Abstract
Focal adhesions play a critical role as centers that transduce signals by cell-matrix interactions and regulate fundamental processes such as proliferation, migration, and differentiation. Focal adhesion kinase (FAK), paxillin, integrin-linked kinase (ILK), and hydrogen peroxide–inducible clone-5 (Hic-5) are major proteins that contribute to these events. In this study, we investigated the expression of focal adhesion proteins in the developing rat kidney. Western blotting analysis revealed that the protein levels of FAK, p-FAK397, paxillin, p-paxillin118, and Hic-5 were high in embryonic kidneys, while ILK expression persisted from the embryonic to the mature stage. Immunohistochemistry revealed that FAK, p-FAK397, paxillin, and p-paxillin118 were strongly expressed in condensed mesenchymal cells and the ureteric bud. They were detected in elongating tubules and immature glomerular cells in the nephrogenic zone. Hic-5 was predominantly expressed in mesenchymal cells as well as immature glomerular endothelial and mesangial cells, suggesting that Hic-5 might be involved in mesenchymal cell development. ILK expression was similar to that of FAK in the developmental stages. Interestingly, ILK was strongly expressed in podocytes in mature glomeruli. ILK might play a role in epithelial cell differentiation as well as kidney growth and morphogenesis. In conclusion, the temporospatially regulated expression of focal adhesion proteins during kidney development might play a role in morphogenesis and cell differentiation.
Keywords: embryonic kidney development, focal adhesion kinase, hydrogen peroxide–inducible clone-5, integrin-linked kinase, paxillin
The interactions of cells with the extracellular matrix (ECM) are critical determinants of the biological processes of the cells. Through these interactions, the microenvironment affects cell behavior and the cell fate, such as proliferation, migration, differentiation, and apoptosis, resulting in morphogenesis and organogenesis (Streuli 2009). The expressions of both ECM proteins and ECM receptors, integrins, are under temporal and spatial control in the developing kidney (Ekblom 1989; Ekblom et al. 1981; Korhonen et al. 1990; Kreidberg et al. 1996; Kreidberg and Symons 2000; Muller et al. 1997). In the 1980s, pioneering studies revealed distinct changes in the expression of ECM accompanied by nephrogenesis, such as fibronectins (Ekblom et al. 1981), interstitial collagens (Ekblom 1989), and laminin (Bonadio et al. 1984). Indeed, these changes might be involved in the integration of cell behavior during kidney development (Kanwar et al. 2004; Wallner et al. 1998). Integrins are heterodimeric transmembrane glycoproteins consisting of α and β subunits. In mammals, 18α and 8β subunits form 24 different dimers, each of which has different ligand-binding and -signaling properties (Hynes 2002; Legate et al. 2009). They signal to the cell interior through adhesion complexes, which are plasmacytoplasmic platforms that are assembled around the cytoplasmic face of clustered, ECM-associated integrins. These signals mediate diverse biological functions including cell polarity, cell migration, and angiogenesis (Damsky and Ilic 2002; Hynes 2002). In addition to ECM proteins, integrins also exhibit spatiotemporal expression in the development of mammalian metanephros (Korhonen et al. 1990; Kreidberg and Symons 2000).
Focal adhesions are integrin-based adhesion complexes that provide anchor points for assembly of the cytoskeleton and control the architecture of the cell. They also control cell fate and the function of cells by influencing proliferation, migration, differentiation, and apoptosis. They recruit both adaptor proteins (paxillin, integrin-linked kinase [ILK], and hydrogen peroxide–inducible clone-5 [Hic-5]) and enzymes (focal adhesion kinase [FAK], Src, and Rho family GTPases), which trigger distal signaling pathways that control cell-fate decisions (Giancotti and Tarone 2003). Thus, focal adhesions might affect complicated processes in kidney morphogenesis by orchestrating different cells such as mesenchymal, endothelial, and epithelial cells (Chatzizacharias et al. 2010; Sorenson and Sheibani 1999).
The role of focal adhesion proteins in kidney development and morphogenesis has not yet been adequately clarified because focal adhesions composed of various proteins are structurally complicated and all of the upstream and downstream proteins, ECM, integrins, and signaling molecules are very diverse and intricate. Unfortunately, the mice lacking focal adhesion proteins including FAK (Ilic et al. 1995), paxillin (Hagel et al. 2002), and ILK (Lange et al. 2009) are lethal in the early stage of the embryonic period. Knockout mice for ECM and integrins, such as fibronectin (Romberger 1997), α5 (Yang et al. 1993), β1 (Fassler and Meyer 1995), α3 (Kreidberg et al. 1996), and α8 integrin (Muller et al. 1997), also show embryonic fatality or compromised kidney morphogenesis. To extend the research on focal adhesions following the pioneering works on ECM and integrins (Ekblom 1989; Ekblom et al. 1981; Korhonen et al. 1990), we examined the expression of four major proteins among focal adhesions, which might be the most important molecules that regulate ECM and integrin signaling.
Materials and Methods
Antibodies
Rabbit polyclonal anti-FAK antibody, rabbit monoclonal anti–p-FAK397 antibody, mouse monoclonal anti-paxillin antibody, rabbit anti–p-paxillin118 antibody, and goat polyclonal anti-CD31 antibody were purchased from Santa Cruz Biotechnology (Santa Cruz, CA), Invitrogen (Carlsbad, CA), Transduction Laboratories (Lexington, KY), and Millipore (Billerica, MA), respectively. Mouse monoclonal anti-ILK antibody and mouse monoclonal anti–Hic-5 antibody were purchased from Upstate (Billerica, MA) and BD Transduction (Franklin Lakes, NJ). Mouse monoclonal anti–α-smooth muscle actin (α-SMA) 1A4 antibody and mouse monoclonal anti–proliferating cell nuclear antigen (PCNA) antibody were purchased from Sigma-Aldrich (St. Louis, MO) and Calbiochem Merck KGaA (Darmstadt, Germany). Fluorescein isothiocyanate (FITC)–labeled donkey anti-rabbit IgG antibody, FITC-labeled donkey anti-mouse IgG antibody, tetramethylrhodamine isothiocyanate (TRITC)–coupled donkey anti-mouse IgG antibody, and TRITC-coupled donkey anti-goat IgG antibody were purchased from Jackson ImmunoResearch Laboratories (West Grove, PA).
Experimental Animals and Sampling
All procedures and protocols used in this study were approved by the Institutional Animal Care and Committee of the University of Tokushima Graduate School. Sprague-Dawley rats were obtained from Japan SLC (Shizuoka, Japan). Pregnancy was determined by the detection of a vaginal plug. Before removal of the embryos, pregnant rats were anesthetized by isoflurane with oxygen gas. Embryos were removed and decapitated on day 13 (E13), 14 (E14), 15 (E15), 16 (E16), and 18 (E18) of gestation. E13, E14, and E15 were fixed in 10% neutral buffered formalin. Kidneys from E16 and E18 were harvested and either fixed with 10% neutral buffered formalin or homogenized in cell lysis buffer containing protease inhibitors (Cell Signaling Technology; Danvers, MA). Kidneys from rats 1 (P1), 7 (P7), 21 (P21), and 42 (P42) days after birth were treated in the same manner.
Western Blotting
Whole kidneys from E16, E18, P1, P7, P21, and P42 were harvested and homogenized using 15 strokes of a motor-driven Teflon (DuPont; Wilmington, DE) pestle in a tightly fitted glass tube in cell lysis buffer containing protease inhibitors (Cell Signaling Technology). After incubation of samples on ice for 15 minutes, insoluble materials were removed by centrifugation (10,500 × g, 10 minutes). The protein content in kidney lysates was measured using a BCA protein assay kit (Pierce Biotechnology; Rockford, IL). Protein samples (30 µg) were separated by 12.5% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose membranes (Amersham Bioscience; Piscataway, NJ). The membranes were probed with primary antibodies and then incubated with horseradish peroxidase–conjugated secondary antibodies. Immunoreactive proteins were detected with an enhanced chemiluminescence detection system (Amersham Corp.; Arlington Heights, IL). Bands were quantified by ImageJ 1.33u (National Institutes of Health; Bethesda, MD).
Histology and Immunohistochemistry
To examine the expression and localization of focal adhesion proteins, paraffin sections (3 µm thick) were deparaffinized and rehydrated. Endogenous peroxidase activity was quenched by incubating sections in 2.0% H2O2/methanol for 30 minutes. To unmask antigens, slides were autoclaved at 121C for 10 minutes in 0.01 M citrate buffer (pH 6.0).
The expressions of focal adhesions were detected by an immunoperoxidase technique as previously described (Kondo et al. 2006). Briefly, the sections were incubated with primary antibodies against FAK, p-FAK397, paxillin, p-paxllin118, Hic-5, and ILK (all antibodies diluted to 1:100) for 24 hours at 4C. After washing, the sections were incubated with biotinylated secondary antibodies, avidin-biotin-peroxidase (ABC Elite) (Vector Laboratories; Burlingame, CA), and immunoreaction products were developed using 3,3′-diaminobenzidine (Dojindo; Kumamoto, Japan). The sections were then counterstained with Mayer’s hematoxylin (Wako; Tokyo, Japan), dehydrated, and coverslipped. Negative control experiments were examined by omitting primary antibodies or using control IgG.
Double-staining experiments were performed using anti-FAK antibody or anti–Hic-5 antibody with anti-PCNA antibody or anti-CD31 antibody, respectively. The sections were incubated with an appropriate FITC-labeled secondary antibody for anti-FAK antibody or anti–Hic-5 antibody and an appropriate TRITC-labeled secondary antibody for anti-PCNA antibody or anti-CD31 antibody.
To recognize the localization of focal adhesions, we defined various cells in developing kidneys with segment-specific markers as follows: endothelial cell, CD31; proximal tubule, Phaseolus vulgaris erythroagglutinin; distal tubule, calbindin D 28k; collecting duct, aquaporin 2 (Togawa et al. 2011).
Statistical Analysis
Values are expressed as means ± standard deviations (SDs). Differences were evaluated with the StatMate 3 software package (ATMS Co. Ltd.; Tokyo, Japan). Comparisons of variables between groups were performed by one-way ANOVA and the Dunnett test. All experiments were repeated at least three times. Values of p < 0.05 were considered statistically significant.
Results
Western Blotting
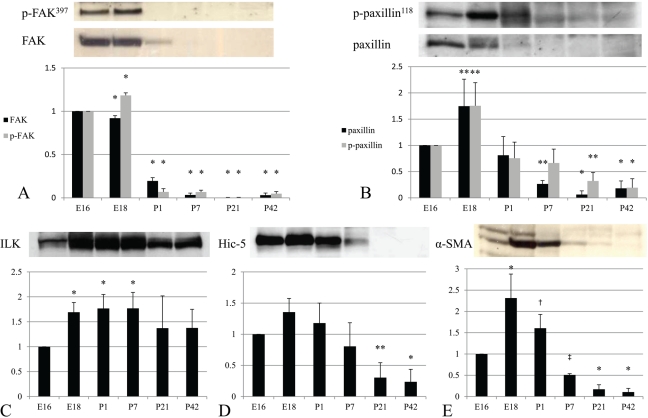
Western blotting was performed to examine protein levels of FAK, p-FAK397, paxillin, p-paxillin118, ILK, Hic-5, and α-SMA in rat kidneys in different stages of development. The expression levels of FAK and p-FAK397 were upregulated in embryonic kidneys (E16 and E18) and significantly decreased after birth. FAK was slightly detected, whereas p-FAK397 was almost absent in P1. They were both undetectable from P7 to mature kidneys (Fig. 1A). Paxillin and p-paxillin118 were especially high in embryonic kidneys and peaked at E18 (Fig. 1B). Similar to FAK, they were decreased after birth and became faint in mature kidneys. Although the levels of FAK and paxillin were very low for detection by Western blotting, their expression could be observed in the following immunohistochemical results, which are summarized in Table 1. Hic-5 was strongly expressed around birth from E16 to P1 and then rapidly decreased and was diminished in mature kidneys (Fig. 1D). The course of Hic-5 expression was similar to that of α-SMA (Fig. 1E). On the other hand, the expression of ILK was continuously observed in all stages of development. An increased level was sustained from E18 to P7, and then this slightly lessened after P21 (Fig. 1C).
Figure 1.
FAK, paxillin, ILK, Hic-5, and α-SMA expression during the development of rat kidneys. FAK, p-FAK397, paxillin, and p-paxillin118 were highly expressed in embryonic kidneys (A and B). ILK expression was detected in embryonic, postnatal, and mature kidneys (C). Hic-5 and α-SMA were highly expressed in E16 to P1 during development (D and E). Results are shown as means ± standard deviations from at least three independent experiments. Significant differences versus E16: ‡p < 0.05; †p < 0.01; **p < 0.001; *p < 0.0001.
Table 1.
Expression of Focal Adhesion Proteins in Developing and Adult Rat Kidneys
| FAK | Paxillin | ILK | Hic-5 | |
|---|---|---|---|---|
| E13–E15 |
||||
| Blastema | ± | ± | + | + |
| Ureteric bud | ++ | ++ | + | − |
| Mesenchymal cells | + | + | + | + |
| E16–E18 |
||||
| Vesicles | + | + | + | ± |
| Comma-, S-shaped bodies | ++ | ++ | ++ | + |
| Gl epithelial cells | ± | + | + | + |
| Gl endothelial cells | ++ | ++ | ++ | ++ |
| Mesangial cells | ++ | ++ | ++ | ++ |
| Ureteric bud branches | ++ | ++ | + | − |
| Mesenchymal cells | + | ± | + | + |
| P1–P7 |
||||
| Vesicles | + | + | + | ± |
| Comma-, S-shaped bodies | + | + | + | ± |
| Gl epithelial cells | ± | + | + | − |
| Gl endothelial cells | + | + | ++ | ++ |
| Mesangial cells | + | + | ++ | + |
| Tubular cells | + | + | ± | − |
| Collecting duct cells | + | ± | + | − |
| Mesenchymal cells | + | ± | + | + |
| P20–P42 |
||||
| Gl visceral epithelial cells | − | − | ++ | − |
| Gl parietal epithelial cells | ± | − | ± | − |
| Gl endothelial cells | − | − | + | − |
| Mesangial cells | − | − | ± | − |
| Proximal tubular cells | + | − | ++ | − |
| Distal tubular cells | − | + | + | − |
| Collecting duct cells | ± | ± | + | − |
| Interstitial cells | − | − | − | VSMC |
Note: – = negative staining; ± = faint staining; + = mild staining; ++ = strong staining; Gl = glomerular; VSMC = vascular smooth muscle cells.
Immunohistochemistry
The localization of each protein is summarized in Table 1. The levels of their expression were graded as follows: – means negative staining, ± means faint staining, + means mild staining, and ++ means strong staining by reference to another previous article (Omori et al. 2000).
Expression of FAK and p-FAK397
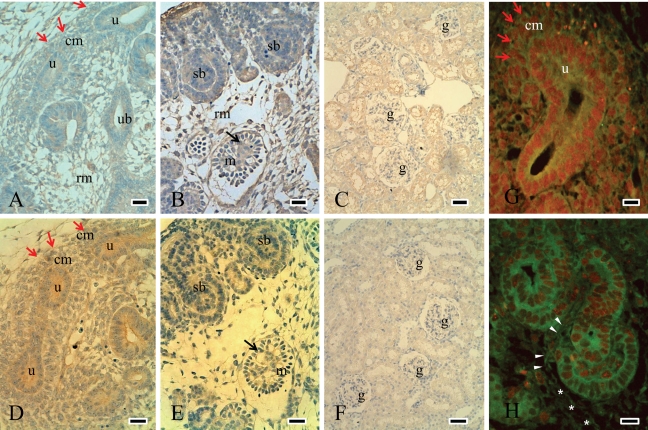
FAK was predominantly expressed at the cell membrane of the ureteric bud and condensed mesenchymal cells around the ureteric bud and weakly observed in mesenchymal cells (Fig. 2A). In the nephrogenic zone, FAK was expressed in comma- and S-shaped bodies and in immature glomerular endothelial and mesangial cells (Fig. 2B). With maturation, FAK expression was decreased and localized in tubules in mature kidneys (Fig. 2C). Similar to FAK expression, p-FAK397 was observed at the cell membrane of the ureteric bud and condensed mesenchymal cells in E16 (Fig. 2D). It was expressed in S-shaped bodies and immature glomerular cells in E18 (Fig. 2E). It was decreased during the course of development and was not observed in mature glomeruli (Fig. 2F).
Figure 2.
Expression of FAK and p-FAK397 during the development of rat kidneys. Expression of FAK and p-FAK397 in E16 was intense at the cell membrane of the ureteric bud and mesenchymal cells, especially in the condensed mesenchyme (red arrows) (A and D). Around the nephrogenic zone in E18, FAK and p-FAK397 were expressed in S-shaped bodies (sb) as well as in immature glomerular endothelial and mesangial cells (black arrows in B and E, which are the serial sections). FAK expression was decreased and localized in tubules in P42 (C), while p-FAK397 was not detected in mature glomeruli of P42 (F). Double-staining experiments were performed to compare the expression of FAK (green, FITC) and PCNA-positive cells (red, TRITC) (G and H). FAK was expressed on the cell membrane of the ureteric bud and condensed mesenchymal cells (red arrows), where the PCNA was positive in E16 (G). In E18, FAK expression was also positive in cleft-invading endothelial and mesangial cells (arrow head) and mesenchymal cells (*), where the PCNA expression was weak (H). rm = renal mesenchymal cells; red arrows and “cm” = condensed mesenchymal cells; ub = ureteric bud; u = ureteric bud tip; sb = S-shaped body; m = immature glomerulus; black arrows in B and E = immature endothelial and mesangial cells; g = glomerulus. Scale bars (A, B, D, and F) = 25 µm; scale bars (C and F) = 50 µm; scale bars (G and H) = 100 µm.
To compare the distribution of FAK with proliferating cells, we investigated PCNA-positive cells by double-staining experiments (Fig. 2G and 2H). PCNA was mainly stained in the ureteric bud and condensed mesenchymal cells around the ureteric bud, and FAK was expressed on the cell membrane of the PCNA-positive cells in E16 (Fig. 2G). PCNA was subsequently expressed in comma- and S-shaped bodies and in elongating epithelial cells in the nephrogenic zone (Fig. 2H). The distribution of PCNA-positive cells was similar to those of FAK, paxillin, and ILK, which might be involved in renal growth and morphogenesis. FAK-positive cells with negative PCNA staining were also observed in E18 by double staining. These cells seemed to be mesenchymal cells, immature endothelial cells, and mesangial cells, which appeared to migrate or invade into the cleft of S-shaped bodies (Fig. 2H).
Expression of Paxillin and P-paxillin118
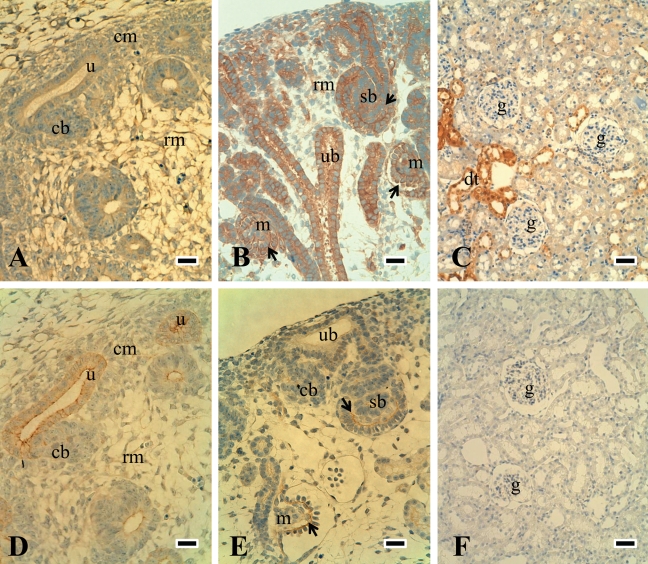
The expression and localization of paxillin and p-paxillin118 were similar to those of FAK, and these were detected mainly in the ureteric bud and weakly in mesenchymal cells in the embryonic stage (Fig. 3A and 3D). They were then expressed in comma- and S-shaped bodies and immature glomerular endothelial and mesangial cells in the nephrogenic zone (Fig. 3B and 3E). With maturation, paxillin expression was decreased and limited to distal tubular cells (Fig. 3C), while p-paxillin118 expression on tubules was very weak (Fig. 3F).
Figure 3.
Expression of paxillin and p-paxillin118 during the development of rat kidneys. Paxillin and p-paxillin118 expression were similar to those of FAK and were detected at the cell membrane of the ureteric bud in E16 (A and D are the serial sections). Both were also observed in immature endothelial (black arrow) and mesangial cells in comma- and S-shaped bodies in the nephrogenic zone of E18 (B and E). Paxillin was decreased and was limited to distal tubules in P42 (C), while p-paxillin118 expression on tubules was very weak (F). rm = renal mesenchymal cells; cm = condensed mesenchymal cells; ub = ureteric bud; u = ureteric bud tips; sb = S-shaped body; m = immature glomerulus; black arrows in B and E = immature endothelial and mesangial cells; dt = distal tubules; g = glomerulus. Scale bars (A, B, D, and F) = 25 µm; scale bars (C and F) = 50 µm.
Expression of ILK
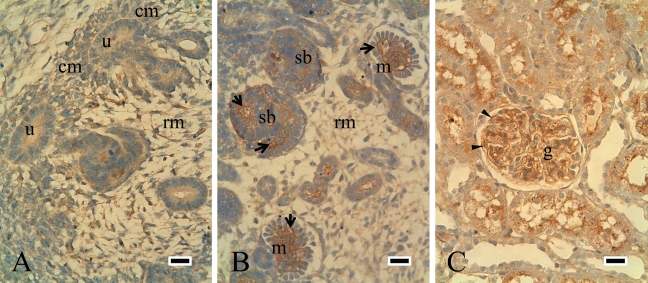
Although ILK expression was similar to that of FAK and paxillin during development, they were very different with maturation. ILK expression was detected in the ureteric bud and mesenchymal cells from E14 to P1 (Fig. 4A). It was mainly expressed in immature endothelial and mesangial cells in the nephrogenic zone (Fig. 4B) and then localized in endothelial cells and podocytes in mature glomeruli (Fig. 4C).
Figure 4.
Expression of ILK during the development of rat kidneys. ILK expression was similar to that of FAK in developing stages. ILK was expressed in the ureteric bud and mesenchymal cells in E15 (A). In E18, ILK was detected in S-shaped bodies as well as in immature endothelial and mesangial cells (B). ILK was strongly expressed in podocytes and epithelial cells in P42 (C). rm = renal mesenchymal cells; cm = condensed mesenchymal cells; u = ureteric bud tips; sb = S-shaped body; m = immature glomerulus; arrow = immature endothelial and mesangial cells; arrowhead = podocytes; g = glomerulus. Scale bars = 25 µm.
Expression of Hic-5
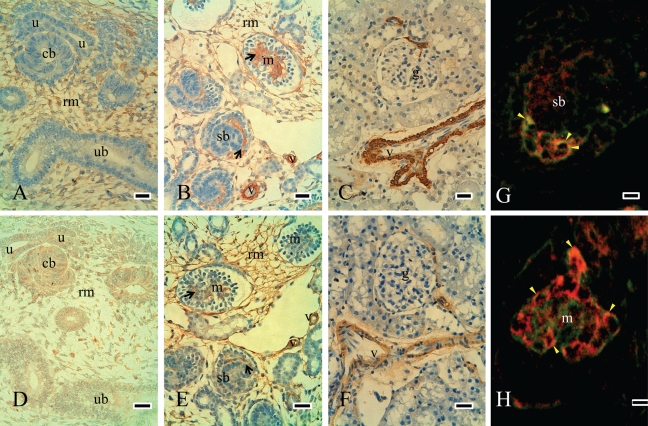
Hic-5 was mainly expressed in mesenchymal cells and immature glomerular endothelial and mesangial cells in embryonic kidneys (Fig. 5A and 5B). Double-staining experiments showed that Hic-5 is mainly expressed in immature mesangial cells and partially co-localized with CD31 in E18 and P1 (Fig. 5G and 5H). The expression of Hic-5 was decreased with maturation, was not observed in glomeruli, and was limited to vascular smooth muscle cells in mature kidneys (Fig. 5C). To elucidate the relationship between Hic-5 and myofibroblastic cells, we investigated cells that were positive for α-SMA (Carey et al. 1992) in serial sections. α-SMA was detected in mesenchymal cells in embryonic kidneys (Fig. 5D). It was also localized in immature glomerular endothelial and mesangial cells and in vascular smooth muscle cells in E18 (Fig. 5E). This expression was then decreased and limited to vascular smooth muscle cells in mature kidneys (Fig. 5F). The distribution of α-SMA–positive cells was similar to that of Hic-5, which might be related to mesenchymal cell development.
Figure 5.
Expression of Hic-5 and α-SMA–positive cells during the development of rat kidneys. Hic-5 was mainly expressed in mesenchymal cells in E16 but was not detected in the ureteric bud or epithelial cells (A). α-SMA–positive cells were detected in mesenchymal cells in E16 (D). Distributions of Hic-5–positive cells were similar to α-SMA–positive cells in E16. In E18, Hic-5 was detected in immature glomerular endothelial and mesangial cells and also in mesenchymal cells (black arrows in B). Most Hic-5 expression was very similar to the distribution of α-SMA–positive cells in E18 (B and E are the serial sections). In mature kidneys, both expressions were not observed in glomeruli and were limited to vascular smooth muscle cells (C and F). Double-staining experiments showed that Hic-5 (green, FITC) and CD31 (red, TRITC) were partially co-localized in immature glomeruli of E18 (yellow arrowhead in G and H). rm = renal mesenchymal cells; u = ureteric bud tips; ub = ureteric bud; sb = S-shaped body; m = immature glomerulus; black arrows in B and E = immature endothelial and mesangial cells; g = glomerulus; v = vascular smooth muscle cells; red in G and H = CD31; green in G and H = Hic-5. Scale bars (A-F) = 25 µm; scale bars (G and H) = 75 µm.
Discussion
This study has four major findings: 1) The expression levels of FAK, p-FAK397, paxillin, p-paxillin118, and Hic-5 were high in the embryonic kidney, whereas the level of ILK expression was maintained in embryonic, postnatal, and mature kidneys. 2) FAK, p-FAK397, paxillin, and p-paxillin118 were strongly expressed in mesenchymal cells and the ureteric bud. In the nephrogenic zone, they were detected in elongating epithelial cells in tubules and collecting ducts and in immature glomerular endothelial and mesangial cells. They were decreased in mature kidneys. 3) Hic-5 was predominantly expressed in mesenchymal cells and immature glomerular endothelial and mesangial cells, similar to α-SMA–positive cells. 4) ILK was expressed similarly to FAK in developing stages. In contrast, ILK was strongly expressed in glomerular endothelial cells and podocytes in mature kidneys.
Kidney development proceeds in sequential steps that involve the dynamic and accurately controlled programming of cellular events. The induction of the metanephric mesenchyme by the ureteric bud promotes aggregation of the condensed mesenchyme around the bud tips. These aggregates undergo mesenchyme-to-epithelial conversion to generate the renal vesicles. Meanwhile, the ureteric buds elongate and reiterate branching and induce new aggregates at the bud tips. By the S-shaped body stage, the nephron is patterned along the proximal-distal axis. Invasion of the proximal cleft by endothelial cells starts the process of glomerulogenesis (Dressler 2006). All the cells that take part in nephrogenesis perform some fundamental activity, including proliferation, migration, polarization, and differentiation through these developmental processes, which are always determined by adhesive interactions between cells and their local microenvironment (Streuli 2009). In all of these developmental processes, focal adhesions are considered to play crucial roles in receiving signals from complex extracellular environments and in conveying intracellular signals that lead to changes in cell behavior.
FAK is a 125-kDa nonreceptor and non–membrane protein tyrosine kinase, which has been identified as a substrate of viral Src oncogene, and is considered to be related to tumor growth (Schaller et al. 1992). FAK is phosphorylated at several tyrosine residues including tyrosine 397 (Tyr397), the first tyrosine residue of its phosphorylation. Clustering of integrins facilitates the autophosphorylation of Tyr397, which increases and regulates the catalytic activity of FAK (van Nimwegen and van de Water 2007). Through multifaceted and diverse molecular connections, FAK can influence the cytoskeletal organization at structures of cell adhesion sites to regulate cell movement (Schlaepfer et al. 2004; Sonoda et al. 2000). FAK expression has been investigated in a variety of human cancers, including primary, metastatic, and recurrent lesions. These studies suggest that FAK may play diverse roles including proliferation and migration in different tumors and/or in different stages of tumor progression (van Nimwegen and van de Water 2007). In this study, we found that many FAK and p-FAK397–positive cells were also positive for PCNA in the ureteric bud and mesenchymal aggregates during early stages as well as in elongating epithelial cells in the nephrogenic zone. These observations suggest that FAK plays a main role in cell proliferation in kidney development. In contrast, FAK expression was also positive in cleft-invading endothelial cells and immature mesangial cells. Because FAK also plays a role in migration as well as proliferation (Sonoda et al. 2000; van Nimwegen and van de Water 2007), we thought that FAK might also be necessary for immature endothelial and mesangial cells to migrate inside vesicles to organize the capillary network of glomeruli. Interestingly, these migratory processes might be associated with the shift of laminin composition and the expression of some integrins including α1 integrin (Abrahamson 2009; Korhonen et al. 1990). In addition, Ma et al. (2010) showed that podocyte-specific deletion of FAK abrogated the proteinuria and foot process effacement induced by glomerular injury, and podocytes isolated from these conditional FAK knockout mice demonstrated reduced spreading and migration. These findings also supported that FAK activation might affect cell motility in unsettled conditions when foot processes by podocytes separate from the ECM in glomerular basement membranes. The decision of a cell to either proliferate or migrate depends on the balance of many stimulatory and inhibitory factors because the property of FAK might be determined by the integrin-ECM proteins with which it interacts (Wozniak et al. 2004).
Paxillin is a 68-kDa protein that contains five leucine- and aspartate-rich LD motifs, which bind vinculin and FAK. Paxillin also contains four LIM domains, which are double zinc-finger motifs that mediate protein-protein interactions. Similar to FAK, multiple tyrosine, serine, and threonine phosphorylation sites exist throughout paxillin molecules; these sites are targeted by a diverse array of kinases that are activated in response to various adhesion stimuli and by growth factors, and its phosphorylation plays a vital role in signaling (Deakin and Turner 2008). Paxillin can be phosphorylated by FAK and may potentially be a downstream component of FAK signaling. Indeed, the localization and expression of paxillin and p-paxillin118 in developing kidneys were similar to those of FAK or p-FAK397. This result supports the idea that the phosphorylation of paxillin through the FAK/Src complex is important for paxillin to act as a docking molecule at focal adhesions and that they have a common role in cell behavior in development, such as cell migration and proliferation. This is well supported by the findings in paxillin- or FAK-deficient mice, which are both fatal before the onset of kidney morphogenesis (Hagel et al. 2002; Ilic et al. 1995). Although both molecules are diminished in mature glomeruli, our results showed different expressions in adult kidneys. FAK was expressed mainly in the proximal tubule, whereas paxillin was limited to distal tubules. This indicates that paxillin might play a different role than FAK in the maintenance of tubular cells.
Hic-5 was first identified in a screen for transforming growth factor beta1 (TGF-β1) and hydrogen peroxide–induced genes (Shibanuma et al. 1994). Hic-5 encodes a 55-kDa protein, which has a structure that is very similar to that of paxillin, and both consist of four LIM proteins and LD proteins (Shibanuma et al. 1994). Hic-5 expression is elevated in platelets (Hagmann et al. 1998), cells of mesenchymal origin, and also in stromal and smooth muscle tissue layers (Brunskill et al. 2001; Cai et al. 2005; Yuminamochi et al. 2003). Brunskill et al. (2001) showed that Hic-5 was strongly and transiently expressed in the early developing heart and then in the smooth muscle layer of developing tissues, including the intestinal tract and bronchial airways in mice. Yuminamochi et al. (2003) showed that there was a difference in the expression of Hic-5 and paxillin in adult human tissues. Paxillin expression was widespread and observed in both nonmuscle and muscle tissue, while Hic-5 was limited to muscle tissues, mainly mononuclear smooth muscle. Our study demonstrated that Hic-5 expression was very different from paxillin expression. We recognized that the expression of Hic-5 was limited to mesenchymal cells, endothelial cells, and mesangial cells in immature glomeruli. The expression of both Hic-5 and paxillin was localized to mesenchymal cells and immature endothelial mesangial cells in embryonic kidneys, whereas paxillin was mainly expressed in epithelial cells. This suggests that Hic-5 may induce or maintain a mesenchymal cell character through competition with paxillin (Mori et al. 2009). Interestingly, the expression of Hic-5 coincides with the distribution of α-SMA–positive cells, suggesting that it may be associated with mesenchymal phenotypes in each developmental stage (Carey et al. 1992).
ILK is a 59-kDa serine/threonine kinase that interacts with cytoplasmic domains of β1 integrins and has been implicated in the regulation of cell adhesion, proliferation, and ECM organization (Wu 2001). ILK is activated by β1 integrin–mediated adhesion to the ECM or stimulation with growth factors such as TGF-β. ILK is considered to function as the effector of PI3-K signaling, which regulates protein kinase B/Akt and glycogen synthase kinase-3 activity, and thereby mediates a wide range of cellular processes. In the present study, the localization of ILK was similar to that of FAK and paxillin in embryonic kidneys, while it was mainly expressed in mature podocytes in adult kidneys. Along with the results of previous studies, the present findings suggest that ILK plays several roles in development. First, it might be involved in the proliferation and migration of epithelial and mesenchymal cells. The concept was supported in a recent work using conditionally deficient mice that ILK expression was selectively knocked down in the ureteric bud (Smeeton et al. 2010). Second, it might be involved in mesenchyme-to-epithelial conversion, which is observed when mesenchymal aggregates change to epithelial cells in renal vesicles (Leung-Hagesteijn et al. 2005). With maturation, its function might switch to the differentiation and maturation of epithelial cells and podocytes (Dai et al. 2006).
ECM, integrins, and focal adhesions are expressed diversely in each developmental stage. Focal adhesions appear to integrate and modulate signals to the cell interior, which results in accurate cell proliferation, migration, and differentiation, as well as signal transduction to achieve normal organ morphogenesis. Because focal adhesions also mediate signals from growth factors such as TGF-β or glial cell–derived neurotrophic factor, the expression and activation of focal adhesions appear to be important for regulating and coordinating diverse signals by both ECM and growth factors during kidney development and morphogenesis. Further studies on the relationship between focal adhesions and stimulatory extracellular factors are needed to elucidate the precise mechanisms of renal development and morphogenesis.
In conclusion, we demonstrated that the expression of focal adhesion proteins is temporospatially regulated during the development of rat kidneys. They might play crucial roles in renal development and morphogenesis.
Acknowledgments
The authors are grateful to Naomi Okamoto, Chizuko Yamamoto, Keita Osumi, Junki Yamajo, and Hiroki Matsumoto for their excellent technical assistance. They also thank Dr. Christine M. Sorenson and Dr. Nader Sheibani (University of Wisconsin–Madison), and Dr. Midori Awazu (Keio University) for their helpful suggestions and Dr. Akito Kobayashi (Brigham and Women’s Hospital) and Dr. Shuta Ishibe (Yale School of Medicine) for providing helpful discussions.
Footnotes
Presented in part at the 42nd Annual Meeting of the American Society of Nephrology; San Diego, CA; October 28–November 1, 2009.
The author(s) declared no potential conflicts of interest with respect to the authorship and publication of this article.
This work was supported in part by Grants-in-Aid for Scientific Research (20591277 and 2059127 to S. Kagami and S. Kondo, respectively). This work was also supported by the Morinaga Foundation for Health and Nutrition.
References
- Abrahamson DR. 2009. Development of kidney glomerular endothelial cells and their role in basement membrane assembly. Organogenesis. 5:275–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonadio JF, Sage H, Cheng F, Bernstein J, Striker GE. 1984. Localization of collagen types IV and V, laminin, and heparan sulfate proteoglycan to the basal lamina of kidney epithelial cells in transfilter metanephric culture. Am J Pathol. 116:289–296 [PMC free article] [PubMed] [Google Scholar]
- Brunskill EW, Witte DP, Yutzey KE, Potter SS. 2001. Novel cell lines promote the discovery of genes involved in early heart development. Dev Biol. 235:507–520 [DOI] [PubMed] [Google Scholar]
- Cai G, Huang H, Shapiro E, Zhou H, Yeh S, Melamed J, Greco MA, Lee P. 2005. Expression of androgen receptor associated protein 55 (ARA55) in the developing human fetal prostate. J Urol. 173:2190–2193 [DOI] [PubMed] [Google Scholar]
- Carey AV, Carey RM, Gomez RA. 1992. Expression of alpha-smooth muscle actin in the developing kidney vasculature. Hypertension. 19:II168–II175 [DOI] [PubMed] [Google Scholar]
- Chatzizacharias NA, Kouraklis GP, Theocharis SE. 2010. The role of focal adhesion kinase in early development. Histol Histopathol. 25:1039–1055 [DOI] [PubMed] [Google Scholar]
- Dai C, Stolz DB, Bastacky SI, St-Arnaud R, Wu C, Dedhar S, Liu Y. 2006. Essential role of integrin-linked kinase in podocyte biology: bridging the integrin and slit diaphragm signaling. J Am Soc Nephrol. 17:2164–2175 [DOI] [PubMed] [Google Scholar]
- Damsky CH, Ilic D. 2002. Integrin signaling: it’s where the action is. Curr Opin Cell Biol. 14:594–602 [DOI] [PubMed] [Google Scholar]
- Deakin NO, Turner CE. 2008. Paxillin comes of age. J Cell Sci. 121:2435–2444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dressler GR. 2006. The cellular basis of kidney development. Annu Rev Cell Dev Biol. 22:509–529 [DOI] [PubMed] [Google Scholar]
- Ekblom P. 1989. Developmentally regulated conversion of mesenchyme to epithelium. FASEB J. 3:2141–2150 [DOI] [PubMed] [Google Scholar]
- Ekblom P, Lehtonen E, Saxen L, Timpl R. 1981. Shift in collagen type as an early response to induction of the metanephric mesenchyme. J Cell Biol. 89:276–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fassler R, Meyer M. 1995. Consequences of lack of beta 1 integrin gene expression in mice. Genes Dev. 9:1896–1908 [DOI] [PubMed] [Google Scholar]
- Giancotti FG, Tarone G. 2003. Positional control of cell fate through joint integrin/receptor protein kinase signaling. Annu Rev Cell Dev Biol. 19:173–206 [DOI] [PubMed] [Google Scholar]
- Hagel M, George EL, Kim A, Tamimi R, Opitz SL, Turner CE, Imamoto A, Thomas SM. 2002. The adaptor protein paxillin is essential for normal development in the mouse and is a critical transducer of fibronectin signaling. Mol Cell Biol. 22:901–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagmann J, Grob M, Welman A, van Willigen G, Burger MM. 1998. Recruitment of the LIM protein hic-5 to focal contacts of human platelets. J Cell Sci. 111(Pt 15):2181–2188 [DOI] [PubMed] [Google Scholar]
- Hynes RO. 2002. Integrins: bidirectional, allosteric signaling machines. Cell. 110:673–687 [DOI] [PubMed] [Google Scholar]
- Ilic D, Furuta Y, Kanazawa S, Takeda N, Sobue K, Nakatsuji N, Nomura S, Fujimoto J, Okada M, Yamamoto T. 1995. Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature. 377:539–544 [DOI] [PubMed] [Google Scholar]
- Kanwar YS, Wada J, Lin S, Danesh FR, Chugh SS, Yang Q, Banerjee T, Lomasney JW. 2004. Update of extracellular matrix, its receptors, and cell adhesion molecules in mammalian nephrogenesis. Am J Physiol Renal Physiol. 286:F202–F215 [DOI] [PubMed] [Google Scholar]
- Kondo S, Shimizu M, Urushihara M, Tsuchiya K, Yoshizumi M, Tamaki T, Nishiyama A, Kawachi H, Shimizu F, Quinn MT, et al. 2006. Addition of the antioxidant probucol to angiotensin II type I receptor antagonist arrests progressive mesangioproliferative glomerulonephritis in the rat. J Am Soc Nephrol. 17:783–794 [DOI] [PubMed] [Google Scholar]
- Korhonen M, Ylanne J, Laitinen L, Virtanen I. 1990. The alpha 1-alpha 6 subunits of integrins are characteristically expressed in distinct segments of developing and adult human nephron. J Cell Biol. 111:1245–1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreidberg JA, Donovan MJ, Goldstein SL, Rennke H, Shepherd K, Jones RC, Jaenisch R. 1996. Alpha 3 beta 1 integrin has a crucial role in kidney and lung organogenesis. Development. 122:3537–3547 [DOI] [PubMed] [Google Scholar]
- Kreidberg JA, Symons JM. 2000. Integrins in kidney development, function, and disease. Am J Physiol Renal Physiol. 279:F233–F242 [DOI] [PubMed] [Google Scholar]
- Lange A, Wickstrom SA, Jakobson M, Zent R, Sainio K, Fassler R. 2009. Integrin-linked kinase is an adaptor with essential functions during mouse development. Nature. 461:1002–1006 [DOI] [PubMed] [Google Scholar]
- Legate KR, Wickstrom SA, Fassler R. 2009. Genetic and cell biological analysis of integrin outside-in signaling. Genes Dev. 23:397–418 [DOI] [PubMed] [Google Scholar]
- Leung-Hagesteijn C, Hu MC, Mahendra AS, Hartwig S, Klamut HJ, Rosenblum ND, Hannigan GE. 2005. Integrin-linked kinase mediates bone morphogenetic protein 7-dependent renal epithelial cell morphogenesis. Mol Cell Biol. 25:3648–3657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H, Togawa A, Soda K, Zhang J, Lee S, Ma M, Yu Z, Ardito T, Czyzyk J, Diggs L, et al. 2010. Inhibition of podocyte FAK protects against proteinuria and foot process effacement. J Am Soc Nephrol. 2:1145–1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori K, Hirao E, Toya Y, Oshima Y, Ishikawa F, Nose K, Shibanuma M. 2009. Competitive nuclear export of cyclin D1 and Hic-5 regulates anchorage dependence of cell growth and survival. Mol Biol Cell. 20:218–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller U, Wang D, Denda S, Meneses JJ, Pedersen RA, Reichardt LF. 1997. Integrin alpha8beta1 is critically important for epithelial-mesenchymal interactions during kidney morphogenesis. Cell. 88:603–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omori S, Hida M, Ishikura K, Kuramochi S, Awazu M. 2000. Expression of mitogen-activated protein kinase family in rat renal development. Kidney Int. 58:27–37 [DOI] [PubMed] [Google Scholar]
- Romberger DJ. 1997. Fibronectin. Int J Biochem Cell Biol. 29:939–943 [DOI] [PubMed] [Google Scholar]
- Schaller MD, Borgman CA, Cobb BS, Vines RR, Reynolds AB, Parsons JT. 1992. pp125FAK a structurally distinctive protein-tyrosine kinase associated with focal adhesions. Proc Natl Acad Sci U S A. 89:5192–5196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaepfer DD, Mitra SK, Ilic D. 2004. Control of motile and invasive cell phenotypes by focal adhesion kinase. Biochim Biophys Acta. 1692:77–102 [DOI] [PubMed] [Google Scholar]
- Shibanuma M, Mashimo J, Kuroki T, Nose K. 1994. Characterization of the TGF beta 1-inducible hic-5 gene that encodes a putative novel zinc finger protein and its possible involvement in cellular senescence. J Biol Chem. 269:26767–26774 [PubMed] [Google Scholar]
- Smeeton J, Zhang X, Bulus N, Mernaugh G, Lange A, Karner CM, Carroll TJ, Fassler R, Pozzi A, Rosenblum ND, Zent R. 2010. Integrin-linked kinase regulates p38 MAPK-dependent cell cycle arrest in ureteric bud development. Development. 137:3233–3243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonoda Y, Matsumoto Y, Funakoshi M, Yamamoto D, Hanks SK, Kasahara T. 2000. Anti-apoptotic role of focal adhesion kinase (FAK): induction of inhibitor-of-apoptosis proteins and apoptosis suppression by the overexpression of FAK in a human leukemic cell line, HL-60. J Biol Chem. 275:16309–16315 [DOI] [PubMed] [Google Scholar]
- Sorenson CM, Sheibani N. 1999. Focal adhesion kinase, paxillin, and bcl-2: analysis of expression, phosphorylation, and association during morphogenesis. Dev Dyn. 215:371–382 [DOI] [PubMed] [Google Scholar]
- Streuli CH. 2009. Integrins and cell-fate determination. J Cell Sci. 122:171–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Togawa H, Nakanishi K, Mukaiyama H, Hama T, Shima Y, Sako M, Miyajima M, Nozu K, Nishii K, Nagao S, et al. 2011. Epithelial-to-mesenchymal transition in cyst lining epithelial cells in an orthologous PCK rat model of autosomal-recessive polycystic kidney disease. Am J Physiol Renal Physiol. 300:F511–F520 [DOI] [PubMed] [Google Scholar]
- van Nimwegen MJ, van de Water B. 2007. Focal adhesion kinase: a potential target in cancer therapy. Biochem Pharmacol. 73:597–609 [DOI] [PubMed] [Google Scholar]
- Wallner EI, Yang Q, Peterson DR, Wada J, Kanwar YS. 1998. Relevance of extracellular matrix, its receptors, and cell adhesion molecules in mammalian nephrogenesis. Am J Physiol. 275:F467–F477 [DOI] [PubMed] [Google Scholar]
- Wozniak MA, Modzelewska K, Kwong L, Keely PJ. 2004. Focal adhesion regulation of cell behavior. Biochim Biophys Acta. 1692:103–119 [DOI] [PubMed] [Google Scholar]
- Wu C. 2001. ILK interactions. J Cell Sci. 114:2549–2550 [DOI] [PubMed] [Google Scholar]
- Yang JT, Rayburn H, Hynes RO. 1993. Embryonic mesodermal defects in alpha 5 integrin-deficient mice. Development. 119:1093–1105 [DOI] [PubMed] [Google Scholar]
- Yuminamochi T, Yatomi Y, Osada M, Ohmori T, Ishii Y, Nakazawa K, Hosogaya S, Ozaki Y. 2003. Expression of the LIM proteins paxillin and Hic-5 in human tissues. J Histochem Cytochem. 51:513–521 [DOI] [PubMed] [Google Scholar]