Abstract
Lipid bodies (LBs), also known as lipid droplets, have increasingly been recognized as functionally active organelles linked to diverse biological functions and human diseases. These organelles are actively formed in vivo within cells from the immune system, such as macrophages, neutrophils, and eosinophils, in response to different inflammatory conditions and are sites for synthesis and storage of inflammatory mediators. In this review, the authors discuss structural and functional aspects of LBs and current imaging techniques to visualize these organelles in cells engaged in inflammatory processes, including infectious diseases. The dynamic morphological aspects of LBs in leukocytes as inducible, newly formable organelles, elicitable in response to stimuli that lead to cellular activation, contribute to the evolving understanding of LBs as organelles that are critical regulators of different inflammatory diseases, key markers of leukocyte activation, and attractive targets for novel anti-inflammatory therapies.
Keywords: lipid droplet, leukocytes, macrophages, inflammation, innate immunity, eicosanoids, adipose differentiation-related protein, electron microscopy, fluorescence microscopy, imaging techniques
Lipid bodies (LBs), also known as lipid droplets, are lipid-rich organelles distributed in the cytoplasm of most eukaryotic cells. In the past, LBs were largely associated with lipid storage, but it is now recognized that LBs are dynamic and functionally active organelles, involved in a variety of functions such as lipid metabolism, cell signaling, and inflammation (reviewed in Beller et al. 2010; Bozza et al. 2007; Farese and Walther 2009).
LBs are roughly spherical organelles with a distinctive architecture. In contrast to membranous organelles and cytoplasmic vesicles that have an aqueous content surrounded by a phospholipid bilayer membrane, LBs are composed of a neutral lipid core surrounded by a monolayer of phospholipids with associated proteins (reviewed in Bozza et al. 2007). LBs lack, therefore, a delimiting unit membrane structure (Fig. 1). LB-specific structural proteins, the PAT family of proteins—perilipin, adipose differentiation-related protein (ADRP; Brasaemle et al. 2004), and tail-interacting protein of 47 kDa (TIP47; Wolins et al. 2001)—are found at the circumferential rim of LBs. Moreover, a number of small GTPases of the Rab family, considered critical regulators of vesicular traffic and organelle interaction, and a variety of other proteins are described in LBs (Brasaemle et al. 2004; Digel et al. 2010; Wan et al. 2007).
Figure 1.

Lipid bodies (LBs) have a unique architecture. (A) LBs are delimited by a monolayer of phospholipids differing from the classical structural organization (phospholipid bilayer membrane) of all other organelles, cytoplasmic vesicles, and plasma membrane. (B) View of an LB within a human blood eosinophil by conventional transmission electron microscopy. N, nucleus. Scale bar = 500 nm.
We have been investigating LB formation, structure, and function within inflammatory cells by both light (Bozza, Payne, Goulet, et al. 1996; D’Avila et al. 2006; Melo et al. 2003) and electron microscopy (Dvorak 2005; Dvorak et al. 1983; Melo 2009; Melo, Fabrino, et al. 2006; Wan et al. 2007), including wet scanning electron microscopy (SEM; Melo, Sabban, et al. 2006). In this review, we discuss structural and functional aspects of LBs and imaging techniques to visualize these organelles in cells associated with inflammatory responses. The increased knowledge of LBs as inflammatory organelles is important for understanding their potential roles in cells, in both health and disease.
Lipid Body Biogenesis and Metabolism
As noted, LBs are common organelles of eukaryotic cells, but their biogenesis is not fully understood. Although it is accepted that LBs originate from the endoplasmic reticulum (ER), there are different models to explain how these organelles are formed. The classical model of LB biogenesis has neutral lipids accumulating between the cytoplasmic and luminal leaflets of ER membranes followed by the budding off of LBs surrounded by a phospholipid monolayer derived from the cytoplasmic leaflets of ER membranes (Murphy 2001). In another model, proposed from studies using human eosinophils and other leukocytes, LBs would be formed by incorporating multiple loops of ER membranes (both cytoplasmic and luminal leaflets of membranes within the developing LB). Accumulations of neutral lipids would develop among the membranes within LBs (Wan et al. 2007). Once formed, cytoplasmic LBs are likely to increase their volume either by localized lipid synthesis (Kuerschner et al. 2008) or by fusion of LBs (Olofsson et al. 2009).
Stored lipids are mobilized upon cellular demand for energy by the activity of specific lipases and other metabolic enzymes. LBs are the location of key enzymes involved in cholesterol metabolism (squalene epoxidase, 17-β-hydroxysteroid dehydrogenase, lanosterol synthase) and for fatty acid synthesis (acetyl-coenzyme A carboxylase, NADH cytochrome b5 reductase), indicating that both anabolic and catabolic steps in lipid metabolism take place at LBs (Brasaemle et al. 2004; Fujimoto et al. 2004). It was also demonstrated that the PAT domain proteins are essential for lipid storage and metabolism in adipocytes (Londos et al. 2005). However, the origin of the protein coat of LBs and how these proteins attach or insert into the LB monolayer of phospholipids are not completely understood. In addition to structural and metabolic proteins, a diversity of other proteins has been identified within LBs, especially by proteomic studies, suggesting that LBs are indeed multifunctional organelles and sites of regulatory events. The LB protein composition has been discussed in reviews (Digel et al. 2010; Hodges and Wu 2010).
Lipid Body Accumulation within Inflammatory Cells
A major advance in LB biology is the recognition of LBs as inducible, newly formable organelles, elicitable in response to inflammatory stimuli. Different from cells associated with lipid storage and lipid-laden cell lineages that have a large number of cytoplasmic LBs, resting leukocytes have few LBs, but these cells can be rapidly stimulated to form new LBs (reviewed in Bozza et al. 2007).
LB biogenesis is a process that happens in vivo during inflammatory reactions of different causes. Increased cytoplasmic lipid accumulation in leukocytes and other cells has been observed in a number of clinical and experimental inflammatory and infectious diseases, including macrophages in atherosclerotic lesions (Paul et al. 2008; Schmitz and Grandl 2008), leukocytes from joints of patients with inflammatory arthritis (Bozza, Payne, Morham, et al. 1996), bronchoalveolar lavage of patients with acute respiratory distress syndrome (Triggiani et al. 1995), eosinophils in allergic inflammation (Vieira-de-Abreu et al. 2010), blood and peritoneal leukocytes in bacterial sepsis (Pacheco et al. 2002; Pacheco et al. 2007), pleural effusions, skin or granulomas from mycobacterial infection (Cardona et al. 2000; D’Avila et al. 2006; Mattos et al. 2010), and cancerous cells (Accioly et al. 2008).
Tuberculosis caused by Mycobacterium tuberculosis remains one of the leading causes of mortality in the world, with around 2 million deaths each year (World Health Organization 2010). Tuberculosis is characterized by a tight interplay between M. tuberculosis and host cells within cellular aggregates (granulomas). The induction of foamy macrophages—a granuloma-specific cell population characterized by its high lipid content compartmentalized in LBs—has been extensively reported during the progression of tuberculosis caused by M. tuberculosis in both humans and experimental settings (Cardona et al. 2000; Peyron et al. 2008). In experimental studies with Mycobacterium bovis bacillus Calmette-Guérin (BCG), it was found that this pathogen is capable of inducing a dose- and time-dependent increase on LB formation within pleural macrophages (D’Avila et al. 2006). LB formation initiates rapidly, and significantly increased LB numbers are noted within 1 hr, reaching maximum levels within 24 hr, and remaining increased for at least 15 days after BCG infection (D’Avila et al. 2006).
LB accumulation was also identified inside dermal macrophages (Mattos et al. 2010) and Schwann cells from nerves (Mattos et al. 2011) of lepromatous leprosy patients and in both peritoneal and heart macrophages during the acute phase of the experimental infection with the parasite Trypanosoma cruzi (Melo 2009; Melo et al. 2003; Melo, Fabrino, et al. 2006), the causal agent of Chagas disease, an infection with a great impact on public health in Latin America (Fabrino et al. 2011). For example, although control peritoneal macrophages present ~2.19 ± 0.4 (mean ± SEM) LBs/cell, peritoneal macrophages from T. cruzi–infected animals show ~18.09 ± 1.4 at day 12 of infection. Accordingly, inflammatory macrophages recruited to the heart, a target organ of Chagas disease, exhibit a significant increase in LB numbers (Fig. 2; Melo et al. 2003).
Figure 2.

Lipid bodies (LBs) are actively formed within heart macrophages following infection with the parasite Trypanosoma cruzi. (A) A non-activated macrophage shows small numbers of LBs in the cytoplasm. (B) At day 12 of infection, an inflammatory macrophage exhibits an increased number of LBs and surface extensions compared to the control. Note a clear interaction between the macrophage and a lymphocyte. Rats were inoculated with a Y strain of T. cruzi as before (Melo and Machado 2001). Samples were processed for transmission electron microscopy. N, nucleus. Scale bar = 1 µm (A and B). Panels A and B have been reprinted from Melo and Machado (2001) and Melo, Fabrino, et al. (2006), respectively, with permission.
The accumulation of LB-enriched macrophage foam cells in atherosclerotic blood vessel intima, forming the fatty streak, is the hallmark of atherosclerosis. Because it is the earliest recognizable lesion of atherosclerosis, much attention is focused on understanding the etiology of this phenomenon and the roles of foam cells in disease. The formation of foam cells involves complex and multistep mechanisms that depend on different signaling pathways regulating lipid influx, metabolism, storage, and mobilization (Schmitz and Grandl 2008). Uncontrolled modified LDL uptake by macrophages through scavenger receptors causes triglyceride and cholesterol loading, followed by cholesterol esterification mediated by acyl-coenzyme A/cholesterol acyltransferase and storage of cholesteryl esters (CEs) in cytoplasmic LBs (Schmitz and Grandl 2008). Recognition and activation of scavenger receptors, mostly CD36, by modified low-density lipoprotein (LDL) play major roles in lipid accumulation in macrophages (Rahaman et al. 2006). Lipid-derived molecules generated in the process of LDL oxidation are also involved in LB formation, including platelet-activating factor (PAF)–like molecules and azelaoyl-phosphatidylcholine (de Assis et al. 2003).
Monocyte chemoattractant protein (MCP-1/CCL2), a key endogenous mediator involved in the pathogenesis of macrophage-driven inflammation such as atherosclerosis and sepsis, is centrally involved in the regulation of macrophage LB biogenesis in oxidized LDL- and lipopolysaccharide (LPS)–induced inflammation as well as in experimental sepsis (Pacheco et al. 2007; Silva et al. 2009). MCP-1-driven ADRP-enriched LB accumulation is a regulated process that depends on an MCP-1 receptor, CCR2, and downstream signaling through MAP- and PI3-kinases and cytoskeleton dynamics (Pacheco et al. 2007). ADRP has been implicated in atherosclerosis progression as increased ADRP levels are observed in atherogenic plaques (Nuotio et al. 2007), and ADRP gene deletion leads to reduced numbers of LBs in foam cells in atherosclerotic lesions and protection against atherosclerosis in apolipoprotein E–deficient mice (Paul et al. 2008). In addition, adipocytokines, including leptin and resistin, were also shown to modulate LB formation in macrophages and may participate in the mechanisms of foam cell formation (Maya-Monteiro et al. 2008; Xu et al. 2006).
Hepatitis C virus (HCV) is the major causative pathogen associated with liver cirrhosis and hepatocellular carcinoma. Hepatic steatosis, characterized as the intracellular accumulation of lipids into LBs, is a common feature of chronic HCV. In addition to being markers for steatosis, LBs are targeted by the viral core protein and may represent sites for initiating assembly and production of nascent virions (reviewed in McLauchlan 2009). Similarly, increased LBs are observed in macrophages and hepatocytes during dengue virus (DENV) infection, an emerging viral disease transmitted by arthropods to humans in tropical countries (Assuncao-Miranda et al. 2010). Newly formed LBs in DENV-infected cells accumulate the capsid protein on the LB surface, and this localization was shown to be required for infectious particle formation (Assuncao-Miranda et al. 2010; Samsa et al. 2009). Moreover, pharmacological inhibition of LB formation greatly decreases DENV and HCV replication, suggesting LBs as a target for antiviral strategies.
As noted, distinct signaling pathways can trigger LB formation within leukocytes. Specific pathogen- and ligand-initiated, receptor-mediated pathways activate intracellular signaling that leads to enhanced LB formation. For instance, M. bovis BCG induces toll-like receptor 2 (TLR2)–mediated formation of LBs in macrophages (D’Avila et al. 2006); PAF, through its receptor, induces LB formation in neutrophils and eosinophils (Bozza, Payne, Goulet, et al. 1996; Bozza et al. 1997); and the chemokines eotaxin (CCL11) and RANTES (CCL5), acting via CCR3 receptors, stimulate LB formation in eosinophils (Bandeira-Melo et al. 2001). Allergic inflammation induces in vivo biogenesis of LBs within recruited eosinophils and occurs in a mechanism largely mediated by a cross-talk of macrophage migration inhibitory factor (MIF), with eotaxin/RANTES acting via CCR3 receptors and PGD2 (Vieira-de-Abreu et al. 2010). Translational and transcription-dependent mechanisms are also involved in LB formation. Characterized mechanisms involve the activation of the mammalian target of rapamycin (mTOR) pathway of translational control (Maya-Monteiro et al. 2008) and the transcription factors SRBP and peroxisome proliferator-activated receptor (PPAR). PPAR-γ, which directly regulates the expression of several genes participating in fatty acid uptake, lipid storage, and the inflammatory response, including fatty acid synthase and ADRP (Chawla et al. 2001), and a role for PPAR-γ in LB biogenesis have been established in atherosclerotic and infectious-triggered reactions (Almeida et al. 2009; Chawla et al. 2001). A list of stimuli, including pathogens that can induce LB formation in inflammatory and other cells, is shown in Table 1.
Table 1.
Lipid body formation in inflammatory and other cells is induced by different stimuli
| Mammalian Cells | ||||||
|---|---|---|---|---|---|---|
| Stimuli | Eosinophils | Macrophages | Neutrophils | Schwann Cells | Other cells | Refs. |
| unstimuladed | + | + | + | + | + | (Bozza et al. 2007; Mattos et al. 2011; Murphy 2001) |
| Fatty acids | ||||||
| unsaturated Fatty acids | + | + | + | n.d. | n.d. | (Bozza et al. 1996b; Weller et al. 1991, 1999) |
| saturated fatty acids | − | − | − | n.d. | n.d. | |
| Phospholipids | ||||||
| PAF | + | + | + | n.d. | n.d. | (Bozza et al. 1996a, 1997; de Assis et al. 2003) |
| Lyso-PAF | − | n.d. | − | n.d. | n.d. | |
| Eiscosanoids | ||||||
| 5-HETE | + | n.d. | + | n.d. | n.d. | (Bozza et al. 1996a; Mesquita-Santos et al. 2006) |
| PGD2 | + | − | − | n.d. | n.d. | |
| LTB4 | − | n.d. | − | n.d. | n.d. | |
| Hormones | ||||||
| Leptin | − | + | − | n.d. | n.d. | (Maya-Monteiro et al. 2008; Xu et al. 2006) |
| Resistin | − | + | − | n.d. | n.d. | |
| Cytokines | ||||||
| Eotaxin | + | n.d. | − | n.d. | n.d. | (Bandeira-Melo et al. 2001; Bartemes et al. 1999; Pacheco et al. 2007; Vieira-de-Abreu et al. 2010) |
| Rantes | + | n.d. | − | n.d. | n.d. | |
| IL-8 | − | n.d. | − | n.d. | n.d. | |
| IL-5 | + | n.d. | − | n.d. | n.d. | |
| IL-16 | + | n.d. | − | n.d. | n.d | |
| MIF | + | n.d. | n.d | n.d | n.d. | |
| MCP-1/CCL2 | n.d. | + | n.d. | n.d. | n.d. | |
| Bacteria and Derivates | ||||||
| Bacillus subtilis | − | − | − | n.d. | n.d. | (Cao et al., 2007; D’Avila et al. 2006; Mattos et al., 2010, 2011; Pacheco et al., 2002; Peyron et al., 2008) |
| Mycobacterium smegmatis | − | − | − | n.d. | n.d. | |
| Chlamydia pneumoniae | n.d. | + | n.d | n.d. | n.d. | |
| Mycobacterium bovis BCG | + | + | + | n.d. | n.d. | |
| Mycobacterium leprae | n.d. | + | n.d | + | n.d. | |
| Mycobacterium tuberculosis | n.d. | + | n.d. | n.d. | + | |
| LPS/LAM | + | + | + | + | n.d. | |
| Parasites | ||||||
| Trypanosoma cruzi | n.d. | + | n.d. | n.d. | + | (Charron and Sibley 2002; Jackson et al. 2004; Magalhaes et al. 2010; Melo et al. 2003; 2006a) |
| Toxoplasma gondii | n.d. | n.d. | n.d. | n.d. | + | |
| Plasmodium falciparum | n.d. | n.d. | n.d. | n.d. | + | |
| S. mansoni derivates | + | n.d. | n.d. | n.d. | n.d. | |
| Virus | ||||||
| Dengue virus | n.d. | + | n.d | n.d. | n.d. | (Barba et al. 1997; Samsa et al. 2009) |
| Hepatitis C virus | n.d. | n.d. | n.d. | n.d. | + | |
+induced; −, not induced; n.d., not determined; PAF, platelet-activating factor; PG, prostaglandin; BCG, bacillus Calmette-Guérin; LT, leukotriene; LPS, lipopolysaccharide; IL, interleukin; MCP-1/CCL2, monocyte chemoattractant protein; MIF, migration inhibitory factor; LAM, lipoarabinomannan; 5-HETE, 5-eicosatetraenoic acid.
LBs Are Sites for the Production of Inflammatory Mediators
Consistent with the role of leukocytes in inflammation, LBs formed by these cells constitute sites for the production of inflammatory mediators (eicosanoids). LBs within inflammatory cells contain arachidonyl lipids (Fig. 3A), which serve as precursors for eicosanoid synthesis, and all of the enzymes necessary for this synthesis, including cyclooxygenases (COX) (Fig. 3B), prostaglandin E2 (PGE2) synthase, 5- and 15-lypoxygenases (5-LO and 15-LO), and leukotriene C4 (LTC4) synthase, have been localized within LBs in activated cells. Both LB formation and the compartmentalization of enzymes within LBs are highly regulated cellular events involved in the heightened capacity of leukocytes to generate eicosanoids (reviewed in Bozza et al. 2007).
Figure 3.
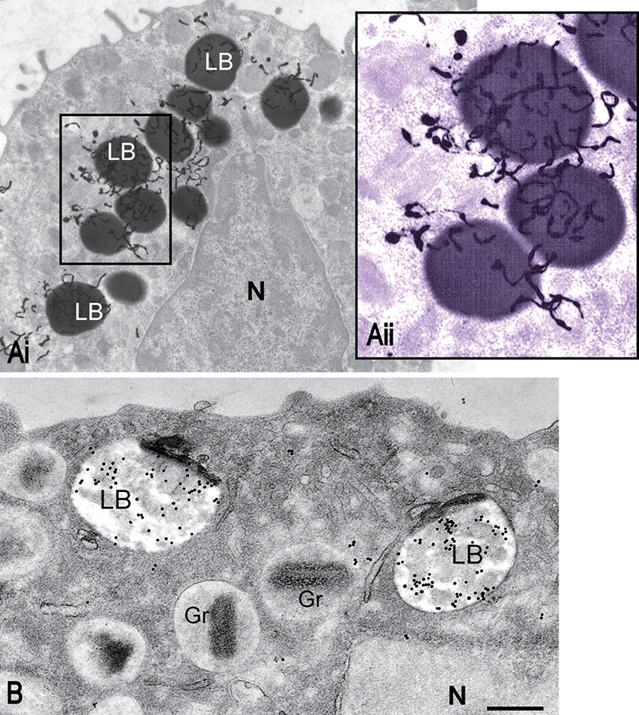
Human lipid bodies (LBs) observed with an ultrastructural method for autoradiography after a pulse of tritiated arachidonic acid (A) or immunogold to detect cyclooxygenase (COX) (B). (Ai) 3H-arachidonate incorporated by a human mast cell is localized predominantly to LBs. In Aii, LBs labeled with numerous silver grains are seen in higher magnification. (B) LBs within an eosinophil are extensively labeled for COX. N, nucleus; Gr, specific granules. Scale bar = 1 µm (Ai), 600 nm (Aii, B). Mast cells and eosinophils were prepared as in Dvorak et al. (1983) and Dvorak et al. (1994), respectively. Panel B is reprinted from Dvorak et al. (1994) with permission. A colored filter was applied on Bii using Adobe Photoshop.
Formation of LBs within inflammatory macrophages was positively correlated with augmented generation PGE2, indicating a role for these organelles in the heightened eicosanoid production observed during acute Chagas disease (Melo et al. 2003). Moreover, by intracellular immunofluorescent detection of immobilized newly formed eicosanoid, we have demonstrated that LBs were the predominant sites for PGE2 synthesis in macrophages infected with BCG (D’Avila et al. 2006). In accordance, foamy macrophages located in pneumonic areas of M. tuberculosis infection in mice were strongly positive for COX-2 and PGE2 synthase staining (Rangel Moreno et al. 2002).
The enhanced capacity of macrophages to generate PGE2 in the course of pathogen infection due to increased LB formation and compartmentalization of signaling to eicosanoid production within LBs may contribute to the mechanisms that intracellular pathogens have evolved to survive in host cells. Indeed, high concentration of PGE2 is a potent inhibitor of Th1-type response and of nitric oxide (NO) production favoring intracellular parasite growth (Freire-de-Lima et al. 2000; Renz et al. 1988). Interestingly, the uptake of apoptotic neutrophils by macrophages observed during infection with M. bovis BCG leads to transforming growth factor–β (TGF-β1) generation and PGE2-derived lipid body formation and may have modulator roles in mycobacterial pathogenesis (D’Avila et al. 2008). LBs could also function as a draining compartment to rapidly uptake and re-acetylate free arachidonic acid with a potentially detrimental outcome for the host cell.
LBs were also established as major intracellular locales for the formation of LTC4 in eosinophils during allergic conditions (Bandeira-Melo et al. 2001), LTB4 in neutrophils and macrophages during sepsis (Pacheco et al. 2002; Pacheco et al. 2007), and LTB4 and LTC4 in oxidized LDL-triggered foamy macrophages (Silva et al. 2009).
Eicosanoid formation within LBs is not limited to leukocytes or to inflammatory conditions. Cells that produce high quantities of eicosanoids under physiological conditions, including granulosa cells of periovulatory follicles involved in the production of PGE2 during ovulation (Seachord et al. 2005) and luteal steroid-producing and interstitial cells involved in regression of the corpus luteum (Arend et al. 2004), were demonstrated to exhibit high numbers of LBs containing eicosanoid-forming enzymes.
In addition to the function of LBs in eicosanoid synthesis, LBs may have other roles in the host response to pathogen infection. Cytokines, chemokines, and growth factors seem to be localized in LBs formed in activated leukocytes (reviewed in Bozza et al. 2007). For instance, evidence for localization of the proinflammatory cytokine tumor necrosis factor–α (TNF-α) in LBs of mast cells, eosinophils, and macrophages was provided by immunogold electron microscopy (EM) in colonic Crohn’s disease (Beil et al. 1995) and by immunofluorescence microscopy after in vivo LPS stimulation of peripheral blood neutrophils and monocytes from septic patients (Pacheco et al. 2002). However, to date, the association of cytokines with LBs is still limited, and whether and how LB-stored cytokines are released and whether they have signaling functions within LBs are questions that remain to be addressed.
Imaging LBs by Light Microscopy
Although LBs are considered structural markers of inflammatory cells in a range of diseases (Bozza et al. 2007), their identification by light microscopy in these and other cells has methodological limitations because LBs are not resistant to drying or to fixation and staining with alcohol-based reagents. For instance, cold methanol fixation extracts the majority of cellular phospholipids and promotes fusion of LBs (DiDonato and Brasaemle 2003), whereas May-Grünwald-Giemsa staining causes dissolution of LBs (Pacheco et al. 2002). In addition, the selection of appropriate fixation and permeabilization methods is critical to preserve LB proteins in situ using immunofluorescence (Ohsaki et al. 2005). Ohsaki and Cols demonstrate that conventional fixation of cultured cells with formaldehyde followed by treatment with Triton X-100 is not appropriate to label ADRP, TIP47, or Rab18 in LBs. On the other hand, these proteins can be visualized by fixation with formaldehyde followed by treatment with digitonin or saponin (Ohsaki et al. 2005). Fixation with a mixture of formaldehyde and a small concentration of glutaraldehyde (0.01%-0.025%) enables preservation of ADRP, even if solubilization with Triton X-100 is used (Ohsaki et al. 2005). In fact, LB proteins are likely solubilized by strong detergents or inadequate fixatives that can cause collapse of the shell of LB-associated proteins (DiDonato and Brasaemle 2003; Ohsaki et al. 2005). However, by using appropriate fixation procedures followed by methods of identification of lipids, LBs can be unmistakably identified in the cytoplasm (Bozza, Payne, Goulet, et al. 1996; DiDonato and Brasaemle 2003; Fowler and Greenspan 1985; Fukumoto and Fujimoto 2002). These techniques usually include fixation with paraformaldehyde or formaldehyde (formalin) and staining with osmium tetroxide (Fig. 4A) or labeling with different fluorescent probes such as BODIPY (Fig. 4B), Nile red (Fig. 4C), and oil red O or 1-pyrenedodecanoic acid (Fig. 4Dii; Melo et al. 2011).
Figure 4.

Cytoplasmic lipid bodies (LBs) imaged by bright-field (A) or fluorescence (B–E) microscopy within pleural macrophages from Mycobacterium bovis–BCG-infected mice. A large number of LBs are seen after staining with osmium tetroxide (A), BODIPY 493/503 (B), Nile red (C), 1-pyrenedodecanoic acid (P-96) (Dii) or immunolabeling for adipose differentiation-related protein (ADRP) (Di, Ei) and prostaglandin E2 (PGE2) (Eii). In Diii, a merged image shows co-localization after ADRP and P96 labeling, with ADRP mainly detected at the LB surface. In Eiii, co-localization of ADRP and (PGE2). Macrophages were infected and prepared by distinct techniques as described by D’Avila et al. (2006). Panels Ei to Eiii have been reprinted from D’Avila et al. (2006) with permission. Scale bar (A–E) = 10 µm.
Osmium tetroxide binds to unsaturated lipids and phospholipid head groups and is reduced by organic materials to elemental osmium, a permanent black substance, easily visible by bright-field microscopy (Fig. 4A). BODIPY lipid probe is an effective dye for highly hydrophobic environments, and for this reason, it is very efficient for LB staining (Fig. 4B; Melo, Sabban, et al. 2006; Wolins et al. 2001). The fluorescence quantum yield of BODIPY dyes is not diminished in water, and BODIPY staining of LBs can be used in parallel with immunofluorescence for proteins of interest (Cocchiaro et al. 2008; D’Avila et al. 2006). Nile red, a phenoxazone dye that fluoresces intensely in hydrophobic lipids, can serve as a sensitive lipophilic stain for LBs (Fig. 4C; Fowler and Greenspan 1985).
Oil red O belongs to the polyazo group of dyes, which also includes the Sudan series of dyes. The principle of the lipid staining is based on the physical properties of the dye that preferentially divide into lipid-rich compartments. Oil red O staining of LBs can be readily visualized in both bright-field and fluorescence microscopy (Koopman et al. 2001). 1-Pyrenedodecanoic acid (P-96; Fig. 4Dii), a fluorescent fatty acid, is readily incorporated into LBs by viable cells (in contrast to the other LB-staining modalities that are applied to fixed cells; Radom et al. 1987). By applying these techniques to activated macrophages and other cells from the immune system such as eosinophils and neutrophils, a high number of LBs are observed in the cytoplasm in response to infection and other inflammatory conditions (Fig. 4; Bozza, Payne, Goulet, et al. 1996; D’Avila et al. 2006; Mattos et al. 2010; Melo et al. 2003; Peyron et al. 2008).
Immunofluorescence labeling for ADRP, the structural protein of LBs considered essential for lipid storage and metabolism, has also been successfully used for identification of LBs within inflammatory and other cells (Fig. 4Di; D’Avila et al. 2006; D’Avila et al. 2008; Turro et al. 2006). Moreover, because LBs within leukocytes are able to form eicosanoids such as LTC4 and PGE2 upon cell activation, an interesting immunofluorescent-based assay (EicosaCell) to localize these mediators has been used in combination with ADRP or BODIPY labeling to investigate the role of LB in eicosanoid synthesis (D’Avila et al. 2006; Fig. 4Eii). This approach consists of intracellular direct detection of eicosanoid-synthesizing compartments by means of a strategy to covalently cross-link and immobilize lipid mediators at their sites of synthesis followed by immunofluorescent-based localization of the targeted eicosanoid (Accioly et al. 2008; Pacheco et al. 2007).
LBs are multifunctional, motile, and highly dynamic organelles. Studies using fluorescence recovery after photobleaching (FRAP), a powerful application of live microscopy, have been performed to understand LB dynamics and the mobility of LB proteins. For example, FRAP has been an important tool to study LB biogenesis and growth within different cells (reviewed in Digel et al. 2010). The intracellular distribution and translocation of LB proteins between the endoplasmic reticulum (ER) and nascent LBs may also be observed with the use of green fluorescent protein (GFP) fusion proteins by means of time-lapse video microscopy (Turro et al. 2006).
Ultrastructural Views of LBs in Inflammatory Cells
As noted, LBs are not surrounded by a typical bilayer membrane but by a monolayer of phospholipids (Fig. 1). This unique feature of LBs—lack of a peripheral true membrane—facilitates the identification of these organelles by transmission electron microscopy (TEM) compared to other intracellular membranous organelles (Melo, Fabrino, et al. 2006). By conventional TEM, LBs within inflammatory cells appear as electron-dense (Figs. 2B and 5A) or electron-lucent (Fig. 5B) organelles depending on the cell type and can change their osmiophilia in response to inflammatory stimuli. In activated macrophages, for example, LB density can consistently change during pathogen infections (D’Avila et al. 2006; Melo, Fabrino, et al. 2006). After 1 hr of the in vivo infection with M. bovis BCG, macrophage LBs are electron dense and morphologically similar to LBs from control cells, but become predominantly light-dense organelles with a peripheral, prominent electron-dense rim after 24 hr of infection (D’Avila et al. 2006). Morphological changes in LBs may reflect differences in lipid composition, stages of formation of new LBs, mobilization, and/or neutral lipid/phospholipid ratio within these organelles (Melo, Fabrino, et al. 2006).
Figure 5.

Lipid bodies (LBs) show distinct densities depending on the cell type. (A) In a macrophage from a rat infected with the parasite Trypanosoma cruzi, giant LBs appear as very electron-dense organelles, whereas in a human monocyte U937 line cell (B), LBs appear to be composed of amorphous light-dense material. In (C), LBs within a heart inflammatory macrophage are seen in association with phagolysosomes (Ph), after infection with T. cruzi. Samples were prepared for transmission electron microscopy as previous works (Melo, Fabrino, et al. 2006; Wan et al. 2007). N, nucleus; C, cardiomyocyte. Scale bar = 1 µm (A), 500 nm (B), 600 nm (C). Panels A and C are reprinted from Melo, Fabrino, et al. (2006) with permission.
LBs, as recognized ultrastructurally best by TEM, also exhibit considerable variations in their size. Our TEM studies with human eosinophils, including stimulation with TNF-α and postfixation with reduced osmium to increase contrast, revealed that small LBs (30–100 nm) can be identified in conjunction with large LBs (Fig. 6). In scoring the diameters of LBs within macrophages from rats experimentally infected with T. cruzi, 74% of LBs had size <0.5 µm in non-infected animals, whereas 54% of LBs from infected animals were >0.5 µm, reaching up to 3 µm. When the parasite burden was increased by animal exposition to gamma irradiation, newly formed LBs within inflammatory macrophages showed significant higher diameters compared to infection alone (Melo, Fabrino, et al. 2006). These findings reveal that not only the number but also the osmiophilia and size of LBs represent structural indicatives of the participation of these organelles in innate immune responses (reviewed in Melo 2009). In addition to TEM, leukocyte LBs can be also detected by wet SEM, which allows cell visualization in a fully hydrated system and facilitates lipid observation (Melo, Sabban, et al. 2006). Although the resolution of wet SEM is lower compared to TEM, it is particularly suitable for the rapid visualization and quantification of LBs (Melo, Sabban, et al. 2006).
Figure 6.

Lipid bodies (LBs) in the cytoplasm of an activated human eosinophil. LBs with different sizes are seen as highly contrasted organelles, whereas the nucleus and other organelles are imaged with less contrast. Arrows indicate very small LBs. Cells were isolated from the peripheral blood, stimulated with the proinflammatory cytokine tumor necrosis factor–α (TNF-α), and processed for transmission electron microscopy using reduced osmium. N, nucleus; Gr, secretory granules. Scale bar = 500 nm.
One intriguing aspect of newly formed LBs revealed by TEM in inflammatory macrophages involved in infections is the ability of these organelles to interact with phagosomes (Fig. 5C) and to relocate in the cytoplasm, suggesting an association between these structures (D’Avila et al. 2006; Melo et al. 2003; Peyron et al. 2008). Quantitative TEM revealed that 47% of LBs were associated with phagolysosomes within Chagas disease–elicited inflammatory macrophages, in addition to being noted within phagolysosomes (Melo, Fabrino, et al. 2006). Similar association between LBs and latex bead–containing phagosomes has been confirmed by high-resolution Raman microscopy in neutrophilic granulocytes (van Manen et al. 2005). The clear association of LB–phagosome points to an ill-understood role of LBs in pathogen control. It has been demonstrated that arachidonic acid has important functions in the induction of apoptosis of mycobacterium-infected macrophages and in actin recruitment to the phagosome, enabling phagosome maturation and increased mycobacterial killing (Anes et al. 2003; Duan et al. 2001). The LB–phagosome association as well as the internalization of LBs by phagosomes during the acute T. cruzi infection may, therefore, represent different stages of phagosome maturation, leading to killing of the parasite (Melo, Fabrino, et al. 2006). On the other hand, the interaction between some pathogens, such as Chlamydia trachomatis (Cocchiaro et al. 2008), Mycobacterium leprae (Mattos et al. 2011), or M. tuberculosis (Peyron et al. 2008), and LBs may be linked to the nutritional needs of the infectious agents, implying that they are accessing and using host lipids for intracellular persistence.
Ultrastructural views of LBs in different cells show that these organelles are also able to associate with the ER, which frequently appears around or even apparently intermingled in the periphery of LBs in conventional thin sections (Fig. 7B; Wan et al. 2007). LBs within leukocytes, such as eosinophils, were also observed by TEM in close association with the nucleus (Fig. 5A) and vesicular compartments, but the meaning of these interactions needs to be delineated (reviewed in Bozza et al. 2007).
Figure 7.

Internal structure of lipid bodies (LBs) in human U937 cells and blood eosinophils. (A) A large and electron-lucent U937 cell LB shows internal membranous structures (arrowheads). (B) An organized membranous network is clearly seen within an osmiophilic eosinophil LB. Note that typical endoplasmic reticulum (ER) cisternae (arrowheads) are seen as part of another LB. Scale bar = 600 nm (A, C), 330 nm (B). Reprinted from Wan et al. (2007) with permission.
Immuno-EM has been a useful tool to localize proteins such as eicosanoid-forming enzymes (Dvorak et al. 1992; Dvorak et al. 1994) (Fig. 3B) and ADRP (Robenek et al. 2005) in LBs from cells of the immune system. Demonstration of eicosanoid-forming enzymes may require an unmasking procedure with 2% sodium metaperiodate before labeling of the thin sections if the postembedding immunogold procedure is used (Dvorak et al. 1992). In this case, highly osmiophilic LBs, such as LBs present in human mast cells and eosinophils, appear as labeled electron-lucent organelles (Fig. 3B; Dvorak et al. 1992; Dvorak et al. 1994). ADRP immunogold EM labeling was demonstrated on both cryothin sections and freeze fracture replicas of macrophages (Robenek et al. 2005). Interestingly, although thin sections showed a prominent ADRP labeling at the surface of LBs, replicas also exhibited a positive ADRP labeling inside the LB core (Robenek et al. 2005). How the polar LB-associated proteins are accommodated among the essentially hydrophobic neutral lipids of the LB core remains to be determined. In fact, although LBs generally appear as homogeneous organelles in TEM images, several studies indicate that the LB core is not a simple mass of lipid esters. Comprehensive examinations of thin sections prepared by conventional TEM have pointed out that LBs in different cell types, including leukocytes, are heterogeneous organelles (reviewed in Digel et al. 2010). By ultrastructural and proteomic studies of human leukocytes, we have defined LBs as organelles with internal ER-like membranes (Fig. 7) and ER luminal proteins (Wan et al. 2007). Loops or sheets of ER-derived membranes within LBs would provide the means for membrane-associated or membrane-spanning proteins to be localized within LBs and not solely in the circumferential membrane monolayer (Wan et al. 2007).
Ultrastructural analyses have also identified the presence of ribosomes attached to the circumferential surfaces of leukocyte LBs and even spread within their electron-dense core content (Wan et al. 2007). The ribosomal localization at and within LBs may be linked to compartmentalized protein synthesis at LBs (Wan et al. 2007). This is fully consonant with prior ultrastructural localization studies of LBs in human mast cells: (1) 3H-uridine was shown to accumulate in LBs, (2) RNA was localized within LBs by hybridization with an RNase-gold probe and by anti-RNA antibody immunogold labeling, (3) poly(A)mRNA was detected within LBs by in situ hybridization with a poly(U) probe, and (4) several human autoimmune sera to ribosomal component proteins immunolabeled LBs (Dvorak 2005). Taken together, ultrastructural studies have been indicating that LBs within different cells, including leukocytes, are heterogeneous organelles with unpredicted functions.
Emerging Imaging Techniques to Study LBs
Because LBs are generally not fluorescent, their study, as noted, usually involves specimen preparation and staining, which limits long-term observation of their dynamics and turnover. Another problem associated with fluorescent labeling of LBs may be the deformation of the LB structure, probably introduced by the sample fixation and staining procedures (Fukumoto and Fujimoto 2002). Therefore, the use of microscopy techniques that detect LBs in unstained samples might be very useful to study the physiological activity of these organelles.
Few methodologies can provide a direct visualization of unstained LBs in situ. Coherent anti-Stokes Raman scattering (CARS) microscopy (Nan et al. 2003), including multiplex CARS microscopy (Rinia et al. 2008), and third harmonic generation (THG) microscopy (Debarre et al. 2006) have been proposed as promising possibilities. These techniques provide high-contrast images of LBs, which can be detected with high specificity in a variety of cells.
The application of CARS and THG microscopy enables not only label-free cellular imaging of lipid composition and packing of individual LBs but also LB imaging in live cells. Moreover, CARS and THG microscopy provide three-dimensional (3D) spatial resolution and can be used to study LBs in a complex environment, such as intact cells and tissues. These techniques should allow monitoring the formation, transport, size, and aggregation of LBs, depending on their composition and structure in cell physiological conditions (Debarre et al. 2006; Nan et al. 2003; Rinia et al. 2008), and may prove useful in understanding physiological processes involved in organ development, inflammatory response, and dysregulated fat metabolism. In addition, the development of new dyes, such as LD540, which allows multicolor imaging of LBs with high specificity and stability in both fixed and living cells, may contribute to the understanding of the different aspects of LBs such as intracellular transport of neutral lipids, LB movement along microtubules tracks, and LB biogenesis (Spandl et al. 2009).
The resolution of new generation techniques, such as CARS and THG microscopy, however, does not enable the detailed observation of the LB substructure, which is still poorly understood. Automated electron tomography (ET), including crioelectron tomography, promises to be a very useful tool to get insights into the 3D architecture of LBs at very high resolution. Because of the advances in instrumentation and methods for data acquisition and reconstruction, the application of this powerful technology has been redefining our understanding of the organization of several organelles and membrane systems (McIntosh et al. 2005; Melo et al. 2008). The advantage of ET is that while conventional TEM studies are usually performed on ~80-nm-thick sections, ET enables the generation of a series of 4-nm-thick digital slices at different depths within the entire section thickness. Thus, it is possible to reveal, within the tomographic volume, structures that cannot be deduced from conventional two-dimensional EM projection images in which they appear overlapped (McIntosh et al. 2005). Therefore, the application of ET to LB research might unveil a yet unknown LB spatial organization in different cells and upon cell activation and enable better understanding of the LB–organelle interactions.
Concluding Remarks and Questions for the Future
The association of LBs with immunopathologies has increasingly been recognized. Detailed studies using different imaging techniques have identified LB accumulation within cells from the immune system in response to varied inflammatory and infectious diseases. In these cells, LBs contribute to the genesis of inflammatory mediators and have been considered structural markers of inflammation (Bozza et al. 2007; Melo, Fabrino, et al. 2006).
Our knowledge of LB biology has advanced significantly in the past decade, but much remains to be learned. Although ensembles of LBs isolated from different cells have been analyzed in great detail, information on the molecular and structural heterogeneity of individual LBs and variations within single LBs is lacking. Electron tomography of cells and molecules as discussed earlier may be helpful in deciphering these aspects. A growing list of proteins has been identified within LBs. In the context of inflammatory cells, these proteins include eicosanoid-forming enzymes and even cytokines. How these and other proteins are spatially arranged within LBs, especially within the hydrophobic core of these organelles, remains to be delineated. Another question regarding LB dynamics during inflammatory responses is the following: How are newly formed inflammatory mediators released from LBs during immune responses? Novel tools for live imaging of LB dynamics (see “Emerging Imaging Techniques to Study LBs”) might accelerate the understanding of these mechanisms and regulatory pathways.
More work will also be needed to understand the meaning of several interactions between LBs and other cell organelles, including peroxisomes and mitocondria, in addition to ER (Beller et al. 2010). One prominent example of these intracellular interactions is the intriguing and intimate LB–phagosome association observed during infectious diseases within specialized cells of the immune system, such as macrophages (D’Avila et al. 2006; Melo et al. 2003; Peyron et al. 2008). Interestingly, LBs can also be recruited to the lumen of phagosomes (Cocchiaro et al. 2008; Mattos et al. 2011). Because phagosomal membrane and luminal contents must undergo considerable remodeling to transform the initially inert environment into a microbicidal one, more attention has been paid to LBs as likely determinant organelles in the phagocytic event. However, whether LBs have a major role in the destruction or intracellular survival of pathogens remains to be defined. Improved imaging technology may help to resolve this issue.
In addition to inflammatory infectious responses, LB accumulation has been linked to other pathologies, for example, neoplasic and emerging metabolic diseases, such as atherosclerosis, diabetes, and obesity (Beller et al. 2010; Farese and Walther 2009). A better understanding of the cell biology of LBs and their functional significance may therefore be crucial for the application of novel therapies addressing different pathophysiological conditions.
Acknowledgments
We thank present and past members of the Laboratory of Cellular Biology (UFJF, Brazil), Laboratory of Immunopharmacology (FIOCRUZ, Brazil), Division of Allergy and Inflammation (Department of Medicine, Beth Israel Deaconess Medical Center [BIDMC], USA), Electron Microscopy Unity (Department of Pathology, BIDMC, USA) for important contributions.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the authorship and/or publication of this article.
The author(s) disclosed receipt of the following financial support for the research and/or authorship of this article: This work was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brazil; grant number 481970/2010-0), Fundação de Amparo a Pesquisa do Estado de Minas Gerais (FAPEMIG, Brazil; grant numbers CBB-APQ-01294-08 and CBB PPM 00508/10), Fundação de Amparo a Pesquisa do Estado do Rio de Janeiro (FAPERJ), and National Institutes of Health, United States (grant numbers AI033372, AI020241, AI051645, AI022571).
References
- Accioly MT, Pacheco P, Maya-Monteiro CM, Carrossini N, Robbs BK, Oliveira SS, Kaufmann C, Morgado-Diaz JA, Bozza PT, Viola JP. 2008. Lipid bodies are reservoirs of cyclooxygenase-2 and sites of prostaglandin-E2 synthesis in colon cancer cells. Cancer Res. 68:1732–1740 [DOI] [PubMed] [Google Scholar]
- Almeida PE, Silva AR, Maya-Monteiro CM, Torocsik D, Avila HD, Dezso B, Magalhaes KG, Castro-Faria-Neto HC, Nagy L, Bozza PT. 2009. Mycobacterium bovis bacillus Calmette-Guerin infection induces TLR2-dependent peroxisome proliferator-activated receptor gamma expression and activation: functions in inflammation, lipid metabolism, and pathogenesis. J Immunol. 183:1337–1345 [DOI] [PubMed] [Google Scholar]
- Anes E, Kuhnel MP, Bos E, Moniz-Pereira J, Habermann A, Griffiths G. 2003. Selected lipids activate phagosome actin assembly and maturation resulting in killing of pathogenic mycobacteria. Nat Cell Biol. 5:793–802 [DOI] [PubMed] [Google Scholar]
- Arend A, Masso R, Masso M, Selstam G. 2004. Electron microscope immunocytochemical localization of cyclooxygenase-1 and -2 in pseudopregnant rat corpus luteum during luteolysis. Prostaglandins Other Lipid Mediat. 74:1–10 [DOI] [PubMed] [Google Scholar]
- Assuncao-Miranda I, Amaral FA, Bozza FA, Fagundes CT, Sousa LP, Souza DG, Pacheco P, Barbosa-Lima G, Gomes RN, Bozza PT, et al. 2010. Contribution of macrophage migration inhibitory factor to the pathogenesis of dengue virus infection. FASEB J. 24:218–228 [DOI] [PubMed] [Google Scholar]
- Bandeira-Melo C, Phoofolo M, Weller PF. 2001. Extranuclear lipid bodies, elicited by CCR3-mediated signaling pathways, are the sites of chemokine-enhanced leukotriene C4 production in eosinophils and basophils. J Biol Chem. 276:22779–22787 [DOI] [PubMed] [Google Scholar]
- Barba G, Harper F, Harada T, Kohara M, Goulinet S, Matsuura Y, Eder G, Schaff Z, Chapman MJ, Miyamura T, et al. 1997. Hepatitis C virus core protein shows a cytoplasmic localization and associates to cellular lipid storage droplets. Proc Natl Acad Sci U S A. 94:1200–1205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartemes KR, McKinney S, Gleich GJ, Kita H. 1999. Endogenous platelet-activating factor is critically involved in effector functions of eosinophils stimulated with IL-5 or IgG. J Immunol. 162:2982–2989 [PubMed] [Google Scholar]
- Beil WJ, Weller PF, Peppercorn MA, Galli SJ, Dvorak AM. 1995. Ultrastructural immunogold localization of subcellular sites of TNF-alpha in colonic Crohn’s disease. J Leukoc Biol. 58:284–298 [DOI] [PubMed] [Google Scholar]
- Beller M, Thiel K, Thul PJ, Jackle H. 2010. Lipid droplets: a dynamic organelle moves into focus. FEBS Lett. 584:2176–2182 [DOI] [PubMed] [Google Scholar]
- Bozza PT, Melo RCN, Bandeira-Melo C. 2007. Leukocyte lipid bodies regulation and function: contribution to allergy and host defense. Pharmacol Ther. 113:30–49 [DOI] [PubMed] [Google Scholar]
- Bozza PT, Payne JL, Goulet JL, Weller PF. 1996. Mechanisms of platelet-activating factor-induced lipid body formation: requisite roles for 5-lipoxygenase and de novo protein synthesis in the compartmentalization of neutrophil lipids. J Exp Med. 183:1515–1525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozza PT, Payne JL, Morham SG, Langenbach R, Smithies O, Weller PF. 1996. Leukocyte lipid body formation and eicosanoid generation: cyclooxygenase-independent inhibition by aspirin. Proc Natl Acad Sci U S A. 93:11091–11096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozza PT, Yu W, Penrose JF, Morgan ES, Dvorak AM, Weller PF. 1997. Eosinophil lipid bodies: specific, inducible intracellular sites for enhanced eicosanoid formation. J Exp Med. 186:909–920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasaemle DL, Dolios G, Shapiro L, Wang R. 2004. Proteomic analysis of proteins associated with lipid droplets of basal and lipolytically stimulated 3T3-L1 adipocytes. J Biol Chem. 279:46835–46842 [DOI] [PubMed] [Google Scholar]
- Cao F, Castrillo A, Tontonoz P, Re F, Byrne GI. 2007. Chlamydia pneumoniae–induced macrophage foam cell formation is mediated by Toll-like receptor 2. Infect Immun. 75:753–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardona PJ, Llatjos R, Gordillo S, Diaz J, Ojanguren I, Ariza A, Ausina V. 2000. Evolution of granulomas in lungs of mice infected aerogenically with Mycobacterium tuberculosis. Scand J Immunol. 52:156–163 [DOI] [PubMed] [Google Scholar]
- Charron AJ, Sibley LD. 2002. Host cells: mobilizable lipid resources for the intracellular parasite Toxoplasma gondii. J Cell Sci. 115:3049–3059 [DOI] [PubMed] [Google Scholar]
- Chawla A, Barak Y, Nagy L, Liao D, Tontonoz P, Evans RM. 2001. PPAR-gamma dependent and independent effects on macrophage-gene expression in lipid metabolism and inflammation. Nat Med. 7:48–52 [DOI] [PubMed] [Google Scholar]
- Cocchiaro JL, Kumar Y, Fischer ER, Hackstadt T, Valdivia RH. 2008. Cytoplasmic lipid droplets are translocated into the lumen of the Chlamydia trachomatis parasitophorous vacuole. Proc Natl Acad Sci U S A. 105:9379–9384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Avila H, Melo RCN, Parreira GG, Werneck-Barroso E, Castro-Faria-Neto HC, Bozza PT. 2006. Mycobacterium bovis bacillus Calmette-Guerin induces TLR2-mediated formation of lipid bodies: intracellular domains for eicosanoid synthesis in vivo. J Immunol. 176:3087–3097 [DOI] [PubMed] [Google Scholar]
- D’Avila H, Roque NR, Cardoso RM, Castro-Faria-Neto HC, Melo RCN, Bozza PT. 2008. Neutrophils recruited to the site of Mycobacterium bovis BCG infection undergo apoptosis and modulate lipid body biogenesis and prostaglandin E production by macrophages. Cell Microbiol. 10:2589–2604 [DOI] [PubMed] [Google Scholar]
- de Assis EF, Silva AR, Caiado LF, Marathe GK, Zimmerman GA, Prescott SM, McIntyre TM, Bozza PT, de Castro-Faria-Neto HC. 2003. Synergism between platelet-activating factor-like phospholipids and peroxisome proliferator-activated receptor gamma agonists generated during low density lipoprotein oxidation that induces lipid body formation in leukocytes. J Immunol. 171:2090–2098 [DOI] [PubMed] [Google Scholar]
- Debarre D, Supatto W, Pena AM, Fabre A, Tordjmann T, Combettes L, Schanne-Klein MC, Beaurepaire E. 2006. Imaging lipid bodies in cells and tissues using third-harmonic generation microscopy. Nat Methods. 3:47–53 [DOI] [PubMed] [Google Scholar]
- DiDonato D, Brasaemle DL. 2003. Fixation methods for the study of lipid droplets by immunofluorescence microscopy. J Histochem Cytochem. 51:773–780 [DOI] [PubMed] [Google Scholar]
- Digel M, Ehehalt R, Fullekrug J. 2010. Lipid droplets lighting up: insights from live microscopy. FEBS Lett. 584:2168–2175 [DOI] [PubMed] [Google Scholar]
- Duan L, Gan H, Arm J, Remold HG. 2001. Cytosolic phospholipase A2 participates with TNF-alpha in the induction of apoptosis of human macrophages infected with Mycobacterium tuberculosis H37Ra. J Immunol. 166:7469–7476 [DOI] [PubMed] [Google Scholar]
- Dvorak AM. 2005. Mast cell secretory granules and lipid bodies contain the necessary machinery important for the in situ synthesis of proteins. Chem Immunol Allergy. 85:252–315 [DOI] [PubMed] [Google Scholar]
- Dvorak AM, Dvorak HF, Peters SP, Shulman ES, MacGlashan DW, Jr, Pyne K, Harvey VS, Galli SJ, Lichtenstein LM. 1983. Lipid bodies: cytoplasmic organelles important to arachidonate metabolism in macrophages and mast cells. J Immunol. 131:2965–2976 [PubMed] [Google Scholar]
- Dvorak AM, Morgan E, Schleimer RP, Ryeom SW, Lichtenstein LM, Weller PF. 1992. Ultrastructural immunogold localization of prostaglandin endoperoxide synthase (cyclooxygenase) to non-membrane-bound cytoplasmic lipid bodies in human lung mast cells, alveolar macrophages, type II pneumocytes, and neutrophils. J Histochem Cytochem. 40:759–769 [DOI] [PubMed] [Google Scholar]
- Dvorak AM, Morgan ES, Tzizik DM, Weller PF. 1994. Prostaglandin endoperoxide synthase (cyclooxygenase): ultrastructural localization to nonmembrane-bound cytoplasmic lipid bodies in human eosinophils and 3T3 fibroblasts. Int Arch Allergy Immunol. 105:245–250 [DOI] [PubMed] [Google Scholar]
- Fabrino DL, Leon LL, Genestra M, Parreira GG, Melo RCN. 2011. Rat models to investigate host macrophage defense against Trypanosoma cruzi. J Innate Immun. 3:71–82 [DOI] [PubMed] [Google Scholar]
- Farese RV, Jr, Walther TC. 2009. Lipid droplets finally get a little R-E-S-P-E-C-T. Cell. 139:855–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler SD, Greenspan P. 1985. Application of Nile red, a fluorescent hydrophobic probe, for the detection of neutral lipid deposits in tissue sections: comparison with oil red O. J Histochem Cytochem. 33:833–836 [DOI] [PubMed] [Google Scholar]
- Freire-de-Lima CG, Nascimento DO, Soares MB, Bozza PT, Castro-Faria-Neto HC, de Mello FG, DosReis GA, Lopes MF. 2000. Uptake of apoptotic cells drives the growth of a pathogenic trypanosome in macrophages. Nature. 403:199–203 [DOI] [PubMed] [Google Scholar]
- Fujimoto Y, Itabe H, Sakai J, Makita M, Noda J, Mori M, Higashi Y, Kojima S, Takano T. 2004. Identification of major proteins in the lipid droplet-enriched fraction isolated from the human hepatocyte cell line HuH7. Biochim Biophys Acta. 1644:47–59 [DOI] [PubMed] [Google Scholar]
- Fukumoto S, Fujimoto T. 2002. Deformation of lipid droplets in fixed samples. Histochem Cell Biol. 118:423–428 [DOI] [PubMed] [Google Scholar]
- Hodges BD, Wu CC. 2010. Proteomic insights into an expanded cellular role for cytoplasmic lipid droplets. J Lipid Res. 51:262–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson KE, Klonis N, Ferguson DJ, Adisa A, Dogovski C, Tilley L. 2004. Food vacuole-associated lipid bodies and heterogeneous lipid environments in the malaria parasite, Plasmodium falciparum. Mol Microbiol. 54:109–122 [DOI] [PubMed] [Google Scholar]
- Koopman R, Schaart G, Hesselink MK. 2001. Optimisation of oil red O staining permits combination with immunofluorescence and automated quantification of lipids. Histochem Cell Biol. 116:63–68 [DOI] [PubMed] [Google Scholar]
- Kuerschner L, Moessinger C, Thiele C. 2008. Imaging of lipid biosynthesis: how a neutral lipid enters lipid droplets. Traffic. 9:338–352 [DOI] [PubMed] [Google Scholar]
- Londos C, Sztalryd C, Tansey JT, Kimmel AR. 2005. Role of PAT proteins in lipid metabolism. Biochimie. 87:45–49 [DOI] [PubMed] [Google Scholar]
- Magalhaes KG, Almeida PE, Atella GC, Maya-Monteiro CM, Castro-Faria-Neto HC, Pelajo-Machado M, Lenzi HL, Bozza MT, Bozza PT. 2010. Schistosomal-derived lysophosphatidylcholine are involved in eosinophil activation and recruitment through Toll-like receptor-2-dependent mechanisms. J Infect Dis. 202:1369–1379 [DOI] [PubMed] [Google Scholar]
- Mattos KA, D’Avila H, Rodrigues LS, Oliveira VG, Sarno EN, Atella GC, Pereira GM, Bozza PT, Pessolani MC. 2010. Lipid droplet formation in leprosy: Toll-like receptor-regulated organelles involved in eicosanoid formation and Mycobacterium leprae pathogenesis. J Leukoc Biol. 87:371–384 [DOI] [PubMed] [Google Scholar]
- Mattos KA, Lara FA, Oliveira VG, Rodrigues LS, D’Avila H, Melo RC, Manso PP, Sarno EN, Bozza PT, Pessolani MC. 2011. Modulation of lipid droplets by Mycobacterium leprae in Schwann cells: a putative mechanism for host lipid acquisition and bacterial survival in phagosomes. Cell Microbiol. 13:259–273 [DOI] [PubMed] [Google Scholar]
- Maya-Monteiro CM, Almeida PE, D’Avila H, Martins AS, Rezende AP, Castro-Faria-Neto H, Bozza PT. 2008. Leptin induces macrophage lipid body formation by a phosphatidylinositol 3-kinase- and mammalian target of rapamycin-dependent mechanism. J Biol Chem. 283:2203–2210 [DOI] [PubMed] [Google Scholar]
- McIntosh R, Nicastro D, Mastronarde D. 2005. New views of cells in 3D: an introduction to electron tomography. Trends Cell Biol. 15:43–51 [DOI] [PubMed] [Google Scholar]
- McLauchlan J. 2009. Lipid droplets and hepatitis C virus infection. Biochim Biophys Acta. 1791:552–559 [DOI] [PubMed] [Google Scholar]
- Melo RCN. 2009. Acute heart inflammation: ultrastructural and functional aspects of macrophages elicited by Trypanosoma cruzi infection. J Cell Mol Med. 13:279–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo RCN, D’Avila H, Bozza PT, Weller PF. 2011. Imaging lipid bodies within leukocytes with different light microscopy techniques. Methods Mol Biol. 689:149–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo RCN, D’Avila H, Fabrino DL, Almeida PE, Bozza PT. 2003. Macrophage lipid body induction by Chagas disease in vivo: putative intracellular domains for eicosanoid formation during infection. Tissue Cell. 35:59–67 [DOI] [PubMed] [Google Scholar]
- Melo RCN, Dvorak AM, Weller PF. 2008. Electron tomography and immunonanogold electron microscopy for investigating intracellular trafficking and secretion in human eosinophils. J Cell Mol Med. 12:1416–1419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo RCN, Fabrino DL, Dias FF, Parreira GG. 2006. Lipid bodies: Structural markers of inflammatory macrophages in innate immunity. Inflamm Res. 55:342–348 [DOI] [PubMed] [Google Scholar]
- Melo RCN, Machado CR. 2001. Trypanosoma cruzi: peripheral blood monocytes and heart macrophages in the resistance to acute experimental infection in rats. Exp Parasitol. 97:15–23 [DOI] [PubMed] [Google Scholar]
- Melo RCN, Sabban A, Weller PF. 2006. Leukocyte lipid bodies: inflammation-related organelles are rapidly detected by wet scanning electron microscopy. J Lipid Res. 47:2589–2594 [DOI] [PubMed] [Google Scholar]
- Mesquita-Santos FP, Vieira-de-Abreu A, Calheiros AS, Figueiredo IH, Castro-Faria-Neto HC, Weller PF, Bozza PT, Diaz BL, Bandeira-Melo C. 2006. Cutting edge: prostaglandin D2 enhances leukotriene C4 synthesis by eosinophils during allergic inflammation: synergistic in vivo role of endogenous eotaxin. J Immunol. 176:1326–1330 [DOI] [PubMed] [Google Scholar]
- Murphy DJ. 2001. The biogenesis and functions of lipid bodies in animals, plants and microorganisms. Prog Lipid Res. 40:325–438 [DOI] [PubMed] [Google Scholar]
- Nan X, Cheng JX, Xie XS. 2003. Vibrational imaging of lipid droplets in live fibroblast cells with coherent anti-Stokes Raman scattering microscopy. J Lipid Res. 44:2202–2208 [DOI] [PubMed] [Google Scholar]
- Nuotio K, Isoviita PM, Saksi J, Ijas P, Pitkaniemi J, Sonninen R, Soinne L, Saimanen E, Salonen O, Kovanen PT, et al. 2007. Adipophilin expression is increased in symptomatic carotid atherosclerosis: correlation with red blood cells and cholesterol crystals. Stroke. 38:1791–1798 [DOI] [PubMed] [Google Scholar]
- Ohsaki Y, Maeda T, Fujimoto T. 2005. Fixation and permeabilization protocol is critical for the immunolabeling of lipid droplet proteins. Histochem Cell Biol. 124:445–452 [DOI] [PubMed] [Google Scholar]
- Olofsson SO, Bostrom P, Andersson L, Rutberg M, Perman J, Boren J. 2009. Lipid droplets as dynamic organelles connecting storage and efflux of lipids. Biochim Biophys Acta. 1791:448–458 [DOI] [PubMed] [Google Scholar]
- Pacheco P, Bozza FA, Gomes RN, Bozza M, Weller PF, Castro-Faria-Neto HC, Bozza PT. 2002. Lipopolysaccharide-induced leukocyte lipid body formation in vivo: innate immunity elicited intracellular Loci involved in eicosanoid metabolism. J Immunol. 169:6498–6506 [DOI] [PubMed] [Google Scholar]
- Pacheco P, Vieira-de-Abreu A, Gomes RN, Barbosa-Lima G, Wermelinger LB, Maya-Monteiro CM, Silva AR, Bozza MT, Castro-Faria-Neto HC, Bandeira-Melo C, et al. 2007. Monocyte chemoattractant protein-1/CC chemokine ligand 2 controls microtubule-driven biogenesis and leukotriene B4-synthesizing function of macrophage lipid bodies elicited by innate immune response. J Immunol. 179:8500–8508 [DOI] [PubMed] [Google Scholar]
- Paul A, Chang BH, Li L, Yechoor VK, Chan L. 2008. Deficiency of adipose differentiation-related protein impairs foam cell formation and protects against atherosclerosis. Circ Res. 102:1492–1501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyron P, Vaubourgeix J, Poquet Y, Levillain F, Botanch C, Bardou F, Daffe M, Emile JF, Marchou B, Cardona PJ, et al. 2008. Foamy macrophages from tuberculous patients’ granulomas constitute a nutrient-rich reservoir for M. tuberculosis persistence. PLoS Pathog. 4:e1000204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radom J, Salvayre R, Maret A, Negre A, Douste-Blazy L. 1987. Metabolism of 1-pyrenedecanoic acid and accumulation of neutral fluorescent lipids in cultured fibroblasts of multisystemic lipid storage myopathy. Biochim Biophys Acta. 920:131–139 [DOI] [PubMed] [Google Scholar]
- Rahaman SO, Lennon DJ, Febbraio M, Podrez EA, Hazen SL, Silverstein RL. 2006. A CD36-dependent signaling cascade is necessary for macrophage foam cell formation. Cell Metab. 4:211–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangel Moreno J, Estrada Garcia I, De La Luz Garcia Hernandez M, Aguilar Leon D, Marquez R, Hernandez Pando R. 2002. The role of prostaglandin E2 in the immunopathogenesis of experimental pulmonary tuberculosis. Immunology. 106:257–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renz H, Gong JH, Schmidt A, Nain M, Gemsa D. 1988. Release of tumor necrosis factor-alpha from macrophages: enhancement and suppression are dose-dependently regulated by prostaglandin E2 and cyclic nucleotides. J Immunol. 141:2388–2393 [PubMed] [Google Scholar]
- Rinia HA, Burger KN, Bonn M, Muller M. 2008. Quantitative label-free imaging of lipid composition and packing of individual cellular lipid droplets using multiplex CARS microscopy. Biophys J. 95:4908–4914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robenek H, Robenek MJ, Troyer D. 2005. PAT family proteins pervade lipid droplet cores. J Lipid Res. 46:1331–1338 [DOI] [PubMed] [Google Scholar]
- Samsa MM, Mondotte JA, Iglesias NG, Assuncao-Miranda I, Barbosa-Lima G, Da Poian AT, Bozza PT, Gamarnik AV. 2009. Dengue virus capsid protein usurps lipid droplets for viral particle formation. PLoS Pathog. 5:e1000632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz G, Grandl M. 2008. Lipid homeostasis in macrophages: implications for atherosclerosis. Rev Physiol Biochem Pharmacol. 160:93–125 [DOI] [PubMed] [Google Scholar]
- Seachord CL, VandeVoort CA, Duffy DM. 2005. Adipose differentiation-related protein: a gonadotropin- and prostaglandin-regulated protein in primate periovulatory follicles. Biol Reprod. 72:1305–1314 [DOI] [PubMed] [Google Scholar]
- Silva AR, Pacheco P, Vieira-de-Abreu A, Maya-Monteiro CM, D’Alegria B, Magalhaes KG, de Assis EF, Bandeira-Melo C, Castro-Faria-Neto HC, Bozza PT. 2009. Lipid bodies in oxidized LDL-induced foam cells are leukotriene-synthesizing organelles: a MCP-1/CCL2 regulated phenomenon. Biochim Biophys Acta. 1791:1066–1075 [DOI] [PubMed] [Google Scholar]
- Spandl J, White DJ, Peychl J, Thiele C. 2009. Live cell multicolor imaging of lipid droplets with a new dye, LD540. Traffic. 10:1579–1584 [DOI] [PubMed] [Google Scholar]
- Triggiani M, Oriente A, Seeds MC, Bass DA, Marone G, Chilton FH. 1995. Migration of human inflammatory cells into the lung results in the remodeling of arachidonic acid into a triglyceride pool. J Exp Med. 182:1181–1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turro S, Ingelmo-Torres M, Estanyol JM, Tebar F, Fernandez MA, Albor CV, Gaus K, Grewal T, Enrich C, Pol A. 2006. Identification and characterization of associated with lipid droplet protein 1: a novel membrane-associated protein that resides on hepatic lipid droplets. Traffic. 7:1254–1269 [DOI] [PubMed] [Google Scholar]
- van Manen HJ, Kraan YM, Roos D, Otto C. 2005. Single-cell Raman and fluorescence microscopy reveal the association of lipid bodies with phagosomes in leukocytes. Proc Natl Acad Sci U S A. 102:10159–10164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira-de-Abreu A, Calheiros AS, Mesquita-Santos FP, Magalhaes ES, Mourao-Sa D, Castro-Faria-Neto HC, Bozza MT, Bandeira-Melo C, Bozza PT. 2010. Crosstalk between MIF and eotaxin in allergic eosinophil activation forms LTC4-synthesizing lipid bodies. Am J Respir Cell Mol Biol. June 10 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Wan HC, Melo RCN, Jin Z, Dvorak AM, Weller PF. 2007. Roles and origins of leukocyte lipid bodies: proteomic and ultrastructural studies. FASEB J. 21:167–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller PF, Bozza PT, Yu W, Dvorak AM. 1999. Cytoplasmic lipid bodies in eosinophils: central roles in eicosanoid generation. Int Arch Allergy Immunol. 118:450–452 [DOI] [PubMed] [Google Scholar]
- Weller PF, Ryeom SW, Picard ST, Ackerman SJ, Dvorak AM. 1991. Cytoplasmic lipid bodies of neutrophils: formation induced by cis-unsaturated fatty acids and mediated by protein kinase C. J Cell Biol. 113:137–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolins NE, Rubin B, Brasaemle DL. 2001. TIP47 associates with lipid droplets. J Biol Chem. 276:5101–5108 [DOI] [PubMed] [Google Scholar]
- World Health Organization 2010. Global tuberculosis control: key findings from the December 2009 WHO report. Wkly Epidemiol Rec. 85:69–80 [PubMed] [Google Scholar]
- Xu W, Yu L, Zhou W, Luo M. 2006. Resistin increases lipid accumulation and CD36 expression in human macrophages. Biochem Biophys Res Commun. 351:376–382 [DOI] [PubMed] [Google Scholar]


