Abstract
Growing evidence indicates that the melanocortin 1 receptor (MC1R) and its ligand α–melanocyte-stimulating hormone (α-MSH) have other functions in the skin in addition to pigment production. Activation of the MC1R/α-MSH signaling pathway has been implicated in the regulation of both inflammation and extracellular matrix homeostasis. However, little is known about the role of MC1R/α-MSH signaling in the regulation of inflammatory and fibroproliferative responses to cutaneous injury. Although MC1R and α-MSH localization has been described in uninjured skin, their spatial and temporal expression during cutaneous wound repair has not been investigated. In this study, the authors report the localization of MC1R and α-MSH in murine cutaneous wounds, human acute burns, and hypertrophic scars. During murine wound repair, MC1R and α-MSH were detected in inflammatory cells and suprabasal keratinocytes at the leading edge of the migrating epithelial tongue. MC1R and α-MSH protein levels were upregulated in human burn wounds and hypertrophic scars compared to uninjured human skin, where receptor and ligand were absent. In burn wounds and hypertrophic scars, MC1R and α-MSH localized to epidermal keratinocytes and dermal fibroblasts. This spatiotemporal localization of MC1R and α-MSH in cutaneous wounds warrants future investigation into the role of MC1R/α-MSH signaling in the inflammatory and fibroproliferative responses to cutaneous injury. This article contains online supplemental material at http://www.jhc.org. Please visit this article online to view these materials.
Keywords: melanocortin 1 receptor, α–melanocyte-stimulating hormone, cutaneous wound repair, hypertrophic scar, immunohistochemistry
The melanocortin 1 receptor (MC1R) is a major determinant of skin pigmentation (Lin and Fisher 2007). Activation of this G-protein-coupled receptor on melanocytes increases production of photoprotective brown/black eumelanin at the expense of yellow/red pheomelanin. The major agonists of melanocortin 1 receptor are derived from the precursor hormone, proopiomelanocortin (POMC), and include the melanocyte-stimulating hormones (MSHs) and adrenocorticotrophin (ACTH). The agonist, α–melanocyte-stimulating hormone (α-MSH), binds the melanocortin 1 receptor with the highest affinity (Suzuki et al. 1996). The downstream effectors of MC1R signaling, adenylyl cyclase and cyclic AMP, mediate phosphorylation of cAMP responsive element-binding protein (CREB) transcription factors, which participate in the activation of micropthalmia transcription factor (MITF), a key regulator of expression of pigment enzymes.
Growing evidence indicates that MC1R and its ligand α-MSH have other functions in the skin in addition to pigment production. Activation of the MC1R/α-MSH signaling pathway has been implicated in the regulation of both inflammation and extracellular matrix homeostasis. Numerous in vitro studies have demonstrated that α-MSH has anti-inflammatory properties. It downregulates production of proinflammatory cytokines such as interleukin (IL)−1, IL-4, IL-6, IFN-γ, and tumor necrosis factor (TNF)–α (Brzoska et al. 2008) and increases production of the anti-inflammatory cytokine, IL-10, in human monocytes (Bhardwaj et al. 1996) and keratinocytes (Redondo et al. 1998). In dermal fibroblasts, α-MSH binding to MC1R also inhibits TNF-α-stimulated NFκB translocation to the nucleus and reduces intercellular adhesion molecule 1 upregulation (Hill et al. 2006). In addition, α-MSH modulates oxidative stress by decreasing production of nitric oxide in macrophages (Mandrika et al. 2001) and melanocytes (Bohm et al. 2004; Tsatmali et al. 2000). It also appears that α-MSH inhibits synthesis of the extracellular matrix. α-MSH reduces transforming growth factor (TGF)–β1-induced synthesis of collagen types I and III in dermal fibroblasts (Bohm et al. 2004), and a recent report indicates that α-MSH reduces skin fibrosis in a mouse model of scleroderma (Kokot et al. 2009).
Despite these observations, little is known about the role of MC1R/α-MSH signaling in the regulation of inflammatory and fibroproliferative responses to cutaneous injury. Although MC1R and α-MSH localization has been described in uninjured skin, their spatial and temporal expression during cutaneous wound repair has not been investigated. In this study, we determined the localization of MC1R and α-MSH in murine excisional wounds, human acute burns, and hypertrophic scars. Changes in MC1R and α-MSH localization suggest a role in epithelialization, inflammation, and fibroproliferation during cutaneous wound repair.
Materials and Methods
Murine Excisional Wound Model
All animal procedures were in accordance with the Guide for the Care and Use of Laboratory Animals and have been approved by the University of Washington Animal Care Committee. Male C57BL/6J mice (The Jackson Laboratory, Bar Harbor, ME) between 6 and 10 weeks of age were used for the wounding experiments. Mice were housed individually, maintained on a 12-hr light/dark cycle, and allowed ad libitum access to food and water. The creation of two full-thickness 6-mm dorsal punch wounds was adapted from previously described experiments (Muangman et al. 2004; Spenny et al. 2002; Sullivan et al. 2004). Following anesthesia, dorsal hair was clipped and the depilatory agent Nair (Carter Wallace, Inc., NY) was applied to remove the remaining hair stubble. The dorsum was washed with povidone-iodine followed by 70% isopropyl alcohol. A template was used to mark the site for biopsy on each mouse, and then the skin was scored using a sterile 6-mm punch biopsy (Miltex, York, PA), and a full-thickness wound (including the panniculus carnosus muscle) was created using sterile surgical scissors. Wounds were covered with the transparent semi-occlusive dressing Tegaderm (3M, St. Paul, MN), using a thin layer of Mastisol (Ferndale Laboratories, Ferndale, MI) to secure the edges. Postoperative recovery occurred on a 37° C heating pad until animals were sufficiently mobile to be returned individually to their cage. During postoperative recovery, each animal was injected intraperitoneally with warm saline to minimize risk of dehydration. In contrast, anti-inflammatory agents or narcotics were only administered if the animal acted in pain. Any animal that received these pain medications was excluded from the study because these agents alone have a direct effect on wound healing and would thus confound our results.
Wound Processing
Mice were euthanized and wounds were collected on days 3, 7, 14, 21, and 28 after injury; there were three mice per time point. For immunohistochemistry, wounds were fixed in 10% neutral buffered formalin for subsequent processing into paraffin. Uninjured skin (excised to generate the wound) was collected at the time of injury and was also processed as mentioned above.
Statistical Analyses
The Student’s t-test was used to determine statistical significance for the quantitative real-time PCR. A p value less than or equal to 0.05 was considered significant.
Human Uninjured Skin, Burn Wounds, and Hypertrophic Scars
The Institutional Review Board of the University of Washington approved the collection of de-identified discarded human tissue at time of surgical burn excision at the University of Washington Regional Burn Unit (Harunari et al. 2006; Scott et al. 2005). Collected human tissue included uninjured skin, burn wounds, and hypertrophic scars. Human samples were fixed in neutral buffered formalin for subsequent embedding in paraffin. Patient demographics and wound descriptions are listed in Table 1.
Table 1.
Description of Human Uninjured Skin, Burn Wound, and Hypertrophic Scar Tissue Samples
| Tissue Description | Wound Age | Injury Type | Wound Location | Patient Sex | Patient Age, y |
|---|---|---|---|---|---|
| Uninjured | NA | Uninjured | Leg | M | 56 |
| Early burn wound | <1 month | Burn | Leg | M | 35 |
| Hypertrophic scar | 10 months | Burn | Neck | F | 6 |
| Hypertrophic scar | 2 years | Burn | Chest | F | 9 |
NA, not applicable; M, male; F, female.
Immunohistochemistry
Immunohistochemistry experiments were conducted as previously described (Olerud et al. 1998). In this study, 6-mm de-paraffinized sections were blocked first with 0.3% H2O2 for 30 min and then 1.3% goat serum in TBS for another 30 min. Sections were incubated with primary antibody for 1 hr at room temperature. The following primary antibodies were used: rabbit anti–melanocortin receptor 1 affinity purified polyclonal antibody (dilution 1:20; Millipore, Temecula, CA) and rabbit anti-α-MSH (dilution 1:500; Peninsula Laboratories, San Carlos, CA). After incubation in primary antibody, sections were rinsed with TBS and incubated for 1 hr with a biotinylated goat anti-rabbit secondary antibody (dilution 1:400; Vector Labs, Burlingame, CA). Sections were incubated with streptavidin (Vectastain Elite ABC Kit; Vector Labs) and then with 0.12% diaminobenzidene (DAB) as the chromagen. Negative controls were included for both murine and human studies. For the murine wound series, isotype controls were performed. For the human study, tissue was incubated with secondary antibody alone. Importantly, all time points and negative controls were processed at the same time for each antibody in both the mouse and human studies.
Image Capture
Digital images of each tissue section (20× and 40×) were taken with differential interference contrast (DIC) illumination on a Nikon upright Optiphot light microscope with a digital Canon PowerShot S3 IS camera. Digital images were then imported into Adobe Photoshop CS2. There were no alterations to figures with image processing software other than the standard linear adjustment to brightness applied to the entire image.
Results
Localization of the Melanocortin 1 Receptor (MC1R) during Murine Wound Repair
In uninjured murine skin, MC1R protein was detected only in muscle of the panniculus carnosus (Suppl. Fig. S1). In contrast, after cutaneous injury, MC1R protein also localized to inflammatory cells, epidermal keratinocytes, and hair follicles in addition to muscle (Figs. 1 and 2; Suppl. Fig. S1). Three days after wounding, MC1R immunostaining was detected in epidermal keratinocytes at the leading edge of the migrating epithelial tongue (Fig. 1A) and in a subset of inflammatory cells in the wound bed (Figs. 1B and 2A). MC1R was also detected in muscle adjacent to the day 3 wound (Fig. 2B). The localization of MC1R to epidermal keratinocytes was also detected in 7-day wounds with MC1R immunostaining of suprabasal keratinocytes at the wound periphery and in keratinocytes above the wound bed in recently epithelialized wounds (Fig. 1D). Low- and high-magnification images show that MC1R protein localized to a discrete population of suprabasal keratinocytes in both 3-day and 7-day wounds (Figs. 1A,D and 2C) and was not detected in epidermal keratinocytes adjacent to the wound margin (Fig. 1C). MC1R immunostaining was not detected in the wound epidermis of either 14-day or 21-day wounds (Fig. 1G,J). In addition, the wound bed was negative for MC1R immunostaining in wounds at days 7, 14, and 21 (Fig. 1E,H,K).
Figure 1.

Melanocortin 1 receptor (MC1R) localization in murine excisional wounds: 3-day wound (A–C), 7-day wound (D–F), 14-day wound (G–I), and 21-day wound (J–L). Dotted line indicates the dermal-epidermal junction. In 3-day wounds, MC1R immunostaining was detected in suprabasal keratinocytes at the leading edge of the migrating epithelial tongue (A; arrow) and in a subset of inflammatory cells in the wound bed (B; arrow). In 3-day wounds, MC1R immunostaining was absent in uninjured skin adjacent to the wound (C). In day 7 wounds, MC1R localized to suprabasal keratinocytes in the wound epidermis (D; arrow) and hair follicles in adjacent uninjured skin (F; arrow). There was no MC1R immunostaining in the wound bed of day 7 wounds (E). In both day 14 and day 21 wounds, MC1R was only present in hair follicles adjacent to the wound (I and L; arrows); MC1R immunostaining was absent both in suprabasal keratinocytes at the wound edge (G, J) and in the wound bed (H, K). Magnification bar = 50 µm.
Figure 2.

High-magnification images of melanocortin 1 receptor (MC1R) localization in day 3 murine wounds (A–C) and adjacent to day 14 murine wounds (D). Three days after cutaneous injury, MC1R was localized to a subset of inflammatory cells in the wound bed (A; arrow), panniculus carnosus adjacent to the wound (B; arrows), and suprabasal epidermal keratinocytes (C; arrow). Fourteen days after injury, MC1R localized to the outer root sheath of hair follicles adjacent to the wound (D; arrows). Magnification bar = 50 µm.
MC1R protein was detected in the hair follicles adjacent to the wound 7 days after injury (Fig. 1F). This localization to hair follicles was also observed in both 14-day and 21-day wounds (Fig. 1I,L). A high-magnification image of the hair follicle adjacent to a 14-day wound shows that MC1R protein localized to cells in the outer root sheath (Fig. 2D). In contrast, MC1R was not detected in hair follicles in either uninjured skin (Suppl. Fig. S1A) or in hair follicles adjacent to 3-day wounds (Fig. 1C). Blood vessels, fibroblasts, and sebaceous glands were consistently negative for MC1R immunostaining. These data were confirmed in other strains of mice: BKS.Cg-m +/+ Leprdb/J mice (diabetic, db/db) and C57Bl6 m +/+ Leprdb (heterozygous non-diabetic littermates or db/–). In experiments conducted with these strains, an expanded wound series incorporating earlier time points (6, 24, and 48 hr) was included. In these experiments, MC1R was seen in inflammatory cells as early as 24 hr and in the advancing migrating tongue as early as 48 hr after injury (Suppl. Fig. S2).
Localization of α–Melanocyte-Stimulating Hormone (α-MSH) during Murine Wound Repair
We next determined the spatial and temporal localization of the primary ligand for the melanocortin 1 receptor in murine wounds. In both uninjured skin and in skin adjacent to cutaneous wounds, α-MSH was localized to sebaceous glands (Fig. 3 and Suppl. Fig. S3A). In response to cutaneous injury, α-MSH had a similar localization pattern to its receptor, MC1R (Fig. 3 and Suppl. Fig. S3). In 3-day wounds, α-MSH protein localized to both suprabasal keratinocytes of the migrating epithelial tongue (Figs. 3A and 4A) and to inflammatory cells (Figs. 3B and 4B). α-MSH immunostaining in suprabasal keratinocytes in the wound epidermis was also observed in 7-day wounds (Fig. 3D) but was undetectable in the epidermis of both 14-day and 21-day wounds (Fig. 3G,J). Also similar to MC1R was the detection of α-MSH in hair follicles adjacent to both 14-day (Fig. 4C) and 21-day wounds (Suppl. Fig. S4). α-MSH was not detected in the wound bed at days 7, 14, and 21 postinjury (Fig. 3E,H,K). Again, high-magnification images show α-MSH localized to suprabasal keratinocytes in migrating epithelial tongue in 3-day wounds (Fig. 4A), a subset of inflammatory cells in 7-day wounds (Fig. 4B), the outer root sheath of hair follicles adjacent to 14-day (Fig. 4C) and 21-day wounds (Suppl. Fig. S4), and in sebaceous glands adjacent to the wound (Fig. 4D).
Figure 3.
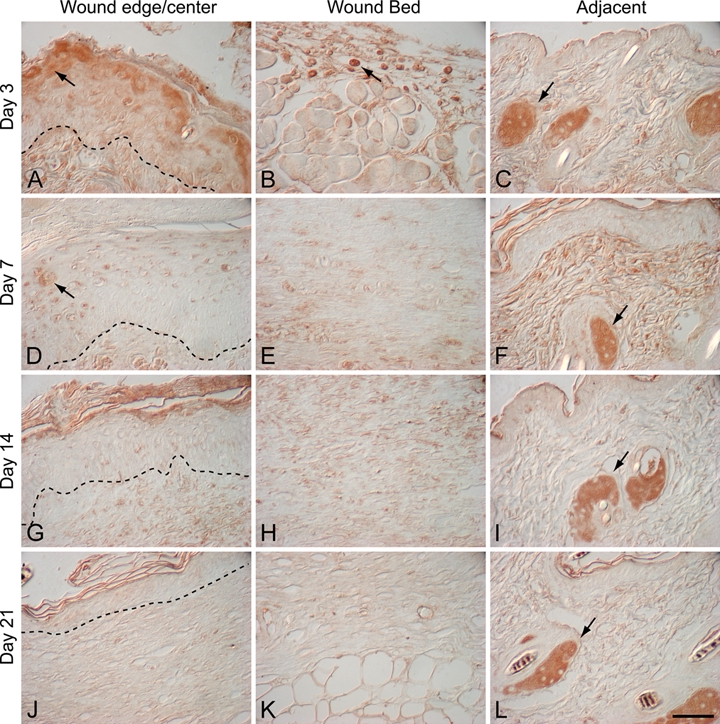
α–Melanocyte-stimulating hormone (α-MSH) localization in murine excisional wounds: 3-day wound (A–C), 7-day wound (D–F), 14-day wound (G–I), and 21-day wound (J–L). Dotted line indicates the dermal-epidermal junction. α-MSH was present in sebaceous glands adjacent to the wound at all time points (C, F, I, L; arrows). In 3-day wounds, α-MSH immunostaining was also detected in suprabasal keratinocytes at the leading edge of the migrating epithelial tongue (A; arrow) and in a subset of inflammatory cells in the wound bed (B; arrow). In day 7 wounds, α-MSH was localized to suprabasal keratinocytes in the wound epidermis (D; arrow) and hair follicles in adjacent uninjured skin (F; arrow). There was no specific α-MSH immunostaining in the wound bed of day 7 wounds (E). In day 14 and day 21 wounds, α-MSH was only present in sebaceous glands adjacent to the wound (I, L; arrows); α-MSH immunostaining was absent both in suprabasal keratinocytes at the wound edge (G, J) and in the wound bed (H, K). Magnification bar = 50 µm.
Figure 4.
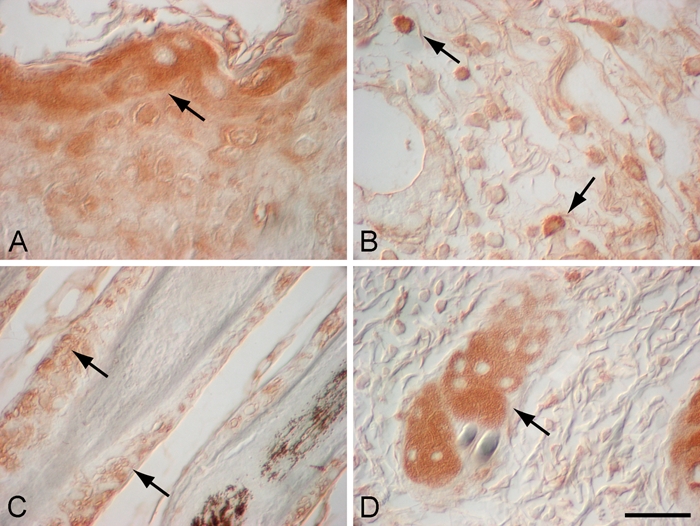
High-magnification images of α–melanocyte-stimulating hormone (α-MSH) localization in day 3 murine wound (A), day 7 murine wound (B), adjacent to day 14 murine wound (C), and adjacent to day 3 wound (D). Three days after cutaneous injury, α-MSH localized to a subset of suprabasal epidermal keratinocytes in the leading edge of migrating epidermal tongue (A; arrow) and in sebaceous glands adjacent to the wound (D; arrow). In day 7 murine wounds, α-MSH was localized to a subset of inflammatory cells in the wound bed after injury (B; arrows). Fourteen days after injury, α-MSH localized to the outer root sheath of hair follicles adjacent to the wound (C; arrows). Magnification bar = 50 µm.
Although similar, the localization of MC1R and its ligand, α-MSH, did differ. Unlike MC1R immunolocalization, α-MSH was not detected consistently in panniculus carnosus muscle. Also, in both uninjured skin and in skin adjacent to cutaneous wounds, robust immunostaining of α-MSH was detected in sebaceous glands (Fig. 3C,F,I,L). In contrast to the discrete localization of MC1R, α-MSH staining was more diffuse in murine skin. Again, the α-MSH localization data in C57BL mice were confirmed in BKS.Cg-m +/+ Leprdb/J mice (diabetic, db/db) and C57Bl6 m +/+ Leprdb (heterozygous non-diabetic littermates or db/–). The only exception was that in db/db and db/– murine wounds, light α-MSH immunostaining was also detected throughout the entire epidermis at all time points in addition to robust α-MSH immunostaining in suprabasal keratinocytes in the wound epidermis in day 3 and day 7 wounds (Suppl. Fig. S2).
Localization of Melanocortin 1 Receptor and α-MSH in Human Burn Wounds and Hypertrophic Scars
Melanocortin 1 receptor and α-MSH protein levels were upregulated in human burn wounds and hypertrophic scars compared to uninjured human skin where both receptor and ligand were absent (Figs. 5–7; Suppl. Figs. S5–S8). In an early burn wound (<1 month postinjury), MC1R protein was detected throughout the epidermis and in dermal stromal cells (Fig. 5B; Suppl. Fig. S5A,D,G). Hypertrophic scar (10 months postinjury) was also positive for MC1R immunostaining in epidermal keratinocytes, hair follicles, dermal fibroblasts, and sebaceous glands (Suppl. Fig. S6). However, in more mature hypertrophic scars (both 2 years and 29 months postinjury), MC1R immunostaining was no longer detected in either the epidermis or the dermis (Fig. 5C; Suppl. Figs. S7A and S8A) and was only observed in hair follicles (Suppl. Figs. S7D and S8D) and in sebaceous glands (Suppl. Fig. S7G). α-MSH had a similar expression pattern to MC1R in burn wounds and hypertrophic scars (Figs. 6 and 7). α-MSH immunostaining was absent in the epidermis and dermis of uninjured skin (Fig. 6A,E) but was robust in epidermal keratinocytes (Fig. 6B) and dermal fibroblasts (Fig. 6F) in an early burn wound (<1 month postinjury). In hypertrophic scars, α-MSH protein localized to hair follicles (Fig. 7B; Suppl. Fig. S7H) and sebaceous glands (Suppl. Fig. S6I) in addition to epidermal keratinocytes (Fig. 6C,D), blood vessels (Fig. 7A), and dermal fibroblasts (Fig. 6G,H). Unlike MC1R, the α-MSH localization pattern was the same in hypertrophic scars at 10 months and up to 2 years postinjury (Fig. 6C,D,G,H; Suppl. Figs. S6 and S7).
Figure 5.
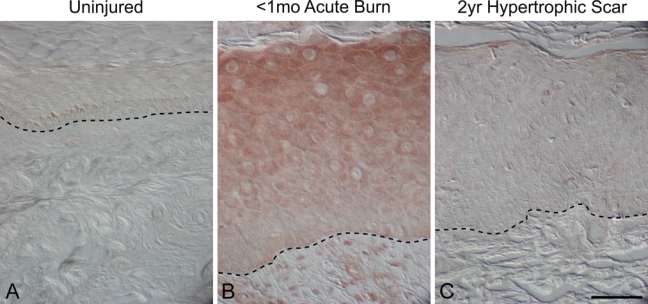
Melanocortin 1 receptor localization (MC1R) in uninjured human skin, early burn wounds (<1 month postinjury), and hypertrophic scars (2 years postinjury). MC1R immunostaining was absent in uninjured skin (A), whereas MC1R localized to epidermal keratinocytes and dermal stromal cells in an early burn wound (B). No MC1R immunostaining was detected in the epidermis and dermis in a hypertrophic scar 2 years after injury (C). Magnification bar = 50 µm.
Figure 7.
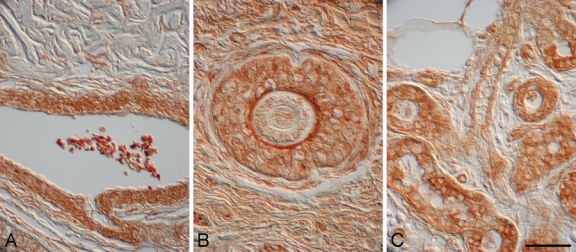
α–Melanocyte-stimulating hormone (α-MSH) localized to blood vessels, hair follicles, and sebaceous glands in human burn wounds and hypertrophic scars. α-MSH immunostaining was detected in blood vessels in a hypertrophic scar 10 months postinjury (A), hair follicles in a hypertrophic scar 2 years postinjury (B), and sebaceous glands in an early burn wound (<1 month postinjury) (C). Magnification bar = 50 µm.
Figure 6.
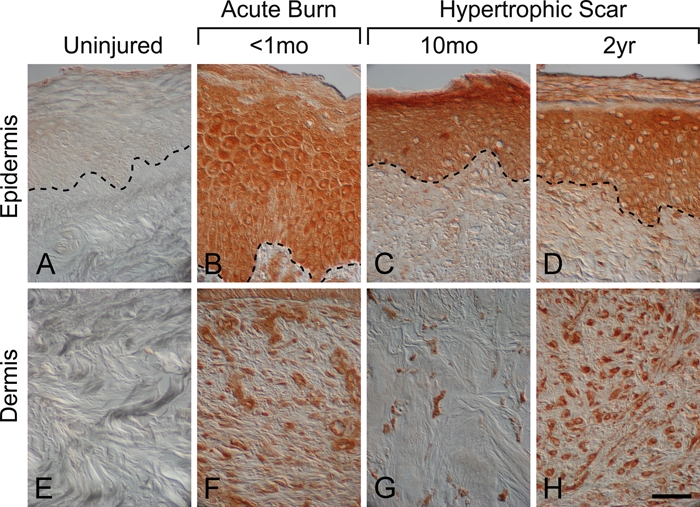
α–Melanocyte-stimulating hormone (α-MSH) localization in human uninjured skin, early burn wounds, and hypertrophic scars. α-MSH immunostaining was absent in uninjured skin (A, E). In contrast, α-MSH immunostaining was robust throughout the epidermis in an early burn wound (<1 month postinjury), (B) a hypertrophic scar 10 months after injury (C), and a hypertrophic scar 2 years postinjury (D). α-MSH immunostaining was also robust in the dermis after cutaneous injury and was localized to dermal fibroblasts in an early burn wound (<1 month post-injury) (F), a hypertrophic scar 10 months after injury (G), and a hypertrophic scar 2 years postinjury (H). Magnification bar = 50 µm.
Discussion
The role of melanocortin 1 receptor and its ligand, α-MSH, in cutaneous wound repair remains to be determined despite evidence implicating α-MSH in the regulation of both inflammation and fibroproliferation (Bohm et al. 2006; Brzoska et al. 2008), known responses to cutaneous injury. In this study, we report the localization of MC1R and α-MSH to inflammatory cells and epidermal keratinocytes in murine cutaneous wounds and to epidermal keratinocytes and dermal fibroblasts in human acute burns and hypertrophic scars. Furthermore, the expression patterns for both MC1r and α-MSH were regulated temporally during wound repair, suggesting contribution to specific responses to cutaneous injury.
The detection of MC1R and α-MSH proteins in inflammatory cells in murine cutaneous wounds was consistent with previous studies reporting expression by macrophages (Star et al. 1995) and neutrophils (Catania et al. 1996). Interestingly, MC1R and α-MSH were localized to a specific subpopulation of inflammatory cells in day 3 murine wounds; further investigation is necessary to identify whether these MC1R/α-MSH-positive cells are macrophages or neutrophils. More work is also needed to determine whether the localization of MC1R and α-MSH to inflammatory cells occurs in human cutaneous wounds during the acute inflammatory response to injury. The functional consequence of MC1R and α-MSH localization to inflammatory cells remains unknown. However, the late timing of the increased expression raises the possibility that MC1R/α-MSH signaling limits or resolves the inflammatory response to cutaneous injury given that numerous in vivo studies have demonstrated that α-MSH exerts anti-inflammatory effects (Brzoska et al. 2008).
Localization of MC1R and α-MSH to epidermal keratinocytes in the healing wound suggests a role in epithelialization, a process requiring keratinocyte proliferation and migration over the injured dermis (Gurtner et al. 2008). In the C57BL mouse, MC1R and α-MSH immunostaining was detected in a specific subset of suprabasal keratinocytes at the leading edge of the migrating epidermal tongue. In humans, robust immunostaining for both MC1R and α-MSH was also observed in the epidermis after cutaneous injury. Further evidence implicating MC1R/α-MSH signaling in epithelialization is a study reporting that treatment of corneal epithelial wounds with a tripeptide derived from α-MSH resulted in accelerated epithelialization (Bonfiglio et al. 2006).
In human burn wounds and hypertrophic scars, MC1R and α-MSH immunostaining was also detected in dermal fibroblasts, known mediators of fibroproliferation during cutaneous wound repair. Fibroproliferative responses to cutaneous injury include wound contraction, extracellular matrix deposition, and remodeling (Martin 1997; Singer and Clark 1999). Importantly, wound fibroblasts are a source of myofibroblasts, which are key contributors to both normal and hypertrophic scar formation (Desmouliere et al. 2005). During normal wound repair, the secretory and contractile myofibroblast undergoes apoptosis after wound contraction and epithelialization are complete; in contrast, myofibroblasts persist in human hypertrophic scars (Ehrlich et al. 1994). It remains to be determined whether the dermal fibroblasts expressing MC1R and α-MSH in the hypertrophic scar are myofibroblasts. Nonetheless, there is both in vitro and in vivo evidence that α-MSH inhibits fibroproliferation in the skin. α-MSH suppresses TGF-β1-induced collagen synthesis and secretion by human dermal fibroblasts in vitro and reduces TGF-β1-induced cutaneous fibrosis in vivo (Bohm et al. 2004). Further confirmation that α-MSH has anti-fibroproliferative activity is provided by a recent study demonstrating that α-MSH also reduces cutaneous fibrosis in a mouse model of scleroderma (Kokot et al. 2009). Despite these intriguing data, it remains to be determined whether MC1R/α-MSH signaling has a role in limiting fibroproliferative responses to cutaneous injury. Also unclear is the role of MC1R/α-MSH signaling in the pathophysiology of hypertrophic scar formation.
In uninjured skin, MC1R and α-MSH immunostaining was absent in the interfollicular epidermis. This observation in mouse, however, may be dependent on hair cycle given that MC1R has been reported to be detected throughout the interfollicular epidermis during hair follicle morphogenesis (Botchkarev et al. 1999). Similarly, α-MSH protein localization to the epidermis of murine uninjured skin may also be regulated by hair follicle cycle (Mazurkiewicz et al. 2000). Indeed, this dependence on hair follicle cycle may explain the observed differences in α-MSH immunostaining of the epidermis between the different mouse strains. In human uninjured skin, the lack of MC1R protein in the epidermis was consistent with a previous study (Bohm et al. 1999); however, our data on α-MSH in uninjured human skin contrasted with a report that α-MSH localized to suprabasal keratinocytes in human uninjured skin (Chakraborty et al. 1999). Resolution of this contradiction requires an increase in sample size with attempts to match age, gender, and ethnicity. Curiously, melanocytes were consistently negative for both MC1R and α-MSH; this may be because of the low levels of these proteins, which were below our level of detection in murine skin, or, in the case of human tissue, where the color of brown/black melanocytes could not be discerned from the brown diaminobenzidene, the chromagen used for visualization of immunostaining.
Despite significant overlap, there were differences in the spatial and temporal localization patterns for MC1R and α-MSH. In uninjured murine skin, only MC1R protein was detected in the panniculus carnosus, which suggests that α-MSH is not the primary ligand for MC1R in this muscle layer. In murine skin, only α-MSH immunostaining was present in sebaceous glands, whereas both MC1R and α-MSH were localized to human sebaceous glands. Our observation of MC1R protein in human sebaceous glands is consistent with a previous report examining MC1R expression in uninjured human skin (Bohm et al. 1999). This difference between species suggests that α-MSH signaling is independent of the melanocortin 1 receptor in murine sebaceous glands. Also of interest is the difference in MC1R and α-MSH localization pattern in human hypertrophic scars 2 years after injury. In this tissue section, MC1R localization was restricted to hair follicles and sebaceous glands, whereas α-MSH immunostaining was present in epidermal keratinocytes, dermal fibroblasts, and blood vessels in addition to hair follicles and sebaceous glands. Again, these data suggest that α-MSH may signal independent of MC1R in the hypertrophic scar.
An important caveat for MC1R localization studies is that the MC1R detected by immunostaining may not be functional given the prevalence of loss-of-function mutations in humans and other mammals (Rees 2003; Sturm 2009). Such mutations are prevalent because the coding region of the MC1R gene is rich in single-nucleotide polymorphisms, many of which cause a single amino acid substitution, resulting in either decreased ligand-receptor binding or decreased activation of adenylyl cyclase (Beaumont et al. 2005; Ringholm et al. 2004; Sanchez-Laorden et al. 2006). The other caveat for this study is that the human burn wounds and hypertrophic scars were not matched for age, gender, or site of injury because of the limited size of our tissue repository. Further investigation is needed to determine whether these factors influence MC1R and α-MSH localization during wound repair after burn injury in humans.
In conclusion, MC1R and its ligand α-MSH appear to contribute to cutaneous responses to injury. Neither protein is ubiquitously or uniformly expressed during cutaneous wound repair. Rather distinct subpopulations of cells express these proteins at specific time points, suggesting complex and distinct roles in the inflammatory and fibroproliferative responses to cutaneous injury.
Acknowledgments
Special thanks to Marcia Usui, Robert Underwood, and Dr. John Olerud in the Department of Dermatology at the University of Washington for providing the uninjured human skin sections.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the authorship and/or publication of this article.
The author(s) disclosed receipt of the following financial support for the research and/or authorship of this article: This work was funded by the Washington State Firefighters Burn Foundation, the National Institutes of Health American Recovery and Reinvestment Act (Grant R01GM056483-11), and in part by the National Institute on Disability and Rehabilitation Research/Office of Special Education and Rehabilitation Services/US Department of Education (Grant H133A070047).
References
- Beaumont KA, Newton RA, Smit DJ, Leonard JH, Stow JL, Sturm RA. 2005. Altered cell surface expression of human MC1R variant receptor alleles associated with red hair and skin cancer risk. Hum Mol Genet. 14:2145–2154 [DOI] [PubMed] [Google Scholar]
- Bhardwaj RS, Schwarz A, Becher E, Mahnke K, Aragane Y, Schwarz T, Luger TA. 1996. Pro-opiomelanocortin-derived peptides induce IL-10 production in human monocytes. J Immunol. 156:2517–2521 [PubMed] [Google Scholar]
- Bohm M, Luger TA, Tobin DJ, Garcia-Borron JC. 2006. Melanocortin receptor ligands: new horizons for skin biology and clinical dermatology. J Invest Dermatol. 126:1966–1975 [DOI] [PubMed] [Google Scholar]
- Bohm M, Metze D, Schulte U, Becher E, Luger TA, Brzoska T. 1999. Detection of melanocortin-1 receptor antigenicity on human skin cells in culture and in situ. Exp Dermatol. 8:453–461 [DOI] [PubMed] [Google Scholar]
- Bohm M, Raghunath M, Sunderkotter C, Schiller M, Stander S, Brzoska T, Cauvet T, Schioth HB, Schwarz T, Luger TA. 2004. Collagen metabolism is a novel target of the neuropeptide alpha-melanocyte-stimulating hormone. J Biol Chem. 279:6959–6966 [DOI] [PubMed] [Google Scholar]
- Bonfiglio V, Camillieri G, Avitabile T, Leggio GM, Drago F. 2006. Effects of the COOH-terminal tripeptide alpha-MSH(11–13) on corneal epithelial wound healing: role of nitric oxide. Exp Eye Res. 83:1366–1372 [DOI] [PubMed] [Google Scholar]
- Botchkarev VA, Botchkareva NV, Slominski A, Roloff B, Luger T, Paus R. 1999. Developmentally regulated expression of alpha-MSH and MC-1 receptor in C57BL/6 mouse skin suggests functions beyond pigmentation. Ann N Y Acad Sci. 885:433–439 [DOI] [PubMed] [Google Scholar]
- Brzoska T, Luger TA, Maaser C, Abels C, Bohm M. 2008. Alpha-Melanocyte-stimulating hormone and related tripeptides: biochemistry, antiinflammatory and protective effects in vitro and in vivo, and future perspectives for the treatment of immune-mediated inflammatory diseases. Endocr Rev. 29:581–602 [DOI] [PubMed] [Google Scholar]
- Catania A, Rajora N, Capsoni F, Minonzio F, Star RA, Lipton JM. 1996. The neuropeptide alpha-MSH has specific receptors on neutrophils and reduces chemotaxis in vitro. Peptides. 17:675–679 [DOI] [PubMed] [Google Scholar]
- Chakraborty AK, Funasaka Y, Pawelek JM, Nagahama M, Ito A, Ichihashi M. 1999. Enhanced expression of melanocortin-1 receptor (MC1-R) in normal human keratinocytes during differentiation: evidence for increased expression of POMC peptides near suprabasal layer of epidermis. J Invest Dermatol. 112:853–860 [DOI] [PubMed] [Google Scholar]
- Desmouliere A, Chaponnier C, Gabbiani G. 2005. Tissue repair, contraction, and the myofibroblast. Wound Repair Regen. 13:7–12 [DOI] [PubMed] [Google Scholar]
- Ehrlich HP, Desmouliere A, Diegelmann RF, Cohen IK, Compton CC, Garner WL, Kapanci Y, Gabbiani G. 1994. Morphological and immunochemical differences between keloid and hypertrophic scar. Am J Pathol. 145:105–113 [PMC free article] [PubMed] [Google Scholar]
- Gurtner GC, Werner S, Barrandon Y, Longaker MT. 2008. Wound repair and regeneration. Nature. 453:314–321 [DOI] [PubMed] [Google Scholar]
- Harunari N, Zhu KQ, Armendariz RT, Deubner H, Muangman P, Carrougher GJ, Isik FF, Gibran NS, Engrav LH. 2006. Histology of the thick scar on the female, red Duroc pig: final similarities to human hypertrophic scar. Burns. 32:669–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill RP, MacNeil S, Haycock JW. 2006. Melanocyte stimulating hormone peptides inhibit TNF-alpha signaling in human dermal fibroblast cells. Peptides. 27:421–430 [DOI] [PubMed] [Google Scholar]
- Kokot A, Sindrilaru A, Schiller M, Sunderkotter C, Kerkhoff C, Eckes B, Scharffetter-Kochanek K, Luger TA, Bohm M. 2009. Alpha-melanocyte-stimulating hormone suppresses bleomycin-induced collagen synthesis and reduces tissue fibrosis in a mouse model of scleroderma: melanocortin peptides as a novel treatment strategy for scleroderma? Arthritis Rheum. 60:592–603 [DOI] [PubMed] [Google Scholar]
- Lin JY, Fisher DE. 2007. Melanocyte biology and skin pigmentation. Nature. 445:843–850 [DOI] [PubMed] [Google Scholar]
- Mandrika I, Muceniece R, Wikberg JE. 2001. Effects of melanocortin peptides on lipopolysaccharide/interferon-gamma-induced NF-kappaB DNA binding and nitric oxide production in macrophage-like RAW 264.7 cells: evidence for dual mechanisms of action. Biochem Pharmacol. 61:613–621 [DOI] [PubMed] [Google Scholar]
- Martin P. 1997. Wound healing—aiming for perfect skin regeneration. Science. 276:75–81 [DOI] [PubMed] [Google Scholar]
- Mazurkiewicz JE, Corliss D, Slominski A. 2000. Spatiotemporal expression, distribution, and processing of POMC and POMC-derived peptides in murine skin. J Histochem Cytochem. 48:905–914 [DOI] [PubMed] [Google Scholar]
- Muangman P, Muffley LA, Anthony JP, Spenny ML, Underwood RA, Olerud JE, Gibran NS. 2004. Nerve growth factor accelerates wound healing in diabetic mice. Wound Repair Regen. 12:44–52 [DOI] [PubMed] [Google Scholar]
- Olerud JE, Chiu DS, Usui ML, Gibran NS, Ansel JC. 1998. Protein gene product 9.5 is expressed by fibroblasts in human cutaneous wounds. J Invest Dermatol. 111:565–572 [DOI] [PubMed] [Google Scholar]
- Redondo P, Garcia-Foncillas J, Okroujnov I, Bandres E. 1998. Alpha-MSH regulates interleukin-10 expression by human keratinocytes. Arch Dermatol Res. 290:425–428 [DOI] [PubMed] [Google Scholar]
- Rees JL. 2003. Genetics of hair and skin color. Annu Rev Genet. 37:67–90 [DOI] [PubMed] [Google Scholar]
- Ringholm A, Klovins J, Rudzish R, Phillips S, Rees JL, Schioth HB. 2004. Pharmacological characterization of loss of function mutations of the human melanocortin 1 receptor that are associated with red hair. J Invest Dermatol. 123:917–923 [DOI] [PubMed] [Google Scholar]
- Sanchez-Laorden BL, Sanchez-Mas J, Turpin MC, Garcia-Borron JC, Jimenez-Cervantes C. 2006. Variant amino acids in different domains of the human melanocortin 1 receptor impair cell surface expression. Cell Mol Biol (Noisy-le-grand). 52:39–46 [PubMed] [Google Scholar]
- Scott JR, Muangman PR, Tamura RN, Zhu KQ, Liang Z, Anthony J, Engrav LH, Gibran NS. 2005. Substance P levels and neutral endopeptidase activity in acute burn wounds and hypertrophic scar. Plast Reconstr Surg. 115:1095–1102 [DOI] [PubMed] [Google Scholar]
- Singer AJ, Clark RA. 1999. Cutaneous wound healing. N Engl J Med. 341:738–746 [DOI] [PubMed] [Google Scholar]
- Spenny ML, Muangman P, Sullivan SR, Bunnett NW, Ansel JC, Olerud JE, Gibran NS. 2002. Neutral endopeptidase inhibition in diabetic wound repair. Wound Repair Regen. 10:295–301 [DOI] [PubMed] [Google Scholar]
- Star RA, Rajora N, Huang J, Stock RC, Catania A, Lipton JM. 1995. Evidence of autocrine modulation of macrophage nitric oxide synthase by alpha-melanocyte-stimulating hormone. Proc Natl Acad Sci U S A. 92:8016–8020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm RA. 2009. Molecular genetics of human pigmentation diversity. Hum Mol Genet. 18:R9–R17 [DOI] [PubMed] [Google Scholar]
- Sullivan SR, Underwood RA, Gibran NS, Sigle RO, Usui ML, Carter WG, Olerud JE. 2004. Validation of a model for the study of multiple wounds in the diabetic mouse (db/db). Plast Reconstr Surg. 113:953–960 [DOI] [PubMed] [Google Scholar]
- Suzuki I, Cone RD, Im S, Nordlund J, Abdel-Malek ZA. 1996. Binding of melanotropic hormones to the melanocortin receptor MC1R on human melanocytes stimulates proliferation and melanogenesis. Endocrinology. 137:1627–1633 [DOI] [PubMed] [Google Scholar]
- Tsatmali M, Graham A, Szatkowski D, Ancans J, Manning P, McNeil CJ, Graham AM, Thody AJ. 2000. Alpha-melanocyte-stimulating hormone modulates nitric oxide production in melanocytes. J Invest Dermatol. 114:520–526 [DOI] [PubMed] [Google Scholar]


