Abstract
Syndecan proteoglycans may be key regulators of tumor invasion and metastasis because this four-member family of transmembrane receptors regulates cell adhesion, proliferation, and differentiation. Their expression can also serve as prognostic markers. In breast carcinomas, syndecan-1 overexpression correlates with poor prognosis and aggressive phenotype. Syndecan-4 is expressed in most breast carcinoma cell lines, but its role in malignancy is unclear. A possible relationship between syndecan-1 and syndecan-4 expression and established prognostic factors in breast carcinomas was examined. Duplicate samples of 114 benign and malignant breast disease cases were stained for the two syndecans. Clinicopathological information was available for all cases. Syndecan-1 was detected in 72.8% of cases, with significant association between its expression and histological tumor type (p<0.05) and high grade tumors (p<0.05). Syndecan-4 was expressed in 66.7% of cases; expression correlated significantly with positive estrogen (p<0.01) and progesterone (p<0.01) receptor status. Independent expression of the two syndecans was noted from an analysis of single and double positive cases. There was a statistical relationship between syndecan-1 presence in high-grade tumors and absence of syndecan-4, whereas syndecan-4 presence in cases positive for estrogen and progesterone receptor associated with syndecan-1 absence. These syndecans may, therefore, have distinct roles in regulating breast carcinoma cell behavior.
Keywords: breast cancer, cell adhesion receptor, proteoglycan, syndecan, heparan sulfate, glycosaminoglycan, estrogen receptor
Breast cancer is the leading cause of cancer-related death in women worldwide (Kamangar et al. 2006). It is not a single disease but is rather composed of distinct subtypes associated with different clinical outcomes (Perou et al. 2000; Sorlie et al. 2001; Bergamaschi et al. 2006; Chin et al. 2006; Vargo-Gogola and Rosen 2007). Until now, breast neoplasms have been classified primarily according to their histopathology; although several histological types and subtypes of mammary carcinoma exist, >95% are either ductal or lobular carcinoma, with the former making up the vast majority (Tavassoli 1999; Rosen 2001). Markers such as estrogen receptor (ER), progesterone receptor (PR), and epidermal growth factor receptor 2 (HER2) are currently used in the clinic for prognostic purposes as well as to stratify patients for appropriately targeted therapies (Harvey et al. 1999; Ross et al. 2005; Subramaniam and Isaacs 2005; Payne et al. 2008). Estimates suggest, however, that treatment failure presently occurs in approximately 30% of breast cancer patients (Mullan and Millikan 2007). A reasonable explanation for this is an incomplete picture of the biologic heterogeneity of breast cancers with respect to molecular alterations, treatment sensitivity, and cellular composition. Beyond the classic clinical parameters (age, node status, tumor size, histological grade) and pathologic parameters (ER, PR, and HER2) routinely used in the clinic, there is an imperative need to identify specific molecular markers related to disease etiology. In turn, this may permit development of therapeutic targets for each patient subgroup, optimizing tailored treatment protocols for individual patients and thereby improving survival.
Cancer-related morbidity and mortality are closely linked to the capacity of tumor cells to invade and metastasize (Hanahan and Weinberg 2000; Chambers et al. 2002). The metastatic dissemination of tumor cells designates the transition from a localized, potentially curable to a generalized, usually incurable disease. The metastatic cascade involves sequential adhesion, motility, and proliferation (Hanahan and Weinberg 2000; Wolf and Friedl 2006). One class of molecules that can influence each of these critical steps is the heparan sulfate proteoglycans (HSPGs; Sanderson et al. 2005; Beauvais and Rapraeger 2004; Fjeldstad and Kolset 2005). HSPGs play diverse roles in tumor biology by mediating adhesion and migration but also regulating cellular responses to mitogenic and angiogenic growth factors (Blackhall et al. 2001). The syndecan and glypican families constitute the major classes of cell surface HSPGs (Bernfield et al. 1999; Mythreye and Blobe 2009), and both have a long evolutionary history. Glypicans, of which there are six in mammals, have a glycosylphosphatidyl inositol anchor and have roles as co-receptors for growth factors and morphogens, in particular (Mythreye and Blobe 2009). The syndecans are a four-member family of cell surface HSPGs, which regulate cell–cell and cell–extracellular matrix (ECM) adhesion, cell migration, and growth factor activity (Bernfield et al. 1999; Couchman 2003, 2010; Morgan et al. 2007; Xian et al. 2010). They are type I membrane proteins with a conserved signature cytoplasmic domain, a single transmembrane domain, and a larger ectodomain that bear heparan sulfate side chains and sometimes chondroitin sulfate chains (Kokenyesi and Bernfield 1994; Couchman 2003). The expression of syndecans and their shedding by cell surface proteases can be altered under certain pathophysiological conditions, including the process of tumor onset, progression, and metastasis (Fitzgerald et al. 2000; Sanderson 2001; Lambaerts et al. 2009; Choi et al. 2010; Manon-Jensen et al. 2010).
Syndecan-1 is the most well-characterized member of the syndecan family and is expressed mainly by epithelial cells (Bernfield et al. 1999; Couchman 2003). Its loss as well as overexpression in carcinoma cells has been associated with malignant progression. Several studies have examined the role of syndecan-1 in breast oncogenesis. Syndecan-1 is upregulated in human breast cancer samples compared to normal breast tissues (Barbareschi et al. 2003; Löfgren et al. 2007). In the in vitro breast cancer models, syndecan-1 can promote tumorigenesis by regulating tumor cell spreading and adhesion (Beauvais and Rapraeger 2003, 2004), proliferation (Maeda et al. 2004), and angiogenesis (Maeda et al. 2006). Two studies have reported syndecan-1 as a marker of poor prognosis in breast cancer (Barbareschi et al. 2003; Leivonen et al. 2004).
Syndecan-4 is widely expressed but usually at low levels in normal tissue, and it is a key adhesion molecule (Couchman 2003; Morgan et al. 2007), unique among the syndecan family members to localize at sites of cell–matrix adhesions, including focal adhesions (Woods and Couchman 1994; Baciu and Goetinck 1995). Syndecan-4 localizes to cell attachment sites where, together with α5β1 integrin, it promotes stress fibers and focal adhesions through activation of protein kinase Cα (PKCα) and coordination of Rho GTPases (Oh et al. 1997; Horowitz et al. 1999; Saoncella et al. 1999; Lim et al. 2003; Dovas et al. 2006; Bass et al. 2007). When overexpressed, syndecan-4 promotes excess focal adhesion formation, resulting in a reduction in cell migration (Longley et al. 1999). On the other hand, wound repair and mesenchymal cell migration are also impaired in syndecan-4 null mice and cells derived from them (Echtermeyer et al. 2001; Midwood et al. 2004). Syndecan-4 is expressed in normal human mammary epithelium, and it is the second most abundant HSPG produced by most breast carcinoma cell lines (Burbach et al. 2003; Baba et al. 2006). Few studies have examined the role of syndecan-4 in breast malignancy, although it can mediate breast cancer cell adhesion and spreading (Beauvais and Rapraeger 2003). The proteoglycan can form a complex with the pro-angiogenic molecule, fibroblast growth factor 2 (FGF2), and its receptor, FGFR-1, to promote breast carcinoma FGF signaling (Mundhenke et al. 2002). As there are limited data regarding syndecan-4 expression in malignant breast tissue, we have evaluated its expression in various tumor types as well as explored a possible prognostic utility. This study confirms earlier reports that syndecan-1 expression is associated with poor prognosis in breast carcinomas, notably where stromal staining was detected. In addition, the data show that human breast carcinomas express syndecan-4 with a statistically significant association between syndecan-4 expression and positive estrogen and progesterone receptor status. Moreover, statistical analysis shows the expression of the two syndecans across the spectrum of carcinomas to be independent of each other.
Materials and Methods
Patients and Tissue Samples
Breast tissue samples from individuals with breast carcinomas (55 cases) and from individuals with nonmalignant breast disease (11 cases) were obtained from patients who underwent surgery at the Turku University Hospital, Turku, Finland. In addition, tissue samples purchased as tissue arrays with breast carcinomas (36 cases) and nonmalignant breast tissue (12 cases, from Pantomics, Inc., San Francisco, CA) were included in the study. The experimental use of the surgical specimens was in accordance with the ethical guidelines of Turku University Hospital. The histological grading of carcinomas was performed according to Elston and Ellis (1991). Clinicopathological information was available for all cases. Patient characteristics are shown in Table 1.
Table 1.
Patient Characteristics
| Median | Range | n (%) | |
|---|---|---|---|
| Age, y | 52 | 16–88 | 114 |
| Histologic type | |||
| Ductal carcinoma | 56 (49.1) | ||
| Lobular carcinoma | 17 (14.9) | ||
| Special type | 3 (2.6) | ||
| Ductal carcinoma in situ | 15 (13.2) | ||
| Atypical ductal hyperplasia | 11 (9.6) | ||
| Nonmalignant | 12 (10.5) | ||
| Tumor size | |||
| T1 | 23 (30.3) | ||
| T2–4 | 53 (69.7) | ||
| Lymph node status | |||
| LN positive | 34 (33.3) | ||
| LN negative | 68 (66.7) | ||
| Histologic grade | |||
| I | 16 (17.6) | ||
| II | 34 (37.4) | ||
| III | 38 (41.8) | ||
| NA | 3 (3.3) | ||
| Hormone receptor status | |||
| ER positive | 49 (53.8) | ||
| ER negative | 31 (34.1) | ||
| PR positive | 41 (45.1) | ||
| PR negative | 39 (42.9) |
ER, estrogen receptor; PR, progesterone receptor; LN, lymph node; NA, not applicable.
Immunoperoxidase Staining
Immunostaining was performed on formalin-fixed paraffin-embedded primary breast tumors. Sections were deparaffinized with xylene and rehydrated through graded alcohols into distilled water. Antigen retrieval was performed in 0.01 M citrate buffer, pH 6.0 (Shi et al. 1995), and specimens were incubated for 20 min at 95C in a steamer followed by cooling down for a further 20 min. Following washing in phosphate-buffered saline (PBS), pH 7.4, nonspecific endogenous peroxidase activity was blocked by treatment with 3% H2O2 in methanol for 5 min at room temperature. The EnVision + System-HRP (DAB) kit (DAKO, Glostrup, Denmark) was used as the detection system. The tissue sections were incubated with the primary antibody diluted in 1% normal goat serum in PBS at room temperature for 1 hr. Monoclonal mouse anti-human antibodies were used for detection of syndecan-1 (clone B-B4; Abcam, Cambridge, UK) and syndecan-4 (clone 5G9; Santa Cruz Biotechnology, Santa Cruz, CA). Slides were counterstained with hematoxylin, dehydrated, and mounted in permanent mounting medium (Eukitt quick-hardening mounting medium; Sigma-Aldrich, St. Louis, MO). As negative staining controls, the primary antibodies were replaced with the primary antibody diluents. Image capturing and analysis were performed on a Olympus BX51 microscope interfaced with a computer using color view soft imaging system Cell^A, version 2.3 software (Olympus Soft Imaging Solutions GmbH Ltd., Münster, Germany).
Immunofluorescence Staining
Sections were deparaffinized with xylene and rehydrated through graded alcohols and distilled water. Heat-induced antigen retrieval was carried out as above. After washing in PBS (pH 7.4), sections were blocked for 30 min in 5% normal goat serum (Chemicon, Temecula, CA) in PBS, followed by incubation with primary antibodies diluted in 1% normal goat serum in PBS for 1.5 hr at room temperature. Slides were washed with PBS and tissue sections were incubated with fluorophore-conjugated secondary antibodies in 1% normal goat serum in PBS. Primary antibodies were anti-syndecan-4 (clone 5G9, mouse mAb; Santa Cruz Biotechnology) and anti-α-smooth muscle actin (clone E184, rabbit mAb; Abcam). Secondary antibodies included Alexa Fluor 488–conjugated donkey anti-mouse IgG (H+L) and Alexa Fluor 568–conjugated goat anti-rabbit IgG (H+L) from Molecular Probes (Invitrogen, Carlsbad, CA). Each slide was incubated with secondary antibody according to the manufacturer’s protocol and stained with 2 µg/ml 4′,6-diamidino-2-phenylindole (DAPI; Molecular Probes, Invitrogen) in PBS as a nuclear counterstain. After washing in PBS, sections were mounted using ProLong Gold Plus mounting medium (Molecular Probes, Invitrogen). Fluorescent images were captured and analyzed on a Zeiss Axioplan-2 microscope (Zeiss, Oberkochen, Germany), and images were processed using MetaMorph (Molecular Devices, Sunnyvale, CA) and Adobe Photoshop CS4 (Adobe, San Jose, CA).
Antigen Absorption
To confirm antibody specificity, antigen absorption experiments were performed for the syndecan-4 antibody, 5G9, and the syndecan-1 antibody, B-B4. The primary antibodies were premixed with the corresponding recombinant proteins at a 1:20 molar ratio in antibody solution buffer (1% normal goat serum in PBS) overnight at 4C. Syndecan-1 ectodomain (residues Q23–D248; NM_002997) and syndecan-4 ectodomain (E19–E142; NM_002999.2) were expressed as fusion proteins with the HepII domain (repeats III12-13, I1812–T1991; NM_019143) of fibronectin. Constructs were inserted into the pET24a vector (Novagen, Merck KGaA, Darmstadt, Germany) for expression in BL21 Escherichia coli. Purification from bacterial lysates was by affinity chromatography on heparin sepharose 6 fast flow resin (GE Healthcare, Piscataway, NJ) equilibrated in 50 mM sodium phosphate (pH 6.0) with elution in a salt gradient to 1.0 M NaCl. In addition, a further test of antibody specificity was performed by adsorbing the primary antibody with the alternate recombinant syndecan core protein (also 1:20 molar ratio). Primary antibody solutions without preadsorption were used as positive controls.
Statistical Analysis
After evaluation of immunohistological staining in a blinded fashion, statistical analyses were carried out using the online available GraphPad Prism software (GraphPad Software, La Jolla, CA). The χ2 test and Fisher’s exact test were used to examine the association between the syndecan-1 and syndecan-4 expression in tumors, as well as clinicopathological parameters, respectively. All p values were two-sided and considered significant when p≤0.05.
Results
Patients and Samples
Syndecan-1 and syndecan-4 expression was studied in duplicate samples of a total of 114 cases of benign and malignant breast disease. Expression was assessed in 12 cases of benign breast disease, 11 cases of atypical ductal hyperplasia (ADH), 15 cases of ductal carcinoma in situ (DCIS), 56 cases of invasive ductal carcinoma (IDC), 17 cases of lobular carcinoma (ILC), and 3 cases of invasive cancer classified as special type. The clinicopathological characteristics of the patients are shown in Table 1. The median age was 52 years (range, 16-88), and all of the patients were females. The majority of patients with primary invasive breast carcinoma (56 of 76) had invasive ductal carcinomas. Cases of ILC (17 of 76) made up 22% of the invasive tumors, whereas 4% of the invasive tumors (3 of 76) were classified as special type. Information regarding histological grading was available for all patients with neoplastic disease (n=91). Information on axillary node status was available for patients with ADH in addition to the group of patients with neoplastic disease (n=102). With respect to tumor grade, hormone receptor status, and percentage of lymph node–positive cases, this cohort appeared representative (Table 1).
Immunohistochemistry
Commercially available monoclonal mouse anti-human antibodies were used for detection of the extracellular core protein of syndecan-1 and syndecan-4. To verify the specificity of the respective antibodies, antigen absorption trials were performed using recombinant bacterial proteins corresponding to the entire core protein ectodomains. Results are shown in Figure 1. Without antigen absorption (Fig. 1A,B), both antibodies stained breast tissue. After antigen absorption with the appropriate recombinant protein (Fig. 1C,D), B-B4 and 5G9 no longer stained adjacent tissue sections to their corresponding positive controls. To further test the specificity, an antigen absorption trial was performed where the recombinant proteins were switched, so that B-B4 was incubated with recombinant syndecan-4 protein, whereas 5G9 was incubated with recombinant syndecan-1 protein (Fig. 1E,F). The staining patterns were identical to untreated positive controls (Fig. 1A,B). These results confirmed the specificity of antibodies B-B4 and 5G9 that were used in the remainder of the study.
Figure 1.
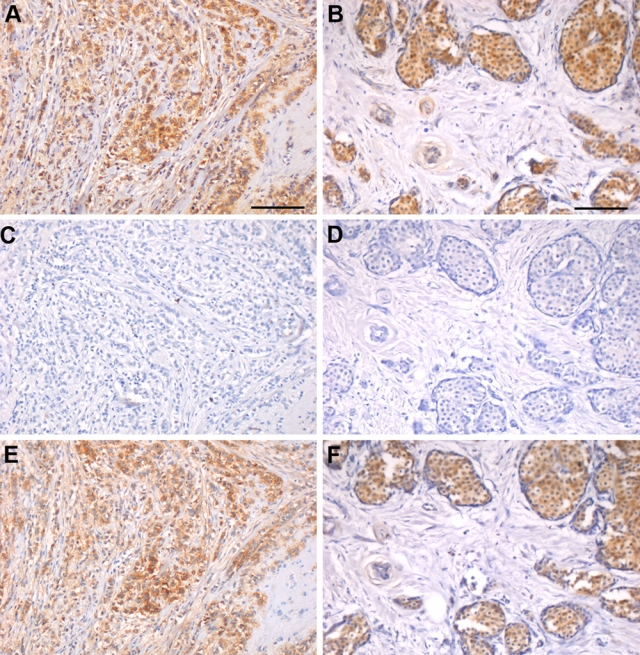
Antibody specificity confirmed by antigen absorption trials. Antigen absorption experiments were performed with recombinant syndecan-1 and syndecan-4 ectodomains on sequential sections of two cases of infiltrating lobular carcinoma. (A, B) Without antigen absorption, both B-B4 (A) and 5G9 (B) stained breast tissue. (C, D) After antigen absorption with the corresponding recombinant proteins, B-B4 (C) and 5G9 (D) no longer stained serial tissue sections. (E, F) Absorbing the antibodies with the alternate recombinant syndecan core proteins revealed a staining pattern with B-B4 (E) and 5G9 (F) similar to the untreated positive controls in A and B, respectively. Bars = 100 µm.
Distribution of Syndecan-1 and Syndecan-4
The results for expression of syndecan-1 and syndecan-4 are summarized in Tables 1 to 5. Staining for syndecan-1 was present in the epithelial cells of benign breast tissue, whereas no detectable staining in the stroma was noted in any sections of benign breast tissue (Fig. 2A). In contrast, staining for syndecan-1 was present in the epithelial cells, cancer cells, and stroma of a considerable proportion of premalignant and malignant breast tumor sections (Fig. 2C,E,G,I). Syndecan-1 staining was frequently concentrated in infiltrating areas of invasive tumors (Fig. 2G,I). Similarly, stromal cell staining for syndecan-1 was of highest intensity in samples with invasive ductal and lobular carcinoma, and in these sections, syndecan-1 association with stromal fibroblasts was predominantly confined to cells adjacent to invasive tumor cells (Fig. 2G,I). Tumors exhibited variable expression of syndecan-1, ranging from weak or complete absence to strong. The staining was graded according to the proportion of tumor that was positive; where <5% of the area was stained, the sample was recorded as negative (Table 3). The immunolabeling appeared to be both cytoplasmic and membranous (Fig. 2). Positive immunoreaction was observed for syndecan-1 in 72.8% of the patient cohort (Table 3); 82.1% of invasive ductal carcinomas and 88.2% of invasive lobular carcinomas presented with positive syndecan-1 immunostaining, and a significant association was detected between syndecan-1 expression and histological tumor type (p=0.032; Table 3). A significant correlation was observed between expression of syndecan-1 and high-grade tumors (p=0.048) and with a trend toward an association of negative PR status with syndecan-1 expression (p=0.085). No significant correlation of high to moderate syndecan-1 expression with age, tumor size, or lymph node status or ER or HER2 status was observed (Table 3).
Table 5.
Positive Epithelial Syndecan-1 and Syndecan-4 Expression versus Concomitantly Positive Epithelial and Stromal Expression According to Clinicopathological Parameters
| Syndecan-4 Expression, n (%) |
p |
Syndecan-1 Expression, n (%) |
p |
||||
|---|---|---|---|---|---|---|---|
| Number of Patients (%) | Positive Epithelial | Positive (Epithelial, Stroma) | 95% CI | Positive Epithelial | Positive (Epithelial, Stroma) | 95% CI | |
| No. (%) | 114 (100) | 45 (39.5) | 30 (26.3) | 31 (27.2) | 55 (48.2) | 0.003* | |
| Age, y | |||||||
| <50 | 45 (39.5) | 21 (46.7) | 7 (15.6) | 0.053 | 12 (26.7) | 24 (53.3) | 0.820 |
| ≥50 | 69 (60.5) | 24 (34.8) | 23 (33.3) | 19 (27.5) | 31 (44.9) | ||
| Tumor size | |||||||
| T1 | 23 (30.3) | 7 (30.4) | 9 (39.1) | 0.551 | 4 (17.4) | 16 (69.6) | 0.728 |
| T2–4 | 53 (69.7) | 18 (34.0) | 15 (28.3) | 7 (13.2) | 37 (69.8) | ||
| Axillary node status | |||||||
| N0 | 68 (66.7) | 26 (38.2) | 15 (22.1) | 0.316 | 19 (27.9) | 31 (45.6) | 0.078 |
| N+ | 34 (33.3) | 13 (38.2) | 14 (41.2) | 5 (14.7) | 23 (67.6) | ||
| Histologic grade | |||||||
| I | 16 (17.6) | 8 (50.0) | 4 (25.0) | 0.374 | 4 (25.0) | 9 (56.3) | 0.939 |
| II | 34 (37.4) | 9 (26.5) | 12 (35.3) | 6 (17.6) | 19 (55.9) | ||
| III | 38 (41.8) | 14 (36.8) | 9 (23.7) | 9 (23.7) | 25 (65.8) | ||
| NA | 3 (3.3) | ||||||
| Histologic type | |||||||
| Ductal carcinoma | 56 (49.1) | 16 (28.6) | 20 (35.7) | 0.129 | 9 (16.1) | 38 (67.9) | 0.00003* |
| Lobular carcinoma | 17 (14.9) | 6 (35.3) | 4 (23.5) | 1 (5.9) | 14 (82.4) | ||
| Special type | 3 (2.6) | 3 (100) | 0 (0.0) | 1 (33.3) | 1 (33.3) | ||
| Ductal carcinoma in situ | 15 (13.2) | 8 (53.3) | 2 (13.3) | 8 (53.3) | 1 (6.7) | ||
| Atypical ductal hyperplasia | 11 (9.6) | 6 (54.5) | 3 (27.3) | 5 (45.5) | 0 (0.0) | ||
| Nonmalignant | 12 (10.5) | 6 (50.0) | 1 (8.3) | 7 (58.3) | 1 (8.3) | ||
| Hormone receptor status | |||||||
| ER positive | 49 (53.8) | 23 (46.9) | 17 (34.7) | 0.087 | 9 (18.4) | 32 (65.3) | 0.436 |
| ER negative | 31 (34.1) | 10 (32.3) | 19 (61.3) | 10 (32.3) | 23 (74.2) | ||
| PR positive | 41 (45.1) | 21 (51.2) | 16 (39.0) | 1.000 | 10 (24.4) | 24 (58.5) | 0.596 |
| PR negative | 39 (42.9) | 12 (30.8) | 10 (25.6) | 9 (23.1) | 31 (79.5) | ||
| HER2 positive | 29 (31.9) | 11 (37.9) | 8 (27.6) | 1.000 | 8 (27.6) | 19 (65.5) | 0.589 |
| HER2 negative | 51 (56.0) | 22 (43.1) | 18 (35.3) | 11 (21.6) | 36 (70.6) | ||
ER, estrogen receptor; PR, progesterone receptor; HER2, epidermal growth factor receptor 2; CI, confidence interval; NA, not applicable.
Statistically significant.
Figure 2.
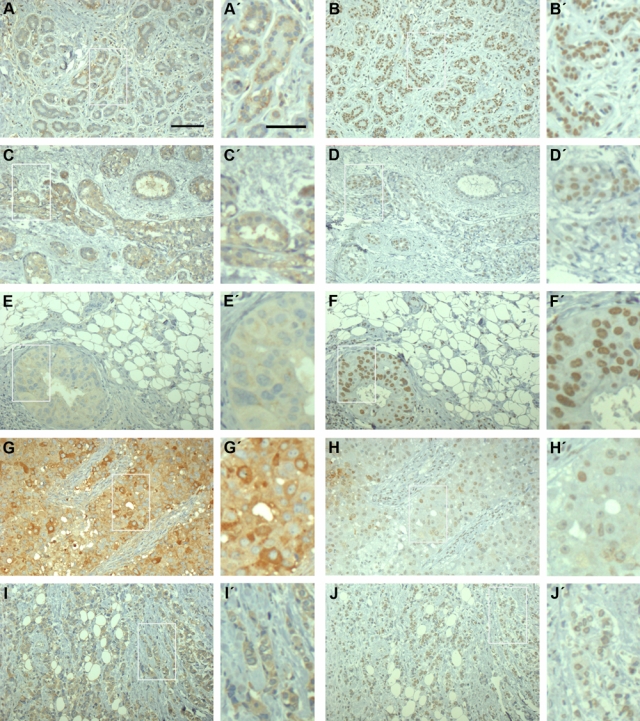
Benign breast tissue and breast carcinomas express syndecan-1 and syndecan-4. Immunohistochemical analysis of the heparan sulfate proteoglycan (HSPG) core proteins in sequential sections of normal breast tissue and human breast carcinoma, respectively. Benign breast lesion (A, B), atypical ductal hyperplasia (C, D), ductal carcinoma in situ (E, F), and infiltrating ductal (G, H) and lobular (I, J) carcinoma were stained for syndecan-1 or syndecan-4 as indicated. In benign breast tissue (A), syndecan-1 was typically limited to the epithelial cells as a cytoplasmic and membranous staining, whereas premalignant and malignant breast tumor tissue displayed both cytoplasmic and membranous staining for syndecan-1 in the epithelial cells and in the stroma. Syndecan-4 immunostaining in benign breast lesion (B), atypical ductal hyperplasia (D), ductal carcinoma in situ (F), and in infiltrating ductal (H) and lobular (J) carcinoma were primarily nuclear or perinuclear. All carcinoma samples were positive for estrogen and progesterone receptors. Bars = 100 µm and inserts = 50 µm.
Table 3.
Association of Syndecan-1 and Syndecan-4 Expression with Clinicopathological Parameters
| Syndecan-4 Expression, n (%) |
p |
Syndecan-1 Expression, n (%) |
p |
||||
|---|---|---|---|---|---|---|---|
| Number of Patients (%) | Positive | Negative | 95% CI | Positive | Negative | 95% CI | |
| No. (%) | 114 (100) | 76 (66.7) | 38 (33.3) | 83 (72.8) | 31 (27.2) | ||
| Age, y | |||||||
| <50 | 45 (39.5) | 28 (62.2) | 17 (37.8) | 35 (77.8) | 10 (22.2) | ||
| ≥50 | 69 (60.5) | 48 (69.6) | 21 (30.4) | 0.425 | 48 (69.6) | 21 (30.4) | 0.393 |
| Tumor size | |||||||
| T1 | 23 (30.3) | 17 (73.9) | 6 (26.1) | 19 (82.6) | 4 (17.4) | ||
| T2–4 | 53 (69.7) | 33 (62.3) | 20 (37.7) | 0.432 | 44 (83.0) | 9 (17.0) | 1.000 |
| Axillary node status | |||||||
| N0 | 68 (66.7) | 42 (61.8) | 26 (38.2) | 49 (72.1) | 19 (27.9) | ||
| N+ | 34 (33.3) | 27 (79.4) | 7 (20.6) | 0.076 | 28 (82.4) | 6 (17.6) | 0.332 |
| Histologic grade | |||||||
| I | 16 (17.6) | 13 (81.3) | 3 (18.8) | 13 (81.3) | 3 (18.8) | ||
| II | 34 (37.4) | 21 (61.8) | 13 (38.2) | 24 (70.6) | 10 (29.4) | ||
| III | 38 (41.8) | 23 (60.5) | 15 (39.6) | 0.329 | 35 (92.1) | 3 (7.9) | 0.048* |
| NA | 3 (3.3) | ||||||
| Histologic type | |||||||
| Ductal carcinoma | 56 (49.1) | 36 (64.3) | 20 (35.7) | 0.712 | 46 (82.1) | 10 (17.9) | 0.032* |
| Lobular carcinoma | 17 (14.9) | 11 (64.7) | 6 (35.3) | 15 (88.2) | 2 (11.8) | ||
| Special type | 3 (2.6) | 3 (100) | 0 | 2 (66.7) | 1 (33.3) | ||
| Ductal carcinoma in situ | 15 (13.2) | 10 (66.7) | 5 (33.3) | 9 (60.0) | 6 (40.0) | ||
| Atypical ductal hyperplasia | 11 (9.6) | 9 (81.8) | 2 (18.2) | 5 (45.5) | 6 (54.5) | ||
| Nonmalignant | 12 (10.5) | 7 (58.3) | 5 (41.7) | 6 (50.0) | 6 (50.0) | ||
| Hormone receptor status | |||||||
| ER positive | 49 (53.8) | 39 (79.6) | 10 (20.4) | 0.003* | 39 (79.6) | 10 (20.4) | 0.772 |
| ER negative | 31 (34.1) | 14 (45.2) | 17 (54.8) | 26 (83.9) | 5 (16.1) | ||
| PR positive | 41 (45.1) | 34 (82.9) | 7 (17.1) | 0.001* | 30 (73.2) | 11 (26.8) | 0.085 |
| PR negative | 39 (42.9) | 19 (48.7) | 20 (51.3) | 35 (89.7) | 4 (10.3) | ||
| HER2 positive | 29 (31.9) | 15 (51.7) | 14 (48.3) | 22 (72.9) | 7 (24.1) | ||
| HER2 negative | 51 (56.0) | 38 (74.5) | 13 (25.5) | 0.05 | 43 (84.3) | 8 (15.7) | 0.383 |
ER, estrogen receptor; PR, progesterone receptor; HER2, epidermal growth factor receptor 2; CI, confidence interval; NA, not applicable.
Statistically significant.
Table 2.
Distribution of Patients According to Epithelial and Stromal Syndecan-1 and Syndecan-4 Expression Levels
| Syndecan-1, n (%) |
Syndecan-4, n (%) |
|||
|---|---|---|---|---|
| Expression Level, % | Epithelial | Stromal | Epithelial | Stromal |
| <5% (negative) | 28 (25) | 58 (51) | 39 (34) | 83 (73) |
| 5%–20% (weak) | 16 (14) | 26 (23) | 8 (7) | 17 (15) |
| 21%–60% (moderate) | 32 (28) | 21 (18) | 43 (38) | 14 (12) |
| >60% (strong) | 38 (33) | 9 (8) | 24 (21) | 0 |
In normal breast tissue, syndecan-4 was present in ductal epithelial cells but weakly or not at all in the adjacent myoepithelia (Figs. 2B and 3A,B). Small blood vessels were positive, but generally the stroma was negative for syndecan-4 (Figs. 2B and 3A). In tumor samples, 66.7% were graded as positive for syndecan-4 (Tables 1–5), but usually the staining was associated with carcinoma cells, not the stroma, unlike syndecan-1 (Figs. 2B,D,F,H,J,L and 3C,D). In addition, intense staining was rare. In immunoperoxidase staining particularly, syndecan-4 appeared to have a nuclear or perinuclear localization. However, immunofluorescent detection of syndecan-4 showed predominantly cytoplasmic distribution in epithelial cells (Fig. 3). It was difficult to ascertain whether this proteoglycan was present at the cell surface where cytoplasmic staining was predominant.
Figure 3.
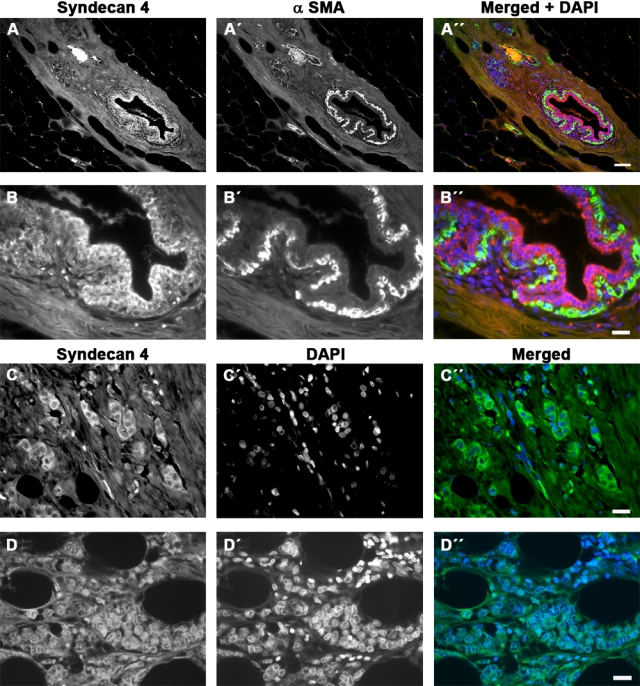
Immunofluorescence detection of syndecan-4 in human breast tissue. (A, B) Low- and high-power view of breast tissue stained for syndecan-4 (red, A, B), α-smooth muscle actin (green, A′, B′), and merged (A″, B″). Nuclei are counterstained with 4′,6-diamidino-2-phenylindole (DAPI) in the merged image. Myoepithelial cells are unstained or weakly stained for syndecan-4. (C, D) Infiltrating ductal (C) and lobular (D) carcinoma stained for syndecan-4 (green, C, D) with nuclei visualized by DAPI staining (C′, D′) and merged (C″, D″). The syndecan is mostly cytoplasmic, with some nuclear or perinuclear localization (D). Both carcinoma samples were positive for estrogen and progesterone receptors. Bars = 200 µm (A) or 50 µm (B–D).
In contrast to syndecan-1, syndecan-4 expression did not correlate with grade (p=0.329) or histological tumor type (p=0.712; Table 3). However, syndecan-4 correlated significantly with positive ER status (p<0.01) and positive PR status (p<0.01), and there was a trend toward an association with negative HER2 status (p=0.05; Table 3).
Because both syndecans were studied in the same tumor series, further data analyses were completed. Cases that were double positive for syndecan-1 and syndecan-4 were compared to double-negative cases. In addition, each tumor was examined for the presence of stromal as well as epithelial syndecan. In no case was stromal staining accompanied by an absence of epithelial staining. Also, each case was examined for single syndecan expression (e.g. presence of syndecan-1 but absence of syndecan-4). These analyses are shown in Tables 3 to 5. Several statistically significant results emerged from the data. Although there were no significant associations of double-positive compared to double-negative staining with any of the disease criteria (Table 3), the single positive data were revealing. There was a highly significant association of syndecan-1-positive, syndecan-4-negative staining with tumor grade and tumor type. Conversely, there was a significant association between ER- and PR-positive status and syndecan-4 presence but syndecan-1 absence (Table 4). This association was also seen where epithelial staining for syndecan-4 alone (no stromal staining) was compared to incidence of complete absence of syndecan (Table 5).
Table 4.
Association of Double-Positive and Single-Positive Syndecan-1 and Syndecan-4 Expression with Clinicopathological Parameters
| Syndecan-1 and Syndecan-4 Expression, n (%) |
p |
p |
|||||
|---|---|---|---|---|---|---|---|
| Number of Patients (%) | Positive | Negative | 95% CI | Syndecan-1 Positive and Syndecan-4 Negative Expression, n (%) | Syndecan-4 Positive and Syndecan-1 Negative Expression, n (%) | 95% CI | |
| No. (%) | 114 (100) | 59 (51.8) | 14 (12.3) | 24 (21.1) | 17 (14.9) | ||
| Age, y | |||||||
| <50 | 45 (39.5) | 23 (51.1) | 5 (11.1) | 12 (26.7) | 5 (11.1) | ||
| ≥50 | 69 (60.5) | 36 (52.2) | 9 (13.0) | 1.000 | 12 (17.4) | 12 (17.4) | 0.217 |
| Tumour size | |||||||
| T1 | 23 (30.3) | 15 (65.2) | 2 (8.7) | 4 (17.4) | 2 (8.7) | ||
| T2–4 | 53 (69.7) | 29 (54.7) | 5 (9.4) | 1.000 | 15 (28.3) | 4 (7.5) | 0.606 |
| Axillary node status | |||||||
| N0 | 68 (66.7) | 31 (45.6) | 8 (11.8) | 18 (26.5) | 11 (16.2) | ||
| N+ | 34 (33.3) | 23 (67.6) | 2 (5.9) | 0.292 | 5 (14.7) | 4 (11.8) | 1.000 |
| Histologic grade | |||||||
| I | 16 (17.6) | 10 (62.5) | 0 | 3 (18.8) | 4 (25.0) | ||
| II | 34 (37.4) | 17 (50.0) | 6 (17.6) | 7 (20.6) | 3 (8.8) | ||
| III | 38 (41.8) | 22 (57.9) | 3 (7.9) | 0.158 | 12 (31.6) | 1 (2.6) | 0.049* |
| NA | 3 (3.3) | ||||||
| Histologic type | |||||||
| Ductal carcinoma | 56 (49.1) | 32 (57.1) | 6 (10.7) | 0.856 | 14 (25.0) | 4 (7.1) | 0.029* |
| Lobular carcinoma | 17 (14.9) | 10 (58.8) | 1 (5.9) | 5 (29.4) | 1 (5.9) | ||
| Special type | 3 (2.6) | 2 (66.7) | 0 | 0 | 1 (33.3) | ||
| Ductal carcinoma in situ | 15 (13.2) | 6 (40.0) | 2 (13.3) | 3 (20.0) | 4 (26.7) | ||
| Atypical ductal hyperplasia | 11 (9.6) | 4 (36.4) | 1 (9.1) | 1 (9.1) | 5 (45.5) | ||
| Normal breast | 12 (10.5) | 5 (41.7) | 4 (33.3) | 1 (8.3) | 2 (16.7) | ||
| Hormone receptor status | |||||||
| ER positive | 49 (53.8) | 32 (65.3) | 3 (6.1) | 0.198 | 6 (12.2) | 7 (14.3) | 0.050 |
| ER negative | 31 (34.1) | 13 (26.5) | 4 (12.9) | 15 (48.4) | 3 (9.7) | ||
| PR positive | 41 (45.1) | 27 (55.1) | 4 (9.8) | 1.000 | 4 (9.8) | 8 (19.5) | 0.002* |
| PR negative | 39 (42.9) | 18 (36.7) | 3 (7.7) | 17 (43.6) | 2 (5.1) | ||
| HER2 positive | 29 (31.9) | 11 (22.4) | 3 (10.3) | 11 (37.9) | 5 (17.2) | 1.000 | |
| HER2 negative | 51 (56.0) | 34 (69.4) | 4 (7.8) | 0.368 | 10 (19.6) | 5 (9.8) | |
ER, estrogen receptor; PR, progesterone receptor; HER2, epidermal growth factor receptor 2; CI, confidence interval; NA, not applicable.
Statistically significant.
With regard to syndecan-1 and tumor type and grade, there was a highly significant association with staining that was both epithelial and stromal (Table 5). This was not the case where epithelial staining alone was observed (Table 4). In other words, syndecan-1 presence in the stroma correlated with tumor grade and type. There was also a trend in association between syndecan-1 presence and positive nodal status that related to the proteoglycan’s distribution in both epithelial and stromal compartments (Table 5; p=0.078). There was also a trend toward positive nodal status with syndecan-4 expression (Table 3; p=0.076), as well as a trend toward its presence in the stroma of older patients (Table 5; p=0.053).
Discussion
Invasion and metastasis are characteristic of malignant solid tumors, and many mechanisms are involved in these processes. Cell adhesion molecules such as integrins, cadherins and cell surface HSPGs, and ECM components are particularly important in the regulation of cell differentiation, morphology, and migration (Berx and van Roy 2009; Mythreye and Blobe 2009; Streuli and Akhtar 2009). In this study, we analyzed syndecan-1 and syndecan-4 expression in a representative library of breast carcinoma cases. Our study confirms earlier reports that syndecan-1 expression is associated with poor prognosis in breast carcinomas (Barbareschi et al. 2003). Syndecan-1 is the best-studied HSPG, and its expression is frequently altered in cancer. It is a versatile molecule that has been demonstrated to have tumor suppressor and tumor promoter functions depending on the model system or tissue examined (Blackhall et al. 2001).
Barbareschi et al. (2003) showed that syndecan-1 was expressed at high levels in a significant percentage of breast carcinomas and in turn was related to an aggressive phenotype with poor clinical behavior. Baba et al. (2006) associated syndecan-1 expression in breast carcinoma with established poor prognostic factors, including high grade, tumor size, and positive lymph node status. Neither study considered stromal syndecan-1 expression from a prognostic standpoint. Stanley et al. (1999) described the induction of syndecan-1 expression in the stroma of invasive breast carcinomas in a small patient cohort, whereas Leivonen et al. (2004) linked a poorer prognosis in breast carcinoma patients with syndecan-1 in tumor cells but a better prognosis for those lacking syndecan-1 expression within the stroma. Furthermore, they observed that epithelial syndecan-1 expression was associated with negative ER status, whereas stromal syndecan-1 expression was associated with positive ER status. Concomitant expression of epithelial and stromal syndecan-1 identified a group of patients with a significantly poorer prognosis, which led the authors to propose that this constellation may be a predictor of unfavorable outcome in breast cancer.
Here, a significant correlation was observed between expression of syndecan-1 and high-grade tumors (p=0.048), and there was a trend toward an association of negative PR status with syndecan-1 expression (p=0.08). No significant correlation of high to moderate syndecan-1 expression with age, tumor size, or lymph node status or ER or HER2 status was observed (Table 3). Our results are consonant with syndecan-1 as a marker of a poorer prognosis in breast cancer as described previously.
Alexander et al. (2000) showed in a murine model that syndecan-1 expression was essential for wnt-1-induced mammary tumorigenesis and that the HSPG was an essential receptor for an oncogenic growth factor. In vitro breast cancer models suggest that syndecan-1 promotes tumorigenesis by regulating tumor cell spreading and adhesion (Beauvais and Rapraeger 2003, 2004; Burbach et al. 2004), proliferation (Maeda et al. 2004), and angiogenesis (Maeda et al. 2006). In addition, studies in breast and gastric cancer demonstrated an association between increased stromal syndecan-1 expression, loss of epithelial syndecan-1 expression, and an adverse clinical outcome (Stanley et al. 1999; Wiksten et al. 2001). Our data are entirely consistent with these observations. Stromal syndecan-1 was associated with aggressive tumor type and histological grade (Fig. 2, Tables 4 and 5). Breast cancer cells can generate their own nonmalignant stroma to facilitate tumor progression ( Petersen et al. 2003), and the expression of syndecan-1 in the reactive stroma cells has been suggested to create a favorable microenvironment for tumor cell growth and angiogenesis (Loussouarn et al. 2008). Moreover, recent studies have shown that although preoperative chemotherapy results in decreased syndecan-1 (Tokes et al. 2009), response to this treatment is decreased in syndecan-1-positive patients, with none showing complete remission (Götte et al. 2006).
Pro-angiogenic/pro-tumor growth factors and enzymes (e.g., FGF-2 and matrix metalloproteinases [MMPs]) upregulated in solid tumors accelerate syndecan-1 and syndecan-4 shedding in vitro (Subramanian et al. 1997; Fitzgerald et al. 2000; Manon-Jensen et al. 2010). Positive staining in the tumor-associated stroma could originate from shed syndecan-1 from tumor cells or from fibroblasts. However, Mennerich et al. (2004) showed that stromal cells in the reactive stroma of breast carcinomas that stained for syndecan-1 were also positive at the mRNA level.
In contrast to syndecan-1, little is known concerning the role of syndecan-4 in malignancy. Syndecan-4 is expressed by most cell types, and it is an adhesion molecule (Couchman 2003; Morgan et al. 2007) with roles in focal adhesion complex assembly and cell migration (Longley et al. 1999). Syndecan-4 may function as an anti-migratory/anti-invasive tumor suppressor. It is downregulated in colon carcinoma cells (Jayson et al. 1999; Park et al. 2002), but Mundhenke et al. (2002) reported an upregulation in normal breast tissue compared to malignant breast tissue. Syndecan-4-positive and syndecan-4-negative cases were equally abundant in benign breast lesions in our study (Table 3). No significant relationship between syndecan-4 positivity and age, tumor size, or lymph node status was observed (Table 3). Previously, syndecan-4 has been reported to correlate significantly with high histological grade and negative estrogen receptor status (Baba et al. 2006), suggesting it to be a marker of poor prognosis in breast cancer. Our investigation failed to confirm this but instead found syndecan-4 expression to be independent of histological tumor grade (p=0.329) and histological tumor type (p=0.712; Table 3). Different antigen retrieval methods and monoclonal antibodies were used in these studies. Mundhenke et al. (2002) and Baba et al. (2006) used monoclonal anti-syndecan-4 antibody, clone 8G3 (David et al. 1992), whereas the present immunostaining was performed with 5G9 monoclonal antibody. To validate the specificity of the antibodies used in this study, antigen absorption experiments were performed (Fig. 1) with recombinant syndecan-1 and syndecan-4 ectodomains.
Estrogen receptor–alpha (ERα), the progesterone receptor (PgR), and the HER2 oncogene/oncoprotein are the three mandatory prognostic and predictive factors in invasive breast cancer used in routine clinical practice today (Allred 2010). ERα, a nuclear transcription factor activated by estrogen, regulates growth and differentiation of normal breast epithelial cells. It is assessed to predict response to hormonal therapies (Jensen and Jordan 2003). ERα regulates the expression of PgR, whose presence indicates that the estrogen–ERα pathway is intact and functional. PgR is activated by progesterone to regulate several normal cellular functions, including proliferation (Allred 2010). HER2 (also referred to as HER2/neu and erbB2) is a proto-oncogene encoding a tyrosine kinase receptor, but the relationship between HER2 status and clinical outcome is complex and varies with the setting. There is a weak but significant association between poor outcome and positive (i.e., amplified and/or overexpressed) HER2 (Allred 2010). A second estrogen receptor, estrogen receptor–β (ERβ), was discovered in 1996. Its precise biologic role remains unclear, in part due to the presence of several isoforms (Marotti et al. 2010). In this study, syndecan-4 levels correlated significantly with positive ER status (p<0.01) and positive PR status (p<0.01), with a trend toward an association with negative HER2 status (p=0.05; Table 3). Associating syndecan-4 expression with ER positivity suggests it could be related to better outcome in mammary carcinoma. Because elevated syndecan-4 expression is more commonly seen in ER-positive carcinomas, it suggests a hormonal regulation of syndecan-4 expression. Currently, there are very few data concerning the effect of estrogen on proteoglycan expression, but in breast carcinomas, it may promote pathological tumor–stroma interactions through upregulation of heparanase gene expression (Elkin et al. 2003). In the highly metastatic MDA-MB231 mammary carcinoma cell line (ERα negative but ERβ positive), estradiol downregulated syndecan-4 expression (Kousidou et al. 2008). The significance of the association between syndecan-4 expression and ER status is an interesting focus for further investigation.
In this study, the patient material was examined, in serial sections, for both syndecan-1 and syndecan-4. The conclusion is they are independent indicators in breast cancer. It is striking that syndecan-1 positivity in high-grade tumors is associated statistically with absence of syndecan-4. Conversely, syndecan-4 presence in tumors that are ER and PR positive links to an absence of syndecan-1. This outcome is consistent with the distinct expression, localization, and regulation of the two syndecans. Although both are implicated in the regulation of integrin-mediated cell adhesion (Morgan et al. 2007; Beauvais et al. 2009; Couchman 2010), their precise roles appear different, as may be their linkage to signaling and the actin cytoskeleton (Couchman 2010). However, both syndecan-1 and syndecan-4 expression levels have been demonstrated to correlate with FGF2/FGFR1 receptor signaling, suggesting elevated co-receptor activity in breast carcinomas (Mundhenke et al. 2002). Where syndecan-1 and syndecan-4 may converge in distribution is shown in Tables 3 and 5, where both show a trend toward positivity with lymph node involvement, but the data fall short of statistical significance (p=0.076–0.078). Because these tumors have relevance to migration and metastatic behavior, it will be interesting to further study the role of these two proteoglycans in adhesion, migration, and proliferation.
In summary, our study supports the hypothesis that syndecan-1 expression is associated with higher tumor grade and more malignant type in breast carcinomas, particularly where epithelial and stromal compartments are positive. In contrast, our data suggest that syndecan-4 expression associates with positive estrogen and progesterone receptor status and potentially indicates a better prognosis in breast cancer patients. It is, therefore, important to determine the molecular basis of syndecan-4 functions in malignancy. Studies on syndecan-4’s core protein and glycosaminoglycan chains in adhesion and invasion of breast carcinoma are under way.
Acknowledgments
We thank Dr. Reidar Albrechtsen (University of Copenhagen) for helpful assistance in the provision of some of the breast tissue samples.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the authorship and/or publication of this article.
The author(s) disclosed receipt of the following financial support for the research and/or authorship of this article: Support from the Danish National Research Foundation, Fabrikant Vilhelm Pedersen og Hustrus Legat through Novo Nordisk Fonden, and the Department of Biomedical Sciences, University of Copenhagen, is gratefully acknowledged. ML was supported by a Rota System PhD Fellowship from the Copenhagen Graduate School of Health Sciences.
References
- Alexander CM, Reichsman F, Hinkes MT, Lincecum J, Becker KA, Cumberledge S, Bernfield M. 2000. Syndecan-1 is required for Wnt-1-induced mammary tumorigenesis in mice. Nat Genet. 25:329–332 [DOI] [PubMed] [Google Scholar]
- Allred DC. 2010. Issues and updates: evaluating estrogen receptor–alpha, progesterone receptor, and HER2 in breast cancer. Mod Pathol. 23(suppl 2):S52–59 [DOI] [PubMed] [Google Scholar]
- Baba F, Swartz K, van Buren R, Eickhoff J, Zhang Y, Wolberg W, Friedl A. 2006. Syndecan-1 and syndecan-4 are overexpressed in an estrogen receptor–negative, highly proliferative breast carcinoma subtype. Breast Cancer Res Treat. 98:91–98 [DOI] [PubMed] [Google Scholar]
- Baciu PC, Goetinck PF. 1995. Protein kinase C regulates the recruitment of syndecan-4 into focal contacts. Mol Biol Cell. 6:1503–1513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbareschi M, Maisonneuve P, Aldovini D, Cangi MG, Pecciarini L, Angelo Mauri F, Veronese S, Caffo O, Lucenti A, Palma PD, et al. 2003. High syndecan-1 expression in breast carcinoma is related to an aggressive phenotype and to poorer prognosis. Cancer. 98:474–483 [DOI] [PubMed] [Google Scholar]
- Bass MD, Roach KA, Morgan MR, Mostafavi-Pour Z, Schoen T, Muramatsu T, Mayer U, Ballestrem C, Spatz JP, Humphries MJ. 2007. Syndecan-4-dependent Rac1 regulation determines directional migration in response to the extracellular matrix. J Cell Biol. 177:527–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauvais DM, Ell BJ, McWorter AR, Rapraeger AC. 2009. Syndecan-1 regulates alphavbeta3 and alphavbeta5 integrin activation during angiogenesis and is blocked by synstatin, a novel peptide inhibitor. J Exp Med. 206:691–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauvais DM, Rapraeger AC. 2003. Syndecan-1-mediated cell spreading requires signaling by alphavbeta3 integrins in human breast carcinoma cells. Exp Cell Res. 286:219–232 [DOI] [PubMed] [Google Scholar]
- Beauvais DM, Rapraeger AC. 2004. Syndecans in tumor cell adhesion and signalling. Reprod Biol Endocrinol. 2:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergamaschi A, Kim YH, Wang P, Sorlie T, Hernandez-Boussard T, Lonning PE, Tibshirani R, Borresen-Dale AL, Pollack JR. 2006. Distinct patterns of DNA copy number alteration are associated with different clinicopathological features and gene-expression subtypes of breast cancer. Genes Chromosomes Cancer. 45:1033–1040 [DOI] [PubMed] [Google Scholar]
- Bernfield M, Gotte M, Park PW, Reizes O, Fitzgerald ML, Lincecum J, Zako M. 1999. Functions of cell surface heparan sulfate proteoglycans. Annu Rev Biochem. 68:729–777 [DOI] [PubMed] [Google Scholar]
- Berx B, van Roy F. 2009. Involvement of members of the cadherin superfamily in cancer. Cold Spring Harb Perspect Biol. 1(6):a003129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackhall FH, Merry CLR, Davies EJ, Jayson GC. 2001. Heparan sulfate proteoglycans and cancer. Br J Cancer. 85:1094–1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burbach BJ, Friedl A, Mundhenke C, Rapraeger AC. (2003). Syndecan-1 accumulates in lysosomes of poorly differentiated breast carcinoma cells. Matrix Biol. 22:163–177 [DOI] [PubMed] [Google Scholar]
- Burbach BJ, Ji Y, Rapraeger AC. 2004. Syndecan-1 ectodomain regulates matrix-dependent signaling in human breast carcinoma cells. Exp Cell Res. 300:234–247 [DOI] [PubMed] [Google Scholar]
- Chambers AF, Groom AC, MacDonald IC. 2002. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer. 2:563–572 [DOI] [PubMed] [Google Scholar]
- Chin K, DeVries S, Fridlyand J, Spellman PT, Roydasgupta R, Kuo WL, Lapuk A, Neve RM, Qian Z, Ryder T, et al. 2006. Genomic and transcriptional aberrations linked to breast cancer pathophysiologies. Cancer Cell. 10:529–541 [DOI] [PubMed] [Google Scholar]
- Choi S, Lee H, Choi JR, Oh ES. 2010. Shedding: towards a new paradigm of syndecan function in cancer. BMB Rep. 43:305–310 [DOI] [PubMed] [Google Scholar]
- Couchman JR. 2003. Syndecans: proteoglycan regulators of cell-surface microdomains? Nat Rev Mol Cell Biol. 4:926–937 [DOI] [PubMed] [Google Scholar]
- Couchman JR. 2010. Transmembrane signalling proteoglycans. Annu Rev Cell Dev Biol. 26:89–114 [DOI] [PubMed] [Google Scholar]
- David G, Bai XM, Van der Schueren B, Cassiman JJ, Van den Berghe H. 1992. Developmental changes in heparan sulfate expression: in situ detection with mAbs. J Cell Biol. 119:961–975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dovas A, Yoneda A, Couchman JR. 2006. PKCalpha-dependent activation of RhoA by syndecan-4 during focal adhesion formation. J Cell Sci. 119:2837–2846 [DOI] [PubMed] [Google Scholar]
- Echtermeyer F, Streit M, Wilcox-Adelman S, Saoncella S, Denhez F, Detmar M, Goetinck P. 2001. Delayed wound repair and impaired angiogenesis in mice lacking syndecan-4. J Clin Invest. 107:R9–R14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkin M, Cohen I, Zcharia E, Orgel A, Guatta-Rangini Z, Peretz T, Vlodavsky I, Kleinman HK. 2003. Regulation of heparanase gene expression by estrogen in breast cancer. Cancer Res. 63:8821–8826 [PubMed] [Google Scholar]
- Elston CW, Ellis IQ. 1991. Pathological prognostic factors in breast cancer: I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 19:403–410 [DOI] [PubMed] [Google Scholar]
- Fitzgerald ML, Wang Z, Park PW, Murphy G, Bernfield M. 2000. Shedding of syndecan-1 and -4 ectodomains is regulated by multiple signaling pathways and mediated by a TIMP-3-sensitive metalloproteinase. J Cell Biol. 148:811–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjeldstad K, Kolset SO. 2005. Decreasing the metastatic potential in cancers: targeting the heparan sulfate proteoglycans. Curr Drug Targets. 6:665–682 [DOI] [PubMed] [Google Scholar]
- Götte M, Kersting C, Ruggiero M, Tio J, Tulusan AH, Kiesel L, Wülfing P. 2006. Predictive value of syndecan-1 expression for the response to neoadjuvant chemotherapy of primary breast cancer. Anticancer Res. 26:621–627 [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. 2000. The hallmarks of cancer. Cell. 100:57–70 [DOI] [PubMed] [Google Scholar]
- Harvey JM, Clark GM, Osborne CK, Allred DC. 1999. Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol. 17:1474–1481 [DOI] [PubMed] [Google Scholar]
- Horowitz A, Murakami M, Gao Y, Simons M. 1999. Phosphatidylinositol-4,5-bisphosphate mediates the interaction of syndecan-4 with protein kinase C. Biochemistry. 38:15871–15877 [DOI] [PubMed] [Google Scholar]
- Jayson GC, Vives C, Paraskeva C, Schofield K, Coutts J, Fleetwood A, Gallagher JT. 1999. Coordinated modulation of the fibroblast growth factor dual receptor mechanism during transformation from human colon adenoma to carcinoma. Int J Cancer. 82:298–304 [DOI] [PubMed] [Google Scholar]
- Jensen EV, Jordan VC. 2003. The estrogen receptor: a model for molecular medicine. Clin Cancer Res. 9:1980–1989 [PubMed] [Google Scholar]
- Kamangar F, Dores GM, Anderson WF. 2006. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 24:2137–2150 [DOI] [PubMed] [Google Scholar]
- Kokenyesi R, Bernfield M. 1994. Core protein structure and sequence determine the site and presence of heparan sulfate and chondroitin sulfate on syndecan-1. J Biol Chem. 269:12304–12309 [PubMed] [Google Scholar]
- Kousidou OC, Berdiaki A, Kletsas D, Zafiropoulos A, Theocharis AD, Tzanakakis GN, Karamanos NK. 2008. Estradiol-estrogen receptor: a key interplay of the expression of syndecan-2 and metalloproteinase-9 in breast cancer cells. Mol Oncol. 2:223–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambaerts K, Wilcox-Adelman SA, Zimmermann P. 2009. The signaling mechanisms of syndecan heparan sulfate proteoglycans. Curr Opinion Cell Biol. 21:662–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leivonen M, Lundin J, Nordling S, von Boguslawski K, Haglund C. 2004. Prognostic value of syndecan-1 expression in breast cancer. Oncology. 67:11–18 [DOI] [PubMed] [Google Scholar]
- Lim ST, Longley RL, Couchman JR, Woods A. 2003. Direct binding of syndecan-4 cytoplasmic domain to the catalytic domain of protein kinase C alpha (PKC alpha) increases focal adhesion localization of PKC alpha. J Biol Chem. 278:13795–13802 [DOI] [PubMed] [Google Scholar]
- Löfgren L, Sahlin L, Jiang S, Von Schoultz B, Fernstad R, Skoog L, Von Schoultz E. 2007. Expression of syndecan-1 in paired samples of normal and malignant breast tissue from postmenopausal women. Anticancer Res. 27:3045–3050 [PubMed] [Google Scholar]
- Longley RL, Woods A, Fleetwood A, Cowling GJ, Gallagher JT, Couchman JR. 1999. Control of morphology, cytoskeleton and migration by syndecan-4. J Cell Sci. 112:3421–3431 [DOI] [PubMed] [Google Scholar]
- Loussouarn D, Campion L, Sagan C, Frenel JS, Dravet F, Classe JM, Pioud-Martigny R, Berton-Rigaud D, Bourbouloux E, Mosnier JF, et al. 2008. Prognostic impact of syndecan-1 expression in invasive ductal breast carcinomas. Br J Cancer. 98:1993–1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda T, Alexander CM, Friedl A. 2004. Induction of syndecan-1 expression in stromal fibroblasts promotes proliferation of human breast cancer cells. Cancer Res. 64:612–621 [DOI] [PubMed] [Google Scholar]
- Maeda T, Desouky J, Friedl A. 2006. Syndecan-1 expression by stromal fibroblasts promotes breast carcinoma growth in vivo and stimulates tumor angiogenesis. Oncogene. 25:1408–1412 [DOI] [PubMed] [Google Scholar]
- Manon-Jensen T, Itoh Y, Couchman JR. 2010. Proteoglycans in health and disease: the multiple roles of syndecan shedding. FEBS J. 277:3876–3889 [DOI] [PubMed] [Google Scholar]
- Marotti JD, Collins LC, Hu R, Tamimi RM. 2010. Estrogen receptor–beta expression in invasive breast cancer in relation to molecular phenotype: results from the Nurses’ Health Study. Mod Pathol. 23:197–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennerich D, Vogel A, Klaman I, Dahl E, Lichtner RB, Rosenthal A, Ohlenz HD, Thierauch KH, Sommer A. 2004. Shift of syndecan-1 expression from epithelial to stromal cells during progression of solid tumours. Eur J Cancer. 40:1373–1382 [DOI] [PubMed] [Google Scholar]
- Midwood KS, Valenick LV, Hsia HC, Schwarzbauer JE. 2004. Coregulation of fibronectin signaling and matrix contraction by tenascin-C and syndecan-4. Mol Biol Cell. 15:5670–5697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan MR, Humphries MJ, Bass MD. 2007. Synergistic control of cell adhesion by integrins and syndecans. Nat Rev Mol Cell Biol. 8:957–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullan PB, Millikan RC. 2007. Molecular subtyping of breast cancer: opportunities for new therapeutic approaches. Cell Mol Life Sci. 64:3219–3232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundhenke C, Meyer K, Drew S, Friedl A. 2002. Heparan sulfate proteoglycans as regulators of fibroblast growth factor–2 receptor binding in breast carcinomas. Am J Pathol. 160:185–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mythreye K, Blobe GC. 2009. Proteoglycan signalling co-receptors: roles in cell adhesion, migration and invasion. Cell Signal. 21:1548–1558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh ES, Woods A, Couchman JR. 1997. Multimerization of the cytoplasmic domain of syndecan-4 is required for its ability to activate protein kinase C. J Biol Chem. 272:11805–11811 [DOI] [PubMed] [Google Scholar]
- Park H, Kim Y, Lim Y, Han I, Oh ES. (2002). Syndecan-2 mediates adhesion and proliferation of colon carcinoma cells. J Biol Chem 277:29730–29736 [DOI] [PubMed] [Google Scholar]
- Payne SJ, Bowen RL, Jones JL, Wells CA. 2008. Predictive markers in breast cancer: the present. Histopathology. 52:82–90 [DOI] [PubMed] [Google Scholar]
- Perou CM, Sørlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, et al. 2000. Molecular portraits of human breast tumours. Nature. 406:747–752 [DOI] [PubMed] [Google Scholar]
- Petersen OW, Nielsen HL, Gudjonsson T, Villadsen R, Rank F, Niebuhr E, Bissell MJ, Rønnov-Jessen L. 2003. Epithelial to mesenchymal transition in human breast cancer can provide a nonmalignant stroma. Am J Pathol. 162:391–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen PP. 2001. Rosen’s breast pathology. 2nd ed. Philadelphia: Lippincott Williams & Wilkins [Google Scholar]
- Ross JS, Symmans WF, Pusztai L, Hortobagyi GN. 2005. Breast cancer biomarkers. Adv Clin Chem. 40:99–125 [DOI] [PubMed] [Google Scholar]
- Sanderson RD. 2001. Heparan sulfate proteoglycans in invasion and metastasis. Semin Cell Dev Biol. 12:89–98 [DOI] [PubMed] [Google Scholar]
- Sanderson RD, Yang Y, Kelly T, MacLeod V, Dai Y, Theus A. 2005. Enzymatic remodeling of heparan sulfate proteoglycans within the tumor microenvironment: growth regulation and the prospect of new cancer therapies. J Cell Biochem. 96:897–905 [DOI] [PubMed] [Google Scholar]
- Saoncella S, Echtermeyer F, Denhez F, Nowlen JK, Mosher DF, Robinson SD, Hynes RO, Goetinck PF. 1999. Syndecan-4 signals cooperatively with integrins in a Rho-dependent manner in the assembly of focal adhesions and actin stress fibers. Proc Natl Acad Sci U S A. 96:2805–2810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi SR, Imam SA, Young L, Cote RJ, Taylor CR. 1995. Antigen retrieval immunohistochemistry under the influence of pH using monoclonal antibodies. J Histochem Cytochem. 43:193–201 [DOI] [PubMed] [Google Scholar]
- Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, Hastie T, Eisen MB, Van de Rijn M, Jeffrey SS, et al. 2001. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 98:10869–10874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley MJ, Stanley MW, Sanderson RD, Zera R. 1999. Syndecan-1 expression is induced in the stroma of infiltrating breast carcinoma. Am J Clin Pathol. 112:377–383 [DOI] [PubMed] [Google Scholar]
- Streuli CH, Akhtar N. 2009. Signal co-operation between integrins and other receptor systems. Biochem J. 418:491–506 [DOI] [PubMed] [Google Scholar]
- Subramaniam DS, Isaacs C. 2005. Utilizing prognostic and predictive factors in breast cancer. Curr Treat Options Oncol. 6:147–159 [DOI] [PubMed] [Google Scholar]
- Subramanian SV, Fitzgerald ML, Bernfield M. 1997. Regulated shedding of syndecan-1 and -4 ectodomains by thrombin and growth factor receptor activation. J Biol Chem. 272:14713–14720 [DOI] [PubMed] [Google Scholar]
- Tavassoli FA. 1999. Invasive lobular carcinoma. In: Tavassoli FA. editor. Pathology of the breast. 2nd ed. Stamford, CT: Appleton Lange; p. 426–436 [Google Scholar]
- Tokes A-M, Szasz AM, Farkas A, Toth AI, Dank M, Harsanyi L, Molnar BA, Molnar IA, Laszlo Z, Rusz Z, et al. 2009. Stromal matrix protein expression following preoperative systemic therapy in breast cancer. Clin Cancer Res. 15:731–739 [DOI] [PubMed] [Google Scholar]
- Vargo-Gogola T, Rosen JM. 2007. Modelling breast cancer: one size does not fit all. Nat Rev Cancer. 7:659–672 [DOI] [PubMed] [Google Scholar]
- Wiksten JP, Lundin J, Nordling S, Lundin M, Kokkola A, von Boguslawski K, Haglund C. 2001. Epithelial and stromal syndecan-1 expression as predictor of outcome in patients with gastric cancer. Int J Cancer. 95:1–6 [DOI] [PubMed] [Google Scholar]
- Wolf K, Friedl P. 2006. Molecular mechanisms of cancer cell invasion and plasticity. Br J Dermatol. 154(suppl 1):11–15 [DOI] [PubMed] [Google Scholar]
- Woods A, Couchman JR. 1994. Syndecan 4 heparan sulfate proteoglycan is a selectively enriched and widespread focal adhesion component. Mol Biol Cell. 5:183–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xian X, Gopal S, Couchman JR. 2010. Syndecans as receptors and organizers of the extracellular matrix. Cell Tissue Res. 339:31–46 [DOI] [PubMed] [Google Scholar]


