Abstract
The loss of antigenicity in archival formalin-fixed paraffin-embedded (FFPE) tissue sections negatively affects both diagnostic histopathology and advanced molecular studies. The mechanisms underlying antigenicity loss in FFPE tissues remain unclear. The authors hypothesize that water is a crucial contributor to protein degradation and decrement of immunoreactivity in FFPE tissues. To test their hypothesis, they examined fixation time, processing time, and humidity of storage environment on protein integrity and antigenicity by immunohistochemistry, Western blotting, and protein extraction. This study revealed that inadequate tissue processing, resulting in retention of endogenous water in tissue sections, results in antigen degradation. Exposure to high humidity during storage results in significant protein degradation and reduced immunoreactivity, and the effects of storage humidity are temperature dependent. Slides stored under vacuum with desiccant do not protect against the effects of residual water from inadequate tissue processing. These results support that the presence of water, both endogenously and exogenously, plays a central role in antigenicity loss. Optimal tissue processing is essential. The parameters of optimal storage of unstained slides remain to be defined, as they are directly affected by preanalytic variables. Nevertheless, minimization of exposure to water is required for antigen preservation in FFPE tissue sections. This article contains online supplemental material at http://www.jhc.org. Please visit this article online to view these materials.
Keywords: Antigenicity, formalin fixed, paraffin embedded, immunohistochemistry, protein array, tissue processing
Formalin-fixed paraffin-embedded (FFPE) tissue is the most common method of tissue preparation for diagnostic histopathology and results in paraffin blocks that are retained for archival purposes. These archival collections of tissue are important for ongoing patient care, as well as an invaluable resource for biomedical research. However, antigen alterations or loss, over time in stored slides and blocks, plague protein-based assays, including immunohistochemistry, and have been demonstrated for nucleic acid–based assays as well (Wester et al. 2000; Srinivasan et al. 2002; Chung, Braunschweig, et al. 2008; Hewitt et al. 2008; Nirmalan et al. 2009). This problem results in variable and poorly understood differences in the detection of biomolecules in diagnostic assays. The variable nature of this challenge has been particularly problematic, as no quality metrics are available to define or measure the loss of antigenicity.
It has been shown that storage-related immnuoreactivity differences are not limited to antigens with a specific subcellular localization. Antigens that are located respectively in the nucleus (endoplasmic reticulum [ER]), in the cytoplasm, or within the cell membrane may all be affected by this problem (Bertheau et al. 1998). Recognition of antigen degradation appears to be marker dependent. Some markers (epithelial membrane antigen, smooth muscle antigen, S-100, CD45, CD20, and CD30) have been consistently detected after a year of storage time, whereas others (p53, estrogen receptor, progesterone receptor, and HER-2/neu) have frequently, but not universally, shown a decrease in staining intensity and number of positive cells (Jacobs et al. 1996; Bertheau et al. 1998; Wester et al. 2000; Fergenbaum et al. 2004).
The mechanisms that influence antigenicity preservation and loss have not been well characterized. Antigen degradation occurs in unstained slides, whereas degradation occurs at a slower rate in paraffin blocks if they are properly fixed, processed, and stored (Camp et al. 2000; Specht et al. 2001; Cronin et al. 2004; Fergenbaum et al. 2004; Mirlacher et al. 2004; Hewitt et al. 2008). Exposure of the section to air, resulting in “oxidation,” has been presumed to be related to antigen alteration (Sauter and Mirlacher 2002; Blind et al. 2008). However, additional paraffin coating of the slides did not significantly protect against antigen loss (Jacobs et al. 1996). Interestingly, the addition of wax-soluble antioxidants, such as butylated hydroxytoluene (BHT) and butylated hydroxyanisole (BHA), has been shown to diminish, not increase, the protective effects of paraffin coating alone (DiVito et al. 2004). Storage temperature may be another important factor, as low temperature seems to prevent a decrease in immunostaining to some degree (Jacobs et al. 1996). Previous studies also showed that degradation is considerably worse for poorly fixed and processed tissue (Arnold et al. 1996; De Marzo et al. 2002).
We hypothesize that the presence of both endogenous water and ambient water is crucial to protein degradation and diminution of immunoreactivity in FFPE tissue sections. In an attempt to test our hypothesis, we examined the impact of inadequate processing in FFPE tissues after tissue processing on antigen degradation. To determine the effect of ambient water on protein integrity and immunoreactivity, two storage conditions, vacuum-pack with desiccant and humidity chamber at different temperatures, were further investigated.
Materials and Methods
Tissue Specimens and Storage Conditions
Mice were obtained from the Small Animals Section, Veterinary Resources Branch, National Institutes of Health (NIH), Bethesda, Maryland. Animals were housed and euthanized in accordance with the NIH guidelines for care and use of laboratory animals (Chung, Braunschweig, et al. 2008). To determine the impact of fixation and tissue processing time on antigen degradation, tissue specimens were fixed in 10% neutral phosphate-buffered formalin (NBF; formalin/tissue ratio, 10:1) for either 12 hr or 24 hr, respectively, and processed for either 4 hr (15 min/stage) or 8 hr (30 min/stage) according to a defined paraffin-embedding procedure with a Tissue-Tek VIP IV automated tissue processor (Sakura Finetek USA, Inc.; Torrance, CA) before embedding in paraffin and sectioning into 5-µm tissue sections. Tissue slides were either vacuum-packed using a “Seal-a-Meal” vacuum packaging unit (FoodSaver; Jarden Corporation, Providence, RI) with or without desiccant (Drierite, anhydrous calcium sulfate [CaSO4]; W.A. Hammond Company, Xenia, Ohio) or placed in a humidity chamber (HC) and stored at 4C, room temperature, and incubators at 30C and 37C, respectively, for 3 months. The humidity chamber was a plastic container (11 × 8 × 6 cm) containing 100 ml distilled water with the slides placed on a rack above the water and sealed in a plastic sack of the same material used for the vacuum-sealed specimens but in the absence of vacuum.
Protein Extraction from FFPE Tissue
Prior to protein extraction, three 5-µm-thick FFPE mouse kidney tissue sections were trimmed of excess wax and homogenized using a disposable pellet mixer in 150 µl protein extraction solution (high pH AgR buffer, pH 9.9; Dako, Carpinteria, CA), 1% NaN3, 1% SDS, 10% glycerol, and protease inhibitor (1 tablet/25 ml; Roche, Indianapolis, IN), followed by incubation for 15 min at 115C within a pressure cooker (Dako) (Chung, Lee, et al. 2008). After incubation, the tissue lysates were centrifuged at 15,000 × g for 30 min at 4C. The supernatants were collected and stored at −80C.
Assessment of Quantity and Quality of Total Protein
To assess protein quality, we used the Agilent 2100 bioanalyzer (Agilent Technologies; Palo Alto, CA) with the Agilent High Sensitivity Protein 250 Kit according to the manufacturer’s instructions. Agilent 2100 expert software was used to compare electropherograms.
Immunohistochemistry
Tissue sections were deparaffinized in xylene and hydrated in serial alcohol solutions, respectively. Endogenous peroxidase was blocked by incubation in 3% H2O2 for 10 min. For Aquaporin 1 (AQP1) staining, the antigen retrieval was performed in a steam pressure cooker with prewarmed antigen retrieval buffer pH 6 (Dako) at 95C for 15 min. To minimize nonspecific staining, the section was incubated with protein block (Dako) for 15 min. After washing with TBST (50 mM Tris [pH 7.5], 150 mM NaCl, 0.05% Tween-20), the specimen was incubated with anti-AQP1 antibody (rabbit polyclonal, cat. no. sc-20810; Santa Cruz Biotechnology, Santa Cruz, CA; dilution 1:50) for 1 hr at room temperature. Antigen–antibody reactions were detected with DAKO Envision+ peroxidase kit (Dako). The stain was visualized using 3,3′-diaminobenzidine plus (Dako) and was lightly counterstained with hematoxylin, dehydrated in ethanol, and cleared in xylene. The slides were coverslipped and observed under a light microscope (Axioplot; Carl Zeiss, Jena, Germany). To examine the morphological change, hematoxylin and eosin (H&E) staining was also performed on each tissue section.
SDS-PAGE and Western Blotting
The protein extracts containing 5 µg of protein from FFPE tissue sections were subjected to 4% to 12% NuPAGE Novex Bis-Tris polyacrylamide gel and transferred onto nitrocellulose membrane according to the manufacturer’s protocol (Invitrogen, Carlsbad, CA). The membranes were blocked with 5% nonfat dry milk in TBST (50 mM Tris [pH 7.5], 150 mM NaCl, 0.05% Tween-20) for 1 hr, washed, and subsequently incubated overnight at 4C in TBST with 5% BSA containing anti–glyceraldehyde-3-phosphate dehydrogenase (GAPDH) monoclonal antibody (Clone 6C5; Calbiochem, Madison, WI; 1:500). Specific molecules were detected with horseradish peroxidase (HRP)–labeled anti-mouse secondary antibodies (cat. no. 32430; Chemicon International, Temecula, CA) and enhanced with SuperSignal Chemiluminescence kit (Pierce Biotechnology, Rockford, IL). Signal was detected on Kodak Biomax MR X-ray film (Kodak, Rochester, NY).
Well-Based Protein Array
GAPDH expression was also detected using MesoScale Discovery (MSD) Multi-Spot plates (MA2400 96 HB Plate) and an MSD Sector Imager 2400 reader (MSD, Gaithersburg, MD), as described previously (Chung, Lee, et al. 2008). Briefly, protein extract (5 µL) from FFPE tissue specimen at predetermined protein concentrations was added to 96-well plates and allowed to dry at room temperature for 90 min. The antigen-coated plates were preincubated with 5% nonfat dry milk in phosphate-buffered saline + Tween-20 (PBST) before incubation with anti-GAPDH (Calbiochem; 1:1000) at 4C overnight. After washing with PBST, the plates were incubated for 90 min with goat anti-mouse SULFO-TAG antibodies at a dilution of 25 ng/ml (cat. no. R32AC-5; MSD). The plates were then aspirated and washed 3 times with PBST. Finally, MSD-T read buffer was added to the plates, which were read on the MSD Sector Imager 2400.
Statistical Analysis
Statistical differences were calculated using the ANOVA test. The Tukey test was performed for multiple comparisons. p ≤0.05 was regarded as statistically significant. All statistical analyses were performed using the SPSS for Windows (16.0) package (SPSS, Chicago, IL).
Results
Effect of Tissue Fixation and Processing Time on Protein Immunoreactivity
The crosslinking of formalin fixation results in the loss or masking of antigenic determinants (Werner et al. 2000; Dapson 2007). The process of fixation encompasses 3 elements: thickness of tissue, volume of fixative, and time. Failure to optimize all 3 of these factors results in underfixation or overfixation of the tissue and suboptimal biomolecular preservation (De Marzo et al. 2002; Hewitt et al. 2008). The role of deviation in fixation in antigen degradation has not been studied previously in tissue. In our study, the formalin to tissue ratio of 10:1 was applied, as recommended previously (Hewitt et al. 2008). Based on metrics previously defined for optimal fixation times and processing conditions (Chung, Braunschweig, et al. 2008), normal mouse kidneys were fixed in 10% NBF for either 12 hr or 24 hr before being subjected to tissue processing. To evaluate the influences of different factors on antigen degradation, we performed immunohistochemistry (IHC) of AQP1. AQP1 is a widely expressed water channel and abundantly present in the basolateral and apical plasma membranes of the proximal tubules, the descending limb of the loop of Henle, and the descending portion of the vasa recta (Beitz and Schultz 1999). Specimen tissues fixed for 24 hr showed no change of AQP1 immunostaining but retained better tissue morphology compared with tissues fixed for 12 hr (Fig. 1a).
Figure 1.
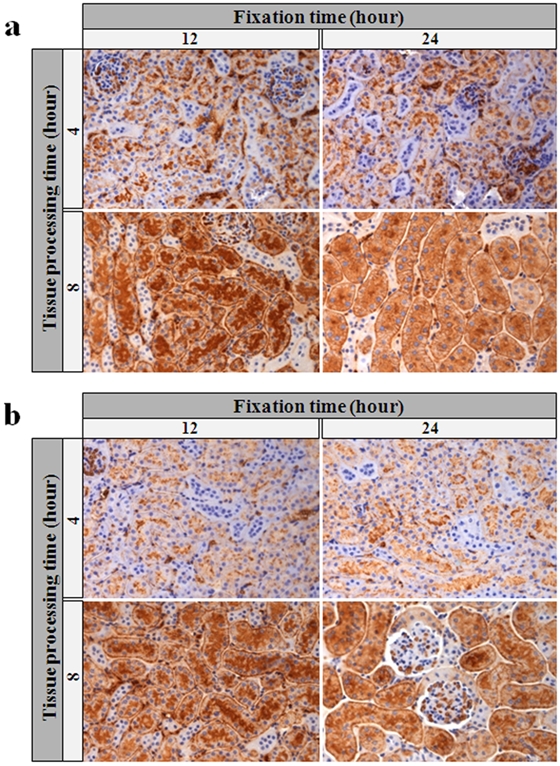
Immunohistochemical analysis of AQP1 expression according to fixation time and tissue processing time. We stained tissue sections of mouse kidney formalin-fixed paraffin-embedded (FFPE) tissue after a 12- or 24-hr fixation period, as well as tested 4-hr or 8-hr tissue processing time after a 0-week (a) and 2-week (b) storage room temperature. All photomicrographs ×250.
The process of converting tissue from a fixed state, in a liquid solution to impregnating in paraffin, is referred to as tissue processing. The contributions of tissue processing to loss of immnuorecognition in tissue sections have not been completely characterized (Grizzle 2009). Previously, we found that longer tissue processing time resulted in better quality of RNA in FFPE (Chung, Braunschweig, et al. 2008), suggesting a reduction in residual water. To further determine the effect of endogenous water in FFPE tissue on protein immunoreactivity, AQP1 expressions were examined by IHC in mouse kidney tissues processed either 4 or 8 hr after 24-hr fixation. As shown in Figure 1a, in comparison with mouse kidney tissues processed for 8 hr, the immunostaining of AQP1 was weaker in tissues processed for 4 hr. Sections from these blocks were stored at room temperature for 2 weeks and stained for AQP1. The stained sections demonstrated further reduction in AQP1 intensity in the tissue processed in 4 hr but not the specimens processed over 8 hr (Fig. 1b). Subsequent experiments used tissue fixation time of 24 hr and tissue processing time of 8 hr.
Effects of Storage Temperature and Humidity on Antigenicity by Immunohistochemistry
Tissue sections of mouse kidney were stored under various conditions for 3 months at 30°C and 37°C either in the presence of desiccant or in a “humidity chamber,” consisting of a sealed environment with added water. Sections were evaluated histologically by H&E staining (Suppl. Fig. S1), and no morphological alterations were observed, but a shift in eosinophilia was appreciable. To determine the effects of storage temperature and humidity on antigenicity of FFPE tissue sections of mouse kidney, AQP1 expressions were examined by IHC (Fig. 2). Strong AQP1 immunostaining was detected in all the tissue sections vacuum-packed with Drierite, regardless of temperatures (Fig. 2a,c,e,g). In contrast, AQP1 staining was much weaker at 30°C and 37°C (Fig. 2b,d,f,h) in tissue slides stored in humid chamber.
Figure 2.
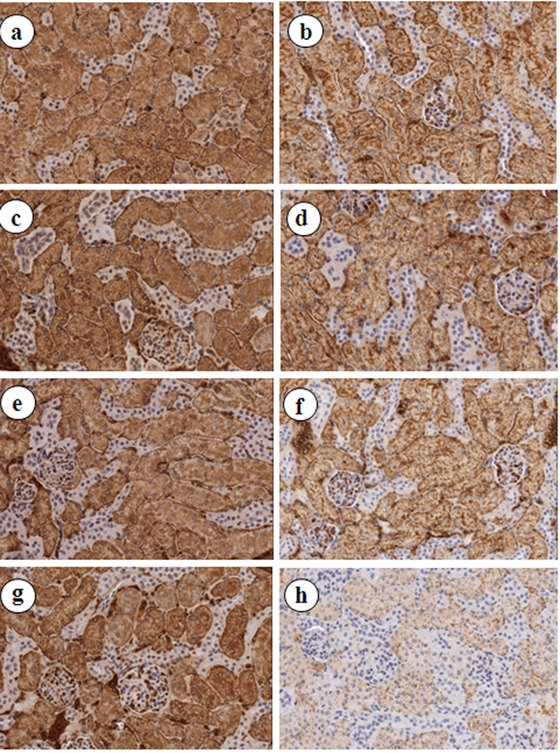
Immunohistochemical analysis of AQP1 expression in formalin-fixed paraffin-embedded (FFPE) tissue sections of mouse kidney under different storage conditions for 3 months. (a) RT-Vac + Drierite, (b) RT-HC, (c) 4C-Vac + Drierite, (d) 4C–HC, (e) 30C-Vac + Drierite, (f) 30C–HC, (g) 37C-Vac + Drierite, (h) 37C–HC. All photomicrographs ×200. HC, humidity chamber; RT, room temperature; Vac, vacuum packed.
Protein Integrity after Storage under Different Storage Conditions
To appreciate the impact of storage temperature and humidity on protein integrity, we evaluated the quality of protein isolated from tissue sections stored for 3 months under various conditions by microcapillary electrophoresis (bioanalyzer; Fig. 3). When stored at the same temperature but different humidity conditions, all specimens with the protein isolated from slides stored with Drierite were less fragmented, by the shift to the right of the curve on the electropherogram, compared to those stored in the humidity chamber (Fig. 3). This result suggested that diminishing the ambient hydration effect by vacuum-packing with desiccant is protective for protein degradation.
Figure 3.
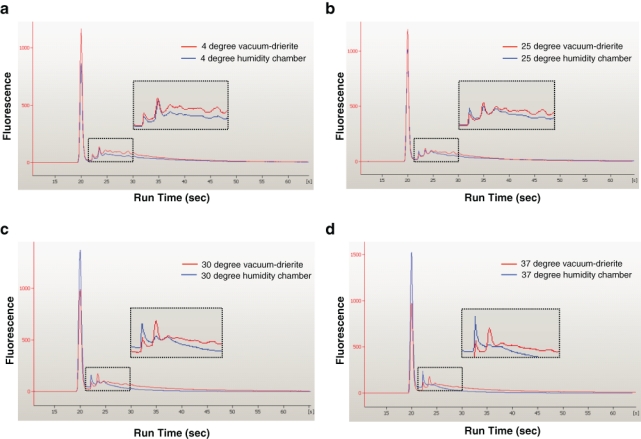
Assessments of protein profiles according to storage conditions. Protein quality was analyzed on the Agilent 2100 bioanalyzer, using 1 µg of denatured total protein extracted from mouse kidney formalin-fixed paraffin-embedded tissue sections under different storage conditions. Representative data are presented as an eletropherogram, in which the first peak on the left is a protein marker.
We further evaluated the immunoreactivity of proteins from mouse kidney FFPE tissue sections by Western blotting (Fig. 4). GAPDH expression was detectable in all the vacuum-packed sections, but the expression levels were reduced with the escalation of storage temperature (Fig. 4a, lanes 1, 3, 5, and 7). On the other hand, for tissue sections in the humidity chamber, GAPDH expressions were only detected in slides stored at 4C and 25C (Fig. 4a, lanes 2 and 4), and there was no detectable expressions of GAPDH in tissue sections stored at 30C and 37C (Fig. 4a, lanes 6 and 8).
Figure 4.
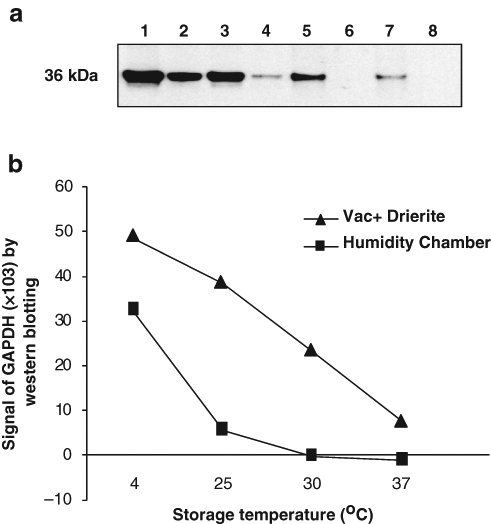
Assessment of protein immunoreactivity to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) by Western blotting. (a) Western Blot by anti-GAPDH antibody. Lane 1, RT-Vac + Drierite; lane 2, RT-HC; lane 3, 4C-Vac + Drierite; lane 4, 4C–HC; lane 5, 30C-Vac + Drierite; lane 6, 30C–HC; lane 7, 37C-Vac + Drierite; lane 8, 37C–HC. (b) Quantitative analysis of Western blot (ImageQuant Program version 5.2, Piscataway, NJ). HC, humidity chamber; RT, room temperature; Vac, vacuum packed.
To confirm and quantify the IHC and Western blotting results, a protein array was also used. Lysates of the protein extracted from tissue sections were immmobilized on a carbon surface of a MSD 96-well plate, used for electrochemiluminescence. Anti-GAPDH antibody was applied and detected by electrochemiluminescence as previously described (Chung, Lee, et al. 2008) to measure protein content of GAPDH. Similarly, an increase of storage temperature significantly decreased GAPDH expression signals from tissue sections in the humidity chamber (Fig. 5).
Figure 5.
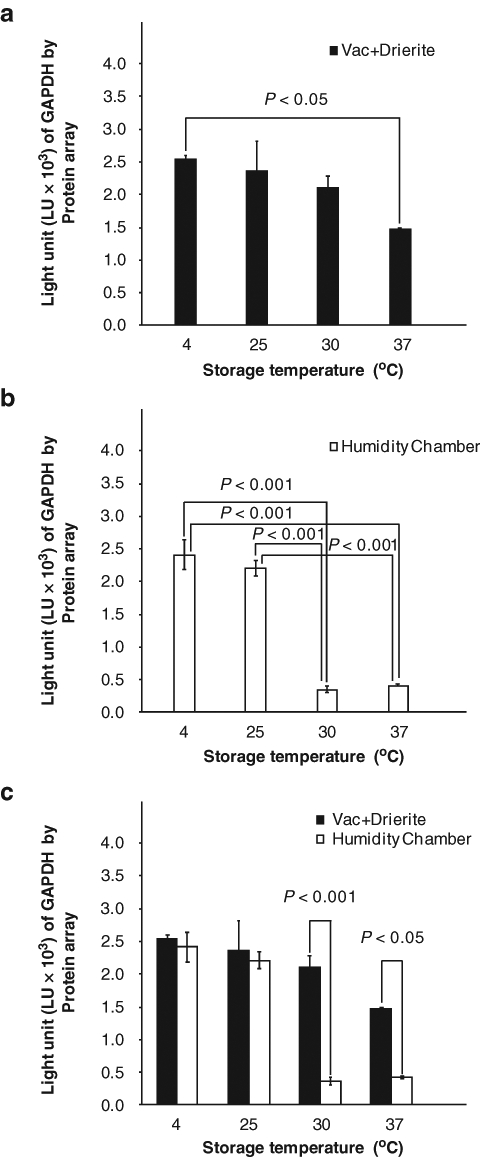
Assessment of protein immunoreactivity to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) in formalin-fixed, paraffin-embedded tissue sections under different storage conditions for 3 months by a novel protein array platform. The expressional values are represented as box graphs as follows: vacuum + Drierite (a), humidity chamber (b), and merged (c). Values shown are mean ± SE.
Discussion
We employed an experimental model system of mouse kidney sections, paired with alteration in tissue fixation, processing and storage, and the application of IHC, Western blots, and protein array technologies as metrics of quality. Our work is the first to interrogate the process of tissue fixation and processing with respect to stability of the biospecimen. Otali et al. (2009) used a cell line model to examine the effects on immunohistochemistry of different steps of the fixation and processing. Our data show that fixation time has a negligible impact on loss of antigenicity. Short processing times result in poor impregnation and loss of antigenicity. We demonstrate that exogenous water can mediate this damage and that temperature is a key factor in the degradation of antigens in the presence of water. The data demonstrate that even with extended processing times, at elevated storage temperatures and dry conditions, tissue sections demonstrate degradation. We hypothesize there is residual water complexed with the proteins and nucleic acids and trapped by the paraffins during tissue processing. Unfortunately, even with optimal conditions, degradation of biomolecules could be detected with sensitive assays.
Paraffin embedding of formalin-fixed tissue is an invaluable biospecimen resource for both diagnostic histopathology and advanced molecular studies. Paraffin-embedded blocks under normal storage conditions appear to retain their antigenicity, and nucleic acids are relatively stable for extended periods (Specht et al. 2001; Cronin et al. 2004). Degradation of RNA within paraffin blocks over periods of months to years is well documented (Cronin et al. 2004; Hewitt et al. 2008). However, tissue sections from paraffin blocks may show a considerable diminution of antigenicity, even after a short time (Jacobs et al. 1996; DeVito et al. 2004; Fergenbaum et al. 2004; Chung, Braunschweig, et al. 2008; Hewitt et al. 2008). Nevertheless, a suitable method for storing slides and blocks, which prevents the loss of antigenicity, has not been described (Henson 1996; Jacobs et al. 1996; Bertheau et al. 1998; van den Broek and van de Vijver 2000; Fergenbaum et al. 2004). The lack of a proper storage solution has a major impact on the viability of large cohort studies that rely on the collection and storage of slides for future assays. Loss of antigenicity affects patient care where consultation or pathology review and additional studies are required for routine care of clinical trials (Leyland-Jones et al. 2008). In addition, the advent of tissue microarrays (TMA) as a tool for the study of immunohistochemical stains on large cohorts of tissue specimens arrayed in a single paraffin block has renewed interest in finding methods for cut slide preservation (Fergenbaum et al. 2004). Loss of antigenicity in stored sections arises, reducing the intensity and extent of staining, resulting in false-negative results (Fergenbaum et al. 2004). In clinical practice, it is a common practice to provide cut slides rather than the original block for additional molecular studies, such as new predictive biomarkers (Leyland-Jones et al. 2008).
Oxidation has been assumed responsible for the degradation of antigenicity, and a variety of means for storage of cut sections have been advocated. Several studies assessed the benefit of storing slides at various temperatures and in some cases coated with paraffin. In general, cold storage of slides (4C) was the optimal method of slide storage but still resulted in significant loss of antigenicity (Jacobs et al. 1996; van den Broek and van de Vijver 2000). Paraffin coating was also found to be an ineffective method of antigen preservation on its own and is problematic because the paraffin is challenging to remove and leads to variable staining results (Jacobs et al. 1996). Other investigators store slides in a nitrogen desiccator, which requires special equipment and monitoring and is not completely protective (Wester et al. 2000; DiVito et al. 2004).
In this study, we demonstrate that the presence of water both endogenously and exogenously plays a key role in antigenicity loss. We sought to determine the impact of differences in fixation time on antigenicity loss by applying the models we had developed in studying RNA integrity in FFPE tissue (Chung, Braunschweig, et al. 2008). It has been well appreciated that fixation time has an impact on RNA quality (Chung, Braunschweig, et al. 2008; Hewitt et al. 2008; Leyland-Jones et al. 2008). Here, we demonstrate that although a shorter fixation condition (12 hr) results in an unsatisfactory immunostaining pattern, the protein quality was less affected than RNA quality (Chung, Braunschweig, et al. 2008) and had limited or no impact on the long-term stability of the proteins within the biospecimen. We suspect that the failure to complete fixation leads to further complications downstream in tissue processing, likely related to inadequate dehydration. We demonstrated that tissue processing time directly affects protein quality. Improper tissue processing leads to inadequate replacement of water by paraffin and retaining of endogenous water in FFPE tissue. In agreement with our previous study on tissue processing time and RNA quality (Chung, Braunschweig, et al. 2008), insufficient tissue processing for 4 hr resulted in significantly reduced AQP1 expression in mouse kidney tissue sections, which implied that the retained water in FFPE tissue sections precipitated loss of antigenicity.
To further elucidate the effects of ambient water on protein immnuoreacitivity, we employed two alternative storage conditions, vacuum-pack with desiccant (calcium sulfate, Drierite) and humidity chamber. Our data indicate that proteins extracted from tissue sections of mouse kidney stored for 3 months in a humidity chamber showed loss of proteins with high molecular weight and a higher degree of protein degradation than proteins from sections in vacuum package with desiccant independent of temperature. Consistently, the immunostaining of AQP1 was weaker in tissue sections stored in a humidity chamber at high temperatures, and vacuum package with desiccant provided protection against antigen degradation. The results were further validated by Western blotting and a novel protein array. Assays for RNA demonstrate a similar trend (data not shown).
We also demonstrated a temperature dependence on the degradation of specimens, independent of environment. These results show that lower storage temperatures correlate with better protein quality. It should be noted that the protein quality of the sections stored under dry conditions at room temperature were superior to the quality of sections stored at 4C under humid conditions. When comparing the quality of tissue processed for different times within this system, underprocessed (4 hr) tissue demonstrated a greater impact of temperature on degradation (data not shown).
On the basis of the observation that there was an increased eosinophilia in the tissue sections with the greatest degradation, as measured by the AQP1 IHC and protein array data, we sought to determine the nature of this alteration. Examination of the eosin-associated transmission spectra using a CRi Nuance (Woburn, MA) camera demonstrated no shift, but measurement of the fluorescent emission of the eosin-stained sections demonstrated an increase in fluorescence, which correlated with the degradation of protein (data not shown). In fact, the relative eosinophilia of the tissue is a reflection of the protein integrity, where hydrolysis of proteins provides a net increase in the fluorescence of eosin. Unfortunately, the practicality of this assay is limited as the net eosinophilia or fluorescent intensity also was affected by tissue type and section thickness.
Our data are the first to present a mechanistic model of antigen degradation, demonstrating the negative effects of endogenous or exogenous water, resulting in biomolecule degradation, presumably through hydrolysis. Alternative, unidentified chemical processes may be occurring that result in cleavage of the peptide backbone, but the presence of water and an increased rate of cleavage with elevated temperature characterize the process. Although we have identified potential mechanisms of degradation, we have not defined an optimal means of maintaining archival integrity, and further studies will be required. We showed that the presence of water is responsible for the loss of antigenicity in FFPE tissue sections. The process is more appropriately termed hydrolysis. Endogenous water retained in FFPE tissue results in the bulk of damage, but exogenous water contributes. Our data demonstrate that tissue processing is a critical factor in antigen stability, whereas fixation time is not. In addition, degradation is temperature dependent.
The studies presented here were carried out on tissue sections, with an emphasis on protein integrity as an endpoint. RNA has been demonstrated to be equally labile in tissue sections (Hewitt et al. 2008; Leland-Jones et al. 2008) and is well recognized to degrade over time in tissue blocks (Cronin et al. 2004). We suspect the molecular mechanisms are identical, and previous experiments (unpublished data) support this model but demonstrate RNA is more labile than proteins to degradation. This suggests that tissue blocks are susceptible to the same degradation, but the capacity of exogenous water to penetrate the paraffin is reduced, but endogenous water and the effects of temperature remain at play.
Our data help explain the observations of others, where no single set of conditions has been demonstrated to prevent degradation. We suspect that paraffin-dipping and nitrogen gas environments fail because of the effects of endogenous water, and we suspect that vacuum provides similar benefit. Although degradation is temperature dependent, our data are congruent with the observations of some groups that have failed to routinely observe degradation of their specimens, presumably because the material is well processed and has low levels of endogenous water, and the end assays lack sufficient precision to identify a difference by IHC, which is frequently limited to interpretation as the presence or absence of staining. Clearly, storage at lower temperatures demonstrated improved quality, but the experiments are inadequate to make this a recommended storage method, as dry storage conditions at room temperature remain superior compared to wet conditions under refrigeration. In a routine work environment, refrigeration of blocks may prove economically and operationally unmanageable, whereas containment of humidity through a variety of means is likely feasible.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the authorship and/or publication of this article.
The author(s) disclosed receipt of the following financial support for the research and/or authorship of this article: This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
References
- Arnold MM, Srivastava S, Fredenburgh J, Stockard CR, Myers RB, Grizzle WE. 1996. Effects of fixation and tissue processing on immunohistochemical demonstration of specific antigens. Biotech Histochem. 71:224–230 [DOI] [PubMed] [Google Scholar]
- Bertheau P, Cazals-Hatem D, Meignin V, de Roquancourt A, Verola O, Lesourd A, Sene C, Brocheiou C, Janin A. 1998. Variability of immunohistochemical reactivity on stored paraffin slides. J Clin Pathol. 51:370–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beitz E, Schultz JE. 1999. The mammalian aquaporin water channel family: a promising new drug target. Curr Med Chem. 6:457–467 [PubMed] [Google Scholar]
- Blind C, Koepenik A, Pacyna-Gengelbach M, Fernahl G, Deutschmann N, Dietel M, Krenn V, Petersen I. 2008. Antigenicity testing by immunohistochemistry after tissue oxidation. J Clin Pathol. 61:79–83 [DOI] [PubMed] [Google Scholar]
- Camp RL, Charette LA, Rimm DL. 2000. Validation of tissue microarray technology in breast carcinoma. Lab Invest. 80:1943–1949 [DOI] [PubMed] [Google Scholar]
- Chung JY, Braunschweig T, Williams R, Guerrero N, Hoffmann KM, Kwon M, Song YK, Libutti SK, Hewitt SM. 2008. Factors in tissue handling and processing that impact RNA obtained from formalin-fixed, paraffin-embedded tissue. J Histochem Cytochem. 56:1033–1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung JY, Lee SJ, Kris Y, Braunschweig T, Traicoff JL, Hewitt SM. 2008. A well-based reverse-phase protein array applicable to extracts from formalin-fixed paraffin-embedded tissue. Proteomics Clin Appls. 2:1539–1547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronin M, Pho M, Dutta D, Stephans JC, Shak S, Kiefer MC, Estban JM, Baker JB. 2004. Measurement of gene expression in archival paraffin-embedded tissues: development and performance of a 92-gene reverse transcriptase-polymerase chain reaction assay. Am J Pathol. 164:35–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dapson RW. 2007. Macromolecular changes caused by formalin fixation and antigen retrieval. Biotech Histochem. 82:133–140 [DOI] [PubMed] [Google Scholar]
- De Marzo AM, Fedor HH, Gage WR, Rubin MA. 2002. Inadequate formalin fixation decreases reliability of p27 immunohistochemical staining: probing optimal fixation time using high-density tissue microarrays. Hum Pathol. 33:756–760 [DOI] [PubMed] [Google Scholar]
- DiVito KA, Charette LA, Rimm DL, Camp RL. 2004. Long-term preservation of antigenicity on tissue microarrays. Lab Invest. 84:1071–1078 [DOI] [PubMed] [Google Scholar]
- Fergenbaum JH, Garcia-Closas M, Hewitt SM, Lissowska J, Sakoda LC, Sherman ME. 2004. Loss of antigenicity in stored sections of breast cancer tissue microarrays. Cancer Epidemiol Biomarkers Prev. 13:667–672 [PubMed] [Google Scholar]
- Grizzle WE. 2009. Special symposium: fixation and tissue processing models. Biotech Histochem. 84:185–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson DE. 1996. Loss of p53-immunostaining intensity in breast cancer. J Natl Cancer Inst. 88:1015–1016 [DOI] [PubMed] [Google Scholar]
- Hewitt SM, Lewis FA, Cao Y, Conrad RC, Cronin M, Danenberg KD, Goralski TJ, Langmore JP, Raja RG, Williams PM, et al. 2008. Tissue handling and specimen preparation in surgical pathology: issues concerning the recovery of nucleic acids from formalin-fixed, paraffin-embedded tissue. Arch Pathol Lab Med. 132:1929–1935 [DOI] [PubMed] [Google Scholar]
- Jacobs TW, Prioleau JE, Stillman IE, Schnitt SJ. 1996. Loss of tumor marker-immunostaining intensity on stored paraffin slides of breast cancer. J Natl Cancer Inst. 88:1054–1059 [DOI] [PubMed] [Google Scholar]
- Leyland-Jones BR, Ambrosone CB, Bartlett J, Ellis MJ, Enos RA, Raji A, Pins MR, Zujewski JA, Hewitt SM, Forbes JF, et al. 2008. Recommendations for collection and handling of specimens from group breast cancer clinical trials. J Clin Oncol. 26:5638–5644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirlacher M, Kasper M, Storz M, Knecht Y, Durmuller U, Simon R, Mihatsche MJ, Sauter G. 2004. Influence of slide aging on results of translational research studies using immunohistochemistry. Modern Pathol. 17:1414–1420 [DOI] [PubMed] [Google Scholar]
- Nirmalan NJ, Harnden P, Selby PJ, Banks RE. 2009. Development and validation of a novel protein extraction methodology for quantitation of protein expression in formalin-fixed paraffin-embedded tissues using Western blotting. J Pathol. 217:497–506 [DOI] [PubMed] [Google Scholar]
- Otali D, Stockard CR, Oelschlager DK, Wan W, Manne E, Watts SA, Grizzle WE. 2009. The combined effects of formalin fixation and individual steps in tissue processing on immunorecognition. Biotech Histochem. 84:223–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauter G, Mirlacher M. 2002. Tissue microarrays for predictive molecular pathology. J Clin Pathol. 55:575–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Specht K, Richter T, Muller U, Walch A, Werner M, Hofler H. 2001. Quantitative gene expression analysis in microdissected archival formalin-fixed and paraffin-embedded tumor tissue. Am J Pathol. 158:419–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan M, Sedmak D, Jewell S. 2002. Effect of fixatives and tissue processing on the content and integrity of nucleic acids. Am J Pathol. 161:1961–1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Broek LJ, van de Vijver MJ. 2000. Assessment of problems in diagnostic and research immunohistochemistry associated with epitope instability in stored paraffin sections. Appl Immunohistochem Mol Morphol. 8:316–321 [PubMed] [Google Scholar]
- Werner M, Chott A, Fabiano A, Battifora H. 2000. Effect of formalin tissue fixation and processing on immunohistochemistry. Am J Surg Pathol. 24:1016–1019 [DOI] [PubMed] [Google Scholar]
- Wester K, Wahlund E, Sundstrom C, Ranefall P, Bengtsson E, Russell PJ, Ow KT, Malmstrom PU, Busch C. 2000. Paraffin section storage and immunohistochemistry: effects of time, temperature, fixation, and retrieval protocol with emphasis on p53 protein and MIB1 antigen. Appl Immunohistochem Mol Morphol. 8:61–70 [PubMed] [Google Scholar]


