Abstract
Few studies have examined functional adrenal zonation throughout human life. Adrenals from 61 surgical/autopsy patients from 1 day old to 92 years old who had no clinical endocrinological/mineralocorticoid abnormalities were assessed for immunohistochemically defined adrenal zonation. The zona glomerulosa (zG) was well developed in all 11 patients ranging in age from newborn to the 30s. After 40 years of age, however, the zG occupied less than one-quarter of the adrenal circumference, suggestive of zG involution. The other subcapsular areas were occupied by the progenitor zone (zP), which expressed neither cytochrome P450aldo nor P45011β but 3β-hydroxysteroid dehydrogenase and P450scc, although some autopsy cases had adrenals with zG zonation because of secondary aldosteronism, and others who had experienced severe stresses showed subcapsular zona fasciculata (zF). In conclusion, the adrenal cortex consists of homogeneous zG-topped columns from birth to adolescence. Subsequently, in the fifth decade of life, the cortex is reconstituted by integration of three types of cortical columns: scattered zG-topped columns and zonal zP-topped columns, the latter having the ability for bidirectional differentiation into either zG-topped columns or zF-topped columns, according to secondary aldosteronism or the presence of severe stresses. Such adrenocortical remodeling is ascribed to high-sodium/low-potassium diets.
Keywords: human, immunohistochemistry, adrenal zonation, involution of the zona glomerulosa, progenitor zone, zona fasciculata, aging, adrenocortical remodeling, high sodium/low potassium diet
The human zona glomerulosa (zG) is morphologically atrophic in the “endocrinologically normal” adrenal cortex, in contrast to that of other mammals on the land such as oxen and pigs, as well as rats and mice (Symington 1969; Carney and Lloyd 2007). Studies of the morphology on hematoxylin and eosin (HE)–stained sections have revealed clusters of zG-like small cells discontinuously arranged just under the capsule, with zona fasciculata (zF)–like cells on the top of cords reaching the capsule, suggesting atrophy of the zG in the adrenals of human adults. However, few studies have been performed to examine the changes in the zG of the human adrenals with age using functional immunohistochemistry because of relative unavailability of suitable zG markers and “normal” human adrenal tissues.
We investigated the immunohistochemically defined zG, zF, and zona reticularis (zR) of 61 adrenal glands derived from humans ranging in age from newborn to the 90s (Fig. 1; Aiba and Fujibayashi 2005). We found a well-developed zG in earlier life but a marked decrease of the zG population and replacement by the progenitor zone (zP) after 40 years of age. The results were discussed from the viewpoint of a high-sodium/low-potassium diet of the human being today compared with that of wild mammals on the land and human ancestors (Intersalt Cooperative Research Group 1988; O’Shaughnessy and Karet 2004; Adrogue and Madias 2007; He and MacGregor 2009; He et al. 2009; Bibbins-Domingo et al. 2010).
Figure 1.
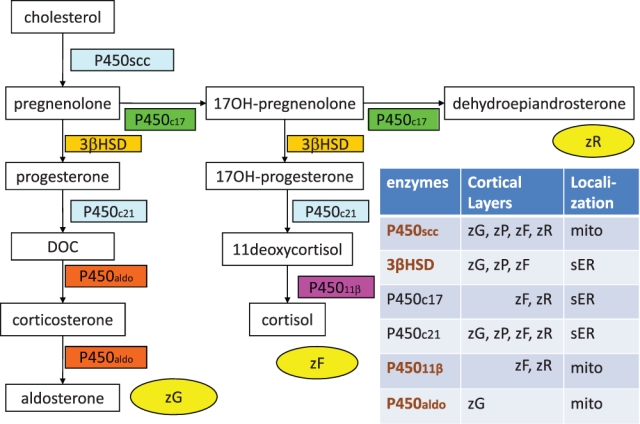
Steroidogenic enzymes and adrenocortical zonation. Aldosterone-producing zona glomerulosa (zG), cortisol-producing zona fasciculata (zF), and dehydroepiandrosterone-producing zona reticularis (zR) require each of the above corticosteroid-biosynthetic pathways, respectively. Conversely, six corticosteroid-metabolizing enzymes are distributed in particular adrenal zones, with intracellular localization of either mitochondria (mito) or smooth endoplasmic reticulum (sER). The zP indicates the progenitor zone, which expresses neither P450aldo, a zG marker, nor P45011β, a marker of the zF-zR, but has some enzymes that are common to both the zG and the zF. It is noteworthy that P450aldo is exclusively expressed in the zG.
Materials and Methods
Patients
Eighteen adrenals resected from patients suffering from renal malignancies without apparent endocrinological diseases (age range, 40–84 years; mean, 65.6 years; group As) and three adrenals resected from patients with a nonfunctioning ganglioneuroma, dehydroepiandrosterone-producing adenoma, and primary pigmented nodular adrenocortical disease (ages 26 years, 4 months, and 10 years, respectively; group Bs) with no apparent abnormality of the renin-angiotensin-aldosterone system (RAAS) were investigated as the surgical case group.
Forty adrenals from autopsy cases with no clinical endocrine diseases were also investigated. Of these, 32 were cases ranging in age from 46 to 92 years (mean, 67.1 years; group Aa), and eight were cases ranging in age from 1 day old to 34 years (mean, 12.7 years; group Ba).
Specimens and Method
Fresh-frozen sections (8 µm) of the adrenal tissues were immunohistochemically stained for cytochrome P450aldo and P45011β using rabbit polyclonal antibodies at 1:4000 and 1:1800 dilution, respectively (courtesy of Dr. Fumiko Mitani, Department of Biochemistry, Keio University School of Medicine, Tokyo, Japan), the specificity of which has been proved (Ogishima et al. 1991; Nishimoto et al. 2010). In addition, tissue sections were immunohistochemically (Aiba et al. 2005; Oxigene, Dallas, TX) at 1:3200 dilution and/or enzyme histochemically (Aiba et al. 1981) stained for 3β-hydroxysteroid dehydrogenase (3βHSD). P450scc and mitochondria (Chemicon, Temecula, CA) at 1:1800 dilution were also immunostained in selected cases. Tissues were reacted with the first antibodies overnight at 4C. For visualization, the EnVision system (DAKO, Copenhagen, Denmark) was employed, using 3,3′-diaminobenzidine (DAKO) as the chromogen and hematoxylin for nuclear counterstaining. Normal rabbit serum and phosphate-buffered saline were employed instead of the first antibodies for the controls. To visualize the zG, zP, and zF on a single specimen of the specific case (see below), one-step double immunostaining was performed; a section was reacted in the medium containing both anti-P450aldo and anti-P45011β antibodies at the same dilutions described above and followed by the same processes described above, including the EnVision system and a peroxidase reaction.
Immunohistochemical Estimation of Adrenal Zonation and Grading of the zG
Adrenal zonation was immunohistochemically defined according to a previous description (Aiba et al. 2005) as follows: The following results for [P450aldo, P45011β, and 3βHSD] were used for describing the zonation: [(++), (−), (++)], [(−), (++), (++)], and [(−), (++), (−)] were classified as the zG, zF, and zR, respectively (Fig. 1). In addition, a new zone with the staining result of [(−), (−), (++)/(+)] was classified as the progenitor zone (zP; Aiba et al. 2005). In this study, the extent of zG occupancy of the adrenal circumference was semiquantitatively evaluated. First, the zG occupancy was estimated as either >50% or ≤50% in each case (Table 1, right columns), and then further grading into one of four grades was performed as follows (Table 1, left columns): zG cells occupying 25% or less (grade 1), 50% or less (grade 2), 75% or less (grade 3), and more than 75% (grade 4) of the adrenal circumference on the glass slide. The grade in each case was correlated with the age of the patient.
Table 1.
Extent of Zona Glomerulosa Cell Occupancy of the Adrenal Circumference
| G1 | G2 | G3 | G4 | G1 + G2 | G3 + G4 | p Value | |
|---|---|---|---|---|---|---|---|
| Surgical | |||||||
| As ≥40 y | 17 | 1 | 0 | 0 | 18 | 0 | |
| Bs ≤26 y | 0 | 0 | 1 | 2 | 0 | 3 | <0.001* |
| Autopsy | |||||||
| Aa ≥46 y | 22 | 1 | 8 | 1 | 23 | 9 | |
| Ba ≤37 y | 0 | 0 | 2 | 6 | 0 | 8 | <0.001* |
| Surgical + autopsy | |||||||
| As + a ≥40 y | 39 | 2 | 8 | 1 | 41 | 9 | |
| Bs + a ≤37 y | 0 | 0 | 3 | 8 | 0 | 11 | <1 × 10−6* |
| Late adulthood | |||||||
| As (surgical) | 17 | 1 | 0 | 0 | 18 | 0 | |
| Aa (autopsy) | 22 | 1 | 8 | 1 | 23 | 9 | <0.02* |
G1, ≤25%; G2, 25% to ≤50%; G3, 50% to ≤75%; G4, >75%; G1 + G2 ≤50%, G3 + G4 >50%.
Statistically significant (Fisher’s exact test).
Statistical analysis was performed by Fisher’s exact test with p values less than 0.05 significant.
Results
The immunohistochemically defined zG, zP, and zF cells did not correspond to the HE staining–defined zG-like cells or zF-like cells; these latter two types of cells could be identified as zG, zP, or zF cells on immunohistochemical staining (Fig. 2). ZG cells, which exclusively express P450aldo, either formed clusters in the subcapsular area or developed from the subcapsular area downward to constitute the upper portion of the cords, an arrangement that was particularly but not exclusively found in the late-adulthood adrenals (i.e., 40 years old or older). zP cells expressed neither P450aldo, a zG marker, nor P45011β, a marker of zF and zR, but instead expressed 3βHSD and P450scc, which they shared with both zG and zF cells (Fig. 2a–c). The zP was inconspicuous from birth through preadrenarche into puberty; the cortex showed the zonal arrangement of the zG and zF with an underdeveloped zR until adrenarche (Fig. 2h–o). zP cells appeared from adolescence to young adulthood and gradually increased until the fourth decade of life (Fig. 2p–s). One-step double immunostaining for P450aldo and P45011β of an adrenal section obtained from a 26-year-old patient revealed that the immunonegative zP separated the zG from zF, both of which were immunopositive (Fig. 2r). The localization of the zG in the section was confirmed by positive staining for P450aldo in serial adrenal sections (Fig. 2s).
Figure 2.
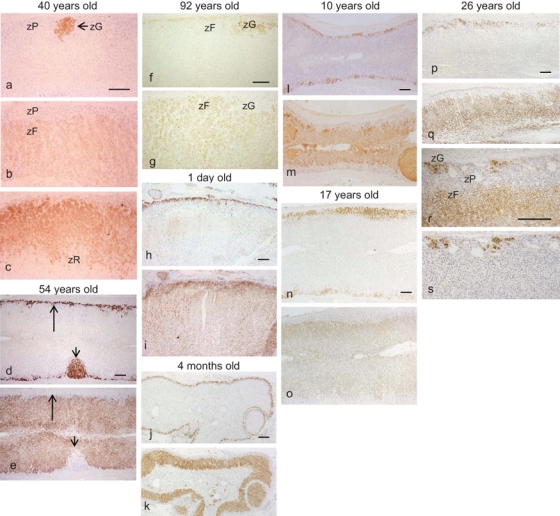
Immunohistochemistry of steroidogenic enzymes according to age: (A) late adulthood (a–g), (B-1) newborns to adolescence (h–o), and (B-2) early adulthood (transitional; p–s). P450aldo (a, d, f, h, j, l, n, p, s), P45011β (b, e, g, i, k, m, o, q), 3βHSD (c), and one-step double immunostaining of P450aldo and P45011β (r). (A) In the adrenals from a 40-year-old patient with renal cell carcinoma (a–c), the great majority of the subcapsular area is replaced by zP cells, which are negative for P450aldo and P45011β but positive for 3βHSD. zG cells occupy less than 25% of the adrenal circumference. The adrenal from a 54-year-old autopsy patient showing secondary aldosteronism and response to severe stress (d, e). Thin-layered zG cells (long arrows) and delta-shaped zG cell clusters (short arrows) may be derived from the zP and the preexisting zG cells, respectively, similar to the findings shown in a–c. Adrenal from a 92-year-old patient with severe stress (f, g). The P450aldo staining pattern is the same as that shown in a, but P45011β immunohistochemistry revealed that the subcapsular cortical zone is replaced by zF cells, except for a few zG clusters due to severe stress. (B-1) ZG/zF zonation is preserved in the adrenals from a newborn (1 day old; h, i), an infant (4 months old; j, k), a preadrenarche subject (10 years old; l, m), and an adolescent subject (17 years old; n, o). (B-2) In the adrenal from a young adult (26 years old), some decrease of the zG with partial replacement by the zP is evident, although the zonal arrangement of the zG is still relatively preserved (p, q). One-step double immunostaining for P450aldo and P45011β reveals that scattered subcapsular immunoreactive cells and lower zonal immunoreactive cells are separated by the immunonegative zonal cells in some areas (r), and staining of serial sections for P450aldo (s) confirms the origin of the cells as being the zG, zF, and zP. zG, zona glomerulosa; zF, zona fasciculata; zP, progenitor zone. Bar = 300 µm.
In surgical specimens of adults aged 40 years or older, the zP cells constituted the uppermost zone of the adrenal cortex, with only scattered zG clusters (Fig. 2a–c). The same was true in the great majority of autopsy cases as well. In some autopsy cases, however, the uppermost portion of the cortex was replaced by zG cells due possibly to secondary aldosteronism (enhanced RAAS activity and/or hyperkalemia; Fig. 2d,e). In some others who had experienced severe stresses such as sepsis, zF cells were the predominant cell type in the subcapsular area (Fig. 2f,g).
Semiquantitatively (Table 1), all of the adrenals from patients who were 37 years old or younger, irrespective of whether they were from surgical or autopsy cases, showed either intact zonation of the zG (i.e., G4; 2 Bs and 6 Ba cases) or the zG occupying more than half of the adrenal circumference (G3; 1 Bs and 2 Ba cases) (relatively intact zonation of the zG). In surgically resected adrenals from adults who were age 40 years or older, all but one of the cases (17 As cases) showed only scattered zG cell clusters (G1), and a great extent of the subcapsular area was occupied by zP cells (a possible involution of the zG and development of the zP). The remaining one case (one As) from an adult older than age 40 years showed G2 (i.e., a zG occupying less than 50% of the adrenal circumferences). Among the 32 autopsy cases of adults who were age 46 years or older, the great majority (23 Aa cases) showed zG changes similar to those observed in the surgical cases (G1 in 22 cases and G2 in 1 case), whereas 8 cases showed G3 and 1 showed G4 (i.e., zG zonation; total, 9 Aa cases), possibly because of secondary aldosteronism. The differences in the adrenal zG involution between the two surgical groups (i.e., the late adult As and the younger Bs group; p<0.001), between two autopsy groups (i.e., the late adult Aa and the younger Ba; p<0.001), and between the two age groups overall (i.e., age 40 years or older: As + a; 37 years or younger: Bs + a; p<1 × 10−6) were statistically significant (Fisher’s exact test). The difference in the finding between the adult surgical cases and adult autopsy cases was also significant (p<0.02).
Finally, we summarized our results in Figure 3a–e according to cell migration theory (see the textbooks and reviews by Symington 1969; Wolkersdorfer and Bornstein 1998; Vinson 2003, 2004; Carney and Lloyd 2007; Kim and Hammer 2007). The adrenal cortices from the age of newborns to adolescence consist of homogeneous columns showing zG cells on the top, followed by zF cells (Fig. 3a). During the third and fourth decades of life, zP cells appear between the zG and zF, or partly occupy the top of the columns, and predominate over the zG cells. Adrenals are composed of three types of cortical columns: the zG cell–topped columns, which may be RAAS sensitive, and the zP cell–topped columns, which can potentially transform into either zG cell–topped ones or zF cell–topped ones, according to the internal environment. At age 40 and later, zP cell–topped columns account for three-quarters or more (grade 1), forming a zonal arrangement with scattered clusters of zG cell–topped columns (Fig. 3b). Under conditions of secondary hyperaldosteronism (ascites, severe chronic heart failure, etc.) or hyperkalemia (chronic renal failure, tumor lysis syndrome, etc.), zP cells differentiate into zG cells, which then show a zonal arrangement (Fig. 3c,d). On the other hand, under severe stresses such as severe sepsis, the zP cells differentiate into zF cells (Fig. 3e), in addition to lipid depletion of the preexisting zF cells.
Figure 3.
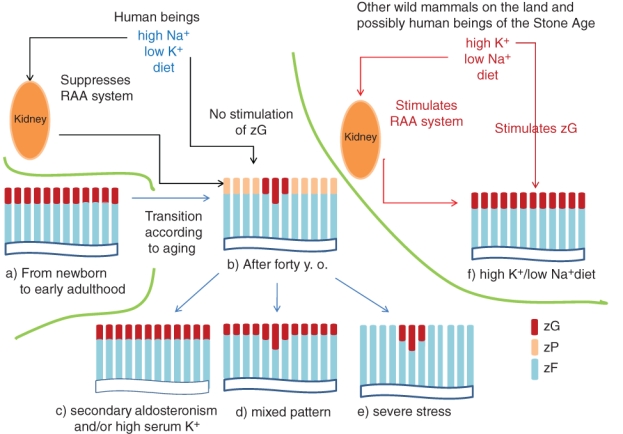
Schematic diagram of the zonation of the upper adrenal areas according to age (a, b), endocrinological environment (c–e), and postulated adrenal zonation in wild mammals living on the land or in human beings of the Stone Age (f). Brown, light brown, and blue indicate the zG, zP, and zF, respectively. The difference between b and f is interpreted by the different dietary salt and potassium content. The zona reticularis (zR) is omitted from this figure because of the complicated staining pattern depending on the age and functions (i.e., adrenarche, stresses, etc.), and this is not the main theme of this article. Although the adrenals are functionally separated according to adrenal zones, they morphologically consist of zG cell–topped, zF cell–followed, and zR cell–bottomed cortical columns according to centripetal/centrifugal theories, the integration of which shows zonal arrangement. zG, zona glomerulosa; zF, zona fasciculata; zP, progenitor zone; RAA, renin-angiotensin-aldosterone.
Discussion
The present study revealed that the immunohistochemically defined zG, zP, and zF cells did not correspond to the HE staining–defined zG-like and zF-like cells. Any of the zG, zP, or zF cells could form subcapsular small cell clusters or occupy the upper portion of the cortical cords, according to age and endocrinological environment (i.e., secondary aldosteronism or severe stresses). Appearance of P450aldo-positive cells in the cortical cords (apparent zF) is not surprising because spironolactone bodies, a marker of aldosterone production (Conn and Hinerman 1977; Aiba et al. 1981), have previously been observed at the upper portion or rarely even the middle portion of the cortical cords (Aiba et al. 1981). Although the zG is relatively well developed in subjects in their 30s or younger, disappearance of most of the zG with a predominance of the zP in the uppermost adrenal cortex is characteristic of the adrenal glands in late adulthood (i.e., age 40 years or older), suggestive of zG involution. Thus, the human late adult adrenal cortex undergoes functional remodeling in addition to structural remodeling, the latter of which has long been morphologically observed (Symington 1969; Carney and Lloyd 2007). These observations might correspond to the descriptions in reports and endocrinological textbooks that the serum aldosterone levels and/or aldosterone production rate are the highest in newborns and then decrease with age (Dillon and Ryness 1975; Dillon et al. 1976; Pratt et al. 1988).
Adrenocortical structure and zonation have been described according to several theories, including zonal theory, cell migration theory (the centripetal and centripetal/centrifugal theories), and transformation field theory; cell migration theory is now the most widely accepted (see the textbooks and reviews by Symington 1969; Wolkersdorfer and Bornstein 1998; Vinson 2004; Carney and Lloyd 2007; Kim and Hammer 2007). The adrenal cortex is considered to be composed of cortical cell columns consisting of zG-, zF- and zR-type cells in both human and nonhuman mammals. With this view of adrenal structure, the present grading system of the extent of zG cell occupancy in the adrenal circumference can be redefined as follows: The extent of zG cell–topped columns relative to zP or zF cell–topped columns can be classified into four grades (Table 1).
Functional adrenal zonation has been studied extensively in rodents, with the major sites of adrenocortical proliferation described as the stem cell zone, progenitor cell zone, undifferentiated cell zone, intermediate zone, and undifferentiated zona glomerulosa (Mitani et al. 1994; Mitani et al. 2003; Peters et al. 1998; McNeill et al. 2005; Brennan et al. 2008). The results of the present study in humans suggest that the zP in humans has a major role in bidirectional differentiation into either the zG or the zF according to the endocrinological environment, rather than in relation to cellular proliferative activity. A few other immunohistochemical studies of human adrenocortical zonation have also been reported (Suzuki et al. 2000; Nishimoto et al. 2010). Nishimoto et al. (2010) reported adrenal zonation in nine adrenals removed with eight renal cell carcinomas and a urinary tract carcinoma, as well as the tumorous and nontumorous parts of 24 aldosteronomas and six cortisol-producing adenomas, using the same antibodies that recognize the same epitopes of P450aldo and P45011β as in our study. Their results of adrenocortical zonation, including the description of undifferentiated cells, are almost compatible with those reported for our late adult surgical cases, although they did not perform the quantitative and chronological descriptions of zG and undifferentiated cells. Because we also employed both adrenals of younger patients, including those of newborns and autopsy patients, we believe that our descriptions are comprehensive. Suzuki et al. (2000) reported adrenal zonation of 24 autopsy cases ranging in age from 7 months to 62 years. Their main concern was immunohistochemical studies of adrenarche. In addition, because their immunohistochemical definition of zG was the absence of P450c17, they could not differentiate between the zG and the zP (Fig. 1).
The results of the present study may serve as a reference for evaluating the zG/zG-zP status and diagnosing/differentiating subtypes of primary aldosteronism, including idiopathic hyperaldosteronism, and secondary aldosteronism. In addition, our results may contribute to the understanding of adrenal zonation in rodents, which has been most extensively studied and clarified particularly from the aspect of cell kinetics when compared with relatively few studies on human adrenals (see below), although adrenal zonal structures are much different among the species.
Finally, we describe a hypothesis that the decrease in the extent of the zG and development of the zP (possible zG involution) might be related to the high-sodium/low-potassium diets of human beings today. Both angiotensin II in RAAS and serum potassium directly stimulate the zG to produce aldosterone, and the former is stimulated or suppressed by low or high dietary sodium, respectively (Dluhy et al. 1972; Williams et al. 1972). The results of the present study might be highly complementary to recent studies of the correlation between the rise in blood pressure with age/hypertension and excessive ingestion of salt (Intersalt Cooperative Research Group 1988; O’Shaughnessy and Karet 2004; Adrogue and Madias 2007; He and MacGregor 2009; He et al. 2009; Bibbins-Domingo et al. 2010). Human beings of the Stone Age or human ancestors, like wild mammals of the land other than human beings, have been estimated to have ingested a high-potassium/low-sodium diet (estimated as low as 1.5–0.1 g/d of salt; Eaton et al. 1996; O’Shaughnessy and Karet 2004; He and MacGregor 2009). RAAS works actively and stimulates the adrenals because of the necessity of sodium reabsorption by the kidney (Fig. 3f). In addition, high dietary potassium may directly stimulate the adrenals in a food-dependent manner (Fig. 3f). Thus, the internal environment is supportive of the maintenance of a zonal arrangement of the zG in human ancestors and wild animals living on land.
On the other hand, human beings today ingest high-sodium/low-potassium diets through cooking or by eating processed foods (Intersalt Cooperative Research Group 1988; O’Shaughnessy and Karet 2004; Adrogue and Madias 2007; He and MacGregor 2009; He et al. 2009; Bibbins-Domingo et al. 2010). The daily salt intake for the average man and woman has been estimated as 10.4 g/d and 7.3 g/d in the United States (US Department of Agriculture, Agricultural Research Service; Bibbins-Domingo et al. 2010) and 11.6 g/d and 9.9 g/d in Japan (Ministry of Health, Labour and Welfare Japan 2010), respectively. The RAAS is relatively suppressed, and the relatively low dietary potassium results in lack of stimulation of the adrenals and involution of the zG in late adulthood (age 40 years or older), and only RAAS-sensitive adrenocortical columns remain in clusters, and potentially bidirectional zP-topped columns predominate and form the subcapsular zP (Fig. 3b). Involution of the zG might be buffered by zG cell hyperplasia along the cortical cords within RAAS-sensitive zG-topped columns (Fig. 3b). In addition, presence of the zP is useful not only as a zG reservoir at the time of secondary aldosteronism but also as a second zF reservoir next to clear cell-type zF cells, lipid depletion of which has been a well-known phenomenon during severe stresses of pandemic infections, severe sepsis, and so on (Fig. 3c; Symington 1969; Aiba et al. 1979). Then, why do adrenals from the younger age group (from newborn to adolescence) not show a decrease of the zG population despite the high-sodium/low-potassium diet? We may explain this based on evolutional medicine, suggesting that plasma volume expansion might be favorable to human development and growth to a reproductive age.
Acknowledgments
We thank Dr. Fumiko Mitani for her kind offering of antibodies and Eiji Iizuka, MT, for his technical assistance.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the authorship and/or publication of this article.
The author(s) received no financial support for the research and authorship of this article.
References
- Adrogue HJ, Madias NE. 2007. Sodium and potassium in the pathogenesis of hypertension. N Engl J Med. 356:1966–1978 [DOI] [PubMed] [Google Scholar]
- Aiba M, Fujibayashi M. 2005. Histopathological diagnosis and prognostic factors in adrenocortical carcinoma. Endocrine Pathol. 16:13–22 [DOI] [PubMed] [Google Scholar]
- Aiba M, Kageyama K, Koide O, Tokugawa H. 1979. Effect of exogenous glucocorticoids given during the terminal stages on the adrenocortical histology. Acta Pathol Jpn. 29:177–182 [DOI] [PubMed] [Google Scholar]
- Aiba M, Suzuki H, Kageyama K, Murai M, Tazaki H, Abe O, Saruta T. 1981. Spironolactone bodies in aldosteronomas and in the attached adrenals: enzyme histochemical study of 19 cases of primary aldosteronism and a case of aldosteronism due to bilateral diffuse hyperplasia of the zona glomerulosa. Am J Pathol. 103:404–410 [PMC free article] [PubMed] [Google Scholar]
- Bibbins-Domingo K, Chertow GM, Coxson PG, Moran A, Lightwood JM, Pletcher MJ, Goldman L. 2010. Projected effect of dietary salt reductions on future cardiovascular disease. N Engl J Med. 362:590–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan CH, Chittka A, Barker S, Vinson GP. 2008. Eph receptors and zonation in the rat adrenal cortex. J Endocrinol. 198:185–191 [DOI] [PubMed] [Google Scholar]
- Carney JA, Lloyd RV. 2007. Adrenal. In: Mills SE, editor. Histology for pathologist. 3rd ed Philadelphia: Lippincott Williams & Wilkins; p. 1167–1188 [Google Scholar]
- Conn JW, Hinerman DL. 1977. Spironolactone-induced inhibition of aldosterone biosynthesis in primary aldosteronism: morphological and functional studies. Metabolism. 26:1293–1307 [DOI] [PubMed] [Google Scholar]
- Dillon MJ, Gillin MEA, Ryness JM, de Swiet M. 1976. Plasma renin activity and aldosterone concentration in the human newborn. Arch Dis Child. 51:537–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon MJ, Ryness JM. 1975. Plasma renin activity and aldosterone concentration in children. Br Med J. 4:316–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dluhy RG, Axelrod L, Underwood RH, Williams GH. 1972. Studies of the control of plasma aldosterone concentration in normal man: II. Effect of dietary potassium and acute potassium infusion. J Clin Invest. 51:1950–1957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton SB, Eaton SB, III, Konner MJ, Shostak M. 1996. An evolutionary perspective enhances understanding of human nutritional requirements. J Nutr. 126:1732–1740 [DOI] [PubMed] [Google Scholar]
- He FJ, MacGregor GA. 2009. A comprehensive review on salt and health and current experience of worldwide salt reduction programmes. J Hum Hypertension. 23:363–384 [DOI] [PubMed] [Google Scholar]
- He FJ, Marciniak M, Visagie E, Markandu ND, Anand V, Dalton RN, MacCregor GA. 2009. Effect of modest salt reduction on blood pressure, urinary albumin, and pulse wave velocity in white, black, and Asian mild hypertensives. Hypertension. 54:482–488 [DOI] [PubMed] [Google Scholar]
- Intersalt Cooperative Research Group 1988. Intersalt: an international study of electrolyte excretion and blood pressure. Results for 24 hour urinary sodium and potassium excretion. Br Med J. 297:319–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim AC, Hammer GD. 2007. Adrenocortical cells with stem/progenitor cell properties: recent advances. Mol Cell Endocrinol. 265–266:10–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeill H, Whitworth E, Vinson GP, Hinson JP. 2005. Distribution of extracellular signal-regulated protein kinase 1 and 2 in the rat adrenal and their activation by angiotensin II. J Endocrinol. 187:149–157 [DOI] [PubMed] [Google Scholar]
- Mitani F, Mukai K, Miyamoto H, Suematsu M, Ishimura Y. 2003. The undifferentiated cell zone is a stem cell zone in adult rat adrenal cortex. Biochim Biophys Acta. 1619:317–324 [DOI] [PubMed] [Google Scholar]
- Ministry of Health, Labour and Welfare, Japan: Summary on results of investigation on national health and nutrient in 2009; Diet and exercise. 2010. (in Japanese). http://www.mhlw.go.jp/stf/houdou/2r9852000000xtwq-img/2r9852000000xu2r.pdf
- Mitani F, Suzuki H, Hata J, Ogishima T, Shimada H, Ishimura Y. 1994. A novel cell layer without corticosteroid-synthesizing enzymes in rat adrenal cortex: histochemical detection and possible physiological role. Endocrinology. 135:431–438 [DOI] [PubMed] [Google Scholar]
- Nishimoto K, Nakagawa K, Li D, Kosaka T, Oya M, Mikami S, Shibata H, Itoh H, Mitani F, Yamazaki T, et al. 2010. Adrenocortical zonation in humans under normal and pathological conditions. J Clin Endocrinol Metab. 95:2296–2305 [DOI] [PubMed] [Google Scholar]
- Ogishima T, Shibata H, Shimada H, Mitani F, Suzuki H, Saruta T, Ishimura Y. 1991. Aldosterone synthase cytochrome P-450 expressed in the adrenals of patients with primary aldosteronism. J Biol Chem. 266:10731–10734 [PubMed] [Google Scholar]
- O’Shaughnessy KM, Karet FE. 2004. Salt handling and hypertension. J Clin Invest. 113:1075–1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters B, Clausmeyer S, Obermuller N, Woyth A, Kranzlin B, Gretz N, Peters J. 1998. Specific regulation of StAR expression in the rat adrenal zona glomerulosa: an in situ hybridization study. J Histochem Cytochem. 46:1215–1221 [DOI] [PubMed] [Google Scholar]
- Pratt JH, Hawthorne JJ, Debono DJ. 1988. Reduced urinary aldosterone excretion rates with normal plasma concentrations of aldosterone in the very elderly. Steroids. 51:163–171 [DOI] [PubMed] [Google Scholar]
- Suzuki T, Sasano H, Takeyama J, Kaneko C, Freije WA, Carr BR, Rainey WE. 2000. Developmental changes in steroidogenic enzymes in human postnatal adrenal cortex: immunohistochemical studies. Clin Endocrinol. 53:739–747 [DOI] [PubMed] [Google Scholar]
- Symington T. 1969. Functional pathology of the human adrenal gland. London: Livingstone [Google Scholar]
- Vinson GP. 2003. Adrenocortical zonation and ACTH. Microsc Res Techn. 61:227–239 [DOI] [PubMed] [Google Scholar]
- Vinson GP. 2004. Glomerulosa function and aldosterone synthesis in the rat. Mol Cell Endocrinol. 217:59–65 [DOI] [PubMed] [Google Scholar]
- Williams GH, Cain JP, Dluhy RG, Underwood RH. 1972. Studies of the control of plasma aldosterone concentration in normal man: I. Response to posture, acute and chronic volume depletion, and sodium loading. J Clin Invest. 51:1731–1742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolkersdorfer GW, Bornstein SR. 1998. Tissue remodeling in the adrenal gland. Biochem Pharmacol. 56:163–171 [DOI] [PubMed] [Google Scholar]


