Abstract
Traditional views hold that immunoglobulin G (IgG) in the human umbilical cord is internalized by human umbilical endothelial cells for passive immunity. In this study, the protein and mRNA transcripts of IgG were found in the cytoplasm of human umbilical endothelial cells by immunohistochemistry, in situ hybridization, and reverse transcription PCR (RT-PCR). The essential enzymes for IgG synthesis and assembling, RAG1 (recombination activating gene 1), RAG2, and variable (V), diversity (D), and joining (J) segments for recombination of IgG, were also found in these cells by RT-PCR and real-time PCR. These results indicate that umbilical endothelial cells are capable of synthesizing IgG with properties similar to those of immune cells and that they may play additional roles besides lining the vessels and transporting IgG.
Keywords: IgG, human, umbilical cord, endothelial cell, expression, V(D)J recombination
In mammals, the umbilical cord connects the embryo or fetus to the fetal site of the placenta. It contains two arteries and one vein, which are embedded in gelatinous connective tissue (Wharton’s jelly) consisting of collagen fibers, myofibroblast-like stroma cells, and proteoglycans (Can and Karahuseyinoglu 2007; Takechi et al. 1993). Both the umbilical cord vein and arteries are lined by a single layer of endothelial cells, which is directly adjacent to the muscularis layer in the umbilical arteries and separated by a thin elastic subintima layer in the umbilical vein (Benirschke and Kaufmann 2000). The muscularis layer consists of an inner longitudinal and an outer circular muscle layer (Zhang S-X 1999). The umbilical vein conducts oxygen, nutrients, and various other substances from the mother to the fetus, whereas the umbilical arteries transport waste materials away from the fetus back to the maternal circulation. The actual exchange of metabolic products between the fetal and maternal circulation occurs in the placenta at the syncytiotrophoblast level. Immunoglobulin G (IgG) is one of the substances that is transferred across the placental barrier (Pitcher-Wilmott et al. 1980) to confer passive immunity to the fetus. Following its transfer across the syncytiotrophoblast layer, IgG is subsequently transported via the umbilical vein to the fetus. The transplacental transfer of IgG is thought to be mediated by the neonatal Fc receptor (FcRn) expressed in syncytiotrophoblasts (Roopenian and Akilesh 2007). It is currently not known whether FcRn is also expressed in the umbilical cord. However, the expression of Fc gamma receptors (FcγRs) has previously been studied in the human umbilical cord by Sedmak et al. (1991) and Lang et al. (1993), who found FcγRs only expressed on immune cells but not on endothelial cells.
Most of the IgG present in the fetal circulation is thought to be produced by the mother (Gitlin and Biasucci 1969). However, a small portion of the IgG circulating in the fetus expresses a non-maternal haplotype. This non-maternal IgG could in part have its origin in the placenta because trophoblasts are capable of producing IgG, which has been demonstrated in a previous study conducted by our group (Zhao Y, Deng R, Chen Z, Korteweg C, Zhang J, Li J,Wang Yun, Wang Yongyu, Lin C, Bluth MH, Niu N, Zhuang Z, Su M, Gu J, unpublished data). In that study, various experiments, including conventional in situ hybridization (ISH), combined immune electron microscopy ISH, and laser capture microdissection followed by RT-PCR on placental tissues and a primary trophoblast cell line, showed the presence of IgG at the mRNA level in trophoblasts, strongly indicating that such cells can produce IgG (Zhao Y, Deng R, Chen Z, Korteweg C, Zhang J, Li J,Wang Yun, Wang Yongyu, Lin C, Bluth MH, Niu N, Zhuang Z, Su M, Gu J unpublished data). In view of the close anatomical relationship between the placenta and the umbilical cord and their common derivation from the same zygote, we hypothesized that cells of the umbilical cord might also synthesize IgG. Using ISH and RT-PCR on umbilical cord tissues and a primary umbilical endothelial cell culture system, we show here that human umbilical endothelial cells (HUECs) have the ability to produce IgG. We also demonstrated mRNA expression of the recombination activating genes -1 and -2 (RAG1 and RAG2) in HUECs. Finally, FcRn was detected on HUECs, whereas none of the FcγR subclasses was expressed on HUECs.
Materials and Methods
Tissues, Sections, and Cell Lines
Umbilical cord tissues were obtained from 10 full-term healthy pregnant women from the first affiliated Hospital of the Medical College of Shantou University (Shantou, P.R. China). All of the samples were divided into two parts. One part of the samples was cut into 1 × 0.5-cm2 specimens washed in PBS, fixed in 4% formalin overnight, and then embedded in paraffin. The sections were cut at the right angle to the long axis of the umbilical cord. The endothelial cells were cut across their entire thickness, which is generally about 3 to 5 µm in length. The tissue sections were prepared at 4 µm thick according to the routine procedure of paraffin sections for immunohistochemistry (IHC) and ISH in our laboratory. The other portion of the samples was cut into small pieces and washed carefully until the suspension became translucent in preparation for RNA extraction of human umbilical cord tissues. The connective tissue adjacent to the large blood vessels was dissected from the umbilical cord and served as a negative control. Primary-cultured human umbilical vein endothelial cells (HUVECs) were a gift from Professor Nanping Wang, Institute of Cardiovascular Science, Peking University (Beijing, P.R. China). This study was in accordance with the Helsinki Declaration, and the procedures were approved by the Research Administrative Committee of Peking University.
To compare the expression levels of IgG in HUVECs with those in pre- and mature B lymphocytes, we used two cell lines—that is, the human pre–B cell line (NALM-6) and Burkitt lymphoma cell line (Raji), respectively. They were cultured in RPMI 1640 (GIBCO, Carlsbad, CA) containing 10% FBS at 37C in a humidified atmosphere with 5% CO2 and were subjected to the same tests as for the endothelial cells.
Human Umbilical Vein Endothelial Cell Preparation
Collagenase treatment was performed to isolate HUVECs, and the cells were cultured on plates coated with collagen (Holland et al. 1988; Jaffe et al. 1973; Wang et al. 1999). Briefly, cords were collected from placentas within 2 hr after delivery. The vein lumina were filled with Hank’s balanced salt solution, containing antibiotic/antimycotic (penicillin, streptomycin, and fungizone). Hank’s balanced salt solution–incubated cords were placed in a 4C refrigerator for 1 hr and then drained, followed by incubation with collagenase for 15 min at 37C. The HUVECs were flushed from the vein vessel together with the collagenase solution. The obtained HUVECs were cultured on plates coated with collagen. The cells were maintained in M199, 20% FBS, 20 mmol/L HEPES (pH 7.4), 1 ng/ml recombinant human fibroblast growth factor, and 90 mg/ml heparin, and antibiotics were added to the supernatant. Only cells with a maximum of six passages were used in this study.
Immunohistochemistry
IHC was performed as previously described (Ye et al. 2007). Briefly, sections were deparaffinized and immersed in gradient alcohol from 100% to 80%. Then, for eliminating endogenous peroxidase activity, sections were immersed in 3% hydrogen peroxide for 30 min. Antigen retrieval was performed by heating the slides at 95C in 0.01 mol/L citrate buffer (pH 6.0) for 15 min and then brought back to neutral pH with PBS. Rabbit anti-human IgG antibody (γ chain specific), mouse anti-human Igκ chain antibody, and mouse anti-human Igλ chain antibody were used to identify the IgG heavy chain, the kappa light chain, and the lambda light chain, respectively. Goat anti-human FcRn antibody was used to identify the neonatal Fc receptor. Antibodies against CD 20, CD38, CD34, and factor VIII were used to identify B lymphocytes, plasma cells, and endothelial cells. The source, dilution, and incubation condition and duration of each primary antibody are listed in Table 1. Tissue sections were incubated with goat anti-rabbit or goat anti-mouse IgG labeled with horseradish peroxidase (PV9000; Zymed Laboratories, South San Francisco, CA). The color reaction was developed with a horseradish peroxidase reaction kit (3-amino-9-ethylcarbazole; Zymed Laboratories), resulting in a red reaction product. Sections were counterstained with hematoxylin. In negative controls, the primary antibody was omitted or replaced by unrelated antibodies, including antibodies to hemagglutinin (HA) and nucleoprotein (NP) of the H5N1 avian influenza virus.
Table 1.
Antibodies Used in This Study
| Primary Antibody | Supplier | Marker for | Dilution or Concentration | Incubation Temperature | Incubation Period |
|---|---|---|---|---|---|
| Rabbit anti-human Igγ | Dako (Carpinteria, CA) | Igγ | 1:300 | 4C | Overnight |
| Mouse anti-human Igκ | Invitrogen (Carlsbad, CA), Zymed Laboratories (South San Francisco, CA) | Igκ | 1:100 | 4C | Overnight |
| Mouse anti-human Igλ | Invitrogen, Zymed Laboratories | Igλ | 1:100 | 4C | Overnight |
| Mouse anti-human CD20 | Invitrogen, Zymed Laboratories | B lymphocyte | 1:100 | 4C | Overnight |
| Mouse anti-human CD34 | Invitrogen, Zymed Laboratories | Endothelial cell | 1:100 | 4C | Overnight |
| Mouse anti-human factor VIII | Invitrogen, Zymed Laboratories | Endothelial cell | 1:100 | 4C | Overnight |
| Mouse anti-human CD38 | Invitrogen, Zymed Laboratories | Plasma cell | 1:100 | 4C | Overnight |
| Mouse anti-human CD16 | Santa Cruz, CA | FcγRI | 1:100 | 4C | Overnight |
| Mouse anti-human CD32 | Santa Cruz, CA | FcγRII | 1:100 | 4C | Overnight |
| Mouse anti-human CD64 | Santa Cruz, CA | FcγRIII | 1:100 | 4C | Overnight |
| Goat anti-human FcRn | Santa Cruz, CA | FcRn | 1:100 | 4C | Overnight |
Immunofluorescence
Immunofluorescence (IF) was performed as described previously by Chen and Gu (2007). Cells were cultured on cover slides and fixed in 4% paraformaldehyde for 15 min. The slides were then immersed in 0.2% Triton X-100 for 15 min. PBS containing 10% normal goat serum was used for 60 min to block background staining. Rabbit anti-human IgG antibody (γ chain specific) or mouse anti-human CD34 antibody was used as the primary antibody. PBS was used for the control slides. After incubating with goat anti-rabbit IgG-FITC or goat anti-mouse IgG-TRITC for 30 min at room temperature, the sections were incubated with Hoechst 33342 (1:1000; Sigma, St. Louis, MO) for 15 min. The positive signals were red for TRITC or green for FITC.
In Situ Hybridization
Sense and antisense probes against mRNA of IGHG1 were prepared as previously described (Chen and Gu 2007). A 351-nucleotide cRNA probe was produced by in vitro transcription and labeled with digoxigenin (Roche Diagnostics, Penzberg, Germany). ISH was performed as described by Gu et al. (2007). In brief, deparaffinized tissue sections and 4% paraformaldehyde fixed cells slides were incubated with 0.1 M HCl for 10 min and immersed in 0.01 M citrate buffer (pH 6.0). Slides were heated at 95C for 20 min and cooled to room temperature. After washing in PBS buffer, sections were fixed in 4% paraformaldehyde for 10 min and then hybridized at 45C for 20 hr with the human IGHG1 antisense cRNA probe. After hybridization, slides were washed in 2× SSC plus 50% formamide for 30 min (50C) and 2× SSC twice for 15 min (37C). Sections were blocked with normal horse serum (1:100) and then incubated with alkaline phosphatase-labeled antidigoxigenin antibody (1:500; Roche Diagnostics) for 1 hr. The reaction products were colorized with nitro blue tetrazolium/5-bromo-4-choloro-3-indolyl phosphate (NBT/BCIP) (Promega, Madison, WI), resulting in a purple-blue signal. Slides were counterstained with methyl green. All solutions were prepared with diethylpyrocarbonate-treated water. In negative controls, the probe was replaced by hybridization solution, corresponding sense probes, or an irrelevant probe of similar nucleotide content and length against the HA of the H5N1 virus.
RNA Extraction and RT-PCR
Trizol Reagent (Invitrogen, Carlsbad, CA) was used to extract total RNA from full-term human umbilical cords and primary cultured HUVECs. Reverse transcription of total RNA was performed using the SuperScript III RT System (Invitrogen) following the manufacturer’s instructions. All extracted RNA was treated with RQ1 RNase-free DNase (Promega). Given the fact that for the constant region of the IgG1 heavy chain (IGHG1) and the recombined variable (V), diversity (D), and joining (J) segments of the IgG heavy chain (VDJH), the sense and antisense primers were localized on different exons, it was easy to discriminate between transcripts and genomic DNA. For the RAG1, RAG2, kappa, and lambda Ig light chains, DNase-treated RNA and cDNA without reverse transcriptase were used to eliminate contamination by genomic DNA. Nested and semi-nested PCR was performed as described in previous studies (Huang et al. 2008; Huang et al. 2009). The primers used in this study are listed in Table 2. DNA sequencing was used to confirm the identity of all PCR products. VDJH PCR products were cloned into a pGEM-T vector (Tiangen, Beijing, China). Three randomly selected clones from the primary culture were subjected to sequencing by an ABI 3100 Genetic Analyzer (Applied Biosystems, Foster City, CA). The sequences of these three clones were then compared with published sequences in BLAST of the GenBank of the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov/BLAST). Peripheral blood lymphocytes were used as positive controls, whereas reaction mixtures with RNA templates without reverse transcriptase and DNase-treated RNA templates served as negative controls. Water was also used as a negative control. The amplification of 18s served as an internal control.
Table 2.
Primer Sequences Used in This Study
| Gene Name | Primer Sequence 5′-3′ | Product Size (Base Pairs) | Located in Different Exons |
|---|---|---|---|
| IGHG1 (human) | External: ACGGCGTGGAGGTGCATAATG (sense) CGGGAGGCGTGGTCTTGTAGTT (antisense) Internal: GACTGGCTGAATGGCAAGGAG (sense) GGCGATGTCGCTGGGATAGAA (antisense) |
201 | Yes |
| VDJH (human) | External: V3, GAGGTGCAGCTCGAGCAGTCAGG (sense) V4f, CAGGTGCAGCTGCTCGAGTCGGG (sense) V6, CAGGTACAGCTCGAGCAGTCAGG (sense) CH1, ACACCGTCACCGGTTCGG (antisense) Internal: The same primers as external (sense) LJH, TGAGGAGACGGTGACC (antisense) |
346/351 | Yes |
| Igκ (human) | TGAGCAAAGCAGACTACGAGA (sense) GGGGTGAGGTGAAAGATGAG (antisense) |
231 | No |
| Igλ (human) | GAGCCTGACGCCTGAG (sense) ATTGAGGGTTTATTGAGTGCAG (antisense) |
223 | No |
| RAG1 (human) for nested PCR | External: TGGATCTTTACCTGAAGATG (sense) CTTGGCTTTCCAGAGAGTCC (antisense) Internal: CACAGCGTTTTGCTGAGCTC (sense) AGCTTGCCTGAGGGTTCATG (antisense) |
327 | No |
| RAG1 (human) for real-time PCR | GCAGCTATTGTCCCTCTTGC (sense) CATTGCACTCTTTTGCTGGA (antisense) |
120 | No |
| RAG2 (human) for nested PCR | External: TGGAAGCAACATGGGAAATG (sense) CATCATCTTCATTATAGGTGTC (antisense) Internal: TTCTTGGCATACCAGGAGAC (sense) CTATTTGCTTCTGCACTG (antisense) |
193 | No |
| RAG2 (human) for real-time PCR | TGCCCTACTTGTGATGTGGA (sense) CAGTAGATCATGGCGGGTTT (antisense) |
80 | No |
| CD19 (human) | TACTATGGCACTGGCTGCTG (sense) CACGTTCCCGTACTGGTTCT (antisense) |
218 | Yes |
| CD38 (human) | AACGGTTTCCCGCAGGTTTGC (sense) ACAACCACAGCGACTGGCTCA (antisense) |
352 | Yes |
| 18s (human) | AAACGGCTACCACATCCAAG (sense) CCTCCAATGGATCCTCGTTA (antisense) |
155 | No |
Real-Time PCR and Quantitative Analysis
Using an Mx3000p instrument, real-time PCR was performed with SYBR Premix Ex Taq (TaKaRa Bio, Inc., Siga, Japan) according to manufacturer’s instructions. Briefly, after pre-denaturation at 95C for 5 min, the amplification reaction was performed for 40 cycles of 5 sec at 94C and 40 cycles of 20 sec at 60C, followed by a final extension period of 1 min at 95C, 1 min at 60C, and 30 sec at 95C. The primers used for real-time PCR are listed in Table 2.
The expression levels of RAG1 and RAG2 in HUVECs were compared with those in Raji and NAML-6 cell lines. For all samples, experiments were performed six times. Amplification of 18s was used for normalization. The relative expressions of RAG in the tested cell types were determined with the method of 2−ΔΔCt (Livak and Schmittgen 2001).
Results
IHC
In this study, IHC was performed to detect IgG proteins with antibodies to the IgG heavy chain (Igγ) and kappa and lambda light chains (Igκ and Igλ). Positive signals were detected in cells of human umbilical vein (Fig. 1B) and arteries (Fig. 1F), and the positive signals were located in the cytoplasm of these cells. IHC was carried out on serial sections, demonstrating colocalization of Igγ, Igκ, Igλ, and CD34 in the same cells, confirming that the IgG-positive cells were HUECs (Fig. 2, arrows). FcRn was also detected in the cytoplasm of HUECs. The signal strength was weaker, and the number of positive cells was lower than that of IgG-containing cells (Fig. 3, arrows). No positive signal of FcγRI, FcγRII, or FcRγIII was detected in HUECs. No CD20-positive B lymphocyte or CD38-positive plasma cell was detected in the umbilical cord tissue (Fig. 4) by careful observation. IHC with antibodies to CD34 and factor VIII, two endothelial cell markers, was carried out on serial sections, and they were found to be colocalized with each other, indicating that the IgG-positive cells were indeed endothelial cells.
Figure 1.
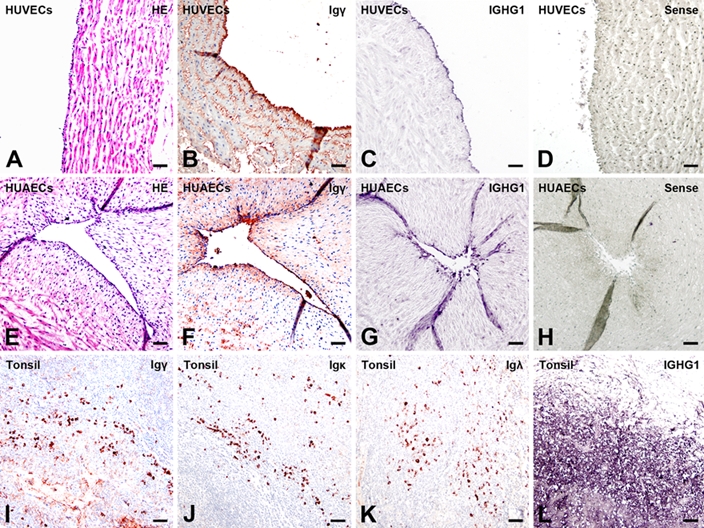
Immunoglobulin G (IgG) immunoreactivities and mRNA transcripts were detected in human umbilical endothelial cells (HUECs). (A–D) human umbilical vein endothelial cells (HUVECs), (E–H) human umbilical arterial endothelial cells (HUAECs), and (I–L) tissues of human tonsils. No abnormalities using hematoxylin and eosin (H&E) staining (A, E). Positive Igγ (B, F) immunohistochemistry (IHC) staining and positive in situ hybridization (ISH) signals (IGHG1 antisense probe) (C, G) found in HUVECs (B, C) and HUAECs (F, G). No signal is seen with an IGHG1 sense probe, confirming the specificity of ISH (D, H). Positive Igγ (I), Igκ (J), and Igλ (K) IHC signals and positive ISH signals with a IGHG1 antisense probe (L) in B lymphocytes of tonsil tissues (bar = 50 µm). IHC: color reaction was developed with a horseradish peroxidase reaction kit (3-amino-9-ethylcarbazole; Zymed Laboratories, South San Francisco, CA), resulting in a red or a dark red color. IHC slides were counterstained with hematoxylin, resulting in a blue color. If the red IHC signals overlay with the blue staining of hematoxylin in the nucleus, they appear dark red or black. ISH: reaction products were colorized with nitro blue tetrazolium/5-bromo-4-choloro-3-indolyl phosphate (NBT/BCIP) (Promega, Madison, WI), resulting in a purple-blue signal. ISH slides were counterstained with methyl green.
Figure 2.
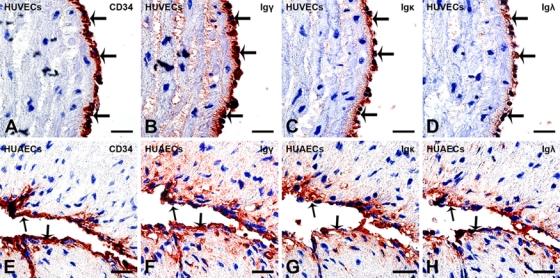
Igγ, Igκ, and Igλ were colocalized with CD34 in human umbilical endothelial cells (HUECs). (A–D) Human umbilical vein endothelial cells (HUVECs) and (E–H) human umbilical arterial endothelial cells (HUAECs). On serial sections, (A, E) CD34 (arrows), (B, F) Igγ (arrows), (C, G) Igκ (arrows), and (D, H) Igλ (arrows) are coexpressed by the same HUECs of the human umbilical vein (A–D) and human umbilical artery (E–H) (bar = 10 µm). Immunohistochemistry (IHC): color reaction was developed with a horseradish peroxidase reaction kit (3-amino-9-ethylcarbazole), resulting in a red or a dark red color. IHC slides were counterstained with hematoxylin, resulting in a blue color. If the red IHC signals overlay with the blue staining of hematoxylin in the nucleus, they appear dark red or black. Slides were counterstained with hematoxylin.
Figure 3.
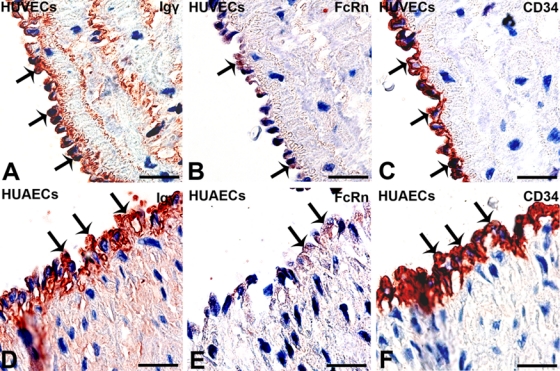
Positive immunohistochemistry (IHC) signals of FcRn and IgG (γ chain specific) were detected in CD34-positive human umbilical endothelial cells (HUECs). On serial sections, (A, D) Igγ (arrows) and (B, E) FcRn (arrows) colocalize in (C, F) CD34-positive (arrows) HUECs in (A–C) human umbilical vein and (D–F) human umbilical artery (bar = 10 µm). Compared with Igγ, the Fc receptor staining is very weak and sparse (arrows). IHC: color reaction was developed with a horseradish peroxidase reaction kit (3-amino-9-ethylcarbazole), resulting in a red or a dark red color. IHC slides were counterstained with hematoxylin, resulting in a blue color. If the red IHC signals overlay with the blue staining of hematoxylin in the nucleus, they appear dark red or black. Slides were counterstained with hematoxylin.
Figure 4.
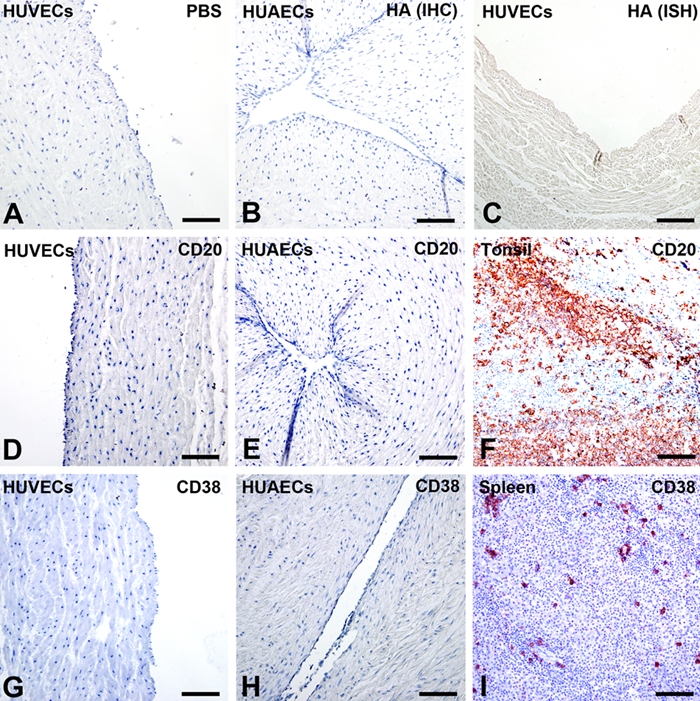
Immunohistochemistry (IHC) with antibodies to CD20 and CD38 and negative controls. (A, C, D, G) Human umbilical vein endothelial cells (HUVECs), (B, E, H) human umbilical arterial endothelial cells (HUAECs), (F) tissue of human tonsil, and (I) tissue of human spleen. In negative controls for IHC, primary antibodies were replaced by PBS (A) or irrelevant antibodies against hemagglutinin (HA) of H5N1 influenza virus (B). In negative controls for ISH, an irrelevant probe against HA of the H5N1 influenza virus of similar nucleotide length was used instead of the IGHG1 antisense probe (C). No positive signals were detected. Neither CD20-positive B lymphocytes (D, E) nor CD38-positive plasma cells (G, H) were identified in the muscular layers of the umbilical vein (D, G) or artery (E, H). Tonsil (F) and spleen (I) tissues served as positive control for CD20 (F) and CD38 (I). (Bar = 50 µm)
IF
IF was performed to detect the IgG protein with antibodies to Igγ. Positive signals were identified in primary cultured HUVECs (Fig. 5D–F), and the positive signals were located in the cytoplasm of these cells. Positive CD34 signals in the cytoplasm of cultured cells indicated that these cells were indeed umbilical endothelial cells (Fig. 5A–C).
Figure 5.
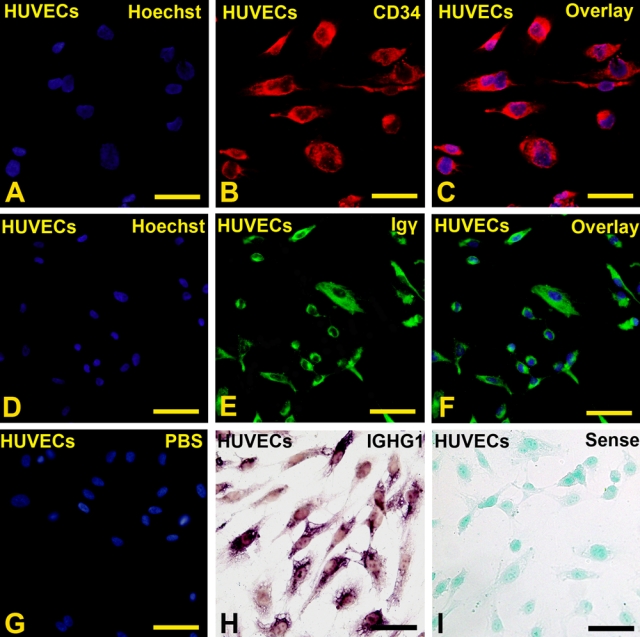
Igγ and IGHG1 were detected in CD34-positive primary cultured human umbilical vein endothelial cells (HUVECs). (A) Positive signals of Hoechst 33342 in the nucleus of CD34-positive cells. (B) Positive signals of CD34 were detected in the cytoplasm of HUVECs. (C) Overlay of A and B. (D) Positive signals of Hoechst 33342 in the nucleus of IgG-positive cells. (E) Positive signals of IgG (γ chain specific) were detected in the cytoplasm of HUVECs. (F) Overlay of D and E. (G) Overlay of negative signals of PBS and positive signals of Hoechst 33342 in the nucleus of PBS-negative cells. (H) Positive in situ hybridization (ISH) signals of IGHG1 were detected in the cytoplasm of HUVECs. (I) IGHG1 sense probe was used as negative control. (Bar = 20 µm)
ISH
ISH was next performed to investigate whether the IgG gene is actually expressed by HUECs. A cRNA probe against IGHG1 was employed, and positive signals were detected in endothelial cells of the umbilical vein (Fig. 1C), umbilical artery (Fig. 1G), and primary cultured HUVECs (Fig. 5H). ISH showed mainly intracytoplasmic staining. IHC and ISH were performed on serial sections, and colocalization of Igγ, IGHG1 mRNA, and CD34 suggests that CD34-positive HUECs have the capacity to produce IgG (Fig. 6, arrows).
Figure 6.
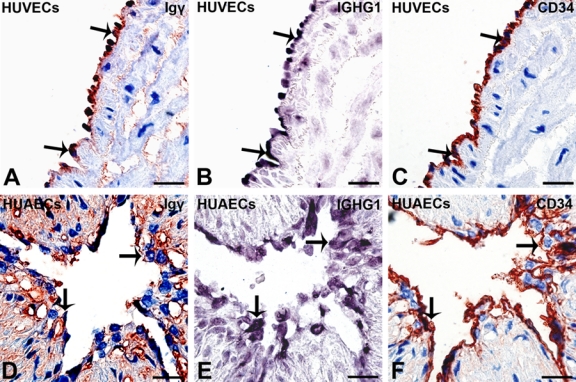
Igγ and IGHG1 were detected in CD34-positive human umbilical endothelial cells (HUECs). On serial sections, (A, D) Igγ (immunohistochemistry [IHC]; arrows) and (B, E) IGHG1 (in situ hybridization [ISH]; arrows) colocalize in (C, F) CD34-positive (arrows) HUECs in (A–C) human umbilical vein and (D–F) human umbilical artery (bar = 10 µm). IHC: color reaction was developed with a horseradish peroxidase reaction kit (3-amino-9-ethylcarbazole; Zymed Laboratories, South San Francisco, CA), resulting in a red or a dark red color. IHC slides were counterstained with hematoxylin, resulting in a blue color. If the red IHC signals overlay with the blue staining of hematoxylin in the nucleus, they appear dark red or black. IHC slides were counterstained with hematoxylin. ISH: the reaction products were colorized with nitro blue tetrazolium/5-bromo-4-choloro-3-indolyl phosphate (NBT/BCIP) (Promega, Madison, WI), resulting in a purple-blue signal. ISH slides were counterstained with methyl green.
RT-PCR and Real-Time PCR
IgG Expression
To further establish that HUECs can express IgG, RT-PCR was performed to detect IgG mRNA in the tissue samples. To exclude possible contamination by fetal lymphocytes, HUVECs from a primary culture system were also generated. Gene transcripts of IGHG1, Igκ, and Igλ were identified in tissues of human umbilical cord and primarily cultured HUVECs (Fig. 7A). Reaction mixtures with RNA templates without reverse transcriptase and DNase-treated RNA templates served as negative controls. The successful amplification of IGHG1, Igκ, and Igλ mRNA, together with the ISH results, strongly suggests that HUECs are capable of producing IgG.
Figure 7.
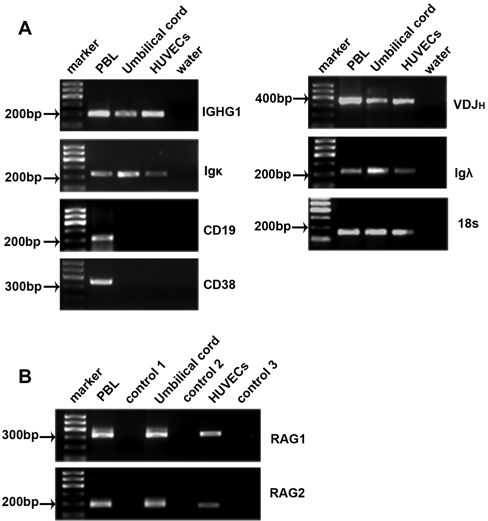
IgG mRNA transcripts were detected in primary cultured human umbilical vein endothelial cells (HUVECs) and tissues of human umbilical cord. (A) mRNA transcripts of IGHG1, VDJH, Igκ, and Igλ were detected in umbilical cord and primary cultured HUVECs. Peripheral blood lymphocytes (PBLs) served as positive control. Absence of CD19 and CD38 transcripts excludes B lymphocyte and plasma cell contamination. (B) Both RAG 1 and 2 were detectable in umbilical cord and cultured HUVECs. Control 1, control 2, and control 3: cDNA without reverse transcriptase as template. Umbilical cord represents tissues of human umbilical cord. RAG, recombination activation gene; VDJH, variable region of the Ig heavy chain; IGHG1, constant region of the IgG1 heavy chain. These data are representative of five experiments.
V(D)J Rearrangement
We also detected the gene transcripts of RAG1 and RAG2 proteins, the two enzymes that together initiate the recombination of the V(D)J segments of Ig chains, both in tissues of human umbilical cord and in the primary culture of HUVECs (Fig. 7B). Raji cell line and NALM-6 cell lines were used to represent mature B cells and pre–B cells, respectively. Real-time PCR was performed to compare the RAG expressions in HUVECs with those in mature B cells and pre–B cells. It was found that the relative expression of RAG1 in HUVECs was approximately 29 times higher than that in Raji cells (Fig. 8A), whereas RAG2 in HUVECs was approximately 4 times higher than that in Raji cells (Fig. 8B). The relative expressions of RAG1 (Fig. 8C) and RAG2 (Fig. 8D) were 205 and 637 times higher, respectively, in pre–B cells compared to that in HUVECs. These data indicate that the expression of RAG in HUVECs is much higher than that in mature B cells but much lower than that in pre–B cells. The connective tissue adjacent to blood vessels, used as a negative control, showed no positive signal of RAG (Fig. 8E). Gene transcripts of recombined VDJH were also detected in tissues of umbilical cord and primary cultured HUVECs (Fig. 7A). Sequencing of three randomly chosen VDJH clones showed that they were highly homologous (87%) to IGHV4-59*01 (Table 3). These findings indicate that rearrangement of V(D)J gene segments might occur in HUECs. Contaminations by B lymphocytes and plasma cells were excluded by using primers for CD19 and CD38 in RT-PCR, respectively (Fig. 7A).
Figure 8.
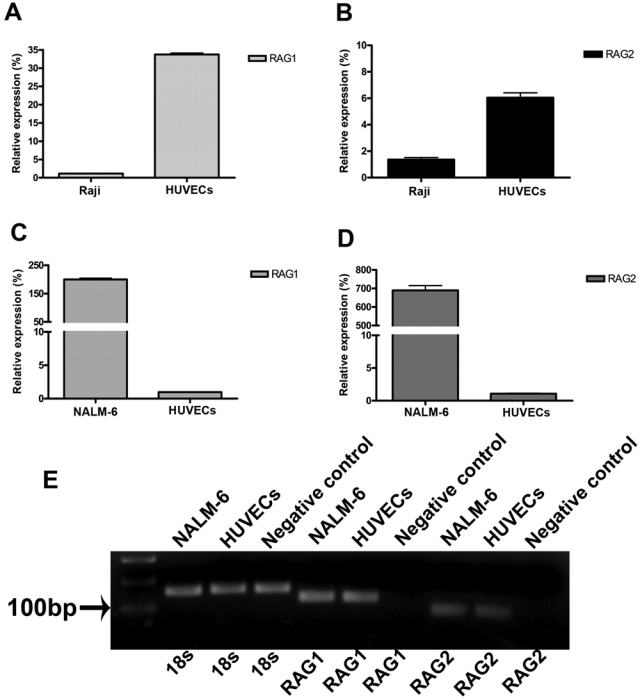
Quantitative analysis of RAG expressions in human umbilical vein endothelial cells (HUVECs), NALM-6 cell line, and Raji cell line. (A) The relative expression of RAG1 in HUVECs is 29 times higher than that in the Raji cell line. (B) The relative expression of RAG2 in HUVECs is 4 times higher than that in the Raji cell line. (C) The relative expression of RAG1 in the NALM-6 cell line is 205 times higher than that in HUVECs. (D) The relative expression of RAG2 in the NALM-6 cell line is 637 times higher than that in HUVECs. (E) Gel electrophoresis of some of the real-time PCR products to indicate the specificity of the reaction. Negative control: total RNA extracted from connective tissues.
Table 3.
Sequences of the PCR Product of VDJH for Primary Cultured Human Umbilical Vein Endothelial Cells
Discussion
The present study demonstrates that HUECs can produce IgG. Both IgG proteins and IGHG1 mRNA transcripts were detected in endothelial cells of the human umbilical vein and arteries. Positive IGHG1 ISH signals were mainly located in the cytoplasm of cells with morphological features of endothelial cells. IHC staining with antibodies to CD34 and factor VIII on consecutive sections confirmed that these cells were indeed endothelial cells. RT-PCR amplified IGHG1 transcripts from umbilical cord tissues further established endogenous expression of IgG. The detection of VDJH gene transcripts suggests that recombination of V(D)J gene segments might take place in the umbilical cord, which is also supported by observation of gene amplification of RAG1 and RAG2, two enzymes required for recombination of V(D)J gene segments. Quantitative analysis demonstrated higher expression levels of RAG1 and RAG2 in HUVECs compared to mature cells and lower levels compared to pre–B cells. Sequencing of three randomly selected VDJH clones showed that they were all closely homologous to the IGHV4-59*01 germ line (http://www.ncbi.nlm.nih.gov/BLAST). To eliminate potential contamination by B lymphocytes and plasma cells, we employed a primary HUVEC culture system, in which the endogenous expression of IgG was ascertained by the techniques of IF, ISH, and RT-PCR. As umbilical cord blood is a well-known source for hematopoietic progenitor and stem cells, and stroma cells isolated from Wharton’s jelly (Troyer and Weiss 2008) support the expansion of such cells, the same primary endothelial cell culturing system was also used to eliminate contamination by hematopoietic progenitor and stem cells (Conrad and Emerson 1998). It is impossible to deduct only from the IHC results whether IgG was actually synthesized in umbilical cord endothelial cells or where it was synthesized. Positive staining of IgG could be the result of absorption or passive adherence of IgG from the surrounding environment. However, together with the results of ISH and RT-PCR obtained from umbilical cord tissue and those of ISH, RT-PCR, and IF observed in the primary culture of HUVECs, we believe that there is sufficient evidence to show that at least some of the IgG detected in the umbilical cord endothelial cells are produced by these cells themselves. The positive IgG signals sometimes were seen over the border of the endothelial cells. There are two possible explanations for this phenomenon. One is that the positive IHC signals could be attributed to serum IgG adhered to endothelial cell surfaces. The other is that IgG produced by the endothelial cells might be released into the surrounding tissue. In this study, we found no B lymphocyte or plasma cell in umbilical cord tissue. This is in agreement with a previous report by Rossi et al. (2003), who indicated that the amount of mature B cells and plasma cells in the fetus was very small. The absence of detectable B lymphoid cells in the tissue samples we examined with a range of techniques strongly supports that the IgG we detected was not from local lymphocytes but from endothelial cells.
Traditional views hold that IgG is expressed only by B lymphocytes and plasma cells. In recent years, our group and others have shown that IgG can also be produced by non-lymphoid cell types. Initial reports described IgG expression by various epithelial cancer types (Babbage et al. 2006; Chen and Gu 2007; Qiu et al. 2003), whereas later studies demonstrated IgG expression in non-cancerous cell types, including neurons of the CNS, testicular spermatogenic cells, epididymal epithelial cells, and epithelial cells of lactating mammary glands (Huang et al. 2008; Huang et al. 2009; Zhang S et al. 2010).
There are two functional classes of IgG receptors—that is, the Fc gamma receptor (FcγR) with three subclasses (FcγRI, FcγRII, and FcγRIII) and the neonatal Fc receptor (FcRn) (Raghavan and Bjorkman 1996). FcγRs are chiefly present on the surface of immune cells, where they mediate effector functions (Raghavan and Bjorkman 1996). FcRn is expressed by antigen presenting cells, as well as epithelial and endothelial cells in various organs, where they are thought to transport IgG and immune complexes across barriers and recycle IgG, extending its half-life (Roopenian and Akilesh 2007). In the placenta, FcRn mediates IgG transfer across the syncytiotrophoblast layer (Roopenian and Akilesh 2007). FcγRs are expressed on Hofbauer cells (Jensen and Matre 1995; Kameda et al. 1991; Sedmak et al. 1991; Stuart et al. 1989) and, in varying degrees, on trophoblasts and endothelial cells (Honig et al. 2005; Jensen and Matre 1995; Kameda et al. 1991; Sedmak et al. 1991; Stuart et al. 1989; Takizawa et al. 2005), where their function is not well defined. In the present study, none of the FcγR subclasses was found to be expressed in the umbilical cord, which is in line with the results of other research groups (Groger et al. 1996; Lang et al. 1993; Sedmak et al. 1991). However, Alberto et al. (2000) described that HUVECs bound heat-aggregated IgG, which appeared to be correlated with the expression of FcγRI, and Cines et al. (1982) reported that herpes simplex virus infection could induce expression of IgG Fc receptors on cultured HUVECs. In contrast to FcγRs, FcRn was expressed by HUECs. Its function in the umbilical cord could relate to the transport of IgG across the endothelial cells. Previous studies have demonstrated that FcRn facilitates the reverse transcytosis into the systemic circulation across the blood-retinal and blood-brain barriers, which are both composed of capillary endothelial cells (Kim et al. 2009; Zhang Y and Pardridge 2001). Analogously, FcRn could transport IgG produced by HUECs into the umbilical vein and arteries.
Based on the original findings made in this study, the following functional significances are conceivable. First, endogenously produced IgG could be involved in the modulation of inflammatory and infectious processes in the umbilical cord. In support of this notion, commercial human immunoglobulins were found to inhibit the proliferation of cultured HUVECs and to downregulate the cytokine-induced expression of adhesion molecules, chemokines, and cytokines in these cells (Xu et al. 1998; Yoon et al. 2006). In addition, locally produced IgG could play a role in the protection against maternal antibodies to paternal major histocompatibility complex (MHC) antigens. Such antibodies are generated in response to fetal lymphocytes entering the maternal bloodstream (Schroder and De la Chapelle 1972) and could in theory cross the placental barrier, causing damage to fetal cells expressing paternal MHC, including endothelial cells and immune cells. These antibodies are thought to be trapped in the placenta in the form of immune complexes composed of maternal antibodies and paternal antigens expressed on stromal and endothelial cells (Simister 1998). In our previous study on IgG expression in the placenta, we suggested that endogenously produced IgG might be anti-idiotypic, targeting the variable region of maternal antipaternal MHC antibodies, thus neutralizing these potential harmful antibodies (Zhao Y, Deng R, Chen Z, Korteweg C, Zhang J, Li J,Wang Yun, Wang Yongyu, Lin C, Bluth MH, Niu N, Zhuang Z, Su M, Gu J, unpublished data). Analogously, IgG produced by HUECs could also be anti-idiotypic with protective properties against antipaternal antibodies. This assumption becomes even more plausible when considering that HUECs themselves are also potential targets for antipaternal MHC antibodies, as they express MHC class I molecules constitutively at high levels (Adams, Lee, Ferguson, et al. 1994; Adams, Lee, Waldman, et al. 1994) and MHC class II at low levels (Krakauer 1996), the latter of which can be increased through induction by various cytokines and microbial pathogens (Adams, Lee, Waldman, et al. 1994; Krakauer 1996). Along the same lines, HUECs could produce asymmetrical antibodies that are incapable of eliciting immune effector functions (Margni and Binaghi 1988). Asymmetrical antibodies are thought to play a role in sustaining pregnancy by blocking paternal MHC antigens expressed on fetal cells (Barrientos et al. 2009; Malan Borel et al. 1991; Margni and Malan Borel 1998). Another possible function of locally produced IgG could involve growth promotion as IgG-stimulated growth of cancer cells in animal and in vitro experiments (Qiu et al. 2003). In fact, umbilical endothelial cells produce various growth factors, including basic fibroblast growth factor, transforming growth factor beta, and platelet-derived growth factor (Hannan et al. 1988; Kourembanas and Faller 1989). Finally, IgG derived from HUECs could play a role in the protection against invading pathogens that could threaten fetal survival.
In conclusion, umbilical endothelial cells can produce IgG, and physiological and pathological significance of this phenomenon is clearly indicated and calls for in-depth further investigation.
Acknowledgments
We acknowledge Yan Zhou and Na Niu for their help in performing part of the experiments, and we also thank Professor Nanping Wang for his help in providing the primary cell culture of human umbilical cord endothelial cells.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the authorship and/or publication of this article.
The author(s) disclosed receipt of the following financial support for the research and/or authorship of this article: This study was supported by the International Collaboration Project (111 Project) (B 07001) and the National Natural Science Foundation of China (Research Fellowship for International Young Scientists; grant number 30950110335). We also acknowledge a research grant support of the National Natural Science Foundation of China to Jiang Gu to study Ig expression in extra-lymphoid cells (no. 30971150).
References
- Adams PW, Lee HS, Ferguson RM, Orosz CG. 1994. Alloantigenicity of human endothelial cells: II. Analysis of interleukin 2 production and proliferation by T cells after contact with allogeneic endothelia. Transplantation. 57:115–122 [DOI] [PubMed] [Google Scholar]
- Adams PW, Lee HS, Waldman WJ, Sedmak DD, Orosz CG. 1994. Alloantigenicity of human endothelial cells: III. Quantitated indirect presentation of endothelial alloantigens to human helper T lymphocytes. Transplantation. 58:476–483 [PubMed] [Google Scholar]
- Alberto MF, Bermejo EI, Lazzari MA. 2000. Receptor expression for IgG constant fraction in human umbilical vein endothelial cells. Thromb Res. 97:505–511 [DOI] [PubMed] [Google Scholar]
- Babbage G, Ottensmeier CH, Blaydes J, Stevenson FK, Sahota SS. 2006. Immunoglobulin heavy chain locus events and expression of activation-induced cytidine deaminase in epithelial breast cancer cell lines. Cancer Res. 66:3996–4000 [DOI] [PubMed] [Google Scholar]
- Barrientos G, Fuchs D, Schrocksnadel K, Ruecke M, Garcia MG, Klapp BF, Raghupathy R, Miranda S, Arck PC, Blois SM. 2009. Low levels of serum asymmetric antibodies as a marker of threatened pregnancy. J Reprod Immunol. 79:201–210 [DOI] [PubMed] [Google Scholar]
- Benirschke K, Kaufmann P. 2000. Pathology of the human placenta. 4th ed. New York: Springer-Verlag [Google Scholar]
- Can A, Karahuseyinoglu S. 2007. Concise review: human umbilical cord stroma with regard to the source of fetus-derived stem cells. Stem Cells. 25:2886–2895 [DOI] [PubMed] [Google Scholar]
- Chen Z, Gu J. 2007. Immunoglobulin G expression in carcinomas and cancer cell lines. Faseb J. 21:2931–2938 [DOI] [PubMed] [Google Scholar]
- Cines DB, Lyss AP, Bina M, Corkey R, Kefalides NA, Friedman HM. 1982. Fc and C3 receptors induced by herpes simplex virus on cultured human endothelial cells. J Clin Invest. 69:123–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad PD, Emerson SG. 1998. Ex vivo expansion of hematopoietic cells from umbilical cord blood for clinical transplantation. J Leukoc Biol. 64:147–155 [DOI] [PubMed] [Google Scholar]
- Gitlin D, Biasucci A. 1969. Development of gamma G, gamma A, gamma M, beta IC-beta IA, C 1 esterase inhibitor, ceruloplasmin, transferrin, hemopexin, haptoglobin, fibrinogen, plasminogen, alpha 1–antitrypsin, orosomucoid, beta-lipoprotein, alpha 2–macroglobulin, and prealbumin in the human conceptus. J Clin Invest. 48:1433–1446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groger M, Sarmay G, Fiebiger E, Wolff K, Petzelbauer P. 1996. Dermal microvascular endothelial cells express CD32 receptors in vivo and in vitro. J Immunol. 156:1549–1556 [PubMed] [Google Scholar]
- Gu J, Xie Z, Gao Z, Liu J, Korteweg C, Ye J, Lau LT, Lu J, Gao Z, Zhang B, et al. 2007. H5N1 infection of the respiratory tract and beyond: a molecular pathology study. Lancet. 370:1137–1145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannan RL, Kourembanas S, Flanders KC, Rogelj SJ, Roberts AB, Faller DV, Klagsbrun M. 1988. Endothelial cells synthesize basic fibroblast growth factor and transforming growth factor beta. Growth Factors. 1:7–17 [DOI] [PubMed] [Google Scholar]
- Holland JA, Pritchard KA, Rogers NJ, Stemerman MB. 1988. Perturbation of cultured human endothelial cells by atherogenic levels of low density lipoprotein. Am J Pathol. 132:474–478 [PMC free article] [PubMed] [Google Scholar]
- Honig A, Rieger L, Kapp M, Dietl J, Kammerer U. 2005. Immunohistochemistry in human placental tissue: pitfalls of antigen detection. J Histochem Cytochem. 53:1413–1420 [DOI] [PubMed] [Google Scholar]
- Huang J, Sun X, Mao Y, Zhu X, Zhang P, Zhang L, Du J, Qiu X. 2008. Expression of immunoglobulin gene with classical V-(D)-J rearrangement in mouse brain neurons. Int J Biochem Cell Biol. 40:1604–1615 [DOI] [PubMed] [Google Scholar]
- Huang J, Zhang L, Ma T, Zhang P, Qiu X. 2009. Expression of immunoglobulin gene with classical V-(D)-J rearrangement in mouse testis and epididymis. J Histochem Cytochem. 57:339–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe EA, Nachman RL, Becker CG, Minick CR. 1973. Culture of human endothelial cells derived from umbilical veins: identification by morphologic and immunologic criteria. J Clin Invest. 52:2745–2756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen TS, Matre R. 1995. Fc gamma-receptor activity in the developing human placenta. Apmis. 103:433–438 [PubMed] [Google Scholar]
- Kameda T, Koyama M, Matsuzaki N, Taniguchi T, Saji F, Tanizawa O. 1991. Localization of three subtypes of Fc gamma receptors in human placenta by immunohistochemical analysis. Placenta. 12:15–26 [DOI] [PubMed] [Google Scholar]
- Kim H, Robinson SB, Csaky KG. 2009. FcRn receptor-mediated pharmacokinetics of therapeutic IgG in the eye. Mol Vis. 15:2803–2812 [PMC free article] [PubMed] [Google Scholar]
- Kourembanas S, Faller DV. 1989. Platelet-derived growth factor production by human umbilical vein endothelial cells is regulated by basic fibroblast growth factor. J Biol Chem. 264:4456–4459 [PubMed] [Google Scholar]
- Krakauer T. 1996. Detection of adhesion of superantigen-activated T lymphocytes to human endothelial cells by ELISA. J Immunoassay. 17:1–12 [DOI] [PubMed] [Google Scholar]
- Lang I, Hartmann M, Blaschitz A, Dohr G, Skofitsch G, Desoye G. 1993. Immunohistochemical evidence for the heterogeneity of maternal and fetal vascular endothelial cells in human full-term placenta. Cell Tissue Res. 274:211–218 [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 25:402–408 [DOI] [PubMed] [Google Scholar]
- Malan Borel I, Gentile T, Angelucci J, Pividori J, Guala MC, Binaghi RA, Margni RA. 1991. IgG asymmetric molecules with antipaternal activity isolated from sera and placenta of pregnant human. J Reprod Immunol. 20:129–140 [DOI] [PubMed] [Google Scholar]
- Margni RA, Binaghi RA. 1988. Nonprecipitating asymmetric antibodies. Annu Rev Immunol. 6:535–554 [DOI] [PubMed] [Google Scholar]
- Margni RA, Malan Borel I. 1998. Paradoxical behavior of asymmetric IgG antibodies. Immunol Rev. 163:77–87 [DOI] [PubMed] [Google Scholar]
- Pitcher-Wilmott RW, Hindocha P, Wood CB. 1980. The placental transfer of IgG subclasses in human pregnancy. Clin Exp Immunol. 41:303–308 [PMC free article] [PubMed] [Google Scholar]
- Qiu X, Zhu X, Zhang L, Mao Y, Zhang J, Hao P, Li G, Lv P, Li Z, Sun X, et al. 2003. Human epithelial cancers secrete immunoglobulin G with unidentified specificity to promote growth and survival of tumor cells. Cancer Res. 63:6488–6495 [PubMed] [Google Scholar]
- Raghavan M, Bjorkman PJ. 1996. Fc receptors and their interactions with immunoglobulins. Annu Rev Cell Dev Biol. 12:181–220 [DOI] [PubMed] [Google Scholar]
- Roopenian DC, Akilesh S. 2007. FcRn: the neonatal Fc receptor comes of age. Nat Rev Immunol. 7:715–725 [DOI] [PubMed] [Google Scholar]
- Rossi MI, Yokota T, Medina KL, Garrett KP, Comp PC, Schipul AH, Jr, Kincade PW. 2003. B lymphopoiesis is active throughout human life, but there are developmental age-related changes. Blood. 101:576–584 [DOI] [PubMed] [Google Scholar]
- Schroder J, De la Chapelle A. 1972. Fetal lymphocytes in the maternal blood. Blood. 39:153–162 [PubMed] [Google Scholar]
- Sedmak DD, Davis DH, Singh U, van de Winkel JG, Anderson CL. 1991. Expression of IgG Fc receptor antigens in placenta and on endothelial cells in humans: an immunohistochemical study. Am J Pathol. 138:175–181 [PMC free article] [PubMed] [Google Scholar]
- Simister NE. 1998. Human placental Fc receptors and the trapping of immune complexes. Vaccine. 16:1451–1455 [DOI] [PubMed] [Google Scholar]
- Stuart SG, Simister NE, Clarkson SB, Kacinski BM, Shapiro M, Mellman I. 1989. Human IgG Fc receptor (hFcRII; CD32) exists as multiple isoforms in macrophages, lymphocytes and IgG-transporting placental epithelium. Embo J. 8:3657–3666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takechi K, Kuwabara Y, Mizuno M. 1993. Ultrastructural and immunohistochemical studies of Wharton’s jelly umbilical cord cells. Placenta. 14:235–245 [DOI] [PubMed] [Google Scholar]
- Takizawa T, Anderson CL, Robinson JM. 2005. A novel Fc gamma R-defined, IgG-containing organelle in placental endothelium. J Immunol. 175:2331–2339 [DOI] [PubMed] [Google Scholar]
- Troyer DL, Weiss ML. 2008. Wharton’s jelly–derived cells are a primitive stromal cell population. Stem Cells. 26:591–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, Verna L, Hardy S, Zhu Y, Ma KS, Birrer MJ, Stemerman MB. 1999. c-Jun triggers apoptosis in human vascular endothelial cells. Circ Res. 85:387–393 [DOI] [PubMed] [Google Scholar]
- Xu C, Poirier B, Van Huyen JP, Lucchiari N, Michel O, Chevalier J, Kaveri S. 1998. Modulation of endothelial cell function by normal polyspecific human intravenous immunoglobulins: a possible mechanism of action in vascular diseases. Am J Pathol. 153:1257–1266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J, Zhang B, Xu J, Chang Q, McNutt MA, Korteweg C, Gong E, Gu J. 2007. Molecular pathology in the lungs of severe acute respiratory syndrome patients. Am J Pathol. 170:538–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon JS, Kim HH, Han JW, Lee Y, Lee JS. 2006. Effects of intravenous immunoglobulin and methylprednisolone on human umbilical vein endothelial cells in vitro. Immunobiology. 211:351–357 [DOI] [PubMed] [Google Scholar]
- Zhang S-X. 1999. An atlas of histology. New York: Springer-Verlag [Google Scholar]
- Zhang S, Mao Y, Huang J, Ma T, Zhang L, Zhu X, Zheng J, Wu L, Yin CC, Qiu X. 2010. Immunoglobulin gene locus events in epithelial cells of lactating mouse mammary glands. Cell Mol Life Sci. 67:985–994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Pardridge WM. 2001. Mediated efflux of IgG molecules from brain to blood across the blood-brain barrier. J Neuroimmunol. 114:168–172 [DOI] [PubMed] [Google Scholar]


