Abstract
Dental pulp elaborates both bone and dentin under pathological conditions such as tooth replantation/transplantation. This study aims to clarify the expression of granulocyte macrophage colony-stimulating factor (GM-CSF) and osteopontin (OPN) in the process of reparative dentin formation by allogenic tooth transplantation using in situ hybridization for OPN and immunohistochemistry for GM-CSF and OPN at both levels of light and electron microscopes. Following the extraction of the mouse molar, the roots and pulp floor were resected and immediately allografted into the sublingual region. On days 1 to 3, immunocompetent cells such as macrophages and dendritic cells expressed both GM-CSF and OPN, and some of them were arranged along the pulp-dentin border and extended their cellular processes into the dentinal tubules. On days 5 to 7, tubular dentin formation commenced next to the preexisting dentin at the pulp horn where nestin-positive odontoblast-like cells were arranged. Until day 14, bone-like tissue formation occurred in the pulp chamber, where OPN-positive osteoblasts surrounded the bone matrix. These results suggest that the secretion of GM-CSF and OPN by immunocompetent cells such as macrophages and dendritic cells plays a role in the maturation of dendritic cells and the differentiation of odontoblasts, respectively, in the regenerated pulp tissue following tooth transplantation.
Keywords: cell differentiation, dental pulp, histocompatibility antigens class II, granulocyte macrophage colony-stimulating factor, odontoblasts, osteopontin, transplantation, mice (inbred ICR)
The dentin-pulp complex is capable of repair after tooth injuries (Nanci 2008). Tooth injuries such as cavity preparation and tooth replantation induce destructive changes in the odontoblasts at the affected site as well as an acute inflammatory reaction (Harada et al. 2008; Nakakura-Ohshima et al. 2003; Ohshima 1990; Ohshima et al. 2003). Once the afflicted odontoblasts die, pulpal mesenchymal cells replace the degenerated cells to differentiate into new odontoblast-like cells, resulting in the formation of reparative dentin (Smith 2002). During the primary dentinogenesis, the differentiation of odontoblasts from the neural crest-derived dental papilla is caused by the expression of signaling molecules and growth factors such as the transforming growth factor (TGF) family, insulin-like growth factor (IGF), and bone morphogenetic protein (BMP) in the inner enamel epithelium. Furthermore, the basement membrane of the inner enamel epithelium plays crucial roles both as a substrate and as a reservoir of signaling molecules (Berkovitz et al. 2009). However, there is neither inner enamel epithelium nor basement membrane along the pulp-dentin border during the reparative dentinogenesis, suggesting that alternative substances or cells are indispensable for inducing the odontoblast differentiation.
Tooth replantation, defined as a therapeutic method in which the dislodged or dislocated tooth is replaced in its original alveolar socket, is now used to treat the complete luxation of teeth whether intentional or accidental. Tooth transplantation is also a common procedure in dentistry for replacing a missing tooth in patients in which implants and other prosthetic replacements are contraindicated for various reasons. These procedures interrupt the nerve and vascular supply to the dental pulp and induce subsequent degeneration of the pulpal cells. In successful cases, the pulpal healing, including reinnervation and revascularization, has been shown to occur in humans (Andreasen et al. 1995) and experimental animal studies (Byers et al. 1992; Hasegawa et al. 2007; Kvinnsland et al. 1991; Nakakura-Ohshima et al. 2003; Ohshima et al. 2001; Rungvechvuttivittaya et al. 1998; Shimizu et al. 2000; Tsukamoto-Tanaka et al. 2006; Unno et al. 2009). Tooth replantation/transplantation induces at least two types of healing patterns in the replanted teeth: dentin and bone tissue formation in the repaired dental pulp (Byers et al. 1992; Hasegawa et al. 2007; Kvinnsland et al. 1991; Ohshima et al. 2001; Rungvechvuttivittaya et al. 1998; Shimizu et al. 2000; Tsukamoto-Tanaka et al. 2006; Unno et al. 2009). Our recent studies have demonstrated that the types of cells appearing along the pulp-dentin border play crucial roles in determining the healing patterns in the replanted teeth: once osteoclast lineage cells appear at the pulp-dentin border, bone matrix deposition can be induced (Hasegawa et al. 2007; Tsukamoto-Tanaka et al. 2006; Unno et al. 2009), whereas the temporal appearance of dendritic cells there induces dentin formation (Nakakura-Ohshima et al. 2003; Shimizu et al. 2000). Thus, the temporal appearance of dendritic cells at the pulp-dentin border is suggestive of a decisive phenomenon to induce the odontoblast differentiation during the pulpal healing process following tooth injuries (Nakakura-Ohshima et al. 2003).
The presence of scaffolds, including the dentin matrix, is indispensable for the differentiation of odontoblasts in the transplanted tooth (Ogawa et al. 2006). Furthermore, our recent study using the allogenic tooth crown transplantation into the sublingual region has demonstrated that an osteopontin (OPN) is deposited in the boundary between the pre- and postoperative dentin in the transplanted tooth (Takamori et al. 2008). The OPN is initially regarded as an RGD-containing adhesive bone matrix protein, and it is a soluble protein capable of stimulating signal transduction pathways (via integrins and CD44 variants) similar to those stimulated by components of the extracellular matrix (Wang and Denhardt 2008). Taking these findings together, an OPN is a possible candidate substance for providing scaffolds to recruit the odontoblast lineage cells in cooperation with the preexisting dentin matrix.
The common myeloid precursors commit to either the osteoclast lineage or the mononucleated phagocyte lineage, which further differentiate into dendritic cells or macrophages, depending on the stimuli received from the external environment. The dendritic cells differentiate from myeloid precursor cells in the presence of granulocyte macrophage colony- stimulating factor (GM-CSF) and interleukin (IL)–4, whereas macrophages are derived from the same myeloid precursor cells in the presence of macrophage colony-stimulating factor (M-CSF; Matsuo and Ray 2004). Therefore, the microenvironment including the secretion of GM-CSF to induce the differentiation and maturation of dendritic cells after tooth injury may result in the differentiation of odontoblasts in cooperation with the deposition of OPN on the preexisting dentin matrix. However, there are no available data on whether the expression of GM-CSF occurs in the replanted/transplanted dental pulp and what type of cells produce and secrete GM-CSF and OPN if it does. This study aimed to clarify the expression of GM-CSF and OPN in the process of reparative dentin formation following allogenic tooth transplantation using in situ hybridization for OPN and immunohistochemistry for GM-CSF and OPN at both levels of light and electron microscopes. Furthermore, we performed double immunohistochemistry using anti-OPN and class II major histocompatibility complex (MHC) antibodies to determine the concern of immunocompetent cells such as dendritic cells and macrophages with the expression of OPN.
Materials and Methods
Allogenic Tooth Crown Transplantation into the Sublingual Region
All experiments were reviewed by the Committee on the Guidelines for Animal Experimentation of Niigata University and performed according to the recommendations or under the conditions proposed by the Review Committee. Crlj:CD1 (ICR) mice, 3 weeks old, were used in this study. The upper-right first molar was extracted with a pair of dental forceps with modification under anesthesia by an intraperitoneal injection of chloral hydrate (the maximum dose of 350 mg/kg), and the roots and pulp floor were resected with a surgical knife. The coronal portion of the resected samples without the periodontal tissue was immediately transplanted into the sublingual region after cutting the ventral side of the tongue of the littermates, and the section was sutured with a nylon suture.
Histological Procedure
Materials were collected in groups of 5 to 10 animals at intervals of 1, 3, 5, 7, and 14 days after allogenic tooth crown transplantation (n = 45). At each stage, the animals were perfused with physiological saline transcardially followed by 4% paraformaldehyde in a 0.1 M phosphate buffer (pH 7.4) under deep anesthesia by an intraperitoneal injection of chloral hydrate (350 mg/kg). The tongues, including the transplanted teeth, were removed en bloc and immersed in the same fixative for an additional 6 hr. Following decalcification in a 10% EDTA-2Na solution for 2 weeks at 4C, the specimens were embedded in paraffin, and sagittal sections of transplants with surrounding lingual tissues were cut at 4 µm. The paraffin sections were mounted on Matsunami adhesive silane (MAS)–coated glass (Matsunami Glass Ind., Osaka, Japan) slides and stained with hematoxylin and eosin (H&E).
Immunohistochemical Analysis
For the immunoperoxidase procedure, the sections were processed for the avidin-biotin peroxidase complex (ABC) method using a rabbit anti-OPN polyclonal antibody diluted to 1:5000 (Cosmo Bio Co., Tokyo, Japan), a goat anti-GM-CSF polyclonal antibody diluted to 1:500 (Santa Cruz Biotechnology, Santa Cruz, CA), and Nichirei Histofine Simple Stain Mouse MAX-PO (Nichirei Biosciences, Inc., Tokyo, Japan) using mouse antinestin monoclonal antibody diluted to 1:50 (Chemicon International, Temecula, CA). The sections were counterstained with 0.05% methylene blue. Immunohistochemical controls were performed by (1) replacing the primary antibodies with non-immune serum or PBS and (2) omitting the streptavidin-peroxidase or the MAX-PO solution. These immunostained sections contained no specific immunoreaction.
For immunohistochemistry with anti-GM-CSF and anti-OPN antibody at the electron-microscopic level, the immunostaining procedure was the same as described above, except for the inhibition of endogenous peroxidase. The immunostained sections were subsequently postfixed in 1% osmium tetroxide reduced with 1.5% potassium ferrocyanide, dehydrated in an ascending series of ethanol, and finally embedded in Epon 812 (Taab, Berkshire, UK). Ultrathin sections (70 nm thick) were double-stained with uranyl acetate and lead citrate and examined with an H-7100 transmission electron microscope (Hitachi High-Technologies Corp., Tokyo, Japan).
For double immunofluorescent staining for OPN and class II MHC, frozen sections were treated by three consecutive incubations with an antimurine class II MHC antibody (diluted 1:500; BMA Biomedicals, Augst, Switzerland), biotinylated anti-rat IgG (diluted 1:200), and Texas red–conjugated streptavidin (diluted 1:250; Vector Laboratories, Inc., Burlingame, CA). After washing with PBS, they were then incubated with an anti-OPN antibody (diluted 1:20,000; Cosmo Bio Co.) and FITC-conjugated anti-rabbit IgG (diluted 1:150; Dako, Carpinteria, CA). The sections were examined with a confocal laser scanning microscope (FV300; Olympus, Tokyo, Japan).
In Situ Hybridization
Section in situ hybridization was conducted as previously described (Nakatomi et al. 2006). Digoxigenin-labeled probe for OPN (Nomura et al. 1993) was prepared according to the manufacturer’s protocol. Following the fixation, the specimens were decalcified with Morse’s solution (10% sodium citrate and 22.5% formic acid) for 24 hr (Shibata et al. 2000), dehydrated through ethanol series and xylene, and embedded in paraffin. Then, 5-µm-thick paraffin sections were mounted on MAS-coated glass slides, deparaffinized, dehydrated, and predigested with proteinase K. The sections were then acetylated with 0.25% acetic anhydride in triethanolamine for 10 min and incubated overnight at 70C with hybridization buffer containing a digoxigenin-labeled probe for OPN. After hybridization, the slides were washed in a series of sodium citrate–sodium chloride solution and treated by two consecutive incubations with blocking reagent (Roche Diagnostics Corp., Indianapolis, IN) and antidigoxigenin antibody (Roche). The sections were stained with 4-nitro-blue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate (Roche).
Results
Histological Changes in Transplanted Teeth
The summarized pulpal changes in the transplanted teeth were as follows (see the previous report by Takamori et al. 2008): All odontoblasts showed the degenerative features and inflammatory reactions, including numerous neutrophils, fibrin networks, and a hemorrhage that occurred in the pulp chamber on day 1; cell proliferation in the pulp chamber became most active on day 3; nestin-positive and OPN-negative newly differentiated odontoblast-like cells were arranged along the pulp-dentin border to deposit the dentin matrix in the pulp horn on days 5 to 7; and bone-like tissue occurred in the pulp chamber apart from the continuous deposition of the dentin matrix on day 14.
Immunoreaction for GM-CSF in Transplanted Teeth
In the control group, GM-CSF-positive reactions were not observed in either the dental pulp or the periodontal ligament (Supplemental Figure S1C). On day 1, the pulp cavity was mainly occupied by inflammatory lesions, and some cells, which extended their cellular processes into the dentinal tubules, showed GM-CSF-positive reactions (Fig. 1A,B). On day 3, GM-CSF-positive reactions were observed in the dentinal tubules deeply and in the cells that arranged along the pulp-dentin border (Fig. 1C,D). On days 5 to 7, the expression of GM-CSF disappeared from the pulp-dentin border and dentinal tubules except for the prolonged inflammatory lesions where GM-CSF-positive cells remained along the pulp-dentin border and extended their cellular processes into the dentinal tubules (Fig. 1E,F). Finally, GM-CSF-positive cells were located beneath the differentiated odontoblast-like cells during days 5 to 14 (Fig. 1E–H).
Figure 1.
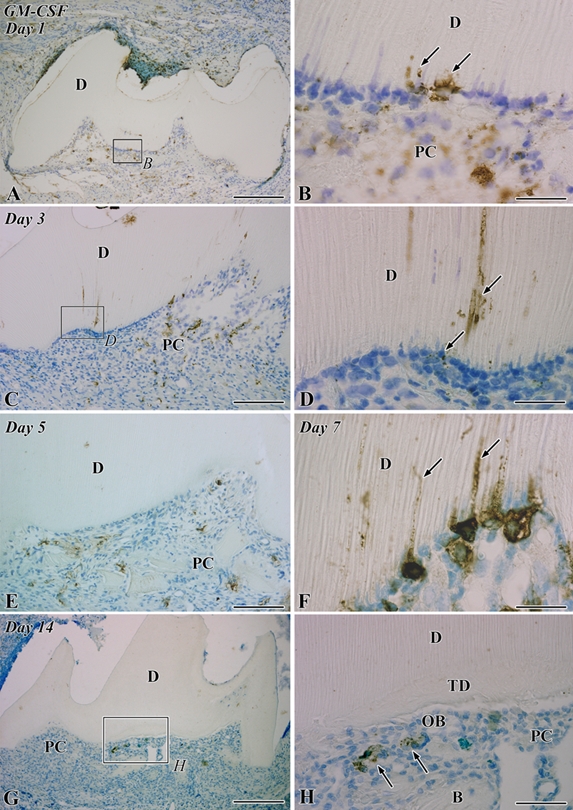
Granulocyte macrophage colony-stimulating factor (GM-CSF) immunoreactivity in the transplanted teeth at 1 (A, B), 3 (C, D), 5 (E), 7 (F), and 14 (G, H) days after operation. B, bone; D, dentin; PC, pulp chamber; TD, tertiary dentin. (A) GM-CSF-positive cells are distributed through the pulp chamber. (B) Higher magnification of the boxed area labeled by B in A. Some cells that extend their cellular processes into the dentinal tubules show GM-CSF-positive reactions (arrows). (C) The distribution pattern of GM-CSF-positive cells is the same as that in the previous stage. (D) Higher magnification of the boxed area labeled by D in C. GM-CSF-positive reactions are observed deeply in the dentinal tubules and in the cells arranging along the pulp-dentin border (arrows). (E) GM-CSF-positive reactions disappear from the pulp-dentin border and dentinal tubules, although GM-CSF-positive cells remain through the pulp chamber. (F) In the prolonged inflammatory lesions, GM-CSF-positive cells accumulate along the pulp-dentin border and extend their cellular processes into the dentinal tubules (arrows). (G) The distribution of GM-CSF-positive cells becomes sparse in the pulp chamber. (H) Higher magnification of the boxed area labeled by H in G. GM-CSF-positive cells (arrows) are located beneath the differentiated odontoblast-like cells (OB). Bars: A, G = 250 µm; C, E = 100 µm; H = 50 µm; B, D, F = 25 µm.
On day 3, the presence of immunocompetent cells such as macrophages and dendritic cells was confirmed judging from the ultrastructural features: These cells possessed peculiar cell organelles such as multivesicular bodies and tubulovesicular structures (Ohshima et al. 1994). Secretory granule- or lysosome-like structures in the Golgi area were positive for GM-CSF in the macrophages and dendritic cells (Fig. 2A,C). Some of these positive cells were arranged along the pulp-dentin border and extended their cellular processes into the dentinal tubules. Typical fibroblasts with a large nucleus and clear nucleoli were negative immunoreactions for GM-CSF (Fig. 2).
Figure 2.
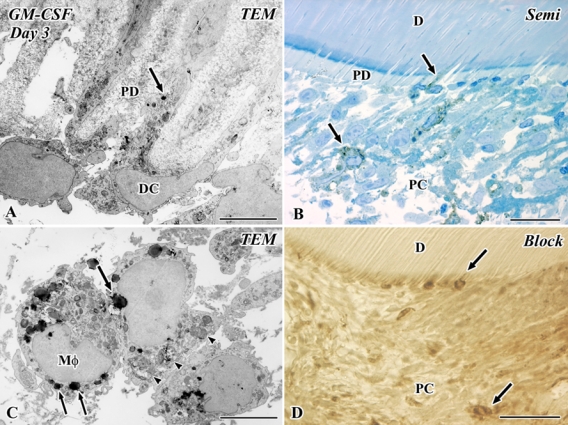
Electron micrographs (A, C) and a semithin section (B), as well as an Epon block after the cutting of ultrathin sections (D) of granulocyte macrophage colony-stimulating factor (GM-CSF) immunoreactivity in the transplanted tooth at 3 days after operation. D, dentin; PC, pulp chamber; PD, predentin; TEM, transmission electron microscope. (A–D) Secretory granule- or lysosome-like structures in the Golgi area are positive for GM-CSF in the macrophages (Mφ) and dendritic cells (DC) (arrows in A and C). Some of these positive cells are arranged along the pulp-dentin border and extend their cellular processes into the dentinal tubules. These cells possess peculiar cell organelles such as multivesticular bodies and tubulovesicular structures, and macrophages contain phagosomes (arrowheads) in addition to these cell organelles (A, C). Bars: D = 50 µm; B = 25 µm; A, C = 5 µm.
Immunoreactions for OPN and Nestin and In Situ Hybridization for OPN in Transplanted Teeth
In the control group, OPN-positive reactions were observed in the osteoblasts, cementoblasts, and the matrix of cementum and bone. The dental pulp lacked OPN immunoreactivity except for the dentinal tubules at the pulp horn showing intense reactivity (Supplemental Fig. S1A). On the other hand, nestin-immunoreactivity was exclusively expressed in the coronal and root odontoblasts, and the other types of cells lacked positive reactions in the dental pulp (Supplemental Fig. S1D). On day 1, OPN-positive reactions were recognized in the fibrin networks of the pulp cavity and the pulp-dentin border (Fig. 3A,B). On day 3, the mineralization front of the preexisting dentin represented continuous OPN-positive reactions, and the cells with an irregular shape appeared along the pulp-dentin border and extended their cellular processes into the dentinal tubules (Fig. 3C,D). We failed to detect OPN-immunopositive reactions in these cells being arranged along the pulp-dentin border because the process for preparing paraffin section may reduce the intensity of their reactions in the cytoplasm. On day 5, the mineralization front of the preexisting dentin represented intense OPN-positive reactions, and the above cells disappeared from the pulp-dentin border (Fig. 3E). On day 7, tubular dentin formation commenced next to the preexisting dentin where nestin-positive odontoblast-like cells were arranged (Fig. 3F,G). On day 14, the continuous OPN immunoreactions were observed at the boundary between the pre- and postoperative dentin, and OPN-positive osteoblasts surrounded the bone matrix (Fig. 3H).
Figure 3.
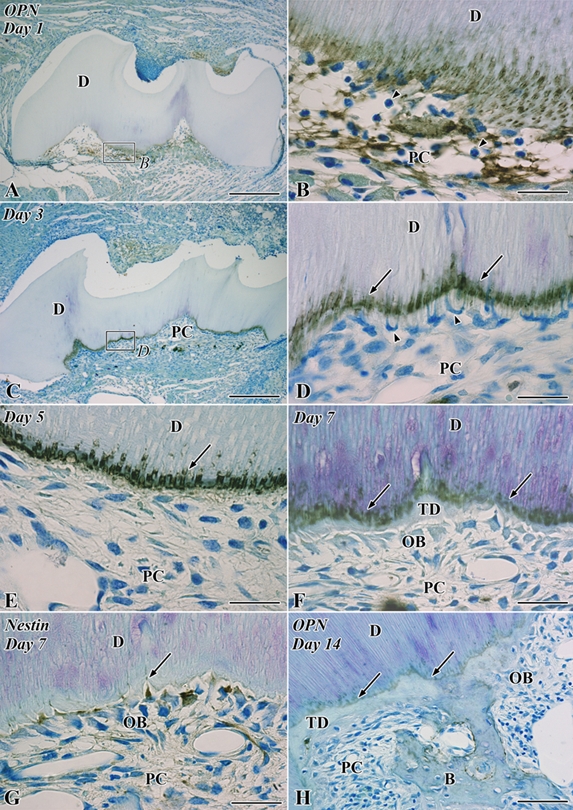
Osteopontin (OPN; A–F, H) and nestin immunoreactivity (G) in the transplanted teeth at 1 (A, B), 3 (C, D), 5 (E), 7 (F, G), and 14 (H) days after operation. B, bone; D, dentin; OB, odontoblast-like cell; PC, pulp chamber; TD, tertiary dentin. (A) Intense OPN-positive reactions are observed throughout the pulp chamber. (B) Higher magnification of the boxed area labeled by B in A. Numerous neutrophils are recognized through the pulp chamber (arrowheads), and OPN-positive reactions are densely distributed in the fibrin networks and the pulp-dentin border, including the dentin matrix. (C) OPN immunoreactions are localized in the pulp-dentin border and certain pulpal cells. (D) Higher magnification of the boxed area labeled by D in C. The mineralization front of the preexisting dentin represents continuous intense OPN-positive reactions (arrows), and the cells with an irregular shape appear along the pulp-dentin border and extend their cellular processes into the dentinal tubules (arrowheads). (E) The mineralization front of the preexisting dentin maintains intense OPN-positive reactions (arrow), and the irregular-shaped cells disappear from the pulp-dentin border. (F) The continuous OPN immunoreactions are observed at the boundary between the preexisting dentin and tertiary dentin (arrows). (G) Nestin-positive odontoblast-like cells are arranged along the pulp-dentin border (arrow). (H) The continuous OPN immunoreactions are recognized both at the boundary between the pre- and postoperative dentin (arrows) and around the bone matrix. Bars: A, C = 250 µm; H = 50 µm; B, D, E–G = 25 µm.
In the semi-thin section, OPN-positive cells were arranged along the pulp-dentin border, and some of them extended their cellular processes into the dentinal tubules on day 3 (Fig. 4B,D). At the ultrastructural level, these OPN-positive cells at the pulp-dentin border were categorized into immunocompetent cells such as macrophages and dendritic cells judging from their ultrastructural features described above (Fig. 4A,C). The OPN-immunopositive reactions were reduced at the level of an electron microscope, whereas clear immunoreactions were observed in the light microscopic level (Fig. 4).
Figure 4.
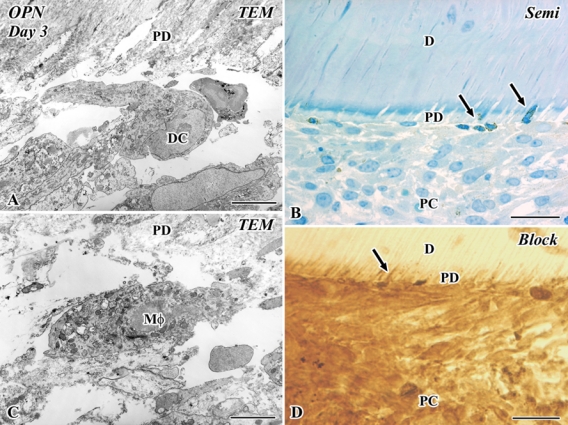
Electron micrographs (A, C), a semi-thin section (B), and an Epon block after the cutting of ultrathin sections (D) of osteopontin (OPN) immunoreactivities in the transplanted tooth at 3 days after operation. D, dentin; PC, pulp chamber; PD, predentin; TEM, transmission electron microscope. (A, C) A macrophage (Mφ) and a dendritic cell (DC) appear at the pulp-dentin border. (B, D) OPN-positive cells are arranged along the pulp-dentin border, and some of them extend their cellular processes into the dentinal tubules (arrows). Bars: B, D = 25 µm; A, C = 5 µm.
The results of in situ hybridization analysis for OPN were as follows. In the control group, dental pulp totally lacked the expression of OPN mRNA, whereas the periphery of alveolar bone showed an intense OPN expression (Supplemental Fig. S1B). On day 1, OPN mRNA-expressing cells were scattered throughout the pulp chamber (Fig. 5A). On days 3 to 7, OPN mRNA-expressing cells were recognized at the pulp-dentin border (Fig. 5B,C,E). In contrast, the newly differentiated nestin-positive odontoblast-like cells were arranged along the pulp-dentin border where OPN-expressing cells had existed on days 5 to 7 (Fig. 5D,F).
Figure 5.
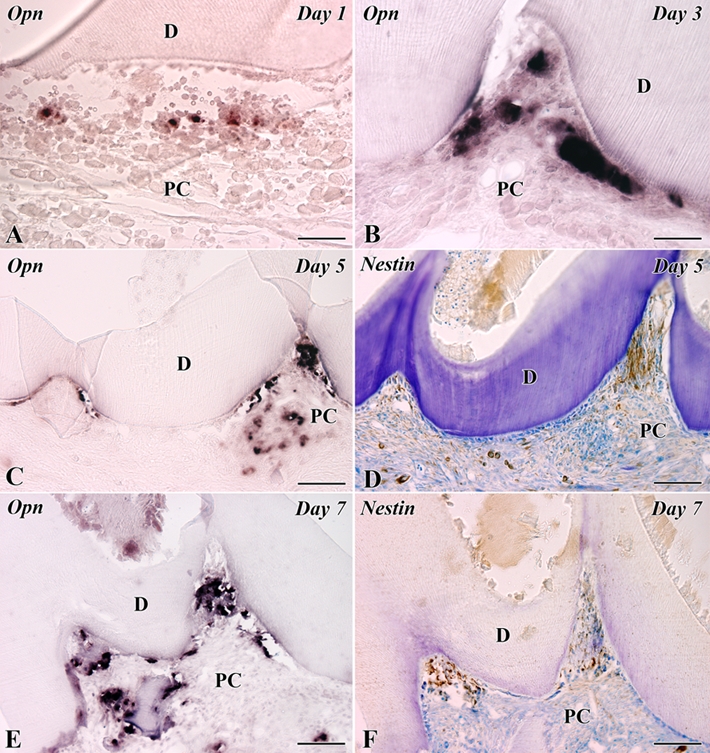
In situ hybridization for osteopontin (OPN) mRNA (A–C, E) and nestin immunoreactivity (D, F) in the transplanted teeth at 1 (A), 3 (B), 5 (C, D), and 7 (E, F) days after operation. D, dentin; PC, pulp chamber. (A) OPN mRNA-expressing cells are scattered throughout the pulp chamber. (B, C, E) OPN mRNA-expressing cells are recognized at the pulp-dentin border. (D, F) The newly differentiated odontoblast-like cells in the pulp horn show intense immunoreactivity for nestin in their cytoplasm. Bars: C–F = 100 µm; A, B = 50 µm.
Double immunofluorescent staining for OPN and class II MHC showed that class II MHC-positive cells with dendritic features appearing along the pulp-dentin border represented a positive reaction for OPN on day 3 (Fig. 6A–C), indicating that OPN-expressing cells were immunocompetent cells such as macrophages and dendritic cells. The anti–class II MHC antibody in this study recognized the premature molecules being produced in the cytoplasm in addition to the mature molecules in the cell membrane (Supplemental Fig. S2).
Figure 6.
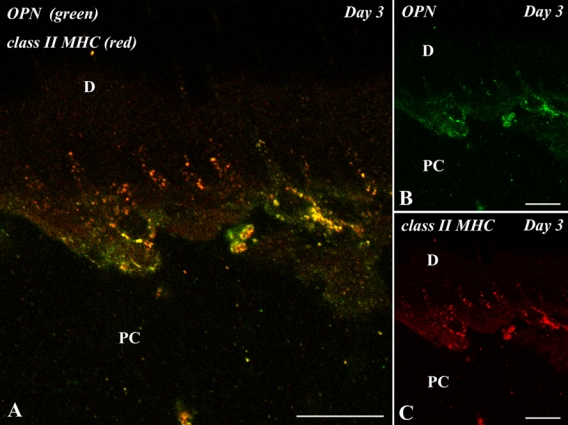
Double immunofluorescent section with anti–osteopontin (OPN) (green) and class II major histocompatibility complex (MHC) (red) antibodies of the pulp-dentin border of the transplanted tooth at 3 days after operation. D, dentin; PC, pulp chamber. (A–C) Class II MHC-positive cells with dendritic features appearing along the pulp-dentin border represent a positive reaction for OPN. Bars = 10 µm.
Discussion
Tooth replantation using mouse molars induces various healing patterns in the replanted teeth: dentin, bone tissue, mixed form of dentin and bone, fibrous tissue formation, and inflammatory reactions (Hasegawa et al. 2007). Thus, the experimental model for tooth replantation is not suitable for the investigation of mechanisms regulating the odontoblast differentiation because the reparative dentinogenesis is not always concomitant with pulpal healing. On the other hand, transplantation of the tooth crown into the sublingual region is a useful experimental model for analyzing the differentiation processes of both odontoblasts and osteoblasts because the deposition of dentin matrix consistently occurs beneath the preexisting dentin, and the bone tissue is induced apart from the dentin matrix (Ogawa et al. 2006; Takamori et al. 2008). Moreover, the immunological rejection rarely occurs between the donor and host of littermates, despite allogenic tooth transplantation. The allogenic transplantation with ROSA reporter mice was available for determination of the derivation of differentiated cells in the previous work (Takamori et al. 2008). Taking these findings together, this study adopts the experimental model for allogenic transplantation of the tooth crown into the sublingual region to achieve the current research objectives.
It is necessary to consider that a variety of signal molecules present in the extracellular milieu at sites of injury play crucial roles in regulating the sequence of events leading to reparative dentinogenesis in the injured pulp tissue. First, several signaling molecules such as TGFβ, BMPs, and fibroblast growth factors (FGFs) are found in the dentin matrix (Cassidy et al. 1997; Roberts-Clark and Smith 2000; Thomadakis et al. 1999). Therefore, a variety of biological effects arising from the dentin matrix dissolution may exist in the pulpal healing process. Second, death of odontoblasts and other pulpal cells may release intracellular contents at the site of injury (Smith 2002). Last, inflammatory cells such as dendritic cells, macrophages, and polymorphonuclear leukocytes recruited to the site of injury will produce cytokines and growth factors, which will modulate cellular events both directly and indirectly. Among the above events, we have focused on two events such as the temporal appearance of dendritic cells along the pulp-dentin border and the deposition of OPN in the calcified front in the absence of both epithelial components and basement membrane in the process of pulpal healing after tooth injury.
With respect to the maturation of dendritic cells, recent studies have demonstrated that GM-CSF primarily acts during inflammation and produces inflammatory dendritic cells (Conti and Gessani 2008; Schmid et al. 2010). This study has clearly shown chronological changes in the expressions of GM-CSF in the process of pulpal healing after tooth transplantation. The intensity of GM-CSF immunoreactions is correlated with the intensity of inflammatory reactions in the pulp cavity: Intense GM-CSF immunoreactions are observed during days 1 to 3, when numerous macrophages and dendritic cells appear in the pulp tissue. Interestingly, GM-CSF-positive dendritic cells extend their cellular processes into the dentinal tubules on day 1, the immunoreactions deep in the dentinal tubules continue until day 3, and subsequently their immunoreactions disappear except for their sparse distribution in the pulp cavity after day 5. However, GM-CSF-positive cells remain at the pulp-dentin border if the inflammatory reactions are retarded for an extended period of time (Fig. 1F). The secretion of GM-CSF around the pulp-dentin border, including the dentinal tubules, may play a role in the differentiation, maturation, and function of dendritic cells in both autocrine and paracrine modes because GM-CSF is a direct player in the generation of functional dendritic cells (Conti and Gessani 2008).
Our recent studies have shown that OPN is deposited in the boundaries between the pre- and postoperative dentin in the transplanted tooth (Takamori et al. 2008) and between the necrotic matrix and reparative dentin following the direct capping of mineral trioxide aggregate (MTA) on the mechanically exposed dental pulp (Kuratate et al. 2008). Thus, OPN may play a triggering role in the initiation of the pulpal reparative process. So far, the cell types that produce and secrete OPN in the process of pulpal healing remain to be clarified. This study has clearly shown the chronological changes in the expression of OPN protein and mRNA, as well as the ultrastructural and cytological features of OPN-positive cells using both immunoelectron microscopy and double immunohistochemistry with anti–class II MHC and OPN antibodies: The immunocompetent cells such as macrophages and dendritic cells are responsible for the secretion of OPN during the pulpal healing process. The findings are supported by the evidence that OPN is secreted by many cell types, including T and B cells, dendritic cells, and macrophages (Rothstein and Guo 2009). In the immune system, OPN modulates immune responses at several levels: Its chemotactic property promotes cell recruitment to sites of inflammation and functions as an adhesion protein to facilitate cell attachment and wound healing. Furthermore, OPN mediates cell activation and cytokine production through interaction with the cellular signaling pathway, and it promotes cell survival by regulating specific programmed cell death pathways (Wang and Denhardt 2008). Thus, it is reasonable to suppose that OPN may play crucial roles in regulating the sequence of events leading to reparative dentinogenesis in the injured pulp tissue (Fig. 7), as a signaling molecule to activate the pulpal healing process and/or scaffold to recruit the odontoblast lineage cells in cooperation with the preexisting dentin matrix sequestering signaling molecules (Goldberg and Smith 2004; Smith and Lesot 2001; Tziafas et al. 2000). This notion may lead to the hypothesis that treatment with OPN, or an antibody to OPN, may be clinically useful in certain specific situations such as the reparative process after tooth injuries. However, a variety of signal molecules present in the extracellular milieu at sites of injury remain to be clarified at present. Further studies are required to understand the roles of GM-CSF and OPN in orchestrating the pulpal healing process after tooth injuries, especially the functional significance of GM-CSF and OPN in the maturation of dendritic cells and the differentiation of odontoblasts, using the loss-of-function and gain-of-function experiments in future.
Figure 7.
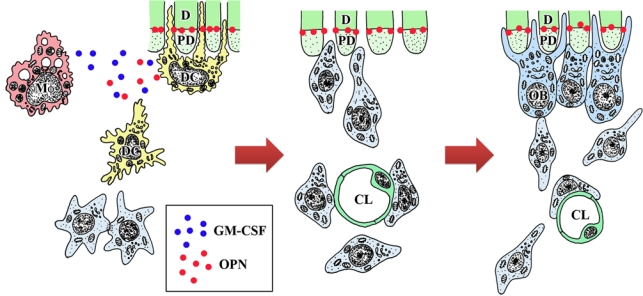
A schematic diagram indicating the putative cell dynamics occurring in the pulp-dentin border in the pulpal healing process following tooth transplantation. CL, capillary lumen; D, dentin; DC, dendritic cells; OB, odontoblast-like cells; Mf, macrophages; PD, predentin. Osteopontin (OPN) is deposited at the dentin-predentin interface because OPN may diffuse in the predentin and adhere to the mineralized matrix. The secretion of granulocyte macrophage colony-stimulating factor (GM-CSF) and OPN by immunocompetent cells such as macrophages and dendritic cells may play a role in the maturation of dendritic cells and the differentiation of odontoblasts, respectively.
Acknowledgments
We are grateful to Dr. S. Nomura for providing riboprobe.
Footnotes
Supplementary material for this article is available on the Journal of Histochemistry & Cytochemistry Web site at http://jhc.sagepub.com/supplemental.
The author(s) declared no potential conflicts of interest with respect to the authorship and/or publication of this article.
The author(s) disclosed receipt of the following financial support for the research and/or authorship of this article: This work was supported in part by Grants-in-Aid for Scientific Research (B) (no. 22390341 to H.O.) and Exploratory Research (no. 20659296 to H.O.) from MEXT and JSPS, and a Grant for Supporting Project for Strategic Research of Nihon Univ Sch Dentistry at Matsudo from MEXT, 2008-2012 (Team: Dental Morphogenesis).
References
- Andreasen JO, Borum MK, Jacobsen HL, Andreasen FM. 1995. Replantation of 400 avulsed permanent incisors: 2. Factors related to pulpal healing. Endod Dent Traumatol. 11:59–68 [DOI] [PubMed] [Google Scholar]
- Berkovitz BKB, Holland GR, Moxham BJ. 2009. Oral anatomy, embryology, and histology. 4th ed. Edinburgh, UK: Mosby [Google Scholar]
- Byers MR, Kvinnsland I, Bothwell M. 1992. Analysis of low affinity nerve growth factor receptor during pulpal healing and regeneration of myelinated and unmyelinated axons in replanted teeth. J Comp Neurol. 326:470–484 [DOI] [PubMed] [Google Scholar]
- Cassidy N, Fahey M, Prime SS, Smith AJ. 1997. Comparative analysis of transforming growth factor-beta isoforms 1-3 in human and rabbit dentine matrices. Arch Oral Biol. 42:219–223 [DOI] [PubMed] [Google Scholar]
- Conti L, Gessani S. 2008. GM-CSF in the generation of dendritic cells from human blood monocyte precursors: recent advances. Immunobiology. 213:859–870 [DOI] [PubMed] [Google Scholar]
- Goldberg M, Smith AJ. 2004. Cells and extracellular matrices of dentin and pulp: a biological basis for repair and tissue engineering. Crit Rev Oral Biol Med. 15:13–27 [DOI] [PubMed] [Google Scholar]
- Harada M, Kenmotsu S, Nakasone N, Nakakura-Ohshima K, Ohshima H. 2008. Cell dynamics in the pulpal healing process following cavity preparation in rat molars. Histochem Cell Biol. 130:773–783 [DOI] [PubMed] [Google Scholar]
- Hasegawa T, Suzuki H, Yoshie H, Ohshima H. 2007. Influence of extended operation time and of occlusal force on determination of pulpal healing pattern in replanted mouse molars. Cell Tissue Res. 329:259–272 [DOI] [PubMed] [Google Scholar]
- Kuratate M, Yoshiba K, Shigetani Y, Yoshiba N, Ohshima H, Okiji T. 2008. Immunohistochemical analysis of nestin, osteopontin, and proliferating cells in the reparative process of exposed dental pulp capped with mineral trioxide aggregate. J Endod. 34:970–974 [DOI] [PubMed] [Google Scholar]
- Kvinnsland I, Heyeraas KJ, Byers MR. 1991. Regeneration of calcitonin gene-related peptide immunoreactive nerves in replanted rat molars and their supporting tissues. Arch Oral Biol. 36:815–826 [DOI] [PubMed] [Google Scholar]
- Matsuo K, Ray N. 2004. Osteoclasts, mononuclear phagocytes, and c-Fos: new insight into osteoimmunology. Keio J Med. 53:78–84 [DOI] [PubMed] [Google Scholar]
- Nakakura-Ohshima K, Watanabe J, Kenmotsu S, Ohshima H. 2003. Possible role of immunocompetent cells and the expression of heat shock protein–25 in the process of pulpal regeneration after tooth injury in rat molars. J Electron Microsc (Tokyo). 52:581–591 [DOI] [PubMed] [Google Scholar]
- Nakatomi M, Morita I, Eto K, Ota MS. 2006. Sonic hedgehog signaling is important in tooth root development. J Dent Res. 85:427–431 [DOI] [PubMed] [Google Scholar]
- Nanci A. 2008. Ten Cate’s oral histology: development, structure, and function. 7th ed. St. Louis, MO: Mosby [Google Scholar]
- Nomura S, Hirakawa K, Nagoshi J, Hirota S, Kim H, Takemura T, Nakase N, Takaoka K, Matsumoto S, Nakajima Y, et al. 1993. Method for detecting the expression of bone matrix protein by in situ hybridization using decalcified mineralized tissue. Acta Cytochem Histochem. 26:303–309 [Google Scholar]
- Ogawa R, Saito C, Jung HS, Ohshima H. 2006. Capacity of dental pulp differentiation after tooth transplantation. Cell Tissue Res. 326:715–724 [DOI] [PubMed] [Google Scholar]
- Ohshima H. 1990. Ultrastructural changes in odontoblasts and pulp capillaries following cavity preparation in rat molars. Arch Histol Cytol. 53:423–438 [DOI] [PubMed] [Google Scholar]
- Ohshima H, Kawahara I, Maeda T, Takano Y. 1994. The relationship between odontoblasts and immunocompetent cells during dentinogenesis in rat incisors: an immunohistochemical study using OX6-monoclonal antibody. Arch Histol Cytol. 57:435-47 [DOI] [PubMed] [Google Scholar]
- Ohshima H, Nakakura-Ohshima K, Takeuchi K, Hoshino M, Takano Y, Maeda T. 2003. Pulpal regeneration after cavity preparation, with special reference to close spatio-relationships between odontoblasts and immunocompetent cells. Microsc Res Tech. 60:483–490 [DOI] [PubMed] [Google Scholar]
- Ohshima H, Nakakura-Ohshima K, Yamamoto H, Maeda T. 2001. Alteration in the expression of heat shock protein (Hsp) 25–immunoreactivity in the dental pulp of rat molars following tooth replantation. Arch Histol Cytol. 64:425–437 [DOI] [PubMed] [Google Scholar]
- Roberts-Clark DJ, Smith AJ. 2000. Angiogenic growth factors in human dentine matrix. Arch Oral Biol. 45:1013–1016 [DOI] [PubMed] [Google Scholar]
- Rothstein TL, Guo B. 2009. Receptor crosstalk: reprogramming B cell receptor signalling to an alternate pathway results in expression and secretion of the autoimmunity-associated cytokine, osteopontin. J Intern Med. 265:632–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rungvechvuttivittaya S, Okiji T, Suda H. 1998. Responses of macrophage-associated antigen-expressing cells in the dental pulp of rat molars to experimental tooth replantation. Arch Oral Biol. 43:701–710 [DOI] [PubMed] [Google Scholar]
- Schmid MA, Kingston D, Boddupalli S, Manz MG. 2010. Instructive cytokine signals in dendritic cell lineage commitment. Immunol Rev. 234:32–44 [DOI] [PubMed] [Google Scholar]
- Shibata Y, Fujita S, Takahashi H, Yamaguchi A, Koji T. 2000. Assessment of decalcifying protocols for detection of specific RNA by non-radioactive in situ hybridization in calcified tissues. Histochem Cell Biol. 113:153–159 [DOI] [PubMed] [Google Scholar]
- Shimizu A, Nakakura-Ohshima K, Noda T, Maeda T, Ohshima H. 2000. Responses of immunocompetent cells in the dental pulp to replantation during the regeneration process in rat molars. Cell Tissue Res. 302:221–233 [DOI] [PubMed] [Google Scholar]
- Smith AJ. 2002. Dentin formation and repair. In: Hargreaves KM, Goodis HE, editors. Seltzer and Bender’s dental pulp. Chicago: Quitessence; p.41–62 [Google Scholar]
- Smith AJ, Lesot H. 2001. Induction and regulation of crown dentinogenesis: embryonic events as a template for dental tissue repair? Crit Rev Oral Biol Med. 12:425–437 [DOI] [PubMed] [Google Scholar]
- Takamori Y, Suzuki H, Nakakura-Ohshima K, Cai J, Cho SW, Jung HS, Ohshima H. 2008. Capacity of dental pulp differentiation in mouse molars as demonstrated by allogenic tooth transplantation. J Histochem Cytochem. 56:1075–1086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomadakis G, Ramoshebi LN, Crooks J, Rueger DC, Ripamonti U. 1999. Immunolocalization of bone morphogenetic protein-2 and -3 and osteogenic protein-1 during murine tooth root morphogenesis and in other craniofacial structures. Eur J Oral Sci. 107:368–377 [DOI] [PubMed] [Google Scholar]
- Tsukamoto-Tanaka H, Ikegame M, Takagi R, Harada H, Ohshima H. 2006. Histochemical and immunocytochemical study of hard tissue formation in dental pulp during the healing process in rat molars after tooth replantation. Cell Tissue Res. 325:219–229 [DOI] [PubMed] [Google Scholar]
- Tziafas D, Smith AJ, Lesot H. 2000. Designing new treatment strategies in vital pulp therapy. J Dent. 28:77–92 [DOI] [PubMed] [Google Scholar]
- Unno H, Suzuki H, Nakakura-Ohshima K, Jung HS, Ohshima H. 2009. Pulpal regeneration following allogenic tooth transplantation into mouse maxilla. Anat Rec (Hoboken). 292:570–579 [DOI] [PubMed] [Google Scholar]
- Wang KX, Denhardt DT. 2008. Osteopontin: role in immune regulation and stress responses. Cytokine Growth Factor Rev. 19:333–345 [DOI] [PubMed] [Google Scholar]


