Abstract
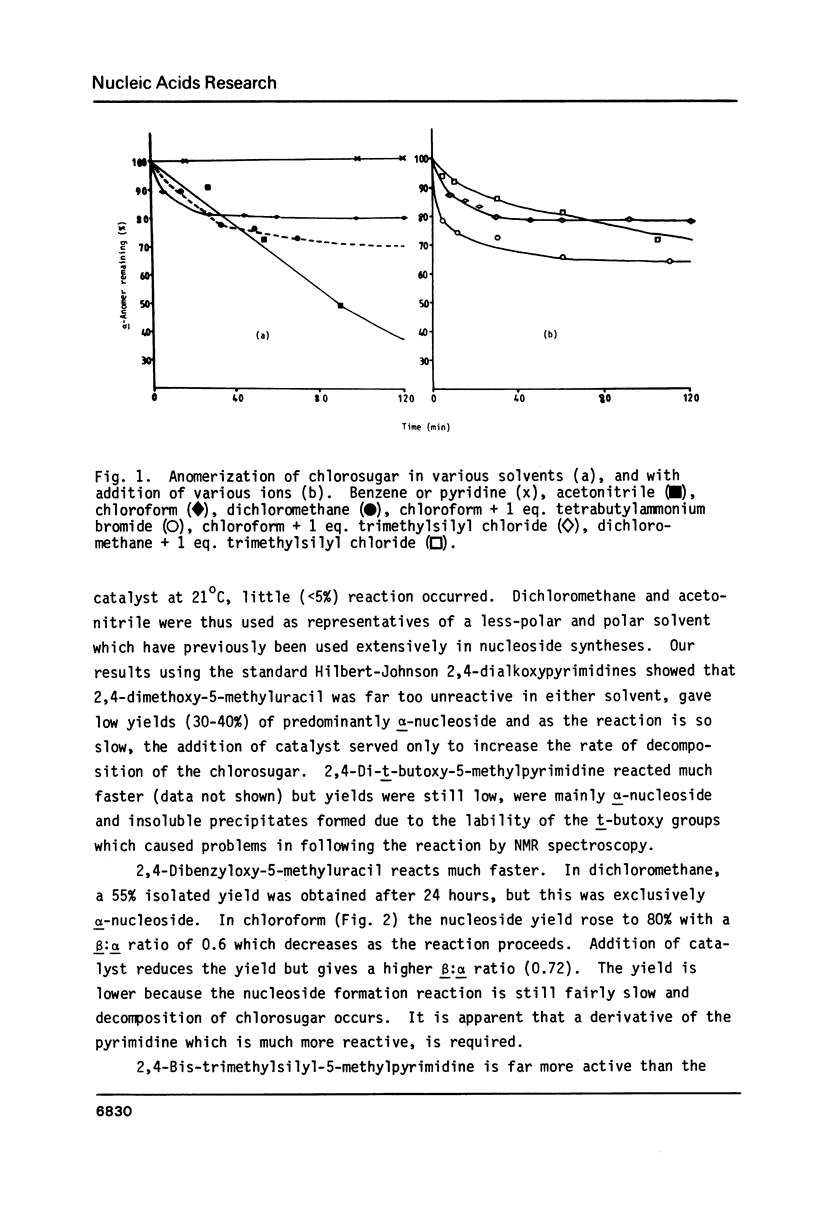
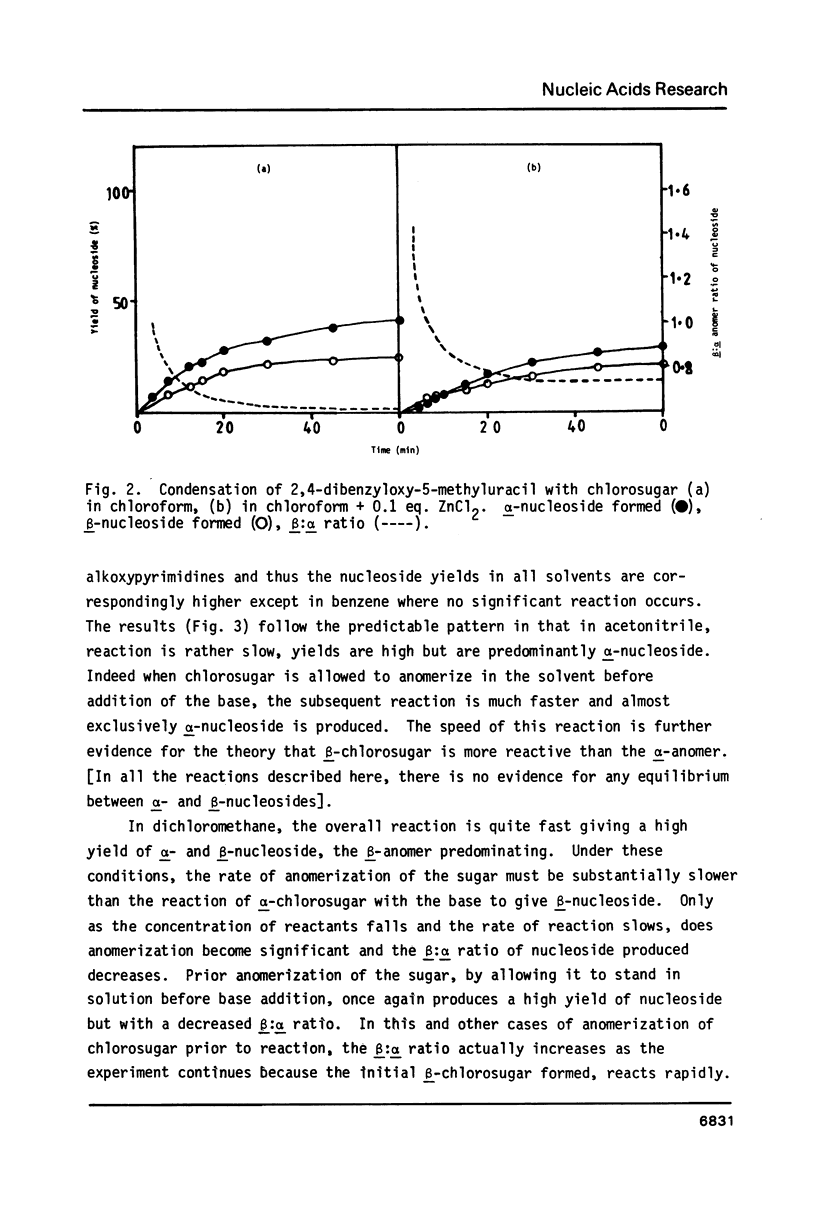
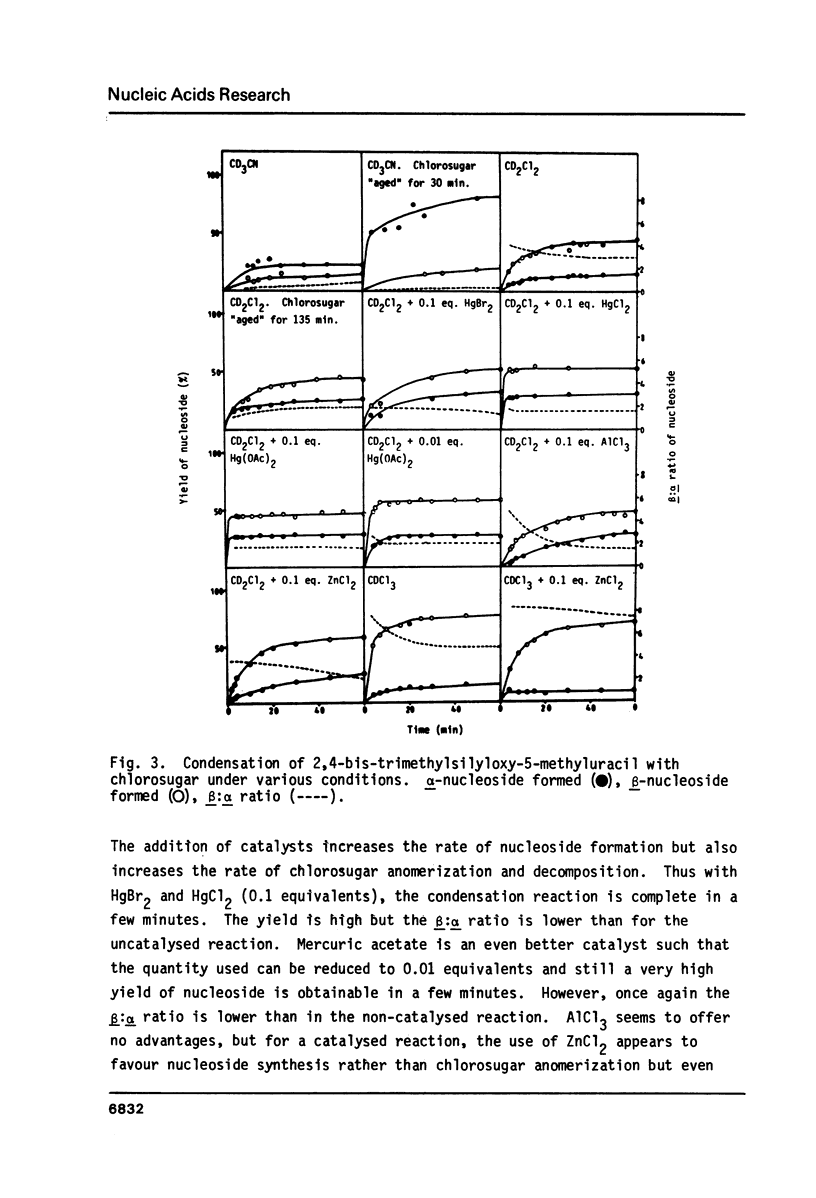
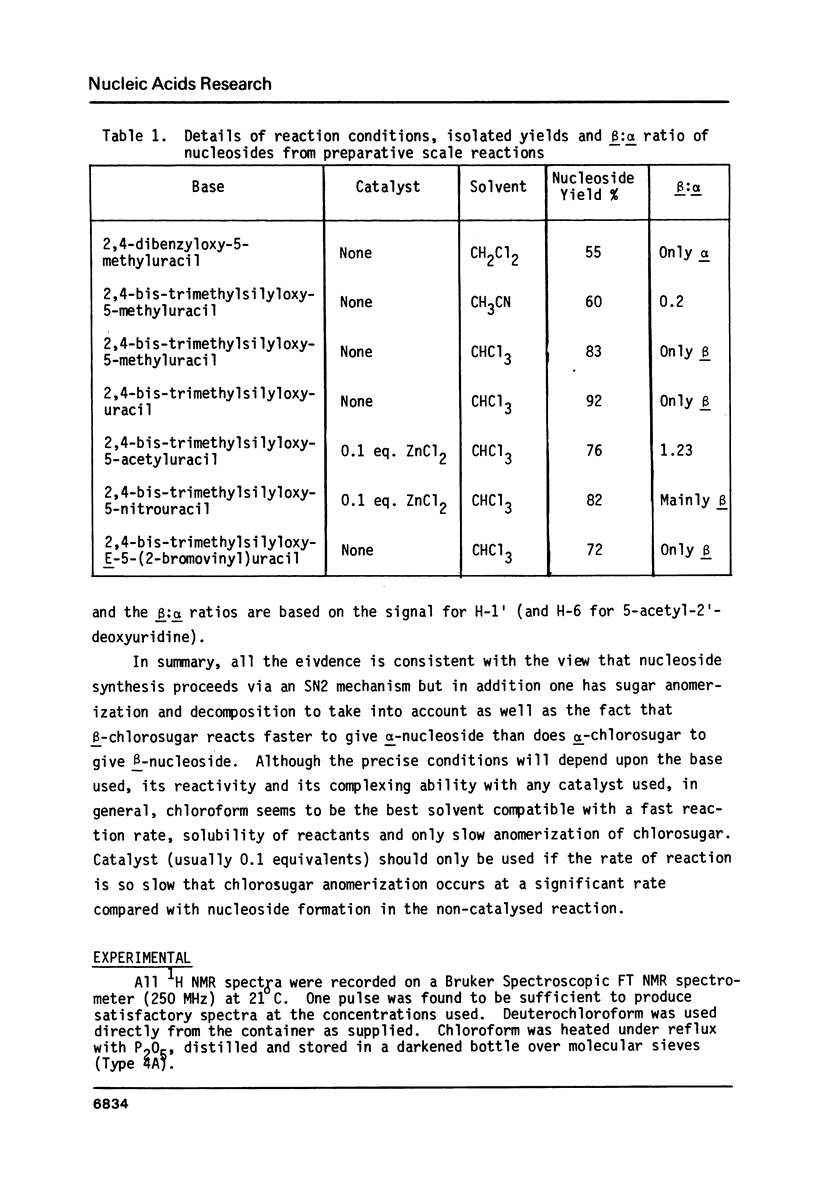
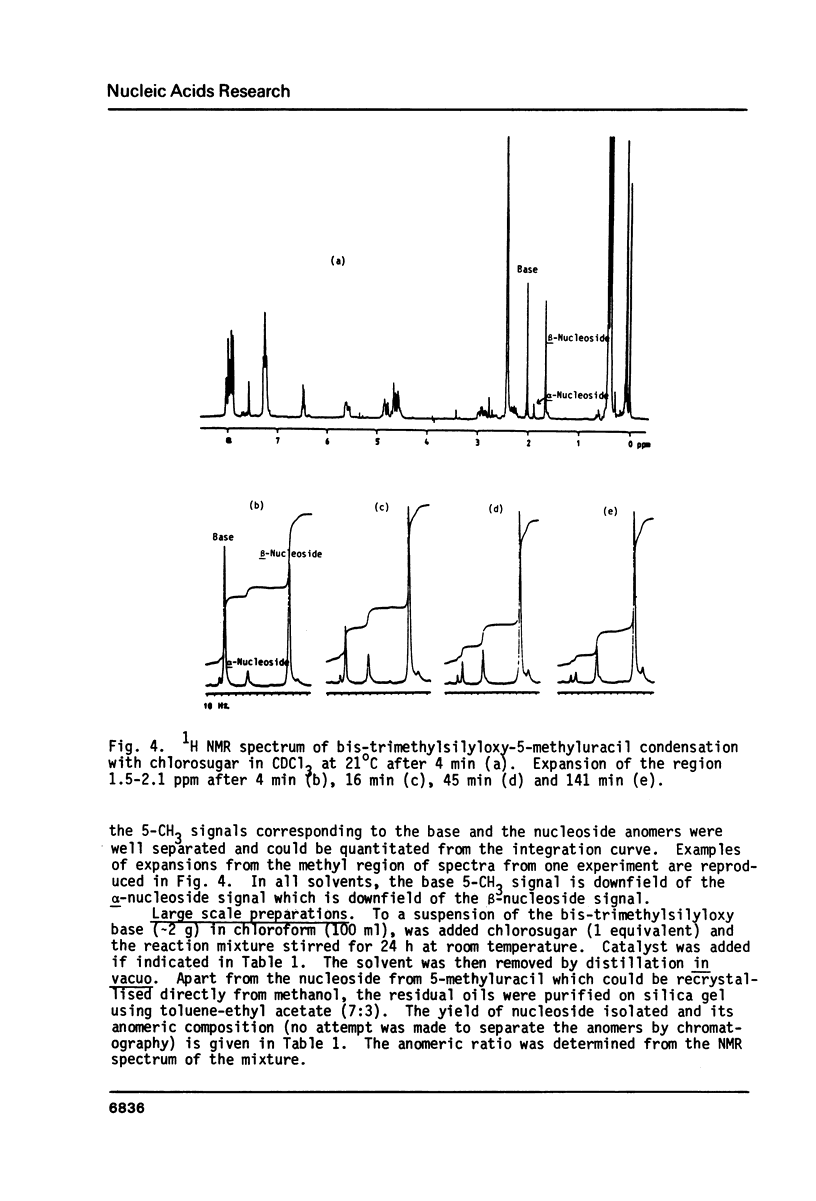
By following the course of the reaction between a suitably-protected base and a chlorosugar in an NMR tube at 250 MHz, it has been shown that the products are consistent with those expected from an SN2 mechanism with inversion of configuration at the anomeric carbon of chlorosugar. In order to achieve high yields of beta-2'-deoxynucleoside, the crystalline alpha-chlorosugar used must react swiftly so that anomerization of the sugar moiety is kept to a minimum. If the base is sufficiently reactive (e.g. 5-methyluracil, uracil), then no catalyst is required and chloroform is the preferred solvent. Using equimolar quantities of the reactants, almost quantitative yields of nucleoside can be obtained in one hour with a beta:alpha ratio greater than 4. With an excess of base, the beta:alpha ratio can be increased even further. With less reactive bases (e.g. 5-nitrouracil, 5-acetyluracil), addition of catalyst can increase the rate of condensation more than the rate of anomerization or decomposition of the sugar. ZnCl2 (0.1 equivalents) has been found to give satisfactory results, although the slower the reaction, inevitably the more alpha-2'-deoxynucleoside is formed. Essentially pure alpha-2'-deoxynucleoside can be isolated in high yield by allowing chlorosugar to anomerize by letting it stand in a polar solvent (acetonitrile) before addition of the base.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Huang G. F., Torrence P. F. Nitration of pyrimidine bases and nucleotides by nitronium tetrafluoroborate. Synthesis of 5-nitro-2'-deoxyuridine. J Org Chem. 1977 Nov 25;42(24):3821–3824. doi: 10.1021/jo00444a006. [DOI] [PubMed] [Google Scholar]
- Johnson T. B., Hilbert G. E. THE SYNTHESIS OF PYRIMIDINE-NUCLEOSIDES. Science. 1929 May 31;69(1796):579–580. doi: 10.1126/science.69.1796.579. [DOI] [PubMed] [Google Scholar]
- Kotick M. P., Szantay C., Bardos T. J. Synthesis of 5-S-substituted 2'-deoxyuridines. Study of the factors influencing stereoselectivity of the silyl modification of the Hilbert-Johnson reaction. J Org Chem. 1969 Dec;34(12):3806–3813. doi: 10.1021/jo01264a015. [DOI] [PubMed] [Google Scholar]
- Niedballa U., Vorbrüggen H. A general synthesis of N-glycosides. 6. On the mechanism of the stannic chloride catalyzed silyl Hilbert-Johnson reaction. J Org Chem. 1976 Jun 11;41(12):2084–2086. doi: 10.1021/jo00874a002. [DOI] [PubMed] [Google Scholar]
- Nuhn P., Zschunke A., Heller D., Wagner G. Zur Konfiguration von 3,5-di-O-p-toluyl-2-desoxy-D-ribofuranosylchlorid. Pharmazie. 1969 Apr;24(4):237–237. [PubMed] [Google Scholar]
- Wittenburg E. Synthese von Thymin-nucleosiden über Silyl-pyrimidin-Verbindungen. Chem Ber. 1968;101(3):1095–1114. doi: 10.1002/cber.19681010346. [DOI] [PubMed] [Google Scholar]



