Abstract
Confocal imaging uses immunohistochemical binding of specific antibodies to visualize tissues, but technical obstacles limit more widespread use of this technique in the imaging of peripheral nerve tissue. These obstacles include same-species antibody cross-reactivity and weak fluorescent signals of individual and co-localized antigens. The aims of this study were to develop new immunohistochemical techniques for imaging of peripheral nerve fibers. Three-millimeter punch skin biopsies of healthy individuals were fixed, frozen, and cut into 50-µm sections. Tissues were stained with a variety of antibody combinations with two signal amplification systems, streptavidin-biotin-fluorochrome (sABC) and tyramide-horseradish peroxidase-fluorochrome (TSA), used simultaneously to augment immunohistochemical signals. The combination of the TSA and sABC amplification systems provided the first successful co-localization of sympathetic adrenergic and sympathetic cholinergic nerve fibers in cutaneous human sweat glands and vasomotor and pilomotor systems. Primary antibodies from the same species were amplified individually without cross-reactivity or elevated background interference. The confocal fluorescent signal-to-noise ratio increased, and image clarity improved. These modifications to signal amplification systems have the potential for widespread use in the study of human neural tissues.
Keywords: autonomic nerve, sensory nerve, skin biopsy, streptavidin-biotin complex, tyramide signal amplification, immunohistochemistry
In vivo structural studies of the human peripheral nervous system for research and diagnostic purposes began with sural nerve biopsies more than 50 years ago (Dyck 1966; Vallat et al. 2009). The introduction of the punch skin biopsy for the assessment of small sensory nerve fibers has reduced reliance on more invasive nerve biopsies in some conditions (Polydefkis et al. 2001; Lauria et al. 2005; Gibbons et al. 2006) and added the possibility of studying populations of autonomic nerve fibers (Kennedy et al. 1994; Donadio et al. 2006; Gibbons et al. 2009). The study of cutaneous tissue stained with the pan-axonal marker protein gene product 9.5 (PGP 9.5), generally by light microscopy, enables visualization of all nerve fibers within the epidermal and dermal tissue layers (McCarthy et al. 1995); however, this nonspecific pan-axonal marker does not differentiate nerve fiber subpopulations. The use of selective biochemical markers in combination with confocal nerve fiber microscopy has led to imaging of cutaneous nerve fiber subpopulations and the structures they innervate, thereby expanding the utility of the skin biopsy (Kennedy et al. 1994; Donadio et al. 2006; Gibbons et al. 2009).
Although anatomic relationships between nerve fibers, blood vessels, sweat glands, and other dermal structures may be displayed using biochemical markers and florescent confocal microscopy (Kennedy et al. 1994; Lauria et al. 2004; Donadio et al. 2006; Nolano et al. 2006; Gibbons et al. 2009), many cutaneous nerves and dermal structures have antigens expressed at low levels and require signal amplification for visualization. The streptavidin-biotin complex (sABC) amplification system is widely used to amplify signals in peripheral cutaneous nerves and has been used to augment visualization of pan-axonal marker PGP 9.5 in skin biopsies (Kennedy et al. 1994; McArthur et al. 1998; Donadio et al. 2006; Lauria and Devigili 2007). Tyramide signal amplification system (TSA) is a less frequently used amplification system that is mediated by horseradish peroxidase (HRP), usually conjugated with secondary antibodies or with streptavidin (Bobrow et al. 1989; Hunyady et al. 1996; Toth and Mezey 2007). To date, this technique has not been used to amplify cutaneous nerve antigen signals.
Despite advances in signal amplification, image acquisition, and analysis, there are still a number of specific challenges to structural investigation of the peripheral sensory and autonomic nerves using punch skin biopsies. First, many primary antibodies used to immunostain peripheral nerve tissues are polyclonal and are raised from the same species, frequently resulting in cross-reactivity (Teramoto et al. 1998). The lack of effective monoclonal antibodies has hindered the ability to co-localize sympathetic adrenergic and sympathetic cholinergic fibers in the same tissue section (Donadio et al. 2006). In contrast to the sABC system, the TSA amplification system can detect two primary antibodies raised from the same species simultaneously (Shindler and Roth 1996), although this system has not been used to stain nerve fibers in human skin biopsies. Second, imaging of multiple co-localized antigens expressed at low levels in the same tissue section is difficult because only a single antigen can be amplified (Toth and Mezey 2007). Both sABC and TSA systems can amplify signal intensity compared with conventional immunostaining (Bobrow et al. 1989, 1991, 1992; van Gijlswijk et al. 1997; Bobrow and Moen 2001) but cannot amplify more than one antigen at a time. For example, in human sweat glands, both sympathetic adrenergic and sympathetic cholinergic fibers are present, but both contain weakly expressed antigens and thus have not been successfully co-localized in the same tissue sections (Donadio et al. 2006). Third, antigens expressed at very low levels may not be visualized in the terminal nerve fibers even with standard amplification using sABC or TSA (Cattoretti et al. 1993; Imam et al. 1995; Shi et al. 1995).
In the present study, we introduce several modifications to signal amplification systems in the study of cutaneous nerve fibers that allow (1) the use of primary antibodies raised from same species in the same tissue section without cross-reactivity, (2) the amplification of two weakly expressed antigens in the same tissue section using two discrete signal amplification systems (i.e., parallel amplification), and (3) and the sequential use of two amplification systems (i.e., serial amplification) on one weakly expressed antigen that cannot be visualized with either amplification system alone.
Materials and Methods
Human Skin Biopsy and Fixation
All subjects reviewed the study protocol and signed an informed consent approved by the Beth Israel Deaconess Medical Center Institutional Review Board. A total of 32 healthy subjects, taking no medications, participated in this study. All subjects had detailed neurological examinations with 3-mm punch skin biopsies performed at the distal leg, distal thigh, and proximal thigh at standard sites after local anesthesia with 2% lidocaine (Holland et al. 1998; Lauria et al. 1998; McArthur et al. 1998; Gibbons et al. 2006). A total of 96 biopsies were evaluated (3 biopsies per subject). Skin biopsy specimens were fixed in 2% paraformaldehyde-lysine-periodate for 18 hr and cryoprotected overnight (20% glycerol and 20% 0.4 M Sorreson buffer). Tissue blocks were cut by freezing microtome into 50-µm-thick sections (Polydefkis et al. 2004).
Immunohistochemistry
All primary and secondary antibodies and reagents used in this study are listed in Table 1. Fluorescent immunostaining protocols are described in detail and briefly illustrated in Figure 1. For all immunostaining protocols, the tissue sections were incubated in 96-well tissue culture plates on a horizontal shaking table at speeds of 50 rolls per minute (Polydefkis et al. 2004). Standard solutions were used in all protocols, unless otherwise mentioned specially, which include block solution (2% powdered milk, 5% nonspecific sera, and 0.01% triton ×100 in 0.5 M Tris buffered saline [TBS], pH 7.6), primary antibody dilution solutions (1% powdered milk, 2.5% nonspecific sera, and 0.01% triton ×100 in TBS, pH 7.6), secondary antibody dilution solution (2.5% nonspecific sera in TBS, pH 7.6), dilution solution for fluorochrome conjugated streptavidin (0.1 M PBS, pH 7.2), and washing buffer (0.5 M TBS with 0.05% triton ×100, pH 7.6). After each step in the incubation, tissue sections were washed by TBS-0.05% triton ×100 three times for 10 min for each, unless otherwise mentioned. Sections were mounted on glass slides and cover-slipped in PBS glycerol (90%).
Table 1.
Antibodies and Reagents Used for Immunohistochemistry
| Antibody and Reagents | Abbreviation | Dilution | Source of Antibodies |
|---|---|---|---|
| Primary antibodies | |||
| Protein gene product 9.5 (mouse) | ms-PGP9.5 | 1:1000 | Ultraclone, Wellow, UK |
| Protein gene product 9.5 (rabbit) | rb-PGP9.5 | 1:10,000 | Ultraclone, Wellow, UK |
| Protein gene product 9.5 (rabbit) | rb-PGP9.5 | 1:1000 | Millipore (Chemicon), MA |
| Calcitonin gene related peptide (rabbit) | rb-CGRP | 1:4000 | Bachem AG, Bubendorf, Switzerland |
| Vasoactive intestinal peptide (rabbit) | rb-VIP | 1:1500 | Incstar Corporation, MN |
| Vasoactive intestinal peptide (mouse) | ms-VIP | 1:1000 | Santa Cruz Biotechnology Inc., CA |
| Tyrosine hydroxylase (rabbit) | rb-TH | 1:1000 | Sigma-Aldrich, MO |
| Tyrosine hydroxylase (mouse) | ms-TH | 1:700 | Millipore (Chemicon), MA |
| Dopamine β hydroxylase (rabbit) | rb-DbH | 1:3000 | Millipore (Chemicon), MA |
| Secondary antibodies | |||
| Donkey anti-rabbit-biotin | DRB | 1:700 | Jackson ImmunoResearch Lab, PA |
| Donkey anti-mouse-biotin | DMB | 1:500 | Jackson ImmunoResearch Lab, PA |
| Donkey anti-rabbit-Cy2 | DR-Cy2 | 1:500 | Jackson ImmunoResearch Lab, PA |
| Donkey anti-rabbit-Cy3 | DR-Cy3 | 1:500 | Jackson ImmunoResearch Lab, PA |
| Donkey anti-mouse Cy2 | DM-Cy2 | 1:500 | Jackson ImmunoResearch Lab, PA |
| Goat anti-rabbit-HRP | GR-HRP | 1:5000 | PerkinElmer, MA |
| Goat anti-mouse-HRP | GM-HRP | 1:5000 | PerkinElmer, MA |
| Reagents | |||
| Streptavidin-Cy2 | SP-Cy2 | 1:700 | Jackson ImmunoResearch Lab, PA |
| Streptavidin-Cy3 | SP-Cy3 | 1:700 | Jackson ImmunoResearch Lab, PA |
| Tyramide-Cy3 | TA-Cy3 | 1:200 | Perkin Elmer, MA |
| Tyramide-FITC | TA-FITC | 1:200 | Perkin Elmer, MA |
| Streptavidin-HRP | SP-HRP | 1:5000 | Jackson ImmunoResearch Lab, PA |
| Ulex Europaeus agglutinin I-FITC | UEAI-FITC | 50 mg/mL | Vector Laboratories, Inc, CA |
Figure 1.
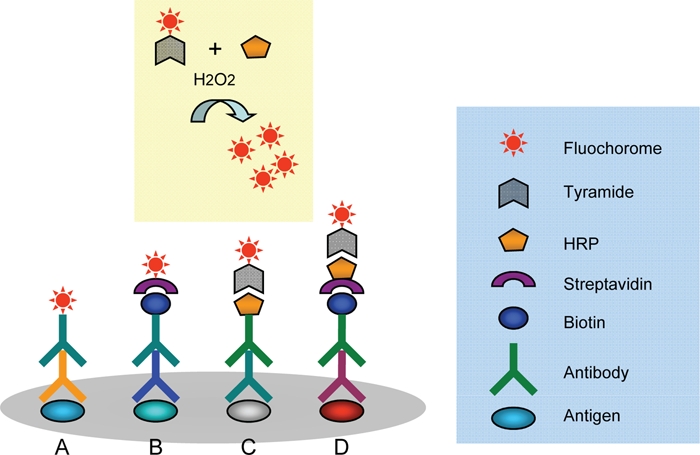
Illustration of four immunofluorescent staining protocols. In the conventional method (A), fluorescent imaging of antigens is achieved by combining a fluorochrome-labeled secondary antibody to the primary antibody without signal amplification. For low levels of antigens, amplification of signal by biotin-streptavidin complex (sABC) can be used to improve the signal intensity (B); in some areas of investigation, tyramide signal amplification system (TSA, C) is also used for this purpose. Tyramide is a substrate for horseradish peroxidase (HRP); in the presence of H2O2, the fluorochrome-labeled reaction product deposits on the immunoreaction sites. For very weak signals, a combination of these methods can dramatically amplify the signal without increasing the background (TSA-sABC, D). To double stain using primary antibodies raised from same species, an antibody visualized with the conventional method (A) is combined with sABC amplification (B). To amplify two distinct antigens, one can be amplified by the sABC system and the other by the TSA system, but the primary antibodies must be from different species.
Immunohistochemistry Staining Protocols
Conventional immunofluorescent staining (protocol A)
The skin biopsy sections were incubated with rabbit or mouse anti–PGP9.5 and other primary antibodies diluted in the antibody dilution solution overnight, at 4C. Subsequently, the sections were visualized by an incubation with fluorochrome (Cy3 or Cy2) conjugated donkey anti-rabbit or donkey anti-mouse for 1 hr at room temperature. All primary antibodies (Table 1) were tested by dilution series for optimal visual thresholding.
sABC amplification (protocol B)
For primary antibodies that could not be visualized by conventional immunostaining, the sABC amplification system was used to enhance antigen signaling. Tissue sections were incubated with primary antibodies (Table 1) in the antibody dilution solutions. Subsequently, sections were incubated with biotinylated secondary antibodies for 2 hr at room temperature. After washout, the sections were visualized by incubation with Cy3 or Cy2 labeled streptavidin for 1 hr at room temperature.
Tyramide-HRP signal amplification (protocol C)
For primary antibodies that could not be adequately visualized on small nerve fibers by conventional immunostaining, the tyramide signal amplification system was used as an alternative to sABC amplification for enhancing antigen signals when antibodies from the same species were to be used and with the sABC system if serial amplification was necessary. The sections were incubated with primary antibodies in the antibody dilution solution overnight at 4C. Subsequently, the sections were incubated with HRP-conjugated secondary antibodies (anti-rabbit or anti-mouse, 1:5000; Perkin Elmer, Wellesley, MA) in PBS for 2 hr at room temperature. After washout, the sections were visualized by an incubation with Cy3 (1:200) or FITC-labeled tyramide (1:50) in a 1× amplification solution (Perkin Elmer) for 10 min at room temperature.
Immunofluoresent staining with primary antibodies raised from the same species
To avoid cross-reactivity when using two primary antibodies raised from the same species, we implemented a technique using a combination of amplification with TSA (protocol C) and a conventional stain (protocol A), as has been previously reported (Shindler and Roth 1996). The technique relies on steric hindrance to block same-species binding sites. Double immunostaining uses HRP-tyramide–amplified fluorescent staining with a diluted first primary antibody and a subsequent conventional fluorescent staining with a second primary antibody, where both primary antibodies are raised from the same species. In this study, rb-TH or rb-VIP was amplified with TSA, and rb-PGP9.5 was visualized without any amplification. The rb-TH or rb-VIP was incubated overnight at 4C at a dilution that was undetectable by Cy3-conjugated second antibodies but was detected by TSA. Subsequently, the tissue sections were incubated with HRP-labeled anti-rabbit (1:5000 in this study) for 2 hr, followed by visualization with tyramide-conjugated Cy3 for 10 min in room temperature (protocol C). After washout (TBS-0.05% tritonx100), the tissue sections were incubated overnight with rabbit anti-PGP9.5 at 4C. Finally, Cy2-conjugated anti-rabbit was applied for 1 hr at room temperature (protocol A).
Amplification of two antigens in the same tissue section with sABC and TSA
To amplify two separate antigens expressed at low levels, a combination of sABC amplification (protocol B) and TSA amplification (protocol C) in the same tissue sections was used. The first primary antibody (e.g., mouse anti-VIP) was applied overnight at 4C, and the tissue was incubated in secondary antibody conjugated with HRP for 1 hr at room temperature. After visualization with a 10-min application of fluorochrome-labeled tyramide (protocol C), the remaining antibody was washed out (TBS-0.05% tritonx100) and the tissue was incubated in the second primary antiserum (e.g., rabbit anti-TH) overnight at 4C. The tissue was then incubated with a biotinylated secondary antibody for 2 hr at room temperature, followed by a 30-min application of fluorochrome-labeled streptavidin (protocol B). In this experiment, a negative control was performed to identify potential cross-reactivity between biotin-streptavidin (sABC) and HRP-tyramide (TSA) amplification systems. The sections with primary antibody-biotinylated second antibody complex were incubated with Cy3-conjugated tyramide for 10 min, and the sections with primary antibody-HRP–labeled second antibody complex were incubated with Cy2-labeled streptavidin for 30 min at working dilution.
Serial amplification of a single antigen with sABC and TSA
To visualize antigens expressed at levels below the visualization threshold using standard amplification systems (sABC or TSA), we adapted the TSA on sABC-based serial signal amplification technique that has been used in the study of other tissue types (Hunyady et al. 1996). The sections were incubated in primary antibody overnight at 4C, and then biotinylated secondary antibodies were applied for 2 hr at room temperature and sections incubated in HRP-labeled streptavidin at a dilution of 1:5000 in PBS for an additional 30 min at room temperature and visualized by a 10-min incubation of Cy3-labeled tyramide diluted 1:100 in 1× amplification solution (Perkin Elmer). To determine the appropriate concentrations of primary antibodies, second antibodies, and reagents used for this sABC-based HRP-tyramide amplification, a dilution series for each of the primary antibodies, biotinylated secondary antibodies, HRP-labeled streptavidin, and fluorochome-labeled tyramide were tested against each other for optimal visualization.
Immunohistochemical Controls
Control studies were performed for all protocols. All antibodies were tested in multiple dilutions to obtain optimal concentrations. In each immunohistochemical experiment, a negative control was included with tissue sections incubated without the addition of the primary or secondary antibody or with the secondary alone with or without TSA.
Confocal Microscopy
All of the stained sections were initially examined under a fluorescent microscope (Zeiss-Axioplan2, Germany), with areas of interest imaged by confocal microscopy (Zeiss, LSM 5 Pascal, Germany). A series of images of optical sections was acquired at 2- to 4-µm intervals throughout the depth of the 50-µm section as a z-stack. The images were analyzed by image software Image Pro Plus (version 6.0). Objective lenses used were Plan-Neofluar 10 × 0.3 and 20 × 0.8. Excitation wavelengths were 488 nm (green), 543 nm (red), and 633 nm (far red, converted to blue). Pinhole set up in three channels were 70, 72, and 74 µm. Image frame sizes were 900 µm2 or 450 µm2, and raw image data was in mdb format.
Results
Staining with Primary Antibodies from the Same Species
We successfully stained tissue with the primary antibodies to PGP 9.5 and VIP or TH, both raised from the same species (rabbit), as shown in Figure 2A and 2B. The VIP or TH was amplified using the TSA system (protocol C), whereas the PGP 9.5 was imaged with conventional staining (protocol A). The fluorescent signal of rb-PGP9.5 was easily visualized on nerve fibers throughout the skin and the VIP co-localized with PGP 9.5 in sweat glands (Figure 2A) but not in the adjacent arrector pili muscles (data not shown). Similarly, the TH-positive nerve fibers co-localized with PGP 9.5 in sweat glands (Figure 2B) and arrector pili muscles but not in the sensory fibers surrounding a hair follicle. The PGP-ir fibers (green) and TH-ir fibers (red) in Figure 2 indicate that the rabbit anti-TH/HRP–labeled anti-rabbit/tyramide-Cy3 complex was sufficiently large to prevent the green fluorescent–labeled secondary antibody from binding to it, thus confirming steric hindrance of TH binding sites. The antibody signals of TH and VIP were clearly separated from PGP 9.5. The results were consistent across tissue sections, biopsy locations, and subjects.
Figure 2.
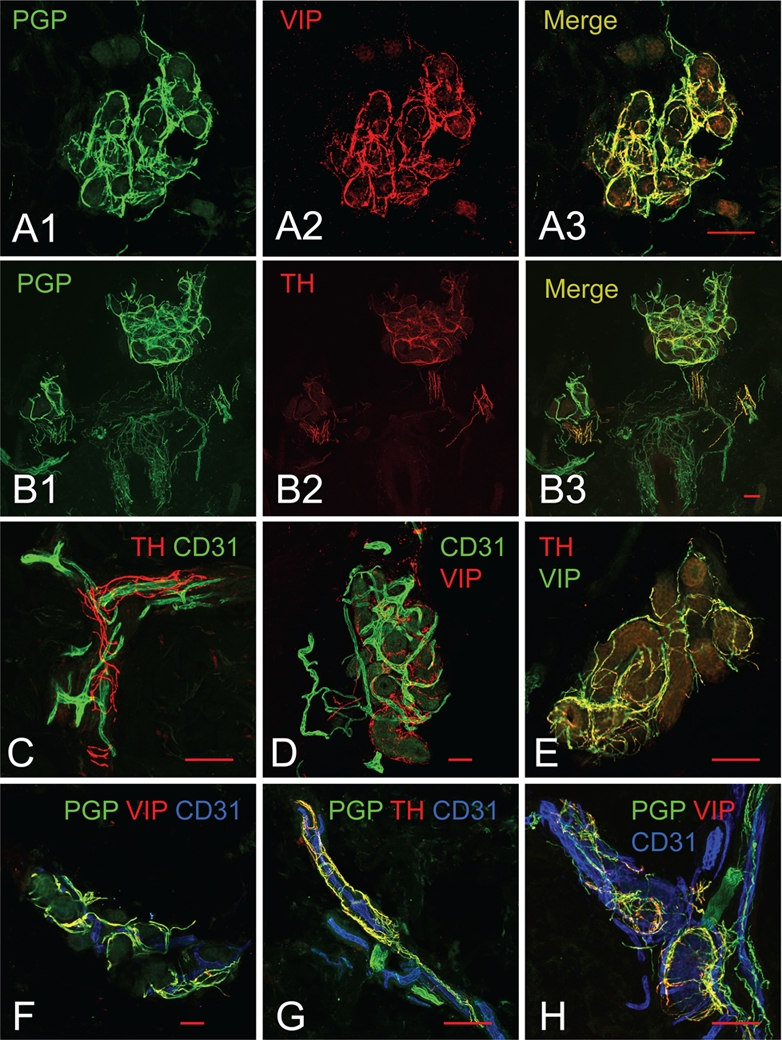
Examples of images obtained through immunostaining protocols. (A, B) Double staining with primary antibodies raised from the same species (rabbit). (A1) Rabbit anti-PGP9.5, stained with the conventional method, reveals all nerve fibers surrounding sweat glands. (A2) Rabbit anti-VIP, amplified by the tyramide signal amplification system (TSA), selectively highlights the sympathetic cholinergic fibers surrounding the sweat glands. (A3) Co-localization of VIP-ir fibers with PGP9.5-ir fibers is observed surrounding sweat glands. (B1) Rabbit anti-PGP9.5, stained with the conventional method, reveals all nerve fibers surrounding sweat glands, hair follicles, and arrector pili muscles. (B2) Rabbit anti-TH, amplified by TSA, selectively highlights the sympathetic adrenergic fibers in the arrector pili muscles and some of the sweat gland tubules. (B3) Co-localization of TH-ir fibers with PGP9.5-ir fibers is observed surrounding sweat glands and arrector pili muscles but not within the sensory fibers surrounding the hair follicle. (C, D) Double staining with parallel signal amplification systems of two weakly expressed antigens. (C) Mouse anti-CD31 is amplified by streptavidin-biotin-fluorochrome (sABC) and rabbit anti-TH is amplified by TSA, thus highlighting the sympathetic adrenergic innervation of the cutaneous vasculature. (D) Mouse anti-CD31 is amplified by sABC and rabbit anti-VIP is amplified by TSA, highlighting the complicated vasculature and cholinergic innervation of sweat glands. (E) Parallel amplification of rabbit anti-VIP and rabbit anti-TH shown with co-localized sympathetic adrenergic and sympathetic cholinergic fibers. (F–H) Parallel amplification of rabbit anti-TH (or -VIP) and mouse anti-CD31 for staining of nerve fibers and blood vessels with the conventional method of nerve fibers using rabbit anti-PGP9.5. (F) The total innervation and subpopulation of sympathetic adrenergic nerves around a sweat gland with the accompanying vasculature. (G) The total innervation and subpopulation of sympathetic adrenergic nerves surrounding a dermal blood vessel. (H) The total innervation and subpopulation of sympathetic cholinergic nerves around a sweat gland with the accompanying vasculature. Scale bars indicate 100 µm. AP, arrector pili muscles; BV, blood vessels; HF, hair follicles; SG, sweat glands.
The negative controls confirmed that protocol errors did not result in florescent staining (i.e., no florescent staining was present without the addition of the primary antibody, secondary antibody, with the secondary alone with or without TSA). Also, no florescent cross-reactivity was observed between HRP-conjugated second antibody and fluorochrome-labeled tyramide.
Visualization of Two Antigens with Parallel Amplification Systems
Blood vessels in skin biopsy tissue were stained with the vascular endothelial marker CD31 (mouse) using the sABC amplification system (protocol B) and stained for TH or VIP (both rabbit) using the TSA amplification system (protocol C). The detailed structure of the blood vessels (CD31) and the sympathetic adrenergic nerve fibers (TH) or sympathetic cholinergic nerve fibers (VIP) surrounding them can be clearly seen only with the amplification of both antibodies (Figure 2C and D). There is no cross-reactivity between the amplification systems.
Sweat glands in skin biopsy tissue were stained with VIP using the sABC amplification system (protocol B) and TH using the TSA amplification system (protocol C). In Figure 2E, a sweat gland with both VIP- and TH-positive nerve fibers wrapping around the glandular tubules can be seen with some co-localization of VIP and TH observed in the merged image.
Serial Amplification of a Single Antigen with TSA and sABC
Calcitonin gene related peptide (CGRP) immunoreactive fibers are observed in subepidermal nerve fiber bundles (Figure 3A) using single amplification with sABC or TSA, but CGRP present in nerve fibers surrounding sweat glands had a low signal-to-background ratio (Figure 3B). However, with serial amplification using both TSA and sABC, the signal strength of CGRP fibers was enhanced, increasing the confocal fluorescent signal-to-noise ratio and improving image clarity (Figure 3C).
Figure 3.
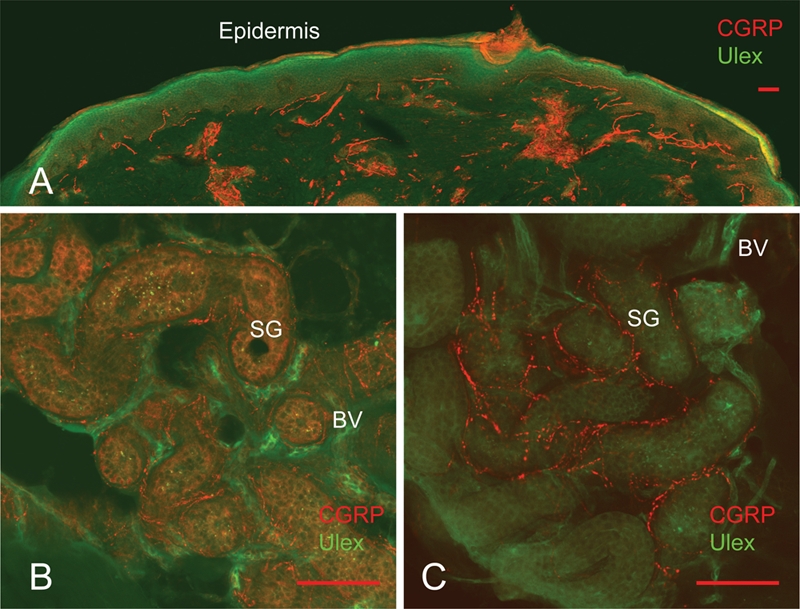
Serial amplification of the weakly expressed antigen calcitonin gene related peptide (CGRP). When rabbit anti-CGRP is amplified by sABC alone the signal of CGRP-ir fibers in subdermal area is strong (A), but within sweat glands the CGRP signal is weak and background is high (B). When the signal of rabbit anti-CGRP is serially amplified by streptavidin-biotin-fluorochrome and the tyramide signal amplification system, the intensity of CGRP-ir fibers in sweat glands becomes greater and the background staining is reduced (C). Scale bars indicate 100 µm.
Discussion
Skin biopsy with the assessment of intraepidermal sensory nerve fiber using the pan-axonal marker, PGP 9.5, is an established clinical and research technique. The skin and associated structures are also innervated by sympathetic and parasympathetic neurons, and recent reports have shown that this technique may also be used to assess these other populations of cutaneous nerve fibers. In this study, we have made several modifications to immunofluoresent protocols to improve the visualization of peripheral nerve antigens associated with sensory and autonomic neurons. First, we have successfully adapted the TSA amplification system to detect simultaneously two antibodies raised from the same species in peripheral nerve tissue, thereby facilitating the study of different populations of cutaneous nerve fibers in the same section (Figure 2A and 2B). This extends the utility of skin biopsies to study different populations of cutaneous nerve fibers, because many primary antibodies are raised from the same species and therefore cannot be used in the same tissue section. Second, we report the first use of the TSA and sABC amplification systems to amplify two discrete antigens in the same tissue section (Figure 2C-D). Using this technique, we show, for the first time, that peripheral nerve fibers express both sympathetic adrenergic and sympathetic cholinergic components (Figure 2E, F, G, H). Finally, we report the first use of the TSA and sABC amplification systems in parallel to double amplify a very low-level antigen. Using this method, we show the first images of CGRP-positive fibers within sweat glands and the complex vasomotor innervation seen within dermal tissues (Figure 3).
TSA is usually conjugated with secondary antibodies or with streptavidin. HRP catalyzes TSA and has been used to amplify signals in a variety of applications, including immunoassays (Bobrow et al. 1991), Western blotting (Wigle et al. 1993), in situ hybridization (Raap et al. 1995), and immunocytochemistry (Toth and Mezey 2007). For most immunohistochemical staining, the sABC system is technically easier to perform and is more widely used and reported. The TSA system has not been previously used in peripheral nerve tissue because the range of antibody concentration used in TSA is very narrow and the technique therefore is more labor intensive and requires additional controls compared with the sABC system.
The introduction of TSA to the sABC-based system allows primary antibodies raised from the same species to be used in the same tissue section without cross-reactivity. Initial reports of the combination of tyramide amplification with conventional fluorolabeling using two different primary antibodies hosted from the same species (Hunyady et al. 1996) have been followed by use in a variety of tissues, but none have used this method in the study of peripheral nerve fibers (Buki et al. 2000; Uchihara et al. 2000; Volante et al. 2000; Nakamura and Uchihara 2004; Toth and Mezey 2007). The tyramide-based amplification system uses the differences in antibody detection thresholds such that two epitopes probed with primary antibodies of the same class from the same species can be detected separately. In this study, we carefully tested the detectable threshold of polyclonal and monoclonal antibodies against TH and VIP by non-amplified conventional immunostaining at different concentrations, where positive nerve fibers were rarely observed. However, with the application of tyramide amplification, VIP-ir and TH-ir nerve fibers could be clearly observed wrapping around sweat glands and errector pili muscles accordingly and co-localized with rb-PGP9.5 fibers (Figure 2). Extensive studies of negative controls revealed no evidence of cross-reactivity between antibodies. One primary antibody was applied at a dilution below the detection limit of a fluorescent-labeled secondary antibody but was still detected with the tyramide-based amplification system.
The combination of the tyramide-based amplification system with the sABC amplification system also enables amplification of two separate antigen signals when used in parallel. Without amplification, we and others (Donadio et al. 2008; Nolano et al. 2008; Donadio et al. 2010) have been unable to visualize co-localization of sympathetic cholinergic or sympathetic adrenergic fibers within sweat glands. Using the parallel amplification technique with sABC and TSA, we report the first co-localization of sympathetic cholinergic fibers and sympathetic adrenergic fibers within sweat glands, arrector pili muscles, and surrounding blood vessels (Figure 2).
Finally, we have successfully amplified a single poorly expressed antigen using both the sABC and TSA systems in a serial double-amplification design, resulting in dramatic augmentation of antigen signal strength. In our study, the method yielded a strong and consistent signal from CGRP-ir fibers in sweat glands that could not be visualized with sABC alone without an increase in background staining (Figure 3). We have also successfully used this technique to image substance P and dopamine β-hydroxylase in the epidermal layer, sweat glands, and arrector pili muscles (data not shown). The serial double-amplification method is also suitable for use in double or triple immunostaining protocols involving peripheral nerve fibers, as shown in Figure 2F to H (Hunyady et al. 1996). This technique may be of particular value in studies of degenerating and regenerating nerve fibers in peripheral neuropathy.
There are still several limitations to the use of these protocols. The use of TSA amplification does result in an approximately 10-fold increase in florescent probe size (which is not seen with sABC amplification) and may potentially influence the observation of protein localization. All studies were conducted using healthy skin; studies of pathologic specimens may not produce the same results. Nevertheless, these results in healthy subjects suggest that these novel methods, when applied to cutaneous structures, will yield insights into sensory and autonomic anatomy in health and disease.
Footnotes
The authors declared no potential conflicts of interest with respect to the authorship and/or publication of this article.
The research was supported by NIH K23 NS050209 (to C.H.G.),the Langer Family Foundation, and the Harriet Lewis Foundation.
References
- Bobrow MN, Harris TD, Shaughnessy KJ, Litt GJ. 1989. Catalyzed reporter deposition, a novel method of signal amplification: application to immunoassays. J Immunol Methods. 125:279-285 [DOI] [PubMed] [Google Scholar]
- Bobrow MN, Litt GJ, Shaughnessy KJ, Mayer PC, Conlon J. 1992. The use of catalyzed reporter deposition as a means of signal amplification in a variety of formats. J Immunol Methods. 150:145-149 [DOI] [PubMed] [Google Scholar]
- Bobrow MN, Moen PT., Jr. 2001. Tyramide signal amplification (TSA) systems for the enhancement of ISH signals in cytogenetics. Curr Protoc Cytom. Chapter 8:Unit 8.9 [DOI] [PubMed] [Google Scholar]
- Bobrow MN, Shaughnessy KJ, Litt GJ. 1991. Catalyzed reporter deposition, a novel method of signal amplification. II. Application to membrane immunoassays. J Immunol Methods. 137:103-112 [DOI] [PubMed] [Google Scholar]
- Buki A, Walker SA, Stone JR, Povlishock JT. 2000. Novel application of tyramide signal amplification (TSA): ultrastructural visualization of double-labeled immunofluorescent axonal profiles. J Histochem Cytochem. 48:153-161 [DOI] [PubMed] [Google Scholar]
- Cattoretti G, Pileri S, Parravicini C, Becker MH, Poggi S, Bifulco C, Key G, D’Amato L, Sabattini E, Feudale E. 1993. Antigen unmasking on formalin-fixed, paraffin-embedded tissue sections. J Pathol. 171:83-98 [DOI] [PubMed] [Google Scholar]
- Donadio V, Cortelli P, Elam M, Di Stasi V, Montagna P, Holmberg B, Giannoccaro MP, Bugiardini E, Avoni P, Baruzzi A, et al. 2010. Autonomic innervation in multiple system atrophy and pure autonomic failure. J Neurol Neurosurg Psychiatry. 81:1327-1335 [DOI] [PubMed] [Google Scholar]
- Donadio V, Nolano M, Elam M, Montagna P, Provitera V, Bugiardini E, Baruzzi A, Santoro L, Liguori R. 2008. Anhidrosis in multiple system atrophy: a preganglionic sudomotor dysfunction? Mov Disord. 23:885-888 [DOI] [PubMed] [Google Scholar]
- Donadio V, Nolano M, Provitera V, Stancanelli A, Lullo F, Liguori R, Santoro L. 2006. Skin sympathetic adrenergic innervation: an immunofluorescence confocal study. Ann Neurol. 59:376-381 [DOI] [PubMed] [Google Scholar]
- Dyck PJ. 1966. Histologic measurements and fine structure of biopsied sural nerve: normal, and in peroneal muscular atrophy, hypertrophic neuropathy, and congenital sensory neuropathy. Mayo Clin Proc. 41:742-774 [PubMed] [Google Scholar]
- Gibbons CH, Griffin JW, Polydefkis M, Bonyhay I, Brown A, Hauer PE, McArthur JC. 2006. The utility of skin biopsy for prediction of progression in suspected small fiber neuropathy. Neurology. 66:256-258 [DOI] [PubMed] [Google Scholar]
- Gibbons CH, Illigens BM, Wang N, Freeman R. 2009. Quantification of sweat gland innervation: a clinical-pathologic correlation. Neurology. 72:1479-1486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland NR, Crawford TO, Hauer P, Cornblath DR, Griffin JW, McArthur JC. 1998. Small-fiber sensory neuropathies: clinical course and neuropathology of idiopathic cases. Ann Neurol. 44:47-59 [DOI] [PubMed] [Google Scholar]
- Hunyady B, Krempels K, Harta G, Mezey E. 1996. Immunohistochemical signal amplification by catalyzed reporter deposition and its application in double immunostaining. J Histochem Cytochem. 44:1353-1362 [DOI] [PubMed] [Google Scholar]
- Imam SA, Young L, Chaiwun B, Taylor CR. 1995. Comparison of two microwave based antigen-retrieval solutions in unmasking epitopes in formalin-fixed tissue for immunostaining. Anticancer Res. 15:1153-1158 [PubMed] [Google Scholar]
- Kennedy WR, Wendelschafer-Crabb G, Brelje TC. 1994. Innervation and vasculature of human sweat glands: an immunohistochemistry-laser scanning confocal fluorescence microscopy study. J Neurosci. 14:6825-6833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauria G, Borgna M, Morbin M, Lombardi R, Mazzoleni G, Sghirlanzoni A, Pareyson D. 2004. Tubule and neurofilament immunoreactivity in human hairy skin: markers for intraepidermal nerve fibers. Muscle Nerve. 30:310-316 [DOI] [PubMed] [Google Scholar]
- Lauria G, Cornblath DR, Johansson O, McArthur JC, Mellgren SI, Nolano M, Rosenberg N, Sommer C. 2005. EFNS guidelines on the use of skin biopsy in the diagnosis of peripheral neuropathy. Eur J Neurol. 12:747-758 [DOI] [PubMed] [Google Scholar]
- Lauria G, Devigili G. 2007. Skin biopsy as a diagnostic tool in peripheral neuropathy. Nat Clin Pract Neurol. 3:546-557 [DOI] [PubMed] [Google Scholar]
- Lauria G, McArthur JC, Hauer PE, Griffin JW, Cornblath DR. 1998. Neuropathological alterations in diabetic truncal neuropathy: evaluation by skin biopsy. J Neurol Neurosurg Psychiatry. 65:762-766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArthur JC, Stocks EA, Hauer P, Cornblath DR, Griffin JW. 1998. Epidermal nerve fiber density: normative reference range and diagnostic efficiency. Arch Neurol. 55:1513-1520 [DOI] [PubMed] [Google Scholar]
- McCarthy BG, Hsieh ST, Stocks A, Hauer P, Macko C, Cornblath DR, Griffin JW, McArthur JC. 1995. Cutaneous innervation in sensory neuropathies: evaluation by skin biopsy. Neurology. 45:1848-1855 [DOI] [PubMed] [Google Scholar]
- Nakamura A, Uchihara T. 2004. Dual enhancement of triple immunofluorescence using two antibodies from the same species. J Neurosci Methods. 135:67-70 [DOI] [PubMed] [Google Scholar]
- Nolano M, Provitera V, Estraneo A, Selim MM, Caporaso G, Stancanelli A, Saltalamacchia AM, Lanzillo B, Santoro L. 2008. Sensory deficit in Parkinson’s disease: evidence of a cutaneous denervation. Brain. 131:1903-1911 [DOI] [PubMed] [Google Scholar]
- Nolano M, Provitera V, Perretti A, Stancanelli A, Saltalamacchia AM, Donadio V, Manganelli F, Lanzillo B, Santoro L. 2006. Ross syndrome: a rare or a misknown disorder of thermoregulation? A skin innervation study on 12 subjects. Brain. 129:2119-2131 [DOI] [PubMed] [Google Scholar]
- Polydefkis M, Hauer P, Griffin JW, McArthur JC. 2001. Skin biopsy as a tool to assess distal small fiber innervation in diabetic neuropathy. Diabetes Technol Ther. 3:23-28 [DOI] [PubMed] [Google Scholar]
- Polydefkis M, Hauer P, Sheth S, Sirdofsky M, Griffin JW, McArthur JC. 2004. The time course of epidermal nerve fibre regeneration: studies in normal controls and in people with diabetes, with and without neuropathy. Brain. 127:1606-1615 [DOI] [PubMed] [Google Scholar]
- Raap AK, van de Corp MP, Vervenne RA, van Gijlswijk RP, Tanke HJ, Wiegant J. 1995. Ultra-sensitive FISH using peroxidase-mediated deposition of biotin- or fluorochrome tyramides. Hum Mol Genet. 4:529-534 [DOI] [PubMed] [Google Scholar]
- Shi SR, Imam SA, Young L, Cote RJ, Taylor CR. 1995. Antigen retrieval immunohistochemistry under the influence of pH using monoclonal antibodies. J Histochem Cytochem. 43:193-201 [DOI] [PubMed] [Google Scholar]
- Shindler KS, Roth KA. 1996. Double immunofluorescent staining using two unconjugated primary antisera raised in the same species. J Histochem Cytochem. 44:1331-1335 [DOI] [PubMed] [Google Scholar]
- Teramoto N, Szekely L, Pokrovskaja K, Hu LF, Yoshino T, Akagi T, Klein G. 1998. Simultaneous detection of two independent antigens by double staining with two mouse monoclonal antibodies. J Virol Methods. 73:89-97 [DOI] [PubMed] [Google Scholar]
- Toth ZE, Mezey E. 2007. Simultaneous visualization of multiple antigens with tyramide signal amplification using antibodies from the same species. J Histochem Cytochem. 55:545-554 [DOI] [PubMed] [Google Scholar]
- Uchihara T, Nakamura A, Nagaoka U, Yamazaki M, Mori O. 2000. Dual enhancement of double immunofluorescent signals by CARD: participation of ubiquitin during formation of neurofibrillary tangles. Histochem Cell Biol. 114:447-451 [DOI] [PubMed] [Google Scholar]
- Vallat JM, Vital A, Magy L, Martin-Negrier ML, Vital C. 2009. An update on nerve biopsy. J Neuropathol Exp Neurol. 68:833-844 [DOI] [PubMed] [Google Scholar]
- van Gijlswijk RP, Zijlmans HJ, Wiegant J, Bobrow MN, Erickson TJ, Adler KE, Tanke HJ, Raap AK. 1997. Fluorochrome-labeled tyramides: use in immunocytochemistry and fluorescence in situ hybridization. J Histochem Cytochem. 45:375-382 [DOI] [PubMed] [Google Scholar]
- Volante M, Pecchioni C, Bussolati G. 2000. Post-incubation heating significantly improves tyramide signal amplification. J Histochem Cytochem. 48:1583-1585 [DOI] [PubMed] [Google Scholar]
- Wigle DA, Radakovic NN, Venance SL, Pang SC. 1993. Enhanced chemiluminescence with catalyzed reporter deposition for increasing the sensitivity of Western blotting. Biotechniques. 14:562-563 [PubMed] [Google Scholar]


