Abstract
The role of the mast cell–specific gangliosides in the modulation of the endocytic pathway of FcεRI was investigated in RBL-2H3 cells and in the ganglioside-deficient cell lines, E5 and D1. MAb BC4, which binds to the α subunit of FcεRI, was used in the analysis of receptor internalization. After incubation with BC4-FITC for 30 min, endocytic vesicles in RBL-2H3 and E5 cells were dispersed in the cytoplasm. After 1 hr, the endocytic vesicles of the RBL-2H3 cells had fused and formed clusters, whereas in the E5 cells, the fusion was slower. In contrast, in D1 cells, the endocytic vesicles were smaller and remained close to the plasma membrane even after 3 hr of incubation. When incubated with BC4-FITC and subsequently imunolabeled for markers of various endocytic compartments, a defect in the endocytic pathway in the E5 and D1 cells became evident. In the D1 cells, this defect was observed at the initial steps of endocytosis. Therefore, the ganglioside derivatives from GD1b are important in the endocytosis of FcεRI in mast cells. Because gangliosides may play a role in mast cell–related disease processes, they provide an attractive target for drug therapy and diagnosis.
Keywords: endocytosis, gangliosides, mast cells, high-affinity IgE receptor, fluorescence microscopy, electron microscopy
Gangliosides, complex glycosphingolipids, are ubiquitous membrane constituents enriched in lipid rafts (Brown and London 1998; Hakomori 1993; Pike et al. 2002). The biological role of gangliosides in cellular regulation is well recognized (Allende and Proia 2002; Fishman 1986; Hannun and Bell 1989; Igarashi et al. 1989; Oliver et al. 1992). Gangliosides are known to function in cell proliferation, adhesion, migration, apoptosis, and cell–cell and cell–substratum interactions and to act as receptors for bacterial toxins. They are also known to regulate cellular differentiation and to serve as differentiation markers in various cell types (Hakomori 1990; Martini et al. 2002; Wang XQ et al. 2002). In addition, gangliosides have been shown to have important regulatory roles in pathological conditions such as cancer (Hakomori 1996b), neurological (Sheikh et al. 1999; Sugiura et al. 2005) and autoimmune disorders (Wang J et al. 2009), and allergies (Flores-Diaz 2005) and inflammation (Lopez and Schnaar 2009).
The α-galactosyl derivatives of the ganglioside GD1b are unique gangliosides present on the surface of rodent mast cells that are specifically recognized by the monoclonal antibody (mAb) AA4 (Guo et al. 1989). These gangliosides have been identified as components of lipid rafts in the plasma membrane of RBL-2H3 cells (Sheets et al. 1999; Silveira e Souza et al. 2008). When these gangliosides are bound by mAb AA4, histamine release is inhibited in a time- and concentration-dependent manner. Furthermore, previous investigations have shown that the gangliosides derived from GD1b are essential for maintaining the structure of lipid rafts as well as for secretory granule release in RBL-2H3 cells (Holowka et al. 2000; Silveira e Souza et al. 2008). Moreover, binding of mAb AA4 also produces morphological and biochemical changes similar to those seen with activation of the high-affinity IgE receptor (FcεRI) (Basciano et al. 1986; Oliver et al. 1992; Stephan et al. 1997; Swaim et al. 1994). It has also been shown that these gangliosides are associated with FcεRI (Basciano et al. 1986) and that they play a crucial role in the initial events of FcεRI activation (Silveira e Souza et al. 2008). Previous results from our laboratory showed that the gangliosides are endocytosed and traffic with the same kinetics as FcεRI (Oliver et al. 2007).
Receptor-mediated endocytosis, including endocytosis of FcεRI, is a temporally and spatially organized process (Ceresa and Schmid 2000; Oliver et al. 2007). Following the binding of a ligand to its receptor, the receptor clusters in lipid rafts in the plasma membrane. The AP2 adaptor complexes, consisting of four adaptins (Boehm and Bonifacino 2001; Pearse 1975), associate with the receptor and recruit clathrin to the plasma membrane, leading to the formation of clathrin-coated vesicles (Bonifacino and Traub 2003), which are responsible for the initial transport of receptors to primary endosomes (Marsh and McMahon 1999). After losing their clathrin coat, the early endosomes express the GTPase Rab5 (Delprato et al. 2004; Rios et al. 2008), syntaxin 1/2 (Bonifacino and Hurley 2008; Carlton et al. 2004; Carlton et al. 2005; Rojas et al. 2008), EEA1 (Simonsen et al. 1998), and Rab4 (Van der Sluijs et al. 1991), among others. The early endosomes have the primary function of sorting internalized cargo to different cellular compartments (Dunn et al. 1989; Mayor et al. 1993). Endosomal contents may be recycled to the plasma membrane (Dautry-Varsat et al. 1983), degraded in lysosomes (Herbst et al. 1994; Mellman 1996), or delivered to the trans-Golgi network (Carlton et al. 2004). The early endosomes fuse with late endosomes (Gruenberg and Stenmark 2004) that express Rab7 (Zhang et al. 2009) or Rab9 (Ganley et al. 2004; Lombardi et al. 1993; Pfeffer 2009; Riederer et al. 1994). The vesicles expressing Rab7 subsequently mature into lysosomes (van Meel and Klumperman 2008), which express CD63 (LAMP-3) (Schmidt et al. 2009), LAMP-1, and LAMP-2 (Escola et al. 1998; Kuronita et al. 2002).
Although the importance of the gangliosides derived from GD1b in activation and mediator release in mast cells is well established, the role of these gangliosides in modulating endocytosis of FcεRI is still not fully understood. The aim of this study was to evaluate the role of the mast cell–specific gangliosides derived from GD1b in the endocytosis of FcεRI. RBL-2H3 cells, a rat mast cell line, and two mast cell lines deficient in gangliosides were used in this study. When these cells were activated via FcεRI, the ganglioside-deficient cell lines displayed defects in the endocytic process. These results indicate that the gangliosides derived from GD1b are essential for the endocytosis of FcεRI.
Materials and Methods
Cell Lines
Mutant RBL-2H3 cells, E5 (B6A4A2III-E5) and D1 (B6A4C1III-D1), were generated by exposure to ethyl methane sulfonate. They were then subcloned, and the sublines deficient in IgE-mediated degranulation as well as in the α-galactosyl derivatives of ganglioside GD1b (Guo et al. 1989) were selected. The E5 and D1 cell lines express 35% and <1%, respectively, of the α-galactosyl derivatives of ganglioside GD1b when compared to RBL-2H3 cells (Silveira e Souza et al. 2008). The E5 cells express 82.9% and D1 cells express <1% of GM1 in relation to the amount in RBL-2H3 cells (Silveira e Souza et al. 2010). All cell lines used in these experiments were grown as monolayers, as previously described (Barsumian et al. 1981).
Conjugation of mAb BC4
mAb BC4 (kindly provided by Reuben Siraganian, National Institutes of Health [NIH], National Institute of Dental and Craniofacial Research [NIDCR], Bethesda, MD), which binds to the α subunit of FcεRI and activates mast cells, was conjugated to FITC (BC4-FITC) using the FluoReporter FITC Protein Labeling Kit and to Qdots using a Qdot Antibody Conjugation Kit according to the manufacturer’s directions. Both kits were purchased from Invitrogen, Molecular Probes (Carlsbad, CA). BC4-FITC was used for fluorescence assays, and BC4-Qdots were evaluated by fluorescence microscopy before using them for electron microscopy.
Antibodies
The following primary antibodies were used: BC4-FITC (15 µg/ml), goat anti-clathrin (10 µg/ml, 15 µg/ml, or 1:200; Santa Cruz Biotechnology, Santa Cruz, CA), mouse anti-α-adaptin (1:500; Clone 8, BD Transduction Laboratories, Franklin Lakes, NJ), rabbit anti-syntaxin2 (SNX2, 1:1000; Haft et al. 1998), mouse anti-Rab5 (1:200; Clone 15, BD Tranduction Laboratories), rabbit anti-Rab7 (1:200; Santa Cruz), and mAb AD1, which recognizes CD63 (5 µg/ml or 1:500; kindly provided by Dr. Reuben Siraganian, NIH, NIDCR, Bethesda, MD). The lower concentrations were used for Western blots and the higher concentrations for immunofluorescence or flow cytometry. For Western blots, the following secondary antibodies, purchased from Jackson ImmunoResearch Laboratories (Port Washington, PA), were used: donkey anti-mouse IgG–horseradish peroxidase (HRP), goat anti-rabbit IgG-HRP, and rabbit anti-goat IgG-HRP (1:20,000). The following secondary antibodies were used for immunofluorescence: goat anti-mouse IgG F(ab)′2–Alexa 594, goat anti-rabbit IgG F(ab)′2–Alexa 594, and donkey anti-goat IgG F(ab)′2–Alexa 594 (1:300). The following secondary antibodies were used for fluorescence-activated cell sorting (FACS): donkey anti-goat IgG–Alexa 488, goat anti-mouse IgG–Alexa 488, and goat anti-rabbit IgG Alexa 488 (1:300). All Alexa-conjugated secondary antibodies were purchased from Invitrogen, Molecular Probes.
Incubation with mAb BC4 Conjugated to FITC
On 13-mm glass coverslips, 3.5 × 104 cells were plated and cultured for 16 hr. The cells were then washed rapidly in PBS, incubated in PBS containing 1% BSA (Sigma-Aldrich, St Louis, MO) and 5 µg/ml normal goat IgG (Jackson ImmunoResearch) for 45 min at 37C, and then incubated with BC4-FITC diluted in PBS + 1% BSA for 5, 15, 30, 60, or 180 min at 37C. After incubation, the cells were washed in PBS and fixed with 2% formaldehyde (Electron Microscopy Sciences, Hatfield, PA) for 20 min. After fixation, the coverslips were rinsed in PBS and mounted on glass slides with Fluormount G (Electron Microscopy Sciences) and examined with a Leica SP-2 scanning confocal microscope (Leica Microsystems, Heidelberg, Germany).
Immunofluorescence
After incubation with BC4-FITC, the cells were immunolabeled with primary antibodies diluted in PBS + 1% BSA for 1 hr at room temperature. For the immunolabeling of clathrin, SNX2, and CD63, the fixed cells were permeabilized with 0.1% saponin (Sigma-Aldrich) diluted in PBS for 15 min at room temperature. After incubation, the cells were rinsed thoroughly in PBS and the samples incubated for 45 min at room temperature with the secondary antibody diluted in PBS. All samples were then rinsed in PBS and coverslips mounted with Fluoromount-G (Electron Microscopy Sciences) and examined with a Leica SP2 or SP5 scanning confocal microscope (Leica Microsystems). Samples incubated without primary antibody served as controls. All controls were negative.
Transmission Electron Microscopy
Cells were plated at 2 × 105 cells/well in six-well tissue culture plates, and 48 hr later, BC4-Qdots were added to the culture medium. The cells were cultured for an additional 15, 30, or 60 min at 37C. Cells were washed in PBS and fixed in 2% glutaraldehyde (Ladd Research Industries, Burlington, VT) plus 2% formaldehyde (EM Sciences) in 0.1 M cacodylate buffer (pH 7.4) containing 0.05% CaCl2 for 1 hr at room temperature. Cells were postfixed in 1% OsO4 (EM Sciences) in 0.1 M cacodylate buffer (pH 7.4) for 2 hr, rinsed in Milli-Q water (Millipore, Billerica, MA), and dehydrated in a graded series of ethanol. Cells were removed from the tissue culture plates with propylene oxide and embedded in EMBED 812 (EM Sciences). Thin sections were cut with a diamond knife, mounted on copper grids, and stained for 10 min each in Reynolds’s lead citrate (Reynolds 1963) and 0.5% aqueous uranyl acetate and examined with a JEOL 100CX transmission electron microscope (JEOL, Tokyo, Japan). The distribution of the Qdots, either associated with the plasma membrane or internalized in cytoplasmic vesicles, was determined for a minimum of sections of 25 cells.
SDS-PAGE and Immunoblotting
For immunoblotting of whole-cell lysates, cell monolayers were washed twice with ice-cold PBS and immediately lysed with hot sample buffer (125 mM Tris-HCl [pH 6.8], 4% SDS, 10% glycerol, 0.006% bromophenol blue, and 1.8% β-mercaptoethanol) for 5 min at 70C. The samples were then sonicated for 10 sec on ice, and protease inhibitor cocktail (Sigma-Aldrich) was added to a final concentration of 1% and the proteins separated electrophoretically on 7.5% or 15% gels and transferred to Hybond membranes (GE-Healthcare Life Sciences, Piscataway, NJ). After transfer, the membranes were blocked for 1 hr at room temperature in TBS (0.05 M Tris-HCl, 0.15 M NaCl [pH 7.5], and 0.05% Tween 20) containing 5% BSA. After blocking, the membranes were incubated for 1 hr at room temperature with the various antibodies diluted in TBS. After washing 10 times with TBS, the membranes were incubated with secondary antibody for 45 min at room temperature. Membranes were then washed in TBS 10 times and developed using chemiluminescence (ECL-GE Healthcare). Mean optical density of the blots was determined using Adobe Photoshop 7.0 (Adobe Systems, San Jose, CA).
Results
Endocytosis and Trafficking of FcεRI Are Affected in Mutants Lacking Gangliosides
To determine whether endocytosis and trafficking of FcεRI are influenced by the lack of the gangliosides derived from GD1b, the three cell lines were incubated for various time intervals with BC4-FITC (Fig. 1). In the RBL-2H3 cells, after 30 min, the BC4-FITC was internalized and present in endocytic vesicles dispersed throughout the cytoplasm. At this same time point, there was a reduced number of endocytic vesicles in the cytoplasm of E5 cells, whereas in the D1 cells, the endocytic vesicles were associated with the plasma membrane. After 60 min of incubation, the endocytic vesicles in the RBL-2H3 cells appeared to be larger and aggregated. In the E5 cells, at 60 min, the distribution of the endocytic vesicles was similar to that seen in the RBL-2H3 cells at 30 min, whereas in the D1 cells, the endocytic vesicles continued to be localized close to the plasma membrane. At 180 min of incubation, in the RBL-2H3 cells, the majority of BC4-FITC was located in large cytoplasmic vesicles reminiscent of lysosomes. At this time, the endocytic vesicles in the E5 cells began to form aggregates, whereas the distribution of the endocytic vesicles in the D1 cells remained unchanged. These results indicate that the gangliosides derived from GD1b are important in the endocytic process.
Figure 1.
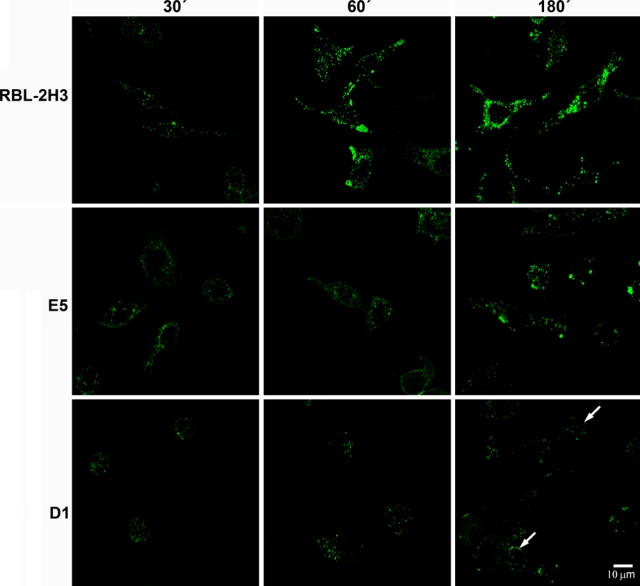
Kinetics of internalization of BC4-FITC. The cell lines were incubated at 37C with BC4-FITC for various times. Internalization of BC4-FITC occurred more rapidly in the RBL-2H3 cells than in E5 or D1 cells. In the D1 cells, BC4-FITC remained largely associated with the plasma membrane (arrows).
Mast Cell–Specific Gangliosides Are Involved in the Initial Steps of Endocytosis
Because the endocytic process appears to be delayed in the E5 and D1 cells, it was of interest to determine at which point in the endocytic pathway the process was perturbed. The three cell lines were incubated with BC4-FITC and subsequently immunostained for various markers of the endocytic pathway. After 5 min of incubation, in RBL-2H3 and E5 cells, clathrin, a marker for clathrin-coated vesicles, co-localized with BC4-FITC in vesicles that were located close to the plasma membrane (Fig. 2). However, in D1 cells, few clathrin-coated vesicles were seen. When the cell lines were immunostained for SNX2, a marker of early endosomes, after 15 min of incubation with BC4-FITC, the RBL-2H3 cells had a greater degree of co-localization of BC4-FITC and SNX2 than the E5 and D1 cells (Fig. 3), and the D1 cells showed less co-localization of BC4-FITC and SNX2 than the E5 cells. The final step in this analysis was to examine the presence of BC4-FITC in lysosomes. CD63 (LAMP-3), which is an integral membrane protein of secretory granules and lysosomes, was used as a marker. In both RBL-2H3 and E5 cells, BC4-FITC co-localized with lysosomes labeled for CD63 (Fig. 4). However, in D1 cells, even after 3 hr of incubation, there was less co-localization of BC4-FITC with lysosomes. Therefore, the endocytic defect seen in the D1 cells appears to occur at the initial steps of endocytosis and possibly results from the inability of D1 cells to form clathrin-coated vesicles.
Figure 2.
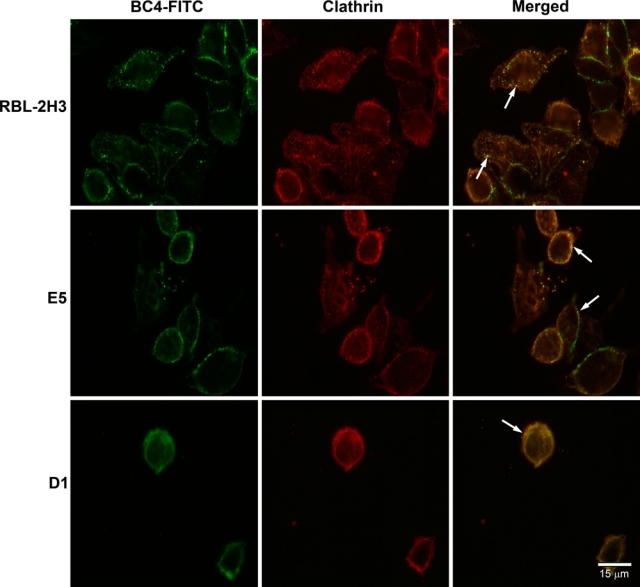
Double labeling of BC4-FITC and clathrin. Following incubation for 5 min at 37C with BC4-FITC, the cells were immunostained for clathrin. In the superimposed images, the BC4-FITC is co-localized with clathrin in endocytic vesicles close to the plasma membrane in RBL-2H3 and E5 cells (arrows). In the D1 cells, the staining pattern of both the BC4-FITC and clathrin is diffuse, and only a small number of double-labeled endocytic vesicles are present (arrows). BC4-FITC, green; clathrin, red.
Figure 3.
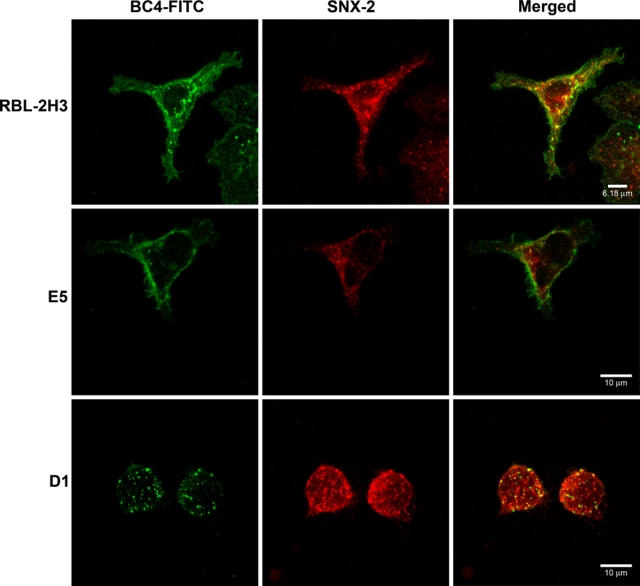
Double labeling of BC4-FITC and SNX2. Cells were incubated for 15 min at 37C with BC4-FITC and then immunostained for SNX2. In the RBL-2H3 cells, BC4-FITC co-localizes with early endosomes labeled with SNX2 (arrows). In contrast, the E5 and D1 cells show little co-localization of BC4-FITC and SNX2. BC4-FITC, green; SNX2, red.
Figure 4.
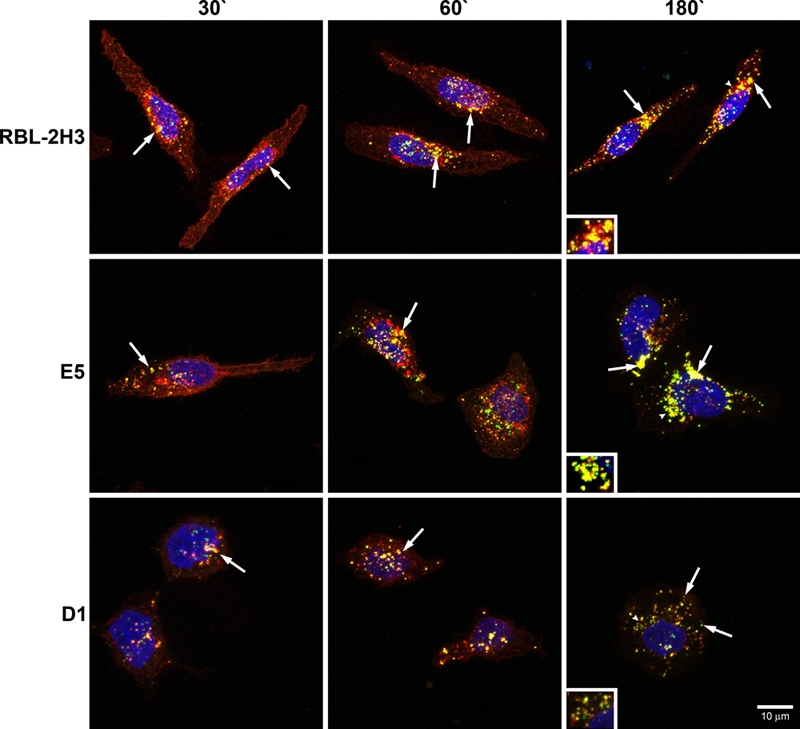
Double labeling of BC4-FITC and CD63. Cells were incubated for various times with BC4-FITC at 37C and subsequently immunostained for CD63. In RBL-2H3 and E5 cells, BC4-FITC is co-localized with lysosomes at all incubation times (arrows). In contrast, the D1 cells show less co-localization of BC4-FITC and CD63 (arrows). Arrowheads indicate original location of insets. Insets are an additional 1.5×. BC4-FITC, green; CD63, red; DAPI, blue.
In Ganglioside-Deficient Cells, FcεRI Remains Close to the Cell Surface
The endocytic pathway of FcεRI was further evaluated by transmission electron microscopy using BC4-Qdots (Fig. 5). In the RBL-2H3 cells, after 15 min of incubation, a few BC4-Qdots could be seen associated with the plasma membrane, but the majority of the BC4-Qdots had already been internalized. With time, there was little change in the distribution of the BC4-Qdots, with most of the BC4-Qdots in cytoplasmic vesicles. In comparison, E5 cells were less efficient in internalizing the BC4-Qdots. At 15 min, the majority of the BC4-Qdots were on the cell surface. They were slowly internalized and at 60 min were distributed both extracellularly and intracellularly. In contrast, in the D1 cells, the majority of the BC4-Qdots remained associated with the cell surface, and by 60 min, the quantity of BC4-Qdots found in cytoplasmic vesicles was reduced in comparison with the RBL-2H3 and E5 cells. The percentage of BC4-Qdots associated with the cell surface in the various cell lines as well as those internalized in cytoplasmic vesicles was then quantified (Fig. 6). In the RBL-2H3 cell at 15 min of incubation, 86.0 ± 2.3% of the cell-associated BC4-Qdots were internalized, and only 14.0 ± 1.0% of the BC4-Qdots were seen associated with the external face of the plasma membrane. These percentages did not change significantly after this time. In the E5 cells after 15 min, only 22.0 ± 1.5% of the BC4-Qdots had been internalized, and 78.0 ± 3.1% remained outside the cells. The percentage of internalized BC4-Qdots increased with time until by 60 min, 47 ± 2.8% had been internalized. The internalization of the BC4-Qdots was also reduced in the D1 cells. At 15 min, 27.0 ± 0.56% of the marker had been internalized, and by 60 min, the percent internalized had increased to only 36.0 ± 1.1%.
Figure 5.
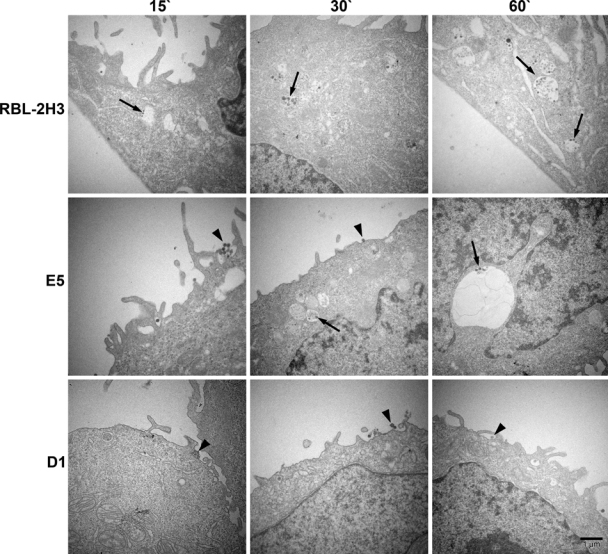
Transmission electron microscopy of BC4-Qdots. The cell lines were incubated with BC4-Qdots for 15, 30, or 60 min at 37C. RBL-2H3 cells rapidly internalized the BC4-Qdots into cytoplasmic vesicles. However, in E5 and D1 cells, the majority of the BC4-Qdots remained associated with the plasma membrane.
Figure 6.
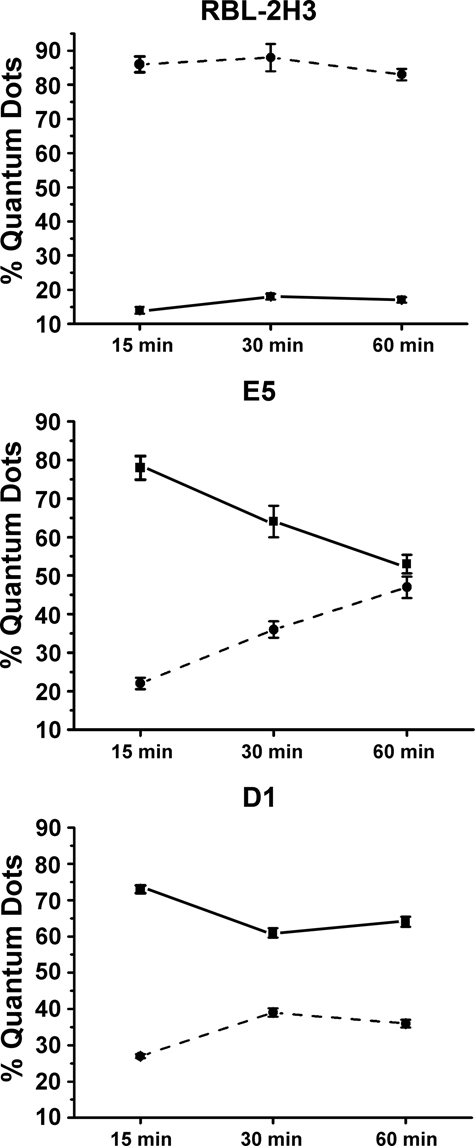
Quantification of the internalization of BC4-Qdots. The cell lines were incubated with BC4-Qdots for 15, 30, or 60 min at 37C and then processed for transmission electron microscopy. The percentage of BC4-Qdots associated with the cell surface or internalized was determined for a minimum of 25 cells at each data point. Results are expressed as % ± SD. Qdots internalized in cytoplasmic vesicles, ---•---; Qdots associated with the external plasma membrane, ▬■▬.
The Defect in Trafficking Is Not Due to Differences in the Expression of Proteins Associated with the Endocytic Pathway
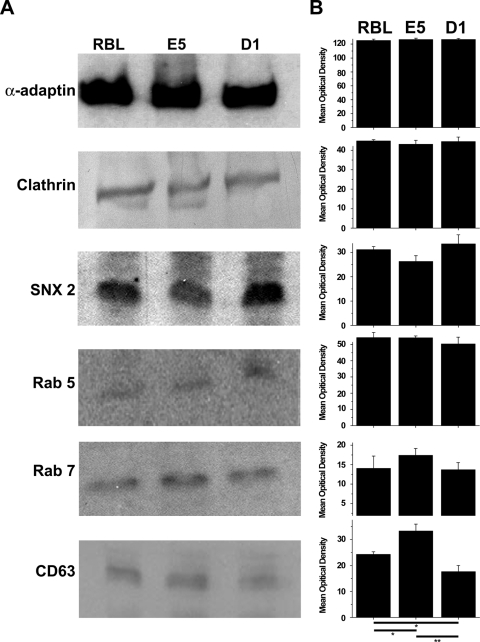
It is possible that the defect in endocytosis is related to the absence or changes in the expression of one or more of the proteins associated with the endocytic pathway. Therefore, the expression of several proteins known to play a role in endocytosis was examined by immunoblotting (Fig. 7) and by FACS (data not shown). Both methods gave the same results. All three cell lines showed similar expression levels of α-adaptin, clathrin, SNX2, Rab5, and Rab7. However, CD63 showed a higher level of expression in E5 cells as compared to RBL-2H3 or D1 cells. The expression of CD63 was lower in the D1 cells than in the RBL-2H3 or E5 cells. Therefore, the defects in the endocytic pathways seen in the ganglioside-deficient cells do not seem to be due to differential expression of these proteins.
Figure 7.
Western blots of proteins associated with the endocytic pathway. Lysates of RBL-2H3, E5, and D1 cells were immunoblotted for α-adaptin, clathrin, SNX2, Rab5, Rab7, and CD63 (A) and the resulting bands quantified (B). The levels of expression of all the proteins were not significantly different among the three cell lines except for CD63. The expression of CD63 was higher in the E5 cells when compared with RBL-2H3 cells (p≤0.05) or D1 cells (p≤0.01). The RBL-2H3 cells expressed more CD63 than the D1 cells (p≤0.05). In A, a representative blot for each protein is shown. In B, the data are expressed as the mean and standard deviation from a minimum of three separate experiments.
Discussion
The present study found that the gangliosides derived from GD1b are essential for internalization of FcεRI in RBL-2H3 cells and that the lack of gangliosides affects the initial endocytic steps. It is known in RBL-2H3 cells that FcεRI is internalized in clathrin-coated vesicles (Oliver et al. 2007; Pfeiffer et al. 1985; Seagrave et al. 1991; Stump et al. 1989). In the E5 and D1 cell lines, FcεRI is also internalized in clathrin-coated vesicles, but the D1 cells form far fewer coated vesicles. Following receptor activation in RBL-2H3 cells, FcεRI clusters in lipid rafts, from which it is internalized in coated vesicles (Oliver et al. 2007; Silveira e Souza et al. 2008). In ganglioside-deficient D1 cells, the receptor remains dispersed on the plasma membrane upon activation, and the lipid rafts continue to be disorganized (Silveira e Souza et al. 2008). In the present study, in the D1 cells, clathrin also remained dispersed following FcεRI activation. The failure of the receptor to cluster in lipid rafts may have interfered with the assembly of the clathrin coat, thus preventing endocytosis of the receptor. In addition, the disorganization of the lipid rafts may have affected the correct binding of the adaptins to the receptor and/or the recruitment of clathrin by the AP2 complex. However, the clathrin-coated vesicles that ultimately formed appeared to transit normally, delivering FcεRI to lysosomes. This impaired formation of coated vesicles in D1 cells does not seem to be specific to the internalization of FcεRI. In preliminary experiments examining the uptake of Lucifer yellow or concanavalin A by fluorescence microscopy or horseradish peroxidase by electron microscopy, the uptake of the tracers into the D1 cells was also diminished.
The results show that the mutant cell lines (E5 and D1) have a defect in the endocytosis of the FcεRI. Other authors also have observed that these mutant cells have an impaired exocytosis as assessed by a decrease in the release of β-hexosaminidase activity, after FcεRI stimulation (Silveira e Souza et al. 2008). Impaired FcεRI-mediated degranulation has also been observed in the parent clone (B6A4C1) of D1 cells (Field et al. 2000; Hong-Geller et al. 2000). The α-galactosyl derivatives of GD1b are also important for the preservation of the structure and organization of RBL-2H3 cells (Silveira e Souza et al. 2010). In this previous study, the authors observed a relationship between ganglioside expression and morphology. A significant alteration in morphology was observed only in D1 cells, which express <1% of gangliosides, and not in E5 cells, which express 35% of gangliosides.
Gangliosides play an important role in modulating not only FcεRI but also other receptors (Holowka and Baird 2001; Holowka et al. 2000; Rivera et al. 2001; Silveira e Souza et al. 2008; Wilson et al. 2000). GM3 inhibits signaling through the epidermal growth factor receptor (EGFR) by preventing dimerization of the receptor and its autophosphorylation (Rebbaa et al. 1996). Phosphorylation of the insulin receptor is also inhibited by the exogenous administration of GM3 (Tagami et al. 2002). It is now well established that selected glycosphingolipids bind to various receptors such as thyrotropin (Mullin et al. 1976), vitronectin (Cheresh et al. 1987), insulin (Kabayama et al. 2007), epidermal growth factor (EGF; Miljan et al. 2002), and platelet-derived growth factor (PDGF; Bremer et al. 1984). Other studies have shown that the ganglioside GM1 also plays a crucial role in modulating excitatory opioid receptor functions (Crain and Shen 1998, 2004). Molecular interaction between the ganglioside GM1 and δ-type opioid receptors has been suggested as a means of regulation of these receptors (Wu, Lu, Alfinito, et al. 1997; Wu, Lu, Ledeen, et al. 1997).
The finding that the E5 cells had an increased expression of CD63 by immunolabeling, Western blot, and FACS analysis was unexpected. Data obtained by transmission electron microscopy in this study as well as that obtained by Silveira e Souza et al. (2008) showed that the lysosomes in E5 cells are larger than those in RBL-2H3 and D1 cells. This increased surface area of the lysosomal membrane in E5 cells could explain the increased expression of CD63, an integral lysosomal membrane protein.
Because gangliosides are related to many disease processes (Hakomori 1996a; Yamashita et al. 1999), they provide an attractive target for drug therapy and diagnosis. Gangliosides play an important role in tumor progression and metastasis (Birkle et al. 2003; Deng et al. 2000). For instance, because of their high abundance in tumors, as compared with the corresponding normal tissue, they may be good targets for cancer immunotherapy (Hakomori 1996a; Livingston et al. 1979). Given the importance of gangliosides in FcεRI signaling and mediator release in mast cells, they may also be good targets for modulating mast cells during allergic and inflammatory processes.
Acknowledgments
We thank Reuben P. Siraganian, MD, PhD, National Institutes of Health (Bethesda, MD), who generously provided the cell lines; Márcia Sirlene Graeff and Lenaldo Branco Rocha for assistance with the confocal microscopy; Maria Dolores S. Ferreira, Tereza P. Maglia, and José Augusto Moulin for assistance with the transmission electron microscopy; and Anderson Roberto de Souza for technical assistance, all from the Department of Cell and Molecular Biology and Pathogenic Bioagents, FMRP-USP, Ribeirão Preto, SP. We also thank Patrícia Vianna Bonini Palma and Camila Cristina Branquinho de Oliveira Menezes, Hemocentro-FMRP-USP, Ribeirão Preto, Brazil, for assistance with the flow cytometry.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the authorship and/or publication of this article.
The author(s) disclosed receipt of the following financial support for the research and/or authorship of this article: Financial support from CAPES (Coordenação de Aperfeiçoamento de Pessoa de Nível Superior), FAEPA (Fundação de Apoio ao Ensino, Pesquisa e Assistência), and FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo) (individual grant 01/10752-2 to CO).
References
- Allende ML, Proia RL. 2002. Lubricating cell signaling pathways with gangliosides. Curr Opin Struct Biol. 12:587–592 [DOI] [PubMed] [Google Scholar]
- Barsumian EL, Isersky C, Petrino MG, Siraganian RP. 1981. IgE-induced histamine release from rat basophilic leukemia cell lines: isolation of releasing and nonreleasing clones. Eur J Immunol. 11:317–323 [DOI] [PubMed] [Google Scholar]
- Basciano LK, Berenstein EH, Kmak L, Siraganian RP. 1986. Monoclonal antibodies that inhibit IgE binding. J Biol Chem. 261:11823–11831 [PubMed] [Google Scholar]
- Birkle S, Zeng G, Gao L, Yu RK, Aubry J. 2003. Role of tumor-associated gangliosides in cancer progression. Biochimie. 85:455–463 [DOI] [PubMed] [Google Scholar]
- Boehm M, Bonifacino JS. 2001. Adaptins: the final recount. Mol Biol Cell. 12:2907–2920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifacino JS, Hurley JH. 2008. Retromer. Curr Opin Cell Biol. 20:427–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifacino JS, Traub LM. 2003. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu Rev Biochem. 72:395–447 [DOI] [PubMed] [Google Scholar]
- Bremer EG, Hakomori S, Bowen-Pope DF, Raines E, Ross R. 1984. Ganglioside-mediated modulation of cell growth, growth factor binding, and receptor phosphorylation. J Biol Chem. 259:6818–6825 [PubMed] [Google Scholar]
- Brown DA, London E. 1998. Functions of lipid rafts in biological membranes. Annu Rev Cell Dev Biol. 14:111–136 [DOI] [PubMed] [Google Scholar]
- Carlton J, Bujny M, Peter BJ, Oorschot VM, Rutherford A, Mellor H, Klumperman J, McMahon HT, Cullen PJ. 2004. Sorting nexin-1 mediates tubular endosome-to-TGN transport through coincidence sensing of high-curvature membranes and 3-phosphoinositides. Curr Biol. 14:1791–1800 [DOI] [PubMed] [Google Scholar]
- Carlton JG, Bujny MV, Peter BJ, Oorschot VM, Rutherford A, Arkell RS, Klumperman J, McMahon HT, Cullen PJ. 2005. Sorting nexin-2 is associated with tubular elements of the early endosome, but is not essential for retromer-mediated endosome-to-TGN transport. J Cell Sci. 118:4527–4539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceresa BP, Schmid SL. 2000. Regulation of signal transduction by endocytosis. Curr Opin Cell Biol. 12:204–210 [DOI] [PubMed] [Google Scholar]
- Cheresh DA, Pytela R, Pierschbacher MD, Klier FG, Ruoslahti E, Reisfeld RA. 1987. An Arg-Gly-Asp-directed receptor on the surface of human melanoma cells exists in an divalent cation-dependent functional complex with the disialoganglioside GD2. J Cell Biol. 105:1163–1173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crain SM, Shen KF. 1998. GM1 ganglioside-induced modulation of opioid receptor-mediated functions. Ann N Y Acad Sci. 845:106–125 [DOI] [PubMed] [Google Scholar]
- Crain SM, Shen KF. 2004. Neuraminidase inhibitor, oseltamivir blocks GM1 ganglioside-regulated excitatory opioid receptor-mediated hyperalgesia, enhances opioid analgesia and attenuates tolerance in mice. Brain Res. 995:260–266 [DOI] [PubMed] [Google Scholar]
- Dautry-Varsat A, Ciechanover A, Lodish HF. 1983. pH and the recycling of transferrin during receptor-mediated endocytosis. Proc Natl Acad Sci U S A. 80:2258–2262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delprato A, Merithew E, Lambright DG. 2004. Structure, exchange determinants, and family-wide rab specificity of the tandem helical bundle and Vps9 domains of Rabex-5. Cell. 118:607–617 [DOI] [PubMed] [Google Scholar]
- Deng W, Li R, Ladisch S. 2000. Influence of cellular ganglioside depletion on tumor formation. J Natl Cancer Inst. 92:912–917 [DOI] [PubMed] [Google Scholar]
- Dunn KW, McGraw TE, Maxfield FR. 1989. Iterative fractionation of recycling receptors from lysosomally destined ligands in an early sorting endosome. J Cell Biol. 109:3303–3314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escola JM, Kleijmeer MJ, Stoorvogel W, Griffith JM, Yoshie O, Geuze HJ. 1998. Selective enrichment of tetraspan proteins on the internal vesicles of multivesicular endosomes and on exosomes secreted by human B-lymphocytes. J Biol Chem. 273:20121–20127 [DOI] [PubMed] [Google Scholar]
- Field KA, Apgar JR, Hong-Geller E, Siraganian RP, Baird B, Holowka D. 2000. Mutant RBL mast cells defective in Fc epsilon RI signaling and lipid raft biosynthesis are reconstituted by activated Rho-family GTPases. Mol Biol Cell. 11:3661–3673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman PH. 1986. Recent advances in identifying the functions of gangliosides. Chem Phys Lipids. 42:137–151 [DOI] [PubMed] [Google Scholar]
- Flores-Diaz M, Alape-Giron A, Clark G, Catimel B, Hirabayashi Y, Nice E, Gutierrez JM, Titball R, Thelestam M. 2005. A cellular deficiency of gangliosides causes hypersensitivity to Clostridium perfringens phospholipase C. J Biol Chem. 280:26680–26689 [DOI] [PubMed] [Google Scholar]
- Ganley IG, Carroll K, Bittova L, Pfeffer S. 2004. Rab9 GTPase regulates late endosome size and requires effector interaction for its stability. Mol Biol Cell. 15:5420–5430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenberg J, Stenmark H. 2004. The biogenesis of multivesicular endosomes. Nat Rev Mol Cell Biol. 5:317–323 [DOI] [PubMed] [Google Scholar]
- Guo NH, Her GR, Reinhold VN, Brennan MJ, Siraganian RP, Ginsburg V. 1989. Monoclonal antibody AA4, which inhibits binding of IgE to high affinity receptors on rat basophilic leukemia cells, binds to novel alpha-galactosyl derivatives of ganglioside GD1b. J Biol Chem. 264:13267–13272 [PubMed] [Google Scholar]
- Haft CR, de la Luz Sierra M, Barr VA, Haft DH, Taylor SI. 1998. Identification of a family of sorting nexin molecules and characterization of their association with receptors. Mol Cell Biol. 18:7278–7287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakomori S. 1990. Bifunctional role of glycosphingolipids: modulators for transmembrane signaling and mediators for cellular interactions. J Biol Chem. 265:18713–18716 [PubMed] [Google Scholar]
- Hakomori S. 1993. Structure and function of sphingoglycolipids in transmembrane signalling and cell-cell interactions. Biochem Soc Trans. 21(Pt 3):583–595 [DOI] [PubMed] [Google Scholar]
- Hakomori S. 1996a. Sphingolipid-dependent protein kinases. Adv Pharmacol. 36:155–171 [DOI] [PubMed] [Google Scholar]
- Hakomori S. 1996b. Tumor malignancy defined by aberrant glycosylation and sphingo(glyco)lipid metabolism. Cancer Res. 56:5309–5318 [PubMed] [Google Scholar]
- Hannun YA, Bell RM. 1989. Functions of sphingolipids and sphingolipid breakdown products in cellular regulation. Science. 243:500–507 [DOI] [PubMed] [Google Scholar]
- Herbst JJ, Opresko LK, Walsh BJ, Lauffenburger DA, Wiley HS. 1994. Regulation of postendocytic trafficking of the epidermal growth factor receptor through endosomal retention. J Biol Chem. 269:12865–12873 [PubMed] [Google Scholar]
- Holowka D, Baird B. 2001. Fc(epsilon)RI as a paradigm for a lipid raft-dependent receptor in hematopoietic cells. Semin Immunol. 13:99–105 [DOI] [PubMed] [Google Scholar]
- Holowka D, Sheets ED, Baird B. 2000. Interactions between Fc(epsilon)RI and lipid raft components are regulated by the actin cytoskeleton. J Cell Sci. 113(Pt 6):1009–1019 [DOI] [PubMed] [Google Scholar]
- Hong-Geller E, Holowka D, Siraganian RP, Baird B, Cerione RA. 2000. Cdc42 and Rac stimulate exocytosis of secretory granules by activating the IP(3)/calcium pathway in RBL-2H3 mast cells. J Cell Biol. 148:481–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi Y, Nojiri H, Hanai N, Hakomori S. 1989. Gangliosides that modulate membrane protein function. Methods Enzymol. 179:521–541 [DOI] [PubMed] [Google Scholar]
- Kabayama K, Sato T, Saito K, Loberto N, Prinetti A, Sonnino S, Kinjo M, Igarashi Y, Inokuchi J. 2007. Dissociation of the insulin receptor and caveolin-1 complex by ganglioside GM3 in the state of insulin resistance. Proc Natl Acad Sci U S A. 104:13678–13683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuronita T, Eskelinen EL, Fujita H, Saftig P, Himeno M, Tanaka Y. 2002. A role for the lysosomal membrane protein LGP85 in the biogenesis and maintenance of endosomal and lysosomal morphology. J Cell Sci. 115:4117–4131 [DOI] [PubMed] [Google Scholar]
- Livingston PO, Shiku H, Bean MA, Pinsky CM, Oettgen HF, Old LJ. 1979. Cell-mediated cytotoxicity for cultured autologous melanoma cells. Int J Cancer. 24:34–44 [DOI] [PubMed] [Google Scholar]
- Lombardi D, Soldati T, Riederer MA, Goda Y, Zerial M, Pfeffer SR. 1993. Rab9 functions in transport between late endosomes and the trans Golgi network. Embo J. 12:677–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez PH, Schnaar RL. 2009. Gangliosides in cell recognition and membrane protein regulation. Curr Opin Struct Biol. 19:549–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh M, McMahon HT. 1999. The structural era of endocytosis. Science. 285:215–220 [DOI] [PubMed] [Google Scholar]
- Martini F, Riondino S, Pignatelli P, Gazzaniga PP, Ferroni P, Lenti L. 2002. Involvement of GD3 in platelet activation: a novel association with Fcgamma receptor. Biochim Biophys Acta. 1583:297–304 [DOI] [PubMed] [Google Scholar]
- Mayor S, Presley JF, Maxfield FR. 1993. Sorting of membrane components from endosomes and subsequent recycling to the cell surface occurs by a bulk flow process. J Cell Biol. 121:1257–1269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellman I. 1996. Endocytosis and molecular sorting. Annu Rev Cell Dev Biol. 12:575–625 [DOI] [PubMed] [Google Scholar]
- Miljan EA, Meuillet EJ, Mania-Farnell B, George D, Yamamoto H, Simon HG, Bremer EG. 2002. Interaction of the extracellular domain of the epidermal growth factor receptor with gangliosides. J Biol Chem. 277:10108–10113 [DOI] [PubMed] [Google Scholar]
- Mullin BR, Fishman PH, Lee G, Aloj SM, Ledley FD, Winand RJ, Kohn LD, Brady RO. 1976. Thyrotropin-ganglioside interactions and their relationship to the structure and function of thyrotropin receptors. Proc Natl Acad Sci U S A. 73:842–846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver C, Fujimura A, Silveira ESAM, Orlandini de Castro R, Siraganian RP, Jamur MC. 2007. Mast cell–specific gangliosides and FcepsilonRI follow the same endocytic pathway from lipid rafts in RBL-2H3 cells. J Histochem Cytochem. 55:315–325 [DOI] [PubMed] [Google Scholar]
- Oliver C, Sahara N, Kitani S, Robbins AR, Mertz LM, Siraganian RP. 1992. Binding of monoclonal antibody AA4 to gangliosides on rat basophilic leukemia cells produces changes similar to those seen with Fc epsilon receptor activation. J Cell Biol. 116:635–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearse BM. 1975. Coated vesicles from pig brain: purification and biochemical characterization. J Mol Biol. 97:93–98 [DOI] [PubMed] [Google Scholar]
- Pfeffer SR. 2009. Multiple routes of protein transport from endosomes to the trans Golgi network. FEBS Lett. 583:3811–3816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer JR, Seagrave JC, Davis BH, Deanin GG, Oliver JM. 1985. Membrane and cytoskeletal changes associated with IgE-mediated serotonin release from rat basophilic leukemia cells. J Cell Biol. 101:2145–2155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike LJ, Han X, Chung KN, Gross RW. 2002. Lipid rafts are enriched in arachidonic acid and plasmenylethanolamine and their composition is independent of caveolin-1 expression: a quantitative electrospray ionization/mass spectrometric analysis. Biochemistry. 41:2075–2088 [DOI] [PubMed] [Google Scholar]
- Rebbaa A, Hurh J, Yamamoto H, Kersey DS, Bremer EG. 1996. Ganglioside GM3 inhibition of EGF receptor mediated signal transduction. Glycobiology. 6:399–406 [DOI] [PubMed] [Google Scholar]
- Reynolds ES. 1963 The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 17:208–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riederer MA, Soldati T, Shapiro AD, Lin J, Pfeffer SR. 1994. Lysosome biogenesis requires Rab9 function and receptor recycling from endosomes to the trans-Golgi network. J Cell Biol. 125:573–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios EJ, Piliponsky AM, Ra C, Kalesnikoff J, Galli SJ. 2008. Rabaptin-5 regulates receptor expression and functional activation in mast cells. Blood. 112:4148–4157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera J, Arudchandran R, Gonzalez-Espinosa C, Manetz TS, Xirasagar S. 2001. A perspective: regulation of IgE receptor-mediated mast cell responses by a LAT-organized plasma membrane-localized signaling complex. Int Arch Allergy Immunol. 124:137–141 [DOI] [PubMed] [Google Scholar]
- Rojas R, van Vlijmen T, Mardones GA, Prabhu Y, Rojas AL, Mohammed S, Heck AJ, Raposo G, van der Sluijs P, Bonifacino JS. 2008. Regulation of retromer recruitment to endosomes by sequential action of Rab5 and Rab7. J Cell Biol. 183:513–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt H, Gelhaus C, Lucius R, Nebendahl M, Leippe M, Janssen O. 2009. Enrichment and analysis of secretory lysosomes from lymphocyte populations. BMC Immunol. 10:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seagrave J, Pfeiffer JR, Wofsy C, Oliver JM. 1991. Relationship of IgE receptor topography to secretion in RBL-2H3 mast cells. J Cell Physiol. 148:139–151 [DOI] [PubMed] [Google Scholar]
- Sheets ED, Holowka D, Baird B. 1999. Membrane organization in immunoglobulin E receptor signaling. Curr Opin Chem Biol. 3:95–99 [DOI] [PubMed] [Google Scholar]
- Sheikh KA, Sun J, Liu Y, Kawai H, Crawford TO, Proia RL, Griffin JW, Schnaar RL. 1999. Mice lacking complex gangliosides develop Wallerian degeneration and myelination defects. Proc Natl Acad Sci U S A. 96:7532–7537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveira e Souza AM, Mazucato VM, de Castro RO, Matioli F, Ciancaglini P, de Paiva Paulino T, Jamur MC, Oliver C. 2008. The alpha-galactosyl derivatives of ganglioside GD(1b) are essential for the organization of lipid rafts in RBL-2H3 mast cells. Exp Cell Res. 314:2515–2528 [DOI] [PubMed] [Google Scholar]
- Silveira e Souza AM, Trindade ES, Jamur MC, Oliver C. 2010. Gangliosides are important for the preservation of the structure and organization of RBL-2H3 mast cells. J Histochem Cytochem. 58:83–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsen A, Lippe R, Christoforidis S, Gaullier JM, Brech A, Callaghan J, Toh BH, Murphy C, Zerial M, Stenmark H. 1998. EEA1 links PI(3)K function to Rab5 regulation of endosome fusion. Nature. 394:494–498 [DOI] [PubMed] [Google Scholar]
- Stephan V, Seibt A, Dukanovic D, Skasa M, Swaim WD, Berenstein EH, Siraganian RP, Wahn V. 1997. Anti-ganglioside monoclonal antibody AA4 selectively inhibits IgE-induced signal transduction pathways in rat basophilic leukemia cells. Mol Immunol. 34:227–235 [DOI] [PubMed] [Google Scholar]
- Stump RF, Pfeiffer JR, Schneebeck MC, Seagrave JC, Oliver JM. 1989. Mapping gold-labeled receptors on cell surfaces by backscattered electron imaging and digital image analysis: studies of the IgE receptor on mast cells. Am J Anat. 185:128–141 [DOI] [PubMed] [Google Scholar]
- Sugiura Y, Furukawa K, Tajima O, Mii S, Honda T, Furukawa K. 2005. Sensory nerve-dominant nerve degeneration and remodeling in the mutant mice lacking complex gangliosides. Neuroscience. 135:1167–1178 [DOI] [PubMed] [Google Scholar]
- Swaim WD, Minoguchi K, Oliver C, Hamawy MM, Kihara H, Stephan V, Berenstein EH, Siraganian RP. 1994. The anti-ganglioside monoclonal antibody AA4 induces protein tyrosine phosphorylations, but not degranulation, in rat basophilic leukemia cells. J Biol Chem. 269:19466–19473 [PubMed] [Google Scholar]
- Tagami S, Inokuchi Ji J, Kabayama K, Yoshimura H, Kitamura F, Uemura S, Ogawa C, Ishii A, Saito M, Ohtsuka Y, et al. 2002. Ganglioside GM3 participates in the pathological conditions of insulin resistance. J Biol Chem. 277:3085–3092 [DOI] [PubMed] [Google Scholar]
- Van Der Sluijs P, Hull M, Zahraoui A, Tavitian A, Goud B, Mellman I. 1991. The small GTP-binding protein rab4 is associated with early endosomes. Proc Natl Acad Sci U S A. 88:6313–6317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Meel E, Klumperman J. 2008. Imaging and imagination: understanding the endo-lysosomal system. Histochem Cell Biol. 129:253–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Lu ZH, Gabius HJ, Rohowsky-Kochan C, Ledeen RW, Wu G. 2009. Cross-linking of GM1 ganglioside by galectin-1 mediates regulatory T cell activity involving TRPC5 channel activation: possible role in suppressing experimental autoimmune encephalomyelitis. J Immunol. 182:4036–4045 [DOI] [PubMed] [Google Scholar]
- Wang XQ, Sun P, Paller AS. 2002. Ganglioside modulation regulates epithelial cell adhesion and spreading via ganglioside-specific effects on signaling. J Biol Chem. 277:40410–40419 [DOI] [PubMed] [Google Scholar]
- Wilson BS, Pfeiffer JR, Oliver JM. 2000. Observing FcepsilonRI signaling from the inside of the mast cell membrane. J Cell Biol. 149:1131–1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, Lu ZH, Alfinito P, Ledeen RW. 1997. Opioid receptor and calcium channel regulation of adenylyl cyclase, modulated by GM1, in NG108-15 cells: competitive interactions. Neurochem Res. 22:1281–1289 [DOI] [PubMed] [Google Scholar]
- Wu G, Lu ZH, Ledeen RW. 1997. Interaction of the delta-opioid receptor with GM1 ganglioside: conversion from inhibitory to excitatory mode. Brain Res Mol Brain Res. 44:341–346 [DOI] [PubMed] [Google Scholar]
- Yamashita T, Wada R, Sasaki T, Deng C, Bierfreund U, Sandhoff K, Proia RL. 1999. A vital role for glycosphingolipid synthesis during development and differentiation. Proc Natl Acad Sci U S A. 96:9142–9147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Chen L, Wang S, Wang T. 2009. Rab7: roles in membrane trafficking and disease. Biosci Rep. 29:193–209 [DOI] [PubMed] [Google Scholar]