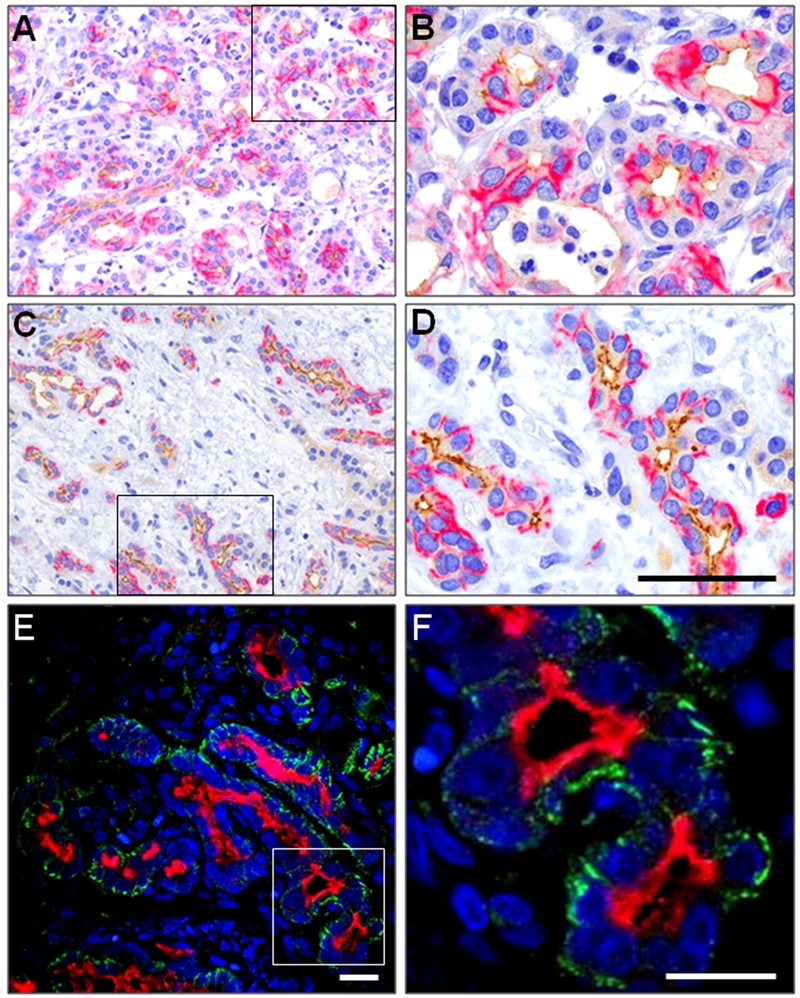
In our series of PDAC, the CD44 expression pattern was complex with major local variations of staining intensity and number of stained tumor cells (Fig. 3). Of the examined 51 cases, 34 (67%) were judged as positive for CD44 in their tumor cells. The positive tumor cells were clustered together as expected in clonally expanding growth. In moderately to well-differentiated carcinomas with tubular or papillary architecture, CD44 expression showed major variation between specimens with similar morphologic features and between different areas within a given specimen (Fig. 3A,C). A varying expression was also observed in less differentiated regions. CD44 positivity dominated in the stromal cells of some tumors (Fig. 3E,F) and in the cancer cells of others (Fig. 3G,H). There were, however, fewer cells expressing CD133 in the less differentiated areas, probably reflecting the decrease of tumor cells in tubular or papillary arrangement.
Figure 3.
Expression of CD44 in pancreatic ductal adenocarcinomas and its relation to CD133. Double-staining with CD44 visualized in red and CD133 in brown. (A, C) Two tumors of moderate to high differentiation grade. Note the highly varying CD44 expression both between (compare A and C) and within (arrowheads in C) the tumor samples. A negative control staining without primary antibody is shown as A′. (B) Higher magnification of the marked area in A. (D) High magnification of a malignant duct with prominent CD44 expression. Note the preference of CD44 staining at the basolateral membrane of tumor cells in B and D and additional apical CD44 positivity in D (arrowheads). CD133 staining of the apical membrane is indicated by arrows. (E, G) Two tumors of low differentiation grade. (F, H) Higher magnification of the marked areas in E and G. Note the large variability of CD44 staining in stromal cells (E, F) and tumor cells (G, H). The arrows indicate CD133 staining of a lumen and an intracellular vacuole (F) and the apical cell membrane of tumor cells (H). Scale bar in H corresponds to 125 µm (A, C, E, G) and 50 µm (B, D, F, H).
In the PDAC cases, simultaneous expression of CD44 and CD133 in tumor cells was characteristic of areas of higher differentiation (Fig. 3B–D). In the positive cell groups, there was a preserved subcellular protein distribution with CD44 located basolaterally (both in the membrane and the cytoplasm) and CD133 expressed in the apical membrane. However, in some ductally arranged cells, we observed CD44 positivity also at the apical membranes (Fig. 3D). When comparing the two cell surface markers, we noted that CD44 was the one varying most in all descriptive variables (intensity, amount and type of positive cells, subcellular localization).
Subcellular CD44 and CD133 Expression Evaluated by Immunofluorescence
From the immunohistochemistry results described above, we concluded that the subcellular distribution of CD44 and CD133 appeared not to overlap. However, the occasional CD44 positivity observed at the apical side of some tumor cells in PDAC (Fig. 3D) motivated us to search for potential co-localization of CD44 and CD133 with a more sensitive method. To this end, we stained pancreatic tissue sections for CD44/CD133 using fluorescent dyes and examined the expression pattern with confocal microscopy. We also now observed that normal, inflamed/atrophic, and malignant pancreatic tissue contained double-positive cells, as illustrated in Figure 2E,F and Figure 4.
Figure 4.
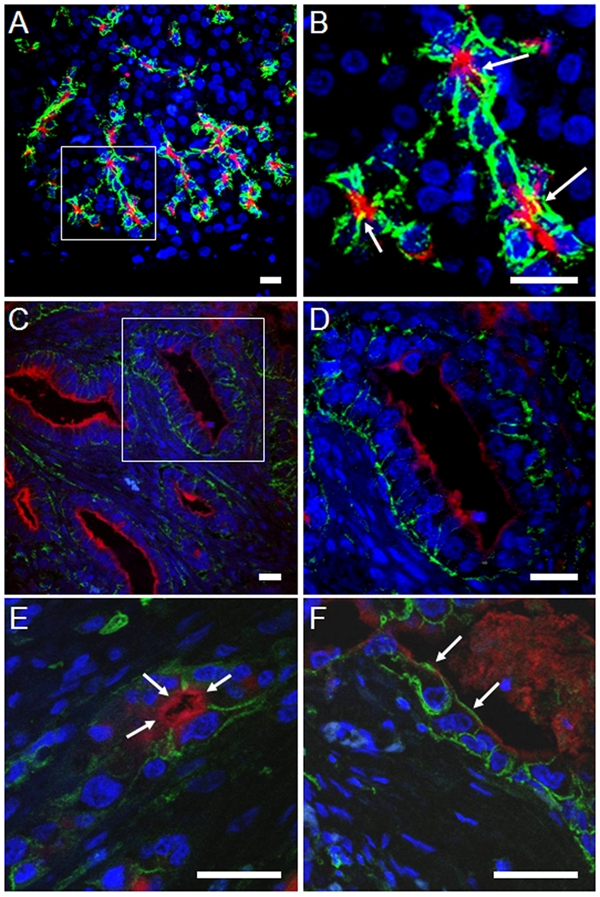
Distribution of the CD44 and CD133 markers in normal and neoplastic (ductal adenocarcinoma) pancreas as judged by immunofluorescence double-staining and confocal microscopy. CD44 is shown in green and CD133 in red. Panels B and D are higher digital magnifications of the marked areas in A and C. Note that CD44 positivity is seen on basolateral membrane surfaces, whereas CD133 is expressed apically/endoluminally. (A, B) Acini of normal pancreas. The small yellow areas in B (arrows) are a result of the stacking of several pictures captured at different levels. Co-localization of CD44 and CD133 was never observed in single layers. A comparable light-microscopy section is shown in panel D of Figure 1. (C–F) CD44 and CD133 expression in pancreatic ductal adenocarcinoma (PDAC) specimens. CD44 is generally seen basolaterally in tumor cells bordering a lumen, and CD133 is expressed apically/endoluminally. (C, D) Example of a well-differentiated PDAC. A comparable light-microscopy section is shown in panel B of Figure 3. (E, F) Examples of moderately differentiated PDACs. The arrows in E indicate endoluminal CD133 expression, not overlapping with positivity for CD44. The arrows in F mark cells with CD44 expression at their apical/endoluminal surface without co-expressing CD133. A light-microscopy section comparable to F is shown in panel D of Figure 3. All scale bars are 20 µm.
In the normal pancreas (Fig. 4A,B), CD44 and CD133 resided closely together in the same cells of the centroacinar regions, with CD44 being present in the basolateral membrane and CD133 apically. In composite confocal pictures consisting of several superimposed layers, a few small areas of the membrane exhibited overlapping yellow color (Fig. 4B). However, co-localization of CD44 and CD133 was never observed in pictures of single layers (not shown). We conclude that CD44 and CD133 do not co-localize on the membranes of normal pancreatic cells.
Similarly, inflamed/atrophic pancreas showed prominent and non-overlapping CD44/CD133 expression when judged by immunofluorescence (Fig. 2E,F). In the series of PDAC, tumor cells expressing both markers were related to tubular or papillary architecture (Fig. 4C–F). CD44 occupied the basolateral and CD133 the apical membrane without co-localization. Notably, in regions where CD44 was present on the apical membrane, CD133 was absent, although the latter marker was present on the apical membrane of neighboring cells (Fig. 4F).
Association of CD44 Expression with Clinical Variables
In our previous report (Immervoll et al. 2008), we did not find a correlation between the level of CD133 expression in PDAC and clinical variables such as TNM stage, differentiation grade, and patient survival. We extended this analysis to the CD44 positivity observed in our series of PDACs, which were scored as described in Materials and Methods. A statistically significant association was found only for CD44 expression and lymph node status (Fig. 5A,B). Of the 17 cases with absent CD44 expression in their tumor cells, 7 (41%) had been staged as N0, compared to 5 (15%) of the 34 cases with weak to strong tumor cell expression (p = 0.036, χ2 test). A similar relationship was found for stromal CD44 expression: Of the 18 cases with weak expression, 8 (44%) had been staged as N0, compared to 4 (12%) of the 33 cases with medium to strong CD44 expression (p = 0.036, χ2 test). However, when CD44 expression was related to patient survival, there was no statistically significant difference between the groups for tumor cell (Fig. 5C) or stromal cell CD44 positivity (not shown).
Figure 5.
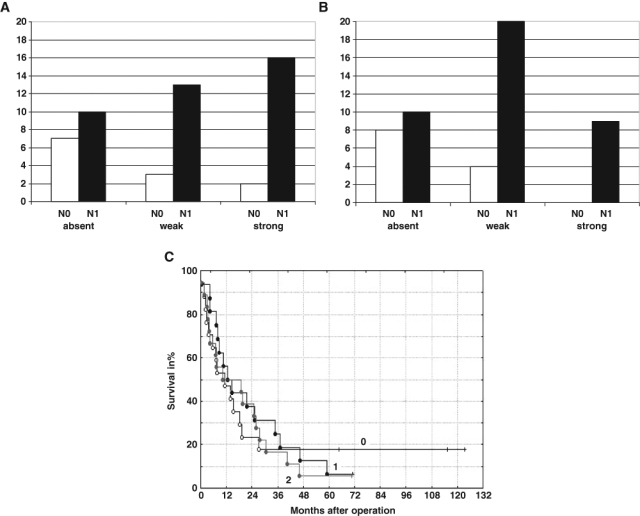
Analysis of CD44 expression in relation to lymph node status (N0 or N1) and patient survival. (A) Number of tumors with absent, weak, and strong CD44 tumor cell expression distributed according to lymph node status. The association between high tumor cell CD44 expression and increased frequency of N1 tumors was statistically significant (see text for details). (B) Number of tumors with weak, medium, and strong stromal CD44 expression distributed according to lymph node status. The association between high stromal CD44 expression and increased frequency of N1 tumors was statistically significant (see text for details). (C) Cumulative proportion survival (Kaplan–Meier) plot for 51 pancreatic adenocarcinomas according to breakdown by tumor cell CD44 expression (absent = 0, weak = 1, strong = 2). Median survival time was 11, 13, and 15 months for groups 0, 1, and 2, respectively. The observed survival times are indicated by circles (complete) or crosses (censored observations).
Discussion
Expression of the surface marker CD44 or CD133 has been used in many studies to enrich cell suspensions from solid tumors for cells with tumor-initiating potential (Table 1). A combination of antibodies against both markers has been applied in studies of colon cancer (Du et al. 2008; Haraguchi et al. 2008) and prostate cancer (Collins et al. 2005). Concerning exocrine pancreatic cancer, the presence of either CD133 or CD44 was employed by Hermann et al. (2007) and Li et al. (2007), respectively, as a sorting criterion in xenograft studies. However, a combined use of these two markers in prospective isolation of subsets of tumor cells or in a morphological characterization by immunohistochemistry has not been published for PDAC, to our knowledge. In a previous article, we investigated the expression of CD133 (Immervoll et al. 2008) and concluded that the population of CD133+ tumor cells appears too large to be specific for a limited, tumor-initiating cell fraction of PDAC. On the background of Hermann et al. (2007) and Li et al. (2007), it is conceivable that such a fraction could correspond to cells simultaneously expressing both the CD44 and the CD133 marker. We therefore performed a systematic investigation of the distribution pattern of CD44 in combination with CD133 in tissue sections of normal and diseased pancreas.
Choice of Antibody
Studies focusing on cancer stem cells have so far applied antibodies against CD133 and CD44 without further analysis of splice variants or other posttranslational events that may change the properties of the proteins. We decided to employ the AC133 and G44-26 monoclonal antibodies, which are used regularly in cell-sorting approaches for the enrichment of cancer-initiating cells from primary human tumors (Table 1). The G44-26 antibody reacts with both the standard and all known variant CD44 forms, and it has been demonstrated to be suitable for both formalin-fixed paraffin-embedded tissue and flow cytometry.
Concerning CD133, immunohistochemistry in PDAC has not shown consistent results, and the staining pattern seems dependent on the antibody, fixation, epitope retrieval, and staining method used (Hermann et al. 2007; Immervoll et al. 2008; Maeda et al. 2008; Smith et al. 2008; Shimizu et al. 2009; Welsch et al. 2009). Kemper et al. (2010) specifically examined possible regulation mechanisms for expression of the AC133 epitope and showed that it is downregulated during cancer stem cell (CSC) differentiation. They concluded that AC133 can be used as a CSC marker. However, it was also emphasized that one should interpret results from the use of the AC133 antibody with care because of a possible epitope masking caused by differential glycosylation and folding of the CD133 protein (Kemper et al. 2010).
The Plasticity of CD44 Expression
In human adult normal tissues (see Suppl. Fig. S1), CD44 was found in many different cell types (epithelia, inflammatory cells, fibroblasts, nerves), whereas CD133 was expressed in fewer cell types, mainly in epithelial cells. In normal and pathologic pancreas, CD44 expression showed a great variability, both in localization and strength of the staining (Figures 1–3). CD133, in contrast, was constantly seen at the apical/endoluminal membrane of normal centroacinar or ductal cells, as well as in ductal or papillary cell groups in those pancreatic tumors that expressed this marker (see also Immervoll et al. 2008).
Published data about CD44 expression in the non-neoplastic pancreas and normal pancreatic cell lines are not completely consistent and may vary according to the antibody used (Gansauge et al. 1995; Castella et al. 1996; Gotoda et al. 1998; Ringel et al. 2001). The explanation could be differences in antibody affinity related to CD44 glycosylation, other posttranslational events, isoform-specific epitopes, or technical differences in epitope retrieval or staining procedure. As mentioned earlier, we stained with an antibody that reacts with all known CD44 forms. Our results were compared with the staining pattern of the CD44 antibody clone DF1485, and we achieved the same staining pattern with both antibodies (not shown). Antibody specificity was also evaluated on frozen sections and by Western blotting (not shown). The pattern that we describe is therefore unlikely to be caused by unspecific staining.
As the normal pancreatic specimens of this study originated from the archives of a diagnostic histopathology laboratory, they cannot be regarded as entirely disease free. It may therefore be questioned whether we have demonstrated a normal expression pattern of CD44 in the pancreas. To further evaluate this issue, we also stained pancreas specimens from seven sudden-death cases with no known pancreatic disease. A varying pattern of CD44 positivity, similar to that described above, was seen in areas of well-preserved tissue (not shown). Accordingly, it is tempting to speculate that normal pancreatic CD44 expression is varying and inducible and that it may be upregulated preceding morphologic changes of inflammation and/or degeneration.
Subcellular Localization of CD44 and CD133
In epithelial monolayers, CD44 is found at the basolateral cell membrane and also in the cytoplasm, whereas CD133 resides at the apical membrane. We did not detect an overlap in the subcellular expression of the two markers in normal or in atrophic/inflamed or neoplastic pancreas (Figures 1–4). Thus, expression of both markers may reflect a well-organized membrane arrangement of a single epithelial cell with one compartment facing the extracellular matrix and lateral cells (CD44 positivity) and the other facing a lumen (CD133 positivity).
CD44 has a multitude of known ligands and functions (Ponta et al. 2003), many of them connected to the extracellular matrix and therefore well in agreement with the protein’s predominant expression at the basolateral membrane. The ligands of CD133 are still unknown. Notably, in some malignant duct cells, we observed CD44 expression on the apical part of the membrane, adjacent to but not overlapping with CD133 positivity (Figs. 2F and 3D). Whether plasma membrane domains, as defined by expression of CD133 and CD44, have a role in cancer should be a topic for further investigation.
CD44 in Pancreatic Ductal Adenocarcinomas
CD44, originally called lymphocyte homing receptor, has a role in malignant disease (Günthert et al. 1995; Jothy 2003; Marhaba and Zoller 2004). It is conceivable that apical expression of CD44, because it is a receptor for extracellular matrix components, could loosen the contact to stromal components, thereby aiding the tumor cells when invading nearby normal tissue. It has also been shown that CD44 can interact with actin (Brown et al. 2005), which is essential for cell motility. Moreover, CD44 expression at the basement membrane could be important for tumor invasion, for example, by recruiting matrix metalloproteinases (Yu and Stamenkovic 1999). Intriguingly, by studying a breast cancer model, Godar et al. (2008) concluded that CD44 promotes tumorigenesis in transformed cells lacking p53 function.
All our PDAC tumors exhibited at least some CD44 positivity in their stroma, and two thirds expressed the marker in their cancer cells. We found an association between CD44 expression, both in tumor and stromal cells, and lymph node status of the patients (Fig. 5). That CD44 may be associated with the metastatic process is now well accepted (Jothy 2003). For example, a CD44 splice variant was found sufficient to induce a metastatic phenotype in a locally growing pancreatic carcinoma cell line (Günthert et al. 1991). Investigations in different tumor types vary concerning the isoform(s) considered relevant for metastatic potential. However, CD44v6 is often implicated (Guriec et al. 1997; Kurozumi et al. 1998; Reeder et al. 1998).
In clinical materials of various cancers, the expression level of CD44 tends to correlate with a poor prognosis, but results are not uniform (Mayer et al. 1993; Tokue et al. 1998; Sato et al. 2000; Watanabe et al. 2005; Huh et al. 2009). We did not find an impact of CD44 expression on patient survival. Earlier reports on pancreas cancer have suggested that poor prognosis is related to overexpression of certain CD44 isoforms (Böttger et al. 1998; Gotoda et al. 1998). It is not straightforward to explain why we found CD44 expression, both in tumor and stromal cells, to be associated with lymph node status but not with survival. One should keep in mind, though, that because of the local variation of CD44 staining, the expression level in a series of TMA samples may not be representative for the whole tumor specimen. A detailed analysis of the expression of certain CD44 protein isoforms, also in association with other tumor or stromal markers, in a larger series of PDAC, would be a natural follow-up of the present study. It will also be interesting to evaluate if the amount and distribution of CD133/CD44 doubly positive cells relate to any clinical variable(s).
CD44 and CD133 as Markers of Pancreatic Tumor-Initiating Cells
We found that a CD44+/CD133+ cell population does exist in pancreatic tissue without morphological signs of overt disease. Moreover, these double-positive cells reside preferentially in the centroacinar region and persist (or even appear to increase in relative number) in chronic inflammation and atrophy. They are also found in PDAC. These observations suggest that, if CD44/CD133 positivity is characteristic for a subpopulation with unique self-renewal potential, the expression of these markers is a necessary but not a sufficient criterion for being a (cancer) stem cell. In other words, additional markers must be sought to strictly define such a subpopulation.
The preferentially centroacinar expression of the CD44+/CD133+ cell population is particularly intriguing on the background of recent studies where this region has been suggested to lodge the cell of origin in pancreatic cancer: Stanger et al. (2005) investigated a mouse model exhibiting pancreas-specific knockout of Pten, which resulted in progressive replacement of the acini with highly proliferative ductal structures and development of ductal malignancy. Zhu et al. (2007) concluded that PanIN lesions, the postulated precursors of PDAC, can develop from acinar cells undergoing acinar-ductal metaplasia in a KrasG12D oncoprotein-expressing animal model. Guerra et al. (2007) found that selective expression of endogenous KrasG12V oncoprotein in embryonic cells of acinar/centroacinar lineage results in PDAC and suggested that differentiation of acinar/centroacinar cells into ductal-like cells precedes this cancer.
In light of these data and the literature listed in Table 1, our present results call for sorting studies in PDAC tumors of cells positive for both CD44 and CD133. It will be most interesting to examine how the isolation of a CD44+/CD133+ subpopulation may affect the tumor-initiating potential of this cancer form. Ji et al. (2009) recently used this approach in a study of the human pancreatic cancer cell line Mia PaCa2. They found that 1% to 2% of the cells expressed both markers. This subpopulation exhibited an increased capability to form tumor spheres in vitro and to initiate tumors by a xenograft assay in mice. Kallifatidis et al. (2009) analyzed two PDAC cases for CD44 and CD133 positivity. In one tumor, 7.5% of the total cellular mass was estimated to express both markers. This case was more resistant toward chemotherapy than the other case, which did not show simultaneous expression of the two markers.
Some caution nevertheless should be exercised as immunohistochemistry does not necessarily directly reflect the cell populations isolated when using the same antibodies in automated cell sorting. The harsh mechanical and enzymatic treatment of tumor specimens prior to cell sorting creates relatively non-physiologic conditions. This may induce an altered pattern of expressed surface molecules and other molecular changes of the cells. It can therefore be expected that the rate of tumor cell recovery after tumor dissociation is far from 100%. Those cells that are enriched as tumorigenic are those that simultaneously are positive for the surface cell marker(s), resistant to the non-physiological conditions, and able to survive after transplantation into an animal. In conventional tissue immunohistochemistry, on the other hand, no cells are lost, but fixation in formalin can mask epitopes and necessitates retrieval methods prior to the application of the primary antibody. Thus, even if identical antibodies are employed, there may not be a 1:1 relationship between the cells enriched by sorting and those detected by immunochemistry.
Conclusion
We have shown that a CD44/CD133 double-positive cell population does exist in the pancreas, as well as in other human tissues. The subcellular membrane distribution of the two markers was never found to overlap in normal or in inflamed/atrophic or neoplastic pancreas. The centroacinar localization of the CD44+/CD133+ cells and their persistence in PDAC suggest that this cell population could be of particular interest when attempting to identify tumor-initiating cells in the pancreas.
Acknowledgments
We thank Dr. Per Øystein Sakariassen, Prof. Ole Didrik Lærum, Dr. Martha Chekenya, and Dr. Bjørn Ove Mæhle for helpful discussions, as well as Marit Nilsen and Torill Halsebakke Bjørge for technical help. We are also grateful to the Department of Pathology, Haukeland University Hospital and to the Department of Pathology, Ålesund Hospital for excellent working conditions and for providing access to archival material.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the authorship and/or publication of this article.
The author(s) disclosed receipt of the following financial support for the research and/or authorship of this article: The study was supported by a PhD fellowship from Helse Vest and by a grant from the Norwegian Cancer Society.
References
- Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. 2003. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 100:3983–3988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aruffo A, Stamenkovic I, Melnick M, Underhill CB, Seed B. 1990. CD44 is the principal cell surface receptor for hyaluronate. Cell. 61:1303–1313 [DOI] [PubMed] [Google Scholar]
- Bao S, Wu Q, Sathornsumetee S, Hao Y, Li Z, Hjelmeland AB, Shi Q, McLendon RE, Bigner DD, Rich JN. 2006. Stem cell-like glioma cells promote tumor angiogenesis through vascular endothelial growth factor. Cancer Res. 66:7843–7848 [DOI] [PubMed] [Google Scholar]
- Böttger T, Maschek H, Gottwald W, Lobo M, Brenner W, Junginger T. 1998. Expression of CD44 and isoforms v4, v5, v6, v7, v10: new prognostic parameters in ductal pancreatic carcinoma? [in German] Chirurg. 69:1089–1092 [DOI] [PubMed] [Google Scholar]
- Brown KL, Birkenhead D, Lai JC, Li L, Li R, Johnson P. 2005. Regulation of hyaluronan binding by F-actin and colocalization of CD44 and phosphorylated ezrin/radixin/moesin (ERM) proteins in myeloid cells. Exp Cell Res. 303:400–414 [DOI] [PubMed] [Google Scholar]
- Bussolati B, Bruno S, Grange C, Buttiglieri S, Deregibus MC, Cantino D, Camussi G. 2005. Isolation of renal progenitor cells from adult human kidney. Am J Pathol. 166:545–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castella EM, Ariza A, Ojanguren I, Mate JL, Roca X, Fernandez-Vasalo A, Navas-Palacios JJ. 1996. Differential expression of CD44v6 in adenocarcinoma of the pancreas: an immunohistochemical study. Virchows Arch. 429:191–195 [DOI] [PubMed] [Google Scholar]
- Collins AT, Berry PA, Hyde C, Stower MJ, Maitland NJ. 2005. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 65:10946–10951 [DOI] [PubMed] [Google Scholar]
- Corbeil D, Marzesco AM, Wilsch-Brauninger M, Huttner WB. 2010. The intriguing links between prominin-1 (CD133), cholesterol-based membrane microdomains, remodeling of apical plasma membrane protrusions, extracellular membrane particles, and (neuro)epithelial cell differentiation. FEBS Lett. 584:1659–1664 [DOI] [PubMed] [Google Scholar]
- Corbeil D, Roper K, Fargeas CA, Joester A, Huttner WB. 2001. Prominin: a story of cholesterol, plasma membrane protrusions and human pathology. Traffic. 2:82–91 [DOI] [PubMed] [Google Scholar]
- Corbeil D, Roper K, Hellwig A, Tavian M, Miraglia S, Watt SM, Simmons PJ, Peault B, Buck DW, Huttner WB. 2000. The human AC133 hematopoietic stem cell antigen is also expressed in epithelial cells and targeted to plasma membrane protrusions. J Biol Chem. 275:5512–5520 [DOI] [PubMed] [Google Scholar]
- Dalerba P, Dylla SJ, Park IK, Liu R, Wang X, Cho RW, Hoey T, Gurney A, Huang EH, Simeone DM, et al. 2007. Phenotypic characterization of human colorectal cancer stem cells. Proc Natl Acad Sci U S A. 104:10158–10163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du L, Wang H, He L, Zhang J, Ni B, Wang X, Jin H, Cahuzac N, Mehrpour M, Lu Y, et al. 2008. CD44 is of functional importance for colorectal cancer stem cells. Clin Cancer Res. 14:6751–6760 [DOI] [PubMed] [Google Scholar]
- Florek M, Haase M, Marzesco AM, Freund D, Ehninger G, Huttner WB, Corbeil D. 2005. Prominin-1/CD133, a neural and hematopoietic stem cell marker, is expressed in adult human differentiated cells and certain types of kidney cancer. Cell Tissue Res. 319:15–26 [DOI] [PubMed] [Google Scholar]
- Fox SB, Fawcett J, Jackson DG, Collins I, Gatter KC, Harris AL, Gearing A, Simmons DL. 1994. Normal human tissues, in addition to some tumors, express multiple different CD44 isoforms. Cancer Res. 54:4539–4546 [PubMed] [Google Scholar]
- Gansauge F, Gansauge S, Zobywalski A, Scharnweber C, Link KH, Nussler AK, Beger HG. 1995. Differential expression of CD44 splice variants in human pancreatic adenocarcinoma and in normal pancreas. Cancer Res. 55:5499–5503 [PubMed] [Google Scholar]
- Gao AC, Lou W, Sleeman JP, Isaacs JT. 1998. Metastasis suppression by the standard CD44 isoform does not require the binding of prostate cancer cells to hyaluronate. Cancer Res. 58:2350–2352 [PubMed] [Google Scholar]
- Godar S, Ince TA, Bell GW, Feldser D, Donaher JL, Bergh J, Liu A, Miu K, Watnick RS, Reinhardt F, et al. 2008. Growth-inhibitory and tumor-suppressive functions of p53 depend on its repression of CD44 expression. Cell. 134:62–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodison S, Urquidi V, Tarin D. 1999. CD44 cell adhesion molecules. Mol Pathol. 52:189–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotoda T, Matsumura Y, Kondo H, Saitoh D, Shimada Y, Kosuge T, Kanai Y, Kakizoe T. 1998. Expression of CD44 variants and its association with survival in pancreatic cancer. Jpn J Cancer Res. 89:1033–1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra C, Schuhmacher AJ, Canamero M, Grippo PJ, Verdaguer L, Perez-Gallego L, Dubus P, Sandgren EP, Barbacid M. 2007. Chronic pancreatitis is essential for induction of pancreatic ductal adenocarcinoma by K-Ras oncogenes in adult mice. Cancer Cell. 11:291–302 [DOI] [PubMed] [Google Scholar]
- Günthert U, Hofmann M, Rudy W, Reber S, Zöller M, Haussmann I, Matzku S, Wenzel A, Ponta H, Herrlich P. 1991. A new variant of glycoprotein CD44 confers metastatic potential to rat carcinoma cells. Cell. 65:13–24 [DOI] [PubMed] [Google Scholar]
- Günthert U, Stauder R, Mayer B, Terpe HJ, Finke L, Friedrichs K. 1995. Are CD44 variant isoforms involved in human tumour progression? Cancer Surv. 24:19–42 [PubMed] [Google Scholar]
- Guriec N, Gairard B, Marcellin L, Wilk A, Calderoli H, Renaud R, Bergerat JP, Oberling F. 1997. CD44 isoforms with exon v6 and metastasis of primary N0M0 breast carcinomas. Breast Cancer Res Treat. 44:261–268 [DOI] [PubMed] [Google Scholar]
- Haraguchi N, Ohkuma M, Sakashita H, Matsuzaki S, Tanaka F, Mimori K, Kamohara Y, Inoue H, Mori M. 2008. CD133+CD44+ population efficiently enriches colon cancer initiating cells. Ann Surg Oncol. 15:2927–2933 [DOI] [PubMed] [Google Scholar]
- Hermann PC, Huber SL, Herrler T, Aicher A, Ellwart JW, Guba M, Bruns CJ, Heeschen C. 2007. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 1:313–323 [DOI] [PubMed] [Google Scholar]
- Hidalgo M. 2010. Pancreatic cancer. N Engl J Med. 362:1605–1617 [DOI] [PubMed] [Google Scholar]
- Hong SP, Wen J, Bang S, Park S, Song SY. 2009. CD44-positive cells are responsible for gemcitabine resistance in pancreatic cancer cells. Int J Cancer. 125:2323–2331 [DOI] [PubMed] [Google Scholar]
- Huh JW, Kim HR, Kim YJ, Lee JH, Park YS, Cho SH, Joo JK. 2009. Expression of standard CD44 in human colorectal carcinoma: association with prognosis. Pathol Int. 59:241–246 [DOI] [PubMed] [Google Scholar]
- Hurt EM, Kawasaki BT, Klarmann GJ, Thomas SB, Farrar WL. 2008. CD44+ CD24(–) prostate cells are early cancer progenitor/stem cells that provide a model for patients with poor prognosis. Br J Cancer. 98:756–765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Immervoll H, Hoem D, Kugarajh K, Steine SJ, Molven A. 2006. Molecular analysis of the EGFR-RAS-RAF pathway in pancreatic ductal adenocarcinomas: lack of mutations in the BRAF and EGFR genes. Virchows Arch. 448:788–796 [DOI] [PubMed] [Google Scholar]
- Immervoll H, Hoem D, Sakariassen PO, Steffensen OJ, Molven A. 2008. Expression of the “stem cell marker” CD133 in pancreas and pancreatic ductal adenocarcinomas. BMC Cancer. 8:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji Q, Hao X, Zhang M, Tang W, Yang M, Li L, Xiang D, Desano JT, Bommer GT, Fan D, et al. 2009. MicroRNA miR-34 inhibits human pancreatic cancer tumor-initiating cells. PLoS One. 4:e6816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jothy S. 2003. CD44 and its partners in metastasis. Clin Exp Metastasis. 20:195–201 [DOI] [PubMed] [Google Scholar]
- Kallifatidis G, Rausch V, Baumann B, Apel A, Beckermann BM, Groth A, Mattern J, Li Z, Kolb A, Moldenhauer G, et al. 2009. Sulforaphane targets pancreatic tumour-initiating cells by NF-kappaB-induced antiapoptotic signalling. Gut. 58:949–963 [DOI] [PubMed] [Google Scholar]
- Karbanová J, Missol-Kolka E, Fonseca AV, Lorra C, Janich P, Hollerová H, Jászai J, Ehrmann J, Kolár Z, Liebers C, et al. 2008. The stem cell marker CD133 (Prominin-1) is expressed in various human glandular epithelia. J Histochem Cytochem. 56:977–993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemper K, Sprick MR, de Bree M, Scopelliti A, Vermeulen L, Hoek M, Zeilstra J, Pals ST, Mehmet H, Stassi G, et al. 2010. The AC133 epitope, but not the CD133 protein, is lost upon cancer stem cell differentiation. Cancer Res. 70:719–729 [DOI] [PubMed] [Google Scholar]
- Klingbeil P, Marhaba R, Jung T, Kirmse R, Ludwig T, Zoller M. 2009. CD44 variant isoforms promote metastasis formation by a tumor cell-matrix cross-talk that supports adhesion and apoptosis resistance. Mol Cancer Res. 7:168–179 [DOI] [PubMed] [Google Scholar]
- Kurozumi K, Nishida T, Nakao K, Nakahara M, Tsujimoto M. 1998. Expression of CD44 variant 6 and lymphatic invasion: importance to lymph node metastasis in gastric cancer. World J Surg. 22:853–857; discussion 857–858 [DOI] [PubMed] [Google Scholar]
- Lardon J, Corbeil D, Huttner WB, Ling Z, Bouwens L. 2008. Stem cell marker prominin-1/AC133 is expressed in duct cells of the adult human pancreas. Pancreas. 36:e1–6 [DOI] [PubMed] [Google Scholar]
- Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, Wicha M, Clarke MF, Simeone DM. 2007. Identification of pancreatic cancer stem cells. Cancer Res. 67:1030–1037 [DOI] [PubMed] [Google Scholar]
- Li CY, Li BX, Liang Y, Peng RQ, Ding Y, Xu DZ, Zhang X, Pan ZZ, Wan DS, Zeng YX, et al. 2009. Higher percentage of CD133+ cells is associated with poor prognosis in colon carcinoma patients with stage IIIB. J Transl Med. 7:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma S, Chan KW, Hu L, Lee TK, Wo JY, Ng IO, Zheng BJ, Guan XY. 2007. Identification and characterization of tumorigenic liver cancer stem/progenitor cells. Gastroenterology. 132:2542–2556 [DOI] [PubMed] [Google Scholar]
- Mackay CR, Terpe HJ, Stauder R, Marston WL, Stark H, Günthert U. 1994. Expression and modulation of CD44 variant isoforms in humans. J Cell Biol. 124:71–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda S, Shinchi H, Kurahara H, Mataki Y, Maemura K, Sato M, Natsugoe S, Aikou T, Takao S. 2008. CD133 expression is correlated with lymph node metastasis and vascular endothelial growth factor-C expression in pancreatic cancer. Br J Cancer. 98:1389–1397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marhaba R, Zoller M. 2004. CD44 in cancer progression: adhesion, migration and growth regulation. J Mol Histol. 35:211–231 [DOI] [PubMed] [Google Scholar]
- Mayer B, Jauch KW, Günthert U, Figdor CG, Schildberg FW, Funke I, Johnson JP. 1993. De-novo expression of CD44 and survival in gastric cancer. Lancet. 342:1019–1022 [DOI] [PubMed] [Google Scholar]
- Mizrak D, Brittan M, Alison MR. 2008. CD133: molecule of the moment. J Pathol. 214:3–9 [DOI] [PubMed] [Google Scholar]
- Monzani E, Facchetti F, Galmozzi E, Corsini E, Benetti A, Cavazzin C, Gritti A, Piccinini A, Porro D, Santinami M, et al. 2007. Melanoma contains CD133 and ABCG2 positive cells with enhanced tumourigenic potential. Eur J Cancer. 43:935–946 [DOI] [PubMed] [Google Scholar]
- Naor D, Wallach-Dayan SB, Zahalka MA, Sionov RV. 2008. Involvement of CD44, a molecule with a thousand faces, in cancer dissemination. Semin Cancer Biol. 18:260–267 [DOI] [PubMed] [Google Scholar]
- O’Brien CA, Pollett A, Gallinger S, Dick JE. 2007. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 445:106–110 [DOI] [PubMed] [Google Scholar]
- Ong CW, Kim LG, Kong HH, Low LY, Iacopetta B, Soong R, Salto-Tellez M. 2010. CD133 expression predicts for non-response to chemotherapy in colorectal cancer. Mod Pathol. 23:450–457 [DOI] [PubMed] [Google Scholar]
- Patrawala L, Calhoun T, Schneider-Broussard R, Li H, Bhatia B, Tang S, Reilly JG, Chandra D, Zhou J, Claypool K, et al. 2006. Highly purified CD44+ prostate cancer cells from xenograft human tumors are enriched in tumorigenic and metastatic progenitor cells. Oncogene. 25:1696–1708 [DOI] [PubMed] [Google Scholar]
- Ponta H, Sherman L, Herrlich PA. 2003. CD44: from adhesion molecules to signalling regulators. Nat Rev Mol Cell Biol. 4:33–45 [DOI] [PubMed] [Google Scholar]
- Prince ME, Sivanandan R, Kaczorowski A, Wolf GT, Kaplan MJ, Dalerba P, Weissman IL, Clarke MF, Ailles LE. 2007. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc Natl Acad Sci U S A. 104:973–978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rall CJ, Rustgi AK. 1995. CD44 isoform expression in primary and metastatic pancreatic adenocarcinoma. Cancer Res. 55:1831–1835 [PubMed] [Google Scholar]
- Reeder JA, Gotley DC, Walsh MD, Fawcett J, Antalis TM. 1998. Expression of antisense CD44 variant 6 inhibits colorectal tumor metastasis and tumor growth in a wound environment. Cancer Res. 58:3719–3726 [PubMed] [Google Scholar]
- Reya T, Morrison SJ, Clarke MF, Weissman IL. 2001. Stem cells, cancer, and cancer stem cells. Nature. 414:105–111 [DOI] [PubMed] [Google Scholar]
- Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, De Maria R. 2007. Identification and expansion of human colon-cancer-initiating cells. Nature. 445:111–115 [DOI] [PubMed] [Google Scholar]
- Ringel J, Jesnowski R, Schmidt C, Kohler HJ, Rychly J, Batra SK, Löhr M. 2001. CD44 in normal human pancreas and pancreatic carcinoma cell lines. Teratog Carcinog Mutagen. 21:97–106 [PubMed] [Google Scholar]
- Rosen JM, Jordan CT. 2009. The increasing complexity of the cancer stem cell paradigm. Science. 324:1670–1673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salnikov AV, Gladkich J, Moldenhauer G, Volm M, Mattern J, Herr I. 2010. CD133 is indicative for a resistance phenotype but does not represent a prognostic marker for survival of non–small cell lung cancer patients. Int J Cancer. 126:950–958 [DOI] [PubMed] [Google Scholar]
- Sato S, Miyauchi M, Takekoshi T, Zhao M, Kudo Y, Ogawa I, Kitagawa S, Fujita M, Takata T. 2000. Reduced expression of CD44 variant 9 is related to lymph node metastasis and poor survival in squamous cell carcinoma of tongue. Oral Oncol. 36:545–549 [DOI] [PubMed] [Google Scholar]
- Shimizu K, Itoh T, Shimizu M, Ku Y, Hori Y. 2009. CD133 expression pattern distinguishes intraductal papillary mucinous neoplasms from ductal adenocarcinomas of the pancreas. Pancreas. 38:e207–214 [DOI] [PubMed] [Google Scholar]
- Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, Dirks PB. 2003. Identification of a cancer stem cell in human brain tumors. Cancer Res. 63:5821–5828 [PubMed] [Google Scholar]
- Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB. 2004. Identification of human brain tumour initiating cells. Nature. 432:396–401 [DOI] [PubMed] [Google Scholar]
- Smith LM, Nesterova A, Ryan MC, Duniho S, Jonas M, Anderson M, Zabinski RF, Sutherland MK, Gerber HP, Van Orden KL, et al. 2008. CD133/prominin-1 is a potential therapeutic target for antibody-drug conjugates in hepatocellular and gastric cancers. Br J Cancer. 99:100–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sneath RJ, Mangham DC. 1998. The normal structure and function of CD44 and its role in neoplasia. Mol Pathol. 51:191–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamenkovic I, Aruffo A, Amiot M, Seed B. 1991. The hematopoietic and epithelial forms of CD44 are distinct polypeptides with different adhesion potentials for hyaluronate-bearing cells. EMBO J. 10:343–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanger BZ, Stiles B, Lauwers GY, Bardeesy N, Mendoza M, Wang Y, Greenwood A, Cheng KH, McLaughlin M, Brown D, et al. 2005. Pten constrains centroacinar cell expansion and malignant transformation in the pancreas. Cancer Cell. 8:185–195 [DOI] [PubMed] [Google Scholar]
- Tanabe KK, Stamenkovic I, Cutler M, Takahashi K. 1995. Restoration of CD44H expression in colon carcinomas reduces tumorigenicity. Ann Surg. 222:493– 501; discussion 501–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokue Y, Matsumura Y, Katsumata N, Watanabe T, Tarin D, Kakizoe T. 1998. CD44 variant isoform expression and breast cancer prognosis. Jpn J Cancer Res. 89:283–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida N, Buck DW, He D, Reitsma MJ, Masek M, Phan TV, Tsukamoto AS, Gage FH, Weissman IL. 2000. Direct isolation of human central nervous system stem cells. Proc Natl Acad Sci U S A. 97:14720–14725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe O, Kinoshita J, Shimizu T, Imamura H, Hirano A, Okabe T, Aiba M, Ogawa K. 2005. Expression of a CD44 variant and VEGF-C and the implications for lymphatic metastasis and long-term prognosis of human breast cancer. J Exp Clin Cancer Res. 24:75–82 [PubMed] [Google Scholar]
- Weigmann A, Corbeil D, Hellwig A, Huttner WB. 1997. Prominin, a novel microvilli-specific polytopic membrane protein of the apical surface of epithelial cells, is targeted to plasmalemmal protrusions of non-epithelial cells. Proc Natl Acad Sci U S A. 94:12425–12430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsch T, Keleg S, Bergmann F, Degrate L, Bauer S, Schmidt J. 2009. Comparative analysis of tumorbiology and CD133 positivity in primary and recurrent pancreatic ductal adenocarcinoma. Clin Exp Metastasis. 26:701–711 [DOI] [PubMed] [Google Scholar]
- Yin AH, Miraglia S, Zanjani ED, Almeida-Porada G, Ogawa M, Leary AG, Olweus J, Kearney J, Buck DW. 1997. AC133, a novel marker for human hematopoietic stem and progenitor cells. Blood. 90:5002–5012 [PubMed] [Google Scholar]
- Yu Q, Stamenkovic I. 1999. Localization of matrix metalloproteinase 9 to the cell surface provides a mechanism for CD44-mediated tumor invasion. Genes Dev. 13:35–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeppernick F, Ahmadi R, Campos B, Dictus C, Helmke BM, Becker N, Lichter P, Unterberg A, Radlwimmer B, Herold-Mende CC. 2008. Stem cell marker CD133 affects clinical outcome in glioma patients. Clin Cancer Res. 14:123–129 [DOI] [PubMed] [Google Scholar]
- Zhu L, Shi G, Schmidt CM, Hruban RH, Konieczny SF. 2007. Acinar cells contribute to the molecular heterogeneity of pancreatic intraepithelial neoplasia. Am J Pathol. 171:263–273 [DOI] [PMC free article] [PubMed] [Google Scholar]


