Abstract
Monocytes are involved in a wide range of physiological and pathological processes, many of which are studied in mouse models. Current protocols to isolate murine monocytes are few and result in unsatisfactory cell yield and purity. Here, we describe a novel approach to efficiently differentiate large numbers of mature inflammatory monocytes from heterogeneous bone marrow cell suspensions. Bone marrow cell suspensions were isolated by flushing femurs and tibias from Balb/c and C57Bl/6 mice, supplemented with macrophage colony–stimulating factor (M-CSF), and were cultured on ultra-low attachment surfaces to inhibit adherence-mediated maturation. Cells were harvested at indicated time points, underwent time-line analysis of the differentiation processes, and were subsequently extensively phenotyped to verify their monocytotic properties. In order to confirm downstream compatibility, we tested for typical monocyte behavior. Our protocol yielded 24 ± 6 × 106 differentiated cells per donor mouse, 10-fold higher than yields obtained using previously described peripheral blood isolation methods. Differentiated cells consisted of approximately 47% ± 12% monocytes, the rest being mature macrophages. We increased monocyte purity to 86% ± 6% by depleting adherent macrophages. Our findings indicate that bone marrow–derived monocytes (BMDMs) are an attractive tool to study, for example, the innate and adaptive immune system, atherosclerosis, and cellular migration during infection. Moreover, BMDM transplantation could be used to test novel, therapeutic in vivo approaches in mice disease models.
Keywords: mouse, monocyte, isolation protocol, bone marrow, cell differentiation, macrophage colony–stimulating factor (M-CSF), pan–macrophage marker F4/80
Monocytes, an important subset of leukocytes, contribute to a wide range of physiological and pathological processes including innate and adaptive immune system functions, tissue remodeling and repair, and vessel growth by angiogenesis and arteriogenesis. Their role in the inflammatory component of atherosclerosis is of particular interest (Girn et al. 2007; Libby et al. 1996). Although investigations of many of these processes rely on wild-type/transgenic mouse strains, the isolation of murine monocytes from peripheral blood remains impractical due to the large numbers of donor mice required to obtain significant blood volume and cell yield. Classic murine monocyte enrichment approaches make use of density gradient centrifugation (Berthold 1981) with subsequent fluorescence-activated cell sorting (FACS) (Heil et al. 2002) or multistep magnetic-activated cell sorting (MACS) (Houthuys et al. 2010; Zhu et al. 2007). Unfortunately, in addition to being difficult, such isolation methods can alter the biological activity of the cells (Auffray et al. 2009; Seeger et al. 2007), a major problem for downstream applications and experimental reproducibility. The difficulty of obtaining pure monocyte cultures greatly impedes progress in many research fields.
Here, we aim to overcome these obstacles by describing both a novel time- and cost-efficient approach for isolating large amounts of murine monocytes from native murine bone marrow suspensions cultured on ultra-low attachment surfaces in the presence of the murine monocytes/macrophage colony–stimulating factor (M-CSF). We further confirm that these cells display the appropriate phenotypic and functional properties, particularly with regard to downstream compatibility and their complex role in vivo.
Materials and Methods
Isolation and Differentiation of Murine Bone Marrow–Derived Monocytes
Bone marrow cell suspensions were isolated by flushing femurs and tibias of 8- to 12-week-old Balb/c and C57Bl/6 mice (Charles River; Sulzfeld, Germany) with complete RPMI1640 (+10% FCS, +1% Pen/Strep) (PAA; Pasching, Austria). Aggregates were dislodged by gentle pipetting, and debris was removed by passaging the suspension through a 70-µm nylon web. Cells were washed twice with medium, adjusted to give a suspension of 106 cells/mL, and seeded on 6-well ultra-low attachment surface plates (Corning Costar; Schiphol-Rijk, the Netherlands). Cells were supplemented with 20 ng/mL rmM-CSF (Strathman/Miltenyi; Bergisch Gladbach, Germany) and cultured in a humidified incubator at 37C and 5% CO2. They were harvested at indicated time points by gentle pipetting and repeated washing of the wells with phosphate buffered saline (PBS), 0.5% bovine serum albumin (BSA), and 2 mM EDTA to detach adherent cells. Nonadherent cells were harvested by using EDTA-free PBS with 0.5% BSA. Furthermore, when indicated, cell samples were depleted of CD117+ stem cells by MACS technology (Miltenyi; Bergisch Gladbach, Germany) according to standard protocols provided by the manufacturer.
This investigation conforms to the Guide for the Care and Use of Laboratory Animals published by the United States National Institutes of Health (NIH Publication No. 85-23, revised 1996). Animal studies were approved by the ethics committee of the regional administrative authority in Dresden (Germany).
FACS Analysis
Cell suspensions were harvested by gentle pipetting and EDTA-mediated detachment and stained with CD4, CD8a, CD11b, CD11c, CD25, CD45, CD45R, CD62L, CD80, CD86, CD115, Gr-1, CD154, MHCII, Ly6-C (eBioscience; San Diego, CA), F4/80, Ly6G, CD192 (AbD Serotec; Duesseldorf, Germany), or CD117 (Miltenyi/Biotec; Bergisch Gladbach, Germany) antibodies. Appropriate unspecific isotypes were used as controls (eBioscience).
Transdifferentiation of Monocytes into Dendritic Cells
Nonadherent M-CSF differentiated bone marrow–derived cells were harvested on day five of culture by gentle pipetting and were subsequently subjected to MACS-mediated depletion of CD117+ stem and progenitor cells. Cell suspensions harvested under these conditions were verified to consist of 86% ± 6% mature monocytes by means of FACS analysis (maturity predominantly estimated based on CD115 und F4/80 expression patterns). They were reseeded on ultra-low attachment cell culture plates in complete RPMI1640 medium supplemented with 20 ng/mL rmGM-CSF and 20 ng/mL rmIL4 (Miltenyi). Controls remained supplemented with rmM-CSF and were treated as adherent cells because further stimulation with M-CSF led to the appearance of strongly adherent macrophages.
Static and Dynamic Adhesion on Endothelial Cells
Interaction between bone marrow–derived monocytes and endothelial cells was analyzed using adhesion assays under both static and dynamic conditions. Endothelial cells were cultured on gelatin-coated surfaces in DMEM high glucose medium supplemented with 10% FCS, 1% Pen/Strep, 1% nonessential amino acids, 1 mM sodium pyruvate, and 0.4% mercaptoethanol (PAA). Immortalized endothelioma cell lines have previously been described to preserve their typical endothelial behavior (Reiss and Engelhardt 1999; Risau et al. 1990; Rohnelt et al. 1997; Wagner and Risau 1994).
Static adhesion experiments were conducted in 24-well plates. Mature endothelioma cells were preincubated with 0, 1, 5, or 10 ng/mL TNFα (Sigma Aldrich; Hamburg, Germany) over four hours. Bone marrow–derived monocytes (BMDMs) were stained with Vybrant DiI (Invitrogen; Karlsruhe, Germany) and harvested by gentle pipetting. 5 ×105 monocytes were seeded on top of intact monolayers in serum-free RPMI1640 medium. Assays were transferred back into the incubator for 90 minutes to allow cell adhesion. Nonadherent cells were depleted by repeated washing. Assays were analyzed with an AxiovertS100 microscope (Carl Zeiss; Jena, Germany) equipped with appropriate filter sets and a RT/SE camera system (Diagnostic Instruments; Sterling Heights, MI). Representative images were taken using SpotAdvanced (Diagnostic Instruments; Sterling Heights, MI) and analyzed using MetaMorph (Molecular Devices; Sunnyvale, CA) by counting adherent DiI-positive cells for seven fields of view (fov). When indicated, monocytes were preincubated with 20 µg/mL functional grade anti-CD11b antibodies (clone M1/70) (eBioscience) to block CD11b/ICAM-1 interaction.
Dynamic adhesion and rolling assays were performed by seeding endothelioma cells in flow chambers (ibidi; Munich, Germany). Shear stress was created by a peristaltic pump aligned in an infusion tubing system. Flow was laminarized by inserting a pressurized tank. Endothelial cells were stimulated with 8 dyn/cm2 for six hours in an incubator and transferred onto the microscope stage. 5 × 105 DiI+ monocytes were homogeneously infused into the system. A time stack of rolling monocytes was recorded over a 10-minute perfusion time. Flow chambers were carefully flushed to remove nonadherent cells, 10 representative images of adherent cells were taken for statistical analysis, and the chamber was transferred back into the incubator for 10 minutes to allow further adherence. The perfusion system was flushed repeatedly and reconnected with the flow chamber. Another time stack was recorded under increasing shear stress in order to monitor the dynamic force detachment of BMDMs. Time-stack videos were analyzed using the motion tracker in ImagePro (Media Cybernetics; Bethesda, MD). Data were exported into Excel (Microsoft; Redmond, WA) and particles classified as being adherent, rolling, or streaming based on their velocity.
Results
Differentiation of Bone Marrow–Derived Monocytes
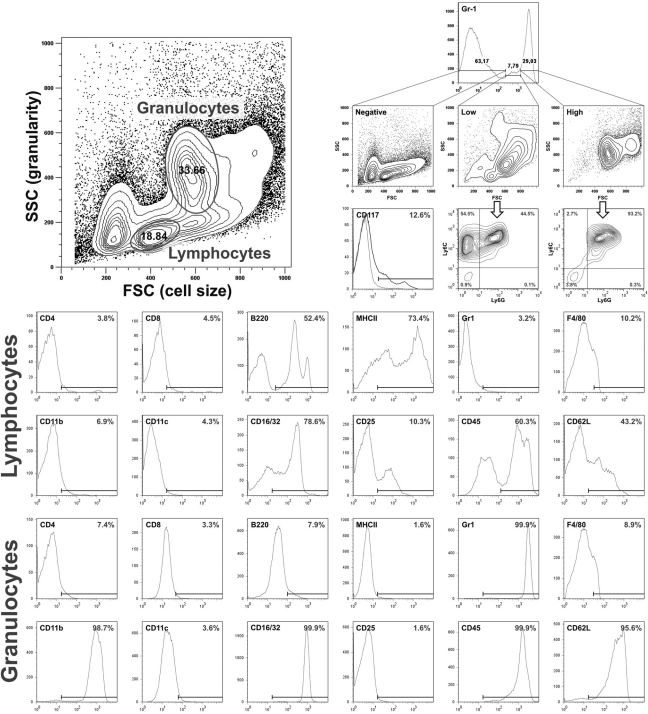
We prepared bone marrow suspensions by flushing femurs of adult mice. FACS-based analysis was used to verify the quality of the isolations and characterize their composition. The cell yield obtained from native bone marrow isolations reached 52 ± 8 × 106 cells per donor mouse (n = 5). The cell suspensions consisted of several distinct populations, including erythroid cells, B-lymphocytes, granulocytes, monocytes, and a wide range of progenitor and stem cells (Fig. 1, representative FACS data for five individual experiments). Most murine progenitor and stem cell lines are characterized by expression of the stem cell factor receptor c-kit and CD117. There were 12% ± 4% of all cells that were CD117+ (Fig. 1). Lymphocytes and granulocytes were identified based on their forward-/side-scatter properties and their gates subsequently analyzed for lineage- specific markers. Lymphocytes were negative for the T-cell markers CD4 and CD8 and granulocyte markers CD11b and Gr-1 but were partially positive for the B-cell markers B220 and MHCII. The lymphocyte content of native suspensions was estimated to be 14% ± 5% based on B220 expression levels (Fig. 1). Granulocytes were 100% positive for the typical markers CD11b and Gr-1 but negative for lymphocyte markers CD4, CD8, and B220, suggesting a homogenous population.
Figure 1.
FACS phenotyping of native bone marrow cell suspensions. Native bone marrow cells were isolated and subsequently analyzed for lymphocyte and granulocyte markers. The indicated bars represent the positive population; gating was performed according to negative isotype-matched controls (gray histograms in some graphs). The white arrow marks a CD115 (M-CSF receptor)–positive subpopulation that represents monocytes within the native cell suspension (25%–30% of cells gated as granulocytes or 5%–10% of all cells, respectively). FACS data are representative for five individual experiments.
Due to the predominant role of M-CSF in the development of the monocytes/macrophage phagocytotic system (MPS), its receptor tyrosine kinase CD115 (colony-stimulating factor-1/MCSF receptor) is used to identify all cells assigned to the MPS in the murine system, including its dedicated progenitors, circulating monocytes, and macrophages (Rohrschneider et al. 1997). The analysis of CD115 expression within the granulocyte population indicated an overlapping monocyte population that was further distinguished by lower expression of the myeloid marker Gr-1 (data not shown). Because Gr-1 (clone RB6-8C5) has been reported to react with both Ly6G on granulocytes and Ly6C on both monocytes and granulocytes (Gordon and Taylor 2005; Hestdal et al. 1991; Taylor et al. 2003), we analyzed Ly6C and Ly6G expression on Gr-1high cells and found 95% ± 3% co-expression of Ly6G and Ly6C, while Gr-1low cells merely showed 45.3% ± 3% co-expression and a second population of Ly6C+Ly6Gneg cells, matching the pattern described for monocytes. Based on this, we estimated an initial granulocyte population of approximately 30% ± 7% and an initial monocyte population of 8% ± 3%, representing only a small population in the suspension (Fig. 1). Having defined the native population, we cultured these cells without further purification or manipulation in suspension on ultra-low attachment plates in the presence of M-CSF.
In order to verify successful monocyte differentiation, M-CSF–supplemented cultures were subjected to longitudinal FACS analysis for general lineage- and monocyte/ macrophage-specific marker expression (n = 5) (Fig. 2). After five days in culture, 94% ± 7% of all cells expressed CD45, indicating that the culture consisted almost entirely of leukocyte lineage cells. Given that CD11b expression steadily increased, these cells were thought to belong to the granulocyte or MPS lineage. Gr-1 expression peaked on day three and subsequently declined, thus increasing the amount of CD11b+Gr-1- cells. This indicates a growing population of cells other than granulocytes. It is also noteworthy that CD11b+Gr1+ cells did not show granulocyte-specific side-scatter properties. Detection of the MPS marker CD115 in Gr-1-/low cells led us to assign them to the monocyte/macrophage lineage, confirming the success of our cell enrichment approach. Differential analysis of Ly6C/G revealed Gr1-positive cells to be Ly6C+Ly6Gneg (data now shown).
Figure 2.
Longitudinal FACS phenotyping of M-CSF–supplemented bone marrow cell suspensions (n = 5). Bone marrow preparations were seeded in the presence of M-CSF and cultured for indicated periods prior to FACS phenotyping.
In addition to CD115, one of the most important antigens used to identify murine macrophages is the F4/80 pan– macrophage marker (Austyn and Gordon 1981; Hirsch et al. 1981). In contrast to CD115, its differential expression during maturation of MPS lineage cells enables the identification of weakly F4/80-positive monocytes and highly F4/80-positive mature macrophages. F4/80 expression profiles are routinely used to estimate the maturation of cells undergoing MPS differentiation (Gordon and Taylor 2005). Differential analysis of F4/80 expression revealed a peak of F4/80 low level–positive cells on day five of culture (47% ± 10%), indicating an accumulation of monocytes. The proportion of highly F4/80-positive cells increased consecutively, suggesting an increasing population of macrophages (31% ± 9% after five days and 70% ± 10% after seven days in culture) accompanied by a loss of the myeloid marker Gr-1 and CD62L (see below). This was verified by the accumulation of strongly adherent cells observed via phase contrast microscopy. Noteworthy, the transient peak of Gr-1 expression preceded the peak of low F4/80 expression, suggesting that Gr-1 expression occurs early during MPS development. The yield of differentiated monocytes and macrophages reached 24 ± 6 × 106 cells per donor mouse when counted after five days in culture, 10-fold outnumbering the yield obtained from peripheral blood isolation (Berthold 1981; Heil et al. 2002; Houthuys et al. 2010; Zhu et al. 2007). Thus, our novel differentiation protocol appears to efficiently differentiate large numbers of mature monocytes.
Subphenotyping of Differentiated Monocytes
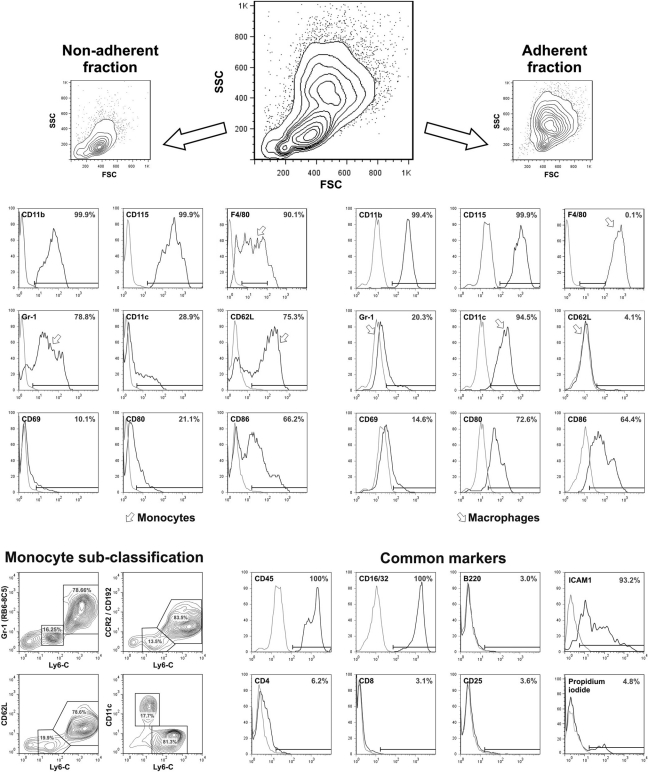
In a final simple step, monocyte purity was further increased by depleting strongly adherent macrophages from the differentiated cultures. By using EDTA-free wash buffer, we were able to selectively harvest the nonadherent cells. Adherent cells were detached in a second washing step using EDTA-containing buffers and less gentle pipetting. To verify the monocytotic features of the differentiated cell preparations, we conducted extensive FACS analysis (Fig. 3, representative FACS data for five individual experiments).
Figure 3.
FACS phenotyping of bone marrow–derived cells after five days of M-CSF–driven differentiation. Analysis of surface antigens for nonadherent and adherent populations. Cells were either harvested completely or separated based on their adhesion properties. Analysis of FSC/SSC properties and F4/80 revealed a significant higher amount of low F4/80-positive monocytes in the nonadherent fraction, while they were entirely absent in the fraction of adherent cells, which depicted strongly F4/80-positive macrophages. Note the expression of monocyte subpopulation–specific markers Gr-1 and CD62L and their downregulation during maturation into macrophages, while macrophage-specific markers F4/80 and CD11c are upregulated. FACS data are representative for five individual experiments. Arrowheads indicate monocyte- and macrophage-specific expression patterns.
All cells within the nonadherent fraction tested positive for the adhesion molecule CD11b, which is expressed by monocytes, macrophages, granulocytes, and natural killer cells. Both granulocytes and natural killer cells, however, are negative for the MPS lineage marker CD115, which we found expressed on all cells within the nonadherent fraction (99.9%). Analysis of the murine macrophage marker F4/80 provided further insight into the degree of maturation, as it is differentially expressed during monocyte maturation into macrophages. We found low F4/80 expression, suggesting that the population contains both monocytes (intermediate expression) and younger MPS lineage cells (F4/80 negative). Gr-1 expression was subclassified using a Ly6-C counterstain, which demonstrated 82% ± 6% Gr-1+Ly6Chigh and 16% ± 4% Gr-1low/negLy6Clow cells. Ly6C expression pattern revealed two distinctive subpopulations that were subjected to further analysis. We found Ly6-Chigh cells to co-express CCR2 (CD192) and CD62L, but not CD11c, which was expressed by Ly6Clow monocytes (Fig. 3, monocyte subclassification section). All subsets expressed low levels of the co-stimulatory molecules CD80 and CD86, both of which play an important role in antigen presentation and lymphocyte co-activation.
All cells within the adherent fraction tested positive for CD11b and CD115, indicating that they belong into the MPS lineage. However, compared to the nonadherent fraction, we found significantly higher expression levels of F4/80, as virtually all cells were gated far beyond the cutoff for F4/80low (99.9% F4/80high). High expression levels of F4/80 indicated a mature macrophage population. This was further supported by the loss of Gr-1 and CD62L expression. These cells also showed higher CD11c and CD80 expression, although the expression level of CD86 remained virtually unchanged (Fig. 3).
The native cell suspension harvested after five days was virtually entirely positive for the pan–leucocyte marker CD45 and the FC receptors CD16/32, indicating that they represent mature leucocytes. ICAM1 was highly expressed on both monocytes and macrophages. They were virtually negative for the lymphocyte markers CD4, CD8, CD25, and B220 (Fig. 3), underlining that they do not belong in the lymphocyte lineage. Cell viability was determined using propidium iodide staining of dead cells and reached >95% viable cells in both fractions (Fig. 3, common marker section).
Together, our results indicate that cell suspensions harvested after five days of culture with M-CSF consisted of approximately 47% ± 12% monocytes; the rest of the population were mature macrophages. By using EDTA-free wash buffer, we were able to selectively harvest the nonadherent cells after five days of differentiation. While 86% ± 6% of nonadherent cells were F4/80 low positive, 99.9% of the cells within the adherent portion expressed high levels of F4/80. This purification step resulted in a monocyte purity up to 90% and depleted macrophages as the major contamination of the culture. The final cell yield reached 11 ± 3 × 106 nonadherent cells per donor mouse. The cells of the nonadherent portion are from now on referred to as “bone marrow–derived monocytes” (BMDMs). BMDMs are classified as CD115+F4/80medGr-1low/neg/Ly6-C+ cells, while mature macrophages are referred to as CD115+F4/ 80highGr-1neg/Ly6-Cneg cells and are predominantly found in the adherent cell fraction. BMDM might be subclassified as Ly6ChighCCR2+CD62L+CD11cneg inflammatory monocytes (80%) and Ly6-ClowCCR2negCD62LnegC-D11c+ resident monocytes, concordant with the subtypes found in peripheral blood. Because both BMDM and macrophages are 99.9% CD11b and CD45 positive, these markers were ignored for plainness reasons.
Interferon Stimulation
MHCII expression is required for the presentation of exogenous antigens. Because monocytes and macrophages are important antigen-presenting cells (Randolph et al. 2008) and antigen presentation is controlled by interferon produced by lymphocytes, we investigated MHC expression in BMDM under interferon stimulation (IFN). Differentiated cell suspensions were stimulated with 8 ng/mL IFNγ for 24 hours before harvesting, and MHCII expression was quantified using FACS analysis. Monocytes and macrophages were gated according to the differential expression of F4/80. While unstimulated BMDM expressed different amounts of MHCII, ranging from none (38% ± 12% of all monocytes) to low (36% ± 4%) to high (23% ± 11%), unstimulated macrophages predominantly expressed low amounts of MHCII (75% ± 11% of all macrophages; n = 3) (Fig. 4). MHCII histogram profiles showed a significant right shift under IFN, indicating higher expression levels (Fig. 4). Strikingly, stimulated BMDM and macrophages showed identical MHCII expression patterns, both featuring approximately 60% MHCIIhigh cells.
Figure 4.
MHCII upregulation during IFN stimulation. Note the right shift of MHCII, ICAM1, CD80, and CD86 expression pattern, which suggest a strong upregulation (n = 3).
IFN-stimulated cells were subjected to extensive FACS phenotyping. We found that CD11c, CD45, CD62L, CD115, Gr-1, and F4/80 expression were not influenced by the stimulation. Expression of co-stimulatory CD80 and CD86, however, was slightly elevated, which might attenuate the activation of T-lymphocytes by IFN-prestimulated antigen presenting cells (APC). We also noticed a compelling upregulation of ICAM1 under interferon γ, while the expression of CD11b, VCAM, and CD62L adhesion molecules remained virtually unchanged. Apparently, stimulation influenced the activation state but showed no effect on monocyte differentiation and maturation. Together, these results demonstrate efficient MHC II and ICAM1 upregulation in response to IFN, suggesting that the differentiated cells are capable of antigen presentation and thereby can fulfill their role in the adaptive immune system in vivo.
LDL Uptake
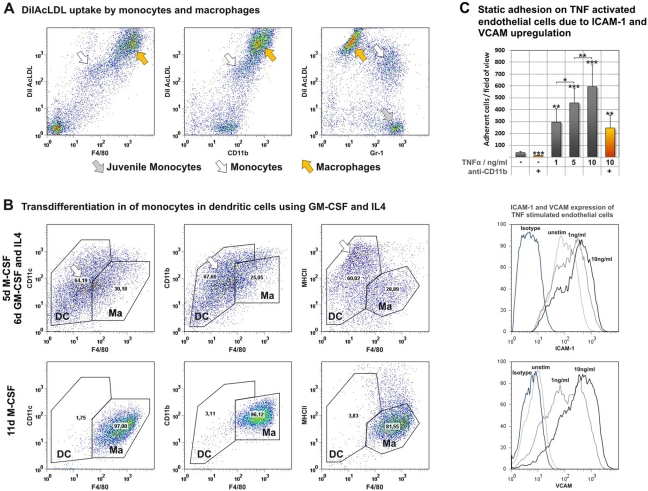
To verify the MPS-like biology of the differentiated cells, we next investigated functional behavior by assaying LDL uptake. The uptake of acetylated LDL is restricted to monocytes, macrophages, and endothelial cells (Brown et al. 1980; Brown et al. 1979; Chen et al. 2009; Wang et al. 2010). Monocytes were incubated with 5 µg/mL acLDL (Invitrogen; Darmstadt, Germany) stained with DiI fluorochromes for 24 hours after four days of M-CSF–driven differentiation before being subjected to FACS phenotyping. Phenotyping revealed a strongly DiIAcLDL-positive portion of CD11b+, F4/80high, and Gr-1- macrophages (Fig. 5A, black arrowheads), although there was also a portion of CD11b+F4/80lowGr-1+ cells consistent with mature monocytes (Fig. 5A, white arrowheads). Strikingly, we found a population of Gr-1–positive, DiIAcLDL-negative cells (Fig. 5A, gray arrowheads). Triple staining revealed these cells to be F4/80 negative, suggesting that they are juvenile monocytes. This assay verified the ability of differentiated monocytes and macrophages to take up acetylated LDL in concordance with peripheral blood monocyte behavior. Furthermore, during the maturation process of MPS progenitors, we found that F4/80 expression appears to correlate with the ability to take up acetylated LDL, highlighting the importance of Gr-1 and F4/80 as both phenotopic and functional maturation markers (Table 1).
Figure 5.
BMDMs were analyzed in vitro for monocyte-like biology. Uptake of acetylated LDL by mature monocytes and macrophages, but not immature myeloid cells (A). Transdifferentiation of monocytes by supplementation with GM-CSF and IL4 (B). Adhesion behavior on endothelial cells stimulated with TNFα and analysis of ICAM-1 and VCAM upregulation on endothelial cells undergoing TNFα stimulation (C).
Table 1.
Summary of Expression Patterns
| F4/80 | CD11b+ | CD115 | AcLDL | Gr1 | Ly6C | CCR2 | CD62L | CD11c | |
|---|---|---|---|---|---|---|---|---|---|
| Juvenile monocytes | − | + | + | − | + | ++ | + | − | − |
| Inflammatory monocytes | + | + | + | + | + | ++ | + | + | − |
| Resident monocytes | + | + | + | + | − | + | − | − | + |
| Macrophages | ++ | + | + | ++ | − | − | + | − | ++ |
Transdifferentiation of BMDM into Dendritic Cells
Monocytes stimulated with GM-CSF and IL4 have been described to differentiate into dendritic cells in vitro (Cody et al. 2005; Inaba et al. 1992; Leon et al. 2004; Reis et al. 2006). Although their abilities as antigen-presenting cells are fundamental to the adaptive immune system (Randolph et al. 2008), many aspects of this differentiation pathway remain unknown. To investigate the ability of bone marrow–derived monocytes to differentiate, cells subjected to our transdifferentiation protocol were harvested after a total of 11 days of cytokine stimulation (11d M-CSF vs 5d M-CSF + 6d GM-CSF/IL4) and characterized by FACS analysis for typical macrophage and dendritic cell markers. Special attention was paid to the F4/80 pan–macrophage antigen because its expression indicates mature MPS lineage cells. Macrophage gates were established based on FACS analysis of cells differentiated with M-CSF over 11 days. We detected strongly adherent F4/80highCD11b+CD11c+ macrophages with low to intermediate MHCII expression levels (Fig. 5B). Compared to this phenotype, the expression patterns of BMDMs cultured with GM-CSF and IL4 revealed a downregulation of F4/80 and an upregulation of CD11c and MHCII, depicting a new F4/80neg/lowCD11c+MHCII+ population that does not match the macrophage phenotype but does align to the patterns previously described for dendritic cells differentiated under similar in vitro conditions (Cody et al. 2005; Fogg et al. 2006).
These FACS findings are supported by phase contrast microscopy detection of nonadherent large cells that have the morphological properties of dendritic cells (Lutz et al. 1999). Our data indicate that monocytes isolated using our novel protocol may be useful to study dendritic cell differentiation processes in vitro.
Adhesion of BMDM on Endothelial Cells
Adhesion of circulating monocytes to endothelial cells primed by inflammatory stimuli is the first step in monocyte extravasation into inflamed tissues. Therefore, we assayed the interaction of BMDM with endothelial cells in vitro using static adhesion assays as well as dynamic rolling assays. Endothelial cells were prestimulated with TNFα, and adhesion molecule upregulation was verified by flow cytometry. ICAM-1 was constitutively expressed on endothelial cells and was only slightly upregulated during TNF stimulation. VCAM-1, however, was not expressed by unstimulated cells and was highly upregulated, even by low TNF concentrations (Fig. 5C). TNF stimulation increased the number of adhering BMDMs in a concentration-dependent manner (unstimulated: 40 ± 9 cells/fov; 1 ng/mL: 296 ± 112 cells/fov; p < 0.01; 5 ng/mL: 457 ± 120 cells/fov; p < 0.001; 10 ng/mL: 596 ± 183 cells/fov; p < 0.001) (Fig. 5C). Adhesion on both stimulated and unstimulated endothelial cells was partially blocked by preincubation of monocytes with functional grade anti-CD11b (clone M1/70) by 60% to 70%. The remaining adhesion was probably mediated through a CD62L/VCAM interaction.
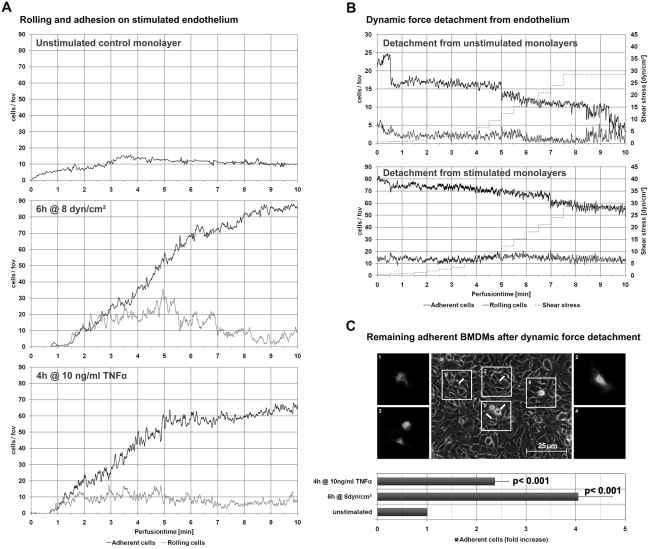
To further investigate adhesion behavior, we analyzed rolling and adhesion on flow-activated endothelium. Rolling experiments were conducted immediately after endothelial cells were stimulated with a laminar shear stress of 8 dyn/cm2 over six hours (Fig. 6A). TNF stimulation of endothelial cells was used as a positive control. Monocytes were virtually unable to role on unstimulated endothelial cells; only a few cells adhered. Dynamic detachment was able to dislodge most of the cells at high shear rates (Fig. 6B). Both TNF and flow stimulation enabled monocytes to role on endothelial monolayers, with increasing numbers of adherent cells observed over 10 minutes (TNF: 2.4- ± 0.3-fold increase, p < 0.0001; flow: 4- ± 0.7-fold increase, p < 0.0001; TNF vs flow, p < 0.05). Shear rates required for detachment were significantly higher, suggesting a stronger adhesion on the monolayer; only approximately 20% of all monocytes could be detached (Fig. 6B).
Figure 6.
BMDM adhesion on endothelial cells primed with laminar shear stress or TNFα (D). Remaining adherent BMDMs after dynamic force detachment were detected by staining and analyzed for their phase contrast behavior. Regions of interest (1–4) with corresponding DiI-positive BMDMs (2-fold magnification of the phase contrast region of interest). Bar = 25 µm.
Monolayers were analyzed for remaining adherent DiI-positive monocytes after dynamic force detachment. Identified by their appearance and behavior in phase contrast microscopy, these cells were found adhering to both the top and bottom of the endothelial cell monolayer. Most remaining adherent cells showed a stretched, phase dark phenotype, suggesting that they transmigrated underneath the monolayer (Fig. 6C), where they were prevented from detachment. Only a few phase light monocytes were observed; thus, not many cells remained adherent to the top of the monolayer (Fig. 6C).
These findings indicate that in vitro differentiated monocytes interact normally with endothelial cells in response to hemodynamic or inflammatory stimuli. It is thus possible that they can be used to study processes including monocyte adhesion and extravasation in vivo, that is, inflammation, wound healing, and vessel growth.
Discussion
Here, we describe the differentiation of murine monocytes from heterogenous bone marrow cell suspensions triggered by M-CSF supplementation. Strikingly, enrichment of the progenitors was unnecessary because the purity of cultures reached unprecedented 99% MPS cells, even without interfering with the native suspensions. Mature macrophages were the major source of contamination. Other cell lines present in our native suspensions, including erythroid cells, granulocytes, and lymphocytes, vanished from cultures driven into differentiation within two to three days, most likely due to the lack of specific growth factors. Cell viability after differentiation was determined to be >95%. Cellular debris from dead cells was not observed in flow cytometry, probably due to the presence of phagocytotic macrophages. Similar differentiation approaches to deplete undesired populations have been previously described to yield satisfactory results for dendritic cells (Lutz et al. 1999).
Using longitudinal FACS phenotyping, we established a timeline of conditions appropriate to accumulate mature monocytes five days after the onset of differentiation. Prolonged cultivation subsequently matured nonadherent monocytes into adherent macrophages. FACS phenotyping revealed major populations of the culture to be CD115+Ly6C+F4/80neg immature monocytes, CD115+Ly6C+ F4/80low mature monocytes, and CD115+Ly6CnegF4/80high macrophages, all of which were positive for other markers, including CD11b and CD45. In addition to the upregulation of the F4/80 maturation marker, we observed a loss of Gr-1 and CD62L expression associated with the differentiation of monocytes into macrophages. It should be noted that murine eosinophil granulocytes have been reported to be Gr-1+F4/80+ and therefore might be seen as potential contamination (McGarry and Stewart 1991). However, their presence is unlikely due to the co-expression of CD115 by BMDMs and macrophages, which is not found on eosinophiles.
The Gr-1 antibody clone RB6-8C5 has been previously described to react with members of the Ly-6 family of proteins, associated with the differentiation of granulocytes (Ly6G and Ly6C) and monocytes (Ly6C) (Fleming et al. 1993; Hestdal et al. 1991). Subanalysis of the RB6-8C5 reactivity revealed a co-expression of Gr-1 and Ly6-C on BMDM, while they were virtually negative for Ly6G, which was found on granulocytes present in the native suspension. Using stained acetylated LDL, we illustrated the importance of F4/80 and Gr-1 as MPS maturation markers, as the expression of F4/80 was associated with uptake of acetylated LDL. Using this assay, we also classified Gr-1 as an early myeloid marker in the BMDM development. We observed a broad concordance between the general expression patterns of BMDMs and those reported for circulating monocytes (Gordon and Taylor 2005; Lagasse and Weissman 1996; Strauss-Ayali et al. 2007). This supports the classification of our differentiated cells into the MPS lineage, a phenotype consistent with circulating monocytes. Recent reviews indicate the presence of distinctive monocyte subpopulations. However, their definition is inconsistent among different authors. While Gordon and Taylor (2005) and Geissmann et al. (2003) suggested a Gr1/Ly6C+CCR2+ inflammatory and Gr-1/Ly6CnegCCR2neg resident subset, more recent work by Robbins and Swirski (2010) and Strauss-Ayali et al. (2007) rather suggests a Gr1negLy6ClowCCR2low/neg resident population. Here, we found phenotypes that matched the ones described by Robbins and Swirski (2010): an inflammatory subset was Ly6-ChighCCR2+CD62L+CD11cneg (80% of all BMDMs); another showed lower, yet still positive Ly6C expression and was determined as Ly6-ClowCCR2negCD62L+CD11c+ resident monocyte subgroup (20% of all BMDMs). The expression of CCR2 is of special interest considering its importance in the recruitment of monocytes into inflamed tissues via the monocyte chemotactic protein 1 (MCP-1).
In addition to the phenotypic analogy to monocytes, we also aimed to verify functional requirements of the differentiated cells in downstream applications. MHCII expression by BMDM is attenuated during IFN. Unstimulated peripheral blood monocytes have been previously described to be MHCII- (Geissmann et al. 2010), although we found that BMDMs express low levels of MHCII, which is typically observed for more mature MPS cells, that is, macrophages. However, because these cells did express typical monocyte markers that are not present on mature macrophages, we concluded that the MHCII expression might be attributed to the differentiation progress driven by M-CSF, which might attenuate the monocyte phenotype. We also noticed a strong ICAM1 upregulation, while CD11b, CD62L, and VCAM expression remained unchanged. The findings from our IFN stimulation experiments are consistent with those previously described (Most et al. 1992; Tang et al. 2001). Furthermore, we verified the capability of BMDMs to differentiate into both macrophages and dendritic cells, revealing their ability to fulfill their role in cellular immunity.
An important aspect of monocyte homing is their rolling and adhesion behavior on endothelium primed by proinflammatory cytokines or hemodynamics. A key aspect of this recruitment is the upregulation of adhesion molecules on endothelial cells by proinflammatory cytokines. Consistent with previous findings, we observed a vast increase in monocyte adhesion on TNF and flow-stimulated endothelium (Bradfield et al. 2008). Using functional grade antibodies, we showed that in vitro adhesion mainly relies on CD11b/CD18–ICAM-1 interaction, also in agreement with previous findings (Ikuta et al. 1991). Our findings indicate that in vitro differentiated monocytes interact normally with endothelial cells in response to hemodynamic or inflammatory stimuli.
Compared to previous purification approaches, that is, using multistep magnetic labeling, the predominant advantage of our novel protocol appears to be the high cell yield that might be obtained from a single donor mouse. While Houthuys et al. (2010) were able to collect 1.4 × 106 cells from pooled peripheral blood of 15 donor mice, our protocol yields 11 ± 3 × 106 BMDM from the marrow of one single donor mouse. Besides the in vitro differentiation period of five days, the actual isolation protocol of harvesting, washing, and seeding the cells merely takes 30 minutes because no additional purification steps are necessary. Considering the economic efficiency, the absence of costly magnetic reagents and lower animal numbers are opposed by costs for growth factors and ultra-low attachment culture plates required in our setting. However, given the high cell yield, low growth factor concentrations, time efficiency, and ethical implications because of the lower demand in animals, our approach appears to be an equal alternative to classic purification methods, especially when downstream experiments require a large number of cells. Additionally, BMDMs remain completely unlabeled because the final enrichment step is based on the physical properties of these cells. This might be of importance for the downstream compatibility of the cells. Considering downstream experiments, we noticed that BMDMs harvested after five days of differentiation keep their phenotype up to three to four days when cultured in M-CSF–free medium on ultra-low attachment suspension culture but differentiate into macrophages within hours when seeded on normal culture surfaces by means of adherence-mediated maturation. These aspects should be considered in the experimental design, especially when pure monocyte behavior is of interest. However, this also applies to monocytes isolated from peripheral blood. Besides the effort that was taken to prove equal phenotype and behavior, BMDMs generated in vitro under growth factor stimulation might not equal peripheral blood isolations by classic FACS/MACS-based methods in some cases. Therefore, the usability of BMDMs should be considered for each individual application, including the deliberation of in vitro stimulation versus antibody labeling, requiring cell yield, purity, and economics.
In summary, we offer an easy-to-use protocol for the isolation of large numbers of murine monocytes from wild-type mice merely by cultivating heterogeneous bone marrow suspensions with M-CSF on ultra-low attachment culture plates. The use of transgenic knockout donor mice, that is, chemokine receptor– or adhesion molecule– deficient strains, might increase applicability of these cells. Based on our functional assays, we find that BMDMs possess all the properties necessary to perform the complex role of monocytes in vivo. This makes them an attractive tool for research on the innate and adaptive immune system, atherosclerosis, cellular migration during infection, and arteriogenesis, among others. Our novel approach of generating monocytes will greatly aid study of the basic biology of these cells.
Acknowledgments
The authors thank Prof. Dr. Med. Hans Schnittler (Institute of Physiology, Technical University of Dresden, Dresden, Germany) for the endothelioma cell line.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the authorship and publication of this article.
This research was funded by the special research field (SFB) 854 of the DFG (Deutsche Forschungsgemeinschaft [German Research Community]).
References
- Auffray C, Fogg DK, Narni-Mancinelli E, Senechal B, Trouillet C, Saederup N, Leemput J, Bigot K, Campisi L, Abitbol M, et al. 2009. CX3CR1+ CD115+ CD135+ common macrophage/DC precursors and the role of CX3CR1 in their response to inflammation. J Exp Med. 206:595–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austyn JM, Gordon S. 1981. F4/80, a monoclonal antibody directed specifically against the mouse macrophage. Eur J Immunol. 11:805–815 [DOI] [PubMed] [Google Scholar]
- Berthold F. 1981. Isolation of human monocytes by Ficoll density gradient centrifugation. Blut. 43:367–371 [DOI] [PubMed] [Google Scholar]
- Bradfield PF, Johnson-Leger CA, Zimmerli C, Imhof BA. 2008. LPS differentially regulates adhesion and transendothelial migration of human monocytes under static and flow conditions. Int Immunol. 20:247–257 [DOI] [PubMed] [Google Scholar]
- Brown MS, Basu SK, Falck JR, Ho YK, Goldstein JL. 1980. The scavenger cell pathway for lipoprotein degradation: specificity of the binding site that mediates the uptake of negatively-charged LDL by macrophages. J Supramol Struct. 13:67–81 [DOI] [PubMed] [Google Scholar]
- Brown MS, Goldstein JL, Krieger M, Ho YK, Anderson RG. 1979. Reversible accumulation of cholesteryl esters in macrophages incubated with acetylated lipoproteins. J Cell Biol. 82:597–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T, Huang Z, Wang L, Wang Y, Wu F, Meng S, Wang C. 2009. MicroRNA-125a-5p partly regulates the inflammatory response, lipid uptake, and ORP9 expression in oxLDL-stimulated monocyte/macrophages. Cardiovasc Res. 83:131–139 [DOI] [PubMed] [Google Scholar]
- Cody V, Shen H, Shlyankevich M, Tigelaar RE, Brandsma JL, Hanlon DJ. 2005. Generation of dendritic cells from rabbit bone marrow mononuclear cell cultures supplemented with hGM-CSF and hIL-4. Vet Immunol Immunopathol. 103:163–172 [DOI] [PubMed] [Google Scholar]
- Fleming TJ, Fleming ML, Malek TR. 1993. Selective expression of Ly-6G on myeloid lineage cells in mouse bone marrow: RB6-8C5 mAb to granulocyte-differentiation antigen (Gr-1) detects members of the Ly-6 family. J Immunol. 151:2399–2408 [PubMed] [Google Scholar]
- Fogg DK, Sibon C, Miled C, Jung S, Aucouturier P, Littman DR, Cumano A, Geissmann F. 2006. A clonogenic bone marrow progenitor specific for macrophages and dendritic cells. Science. 311:83–87 [DOI] [PubMed] [Google Scholar]
- Geissmann F, Jung S, Littman DR. 2003. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 19:71–82 [DOI] [PubMed] [Google Scholar]
- Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. 2010. Development of monocytes, macrophages, and dendritic cells. Science. 327:656–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girn HR, Orsi NM, Homer-Vanniasinkam S. 2007. An overview of cytokine interactions in atherosclerosis and implications for peripheral arterial disease. Vasc Med. 12:299–309 [DOI] [PubMed] [Google Scholar]
- Gordon S, Taylor PR. 2005. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 5:953–964 [DOI] [PubMed] [Google Scholar]
- Heil M, Ziegelhoeffer T, Pipp F, Kostin S, Martin S, Clauss M, Schaper W. 2002. Blood monocyte concentration is critical for enhancement of collateral artery growth. Am J Physiol Heart Circ Physiol. 283:H2411–H2419 [DOI] [PubMed] [Google Scholar]
- Hestdal K, Ruscetti FW, Ihle JN, Jacobsen SE, Dubois CM, Kopp WC, Longo DL, Keller JR. 1991. Characterization and regulation of RB6-8C5 antigen expression on murine bone marrow cells. J Immunol. 147:22–28 [PubMed] [Google Scholar]
- Hirsch S, Austyn JM, Gordon S. 1981. Expression of the macrophage-specific antigen F4/80 during differentiation of mouse bone marrow cells in culture. J Exp Med. 154:713–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houthuys E, Movahedi K, De Baetselier P, Van Ginderachter JA, Brouckaert P. 2010. A method for the isolation and purification of mouse peripheral blood monocytes. J Immunol Methods. 359:1–10 [DOI] [PubMed] [Google Scholar]
- Ikuta S, Kirby JA, Shenton BK, Givan AL, Lennard TW. 1991. Human endothelial cells: effect of TNF-alpha on peripheral blood mononuclear cell adhesion. Immunology. 73:71–76 [PMC free article] [PubMed] [Google Scholar]
- Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman RM. 1992. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 176:1693–1702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagasse E, Weissman IL. 1996. Flow cytometric identification of murine neutrophils and monocytes. J Immunol Methods. 197:139–150 [DOI] [PubMed] [Google Scholar]
- Leon B, Martinez del Hoyo G, Parrillas V, Vargas HH, Sanchez-Mateos P, Longo N, Lopez-Bravo M, Ardavin C. 2004. Dendritic cell differentiation potential of mouse monocytes: monocytes represent immediate precursors of CD8- and CD8+ splenic dendritic cells. Blood. 103:2668–2676 [DOI] [PubMed] [Google Scholar]
- Libby P, Geng YJ, Aikawa M, Schoenbeck U, Mach F, Clinton SK, Sukhova GK, Lee RT. 1996. Macrophages and atherosclerotic plaque stability. Curr Opin Lipidol. 7:330–335 [DOI] [PubMed] [Google Scholar]
- Lutz MB, Kukutsch N, Ogilvie AL, Rossner S, Koch F, Romani N, Schuler G. 1999. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 223:77–92 [DOI] [PubMed] [Google Scholar]
- McGarry MP, Stewart CC. 1991. Murine eosinophil granulocytes bind the murine macrophage-monocyte specific monoclonal antibody F4/80. J Leukoc Biol. 50:471–478 [DOI] [PubMed] [Google Scholar]
- Most J, Schwaeble W, Drach J, Sommerauer A, Dierich MP. 1992. Regulation of the expression of ICAM-1 on human monocytes and monocytic tumor cell lines. J Immunol. 148:1635–1642 [PubMed] [Google Scholar]
- Randolph GJ, Jakubzick C, Qu C. 2008. Antigen presentation by monocytes and monocyte-derived cells. Curr Opin Immunol. 20:52–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis ES, Barbuto JA, Isaac L. 2006. Human monocyte-derived dendritic cells are a source of several complement proteins. Inflamm Res. 55:179–184 [DOI] [PubMed] [Google Scholar]
- Reiss Y, Engelhardt B. 1999. T cell interaction with ICAM-1- deficient endothelium in vitro: transendothelial migration of different T cell populations is mediated by endothelial ICAM-1 and ICAM-2. Int Immunol. 11:1527–1539 [DOI] [PubMed] [Google Scholar]
- Risau W, Engelhardt B, Wekerle H. 1990. Immune function of the blood-brain barrier: incomplete presentation of protein (auto-)antigens by rat brain microvascular endothelium in vitro. J Cell Biol. 110:1757–1766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins CS, Swirski FK. 2010. The multiple roles of monocyte subsets in steady state and inflammation. Cell Mol Life Sci. 67:2685–2693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohnelt RK, Hoch G, Reiss Y, Engelhardt B. 1997. Immunosurveillance modelled in vitro: naive and memory T cells spontaneously migrate across unstimulated microvascular endothelium. Int Immunol. 9:435–450 [DOI] [PubMed] [Google Scholar]
- Rohrschneider LR, Bourette RP, Lioubin MN, Algate PA, Myles GM, Carlberg K. 1997. Growth and differentiation signals regulated by the M-CSF receptor. Mol Reprod Dev. 46:96–103 [DOI] [PubMed] [Google Scholar]
- Seeger FH, Tonn T, Krzossok N, Zeiher AM, Dimmeler S. 2007. Cell isolation procedures matter: a comparison of different isolation protocols of bone marrow mononuclear cells used for cell therapy in patients with acute myocardial infarction. Eur Heart J. 28:766–772 [DOI] [PubMed] [Google Scholar]
- Strauss-Ayali D, Conrad SM, Mosser DM. 2007. Monocyte subpopulations and their differentiation patterns during infection. J Leukoc Biol. 82:244–252 [DOI] [PubMed] [Google Scholar]
- Tang C, Inman MD, van Rooijen N, Yang P, Shen H, Matsumoto K, O’Byrne PM. 2001. The type 1-stimulating activity of lung macrophages inhibits Th2-mediated allergic airway inflammation by an IFN-gamma-dependent mechanism. J Immunol. 166:1471–1481 [DOI] [PubMed] [Google Scholar]
- Taylor PR, Brown GD, Geldhof AB, Martinez-Pomares L, Gordon S. 2003. Pattern recognition receptors and differentiation antigens define murine myeloid cell heterogeneity ex vivo. Eur J Immunol. 33:2090–2097 [DOI] [PubMed] [Google Scholar]
- Wagner EF, Risau W. 1994. Oncogenes in the study of endothelial cell growth and differentiation. Semin Cancer Biol. 5:137–145 [PubMed] [Google Scholar]
- Wang WY, Li J, Yang D, Xu W, Zha RP, Wang YP. 2010. OxLDL stimulates lipoprotein-associated phospholipase A2 expression in THP-1 monocytes via PI3K and p38 MAPK pathways. Cardiovasc Res. 85:845–852 [DOI] [PubMed] [Google Scholar]
- Zhu B, Bando Y, Xiao S, Yang K, Anderson AC, Kuchroo VK, Khoury SJ. 2007. CD11b+Ly-6C(hi) suppressive monocytes in experimental autoimmune encephalomyelitis. J Immunol. 179:5228–5237 [DOI] [PubMed] [Google Scholar]