IFN-γ functions to suppress neutrophil accumulation in the lungs of mice infected with M. tuberculosis, in part by suppressing IL-17 production from CD4+ T cells.
Abstract
Resistance to Mycobacterium tuberculosis requires the host to restrict bacterial replication while preventing an over-exuberant inflammatory response. Interferon (IFN) γ is crucial for activating macrophages and also regulates tissue inflammation. We dissociate these two functions and show that IFN-γ−/− memory CD4+ T cells retain their antimicrobial activity but are unable to suppress inflammation. IFN-γ inhibits CD4+ T cell production of IL-17, which regulates neutrophil recruitment. In addition, IFN-γ directly inhibits pathogenic neutrophil accumulation in the infected lung and impairs neutrophil survival. Regulation of neutrophils is important because their accumulation is detrimental to the host. We suggest that neutrophilia during tuberculosis indicates failed Th1 immunity or loss of IFN-γ responsiveness. These results establish an important antiinflammatory role for IFN-γ in host protection against tuberculosis.
Tuberculosis develops years or even decades after the establishment of latent or subclinical infection by Mycobacterium tuberculosis (Mtb). The immune response to Mtb during latency is poorly characterized; however, granulomas from asymptomatic people are small, often calcified or fibrotic, populated with lymphocytes, and contain rare bacteria. In contrast, granulomas found in people with active tuberculosis are large, caseating, and contain numerous bacteria (Ulrichs and Kaufmann, 2006). The development of clinical symptoms of disease, typically related to chronic lung infection, is a sign of failed immunity. Paradoxically, a robust immune response is detected during active tuberculosis that is thought to be secondary to the greater bacterial and antigen load–driving T cell immunity. In fact, many of the symptoms of tuberculosis are related to the pronounced host inflammatory response that occurs as the immune system tries to regain the upper hand. Tissue damage during tuberculosis primarily arises from the immune response and not as a direct consequence of the bacterium.
Inbred mouse strains vary greatly in their susceptibility to Mtb. C57BL/6 (B6) mice are resistant to low-dose aerosol Mtb infection. They develop small compact lung lesions dominated by lymphocytes and macrophages. Their lesions contain only scattered neutrophils, little or no necrosis, and are not hypoxic (Aly et al., 2006). In contrast, susceptible C3H, DBA/2, and I/St mice succumb within months of Mtb infection. Their pulmonary lesions are less well organized. Neutrophil infiltrates are more prominent, and necrosis, diffuse fibrosis, and hypoxia develop late during infection (Kondratieva et al., 2010). Ultimately, the basis for susceptibility to Mtb among inbred mouse strains is genetic. Pan et al. (2005) identified sst1 as an allelic locus between B6 and C3HeB/FeJ mice that determines the susceptibility to Mtb. Although C3HeB/FeJ (C3H.sst1C3H) mice normally develop large necrotic lesions, congenic C3H.sst1B6 mice survive longer and develop small nonnecrotic lesions that are typical of B6 mice (Pichugin et al., 2009). The striking correlation between susceptibility and the nature of the pathological lesions suggests that the inability to control bacterial replication leads to large necrotic lesions. However, the reverse situation, i.e., the propensity to form large granuloma-like lesions increasing susceptibly to tuberculosis, cannot be excluded.
The formation of large necrotic lesions after low-dose Mtb infection is not unique to susceptible inbred mouse strains. This type of tissue response is observed in a variety of knockout mouse strains that are susceptible to Mtb. An interesting mouse in this regard is the IFN-γR−/− mouse. Mice lacking IFN-γ form large necrotic pulmonary lesions associated with granulocytic infiltrates within weeks of Mtb infection despite a similar bacterial burden as WT mice (Pearl et al., 2001). The IFN-γ signaling axis is crucial for resistance to tuberculosis, and mice lacking IFN-γ, IFN-γR, or STAT1 are extremely susceptible to virulent Mtb infection (Cooper et al., 1993; Flynn et al., 1993). IFN-γ is a powerful immunomodulator that provides crucial signals to BM- and non-BM–derived cells during infection (Desvignes and Ernst, 2009). IFN-γ stimulates inducible nitric oxide synthase and LRG47 production, which are essential for the antibacterial activity of IFN-γ. IFN-γ is also an essential component of the human immune response to tuberculosis, although its mechanism of action may differ from that defined in mice (Jouanguy et al., 1996, 1997; MacMicking et al., 1997, 2003; Madariaga et al., 1998).
In addition to its antimicrobial role, IFN-γ has important immunoregulatory functions. Dalton et al. (2000) first demonstrated that IFN-γ, via NO induction, led to apoptosis of activated CD4+ T cell, thus contributing to T cell homeostasis during systemic Bacillus Calmette-Guerin (BCG) infection. Cooper et al. (2002) recognized that IFN-γ–induced T cell apoptosis has an important role in modulating tissue inflammation during mycobacterial infection in general. In this paper, we consider whether IFN-γ plays a role in dampening the inflammatory response, which promotes host resistance, in addition to its antimicrobial role during tuberculosis infection. This hypothesis is based on two arguments. First, T cells can control Mtb replication independently of IFN-γ (Cowley and Elkins, 2003; Woodworth et al., 2008b; Gallegos et al., 2011), and although these mechanisms may not be as effective as IFN-γ, it is sobering that these other pathways do not protect IFN-γ−/− mice from Mtb infection and thus are unable to compensate for the lack of IFN-γ. This suggests that IFN-γ is playing a crucial role in host resistance independent of its antimicrobial role. Second, IFN-γ−/− mice are more susceptible than mice that lack αβ-TCR+ T cells (Mogues et al., 2001). This raises the possibility that in the absence of IFN-γ, the T cell response adversely affects host resistance to Mtb. There exists substantial precedent for this possibility. Increased tissue inflammation contributes to the susceptibility of IFN-γ−/− mice in experimental autoimmune disease models (Minguela et al., 2007; Sabatino et al., 2008; Pastor et al., 2009). Under these conditions IFN-γ has an antiinflammatory role. The abilities of IFN-γ to inhibit IL-17 production (Cruz et al., 2006) and block neutrophil recruitment (Desvignes and Ernst, 2009) are two functions that are important for its antiinflammatory role. This mechanism may be relevant to tuberculosis in both mice and humans. Patients with active pulmonary tuberculosis have a greater abundance of neutrophils than household contacts (Sutherland et al., 2009; Berry et al., 2010; Eum et al., 2010). Similarly, more neutrophils are recruited to the lungs of susceptible mouse strains after infection (Eruslanov et al., 2005), and neutrophil depletion prolongs their survival (Keller et al., 2006; Dorhoi et al., 2010). We hypothesize that more IL-17 will be made in the absence of IFN-γ or IFN-γR, which will lead to neutrophil recruitment into the lungs of infected mice. We further postulate that neutrophil accumulation will impair the outcome of Mtb infection in IFN-γ−/− and IFN-γR−/− mice independently of the bacterial load.
RESULTS
Relative susceptibility of mice in the absence of IFN-γ signaling or acquired immunity
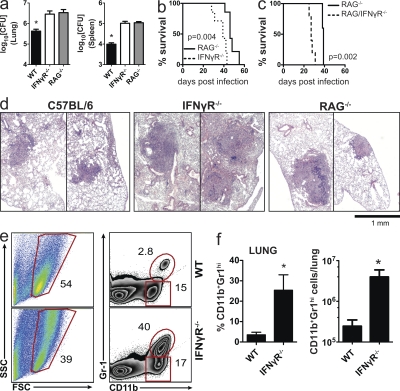
To compare the relative requirement for IFN-γ signaling and acquired immunity in the control of Mtb replication, IFN-γR−/−, RAG2−/−, and WT B6 mice were infected with virulent Mtb by the aerosol route. B6 mice had lower CFU in the lung and spleen compared with the two knockout strains 3.5 wk after infection (P < 0.05). However, the bacterial burdens of IFN-γR−/− and RAG2−/− mice were similar (Fig. 1 a). Despite similar bacterial lung burdens, IFN-γR−/− mice were significantly more susceptible than RAG2−/− mice (median survival time, 39 vs. 46 d, P = 0.004; Fig. 1 b). These results confirm other studies showing that IFN-γ−/− mice are more susceptible than RAG2−/− or αβ-TCR−/− mice (Mogues et al., 2001; Feng et al., 2006). This raised the possibility that T cell responses elicited in the absence of IFN-γ signaling were detrimental to the host, or that IFN-γ production by innate cells (e.g., NK cells), as described by Feng et al. (2006), is quantitatively more important than IFN-γ–independent T cell immunity.
Figure 1.
IFN-γR−/− mice are more susceptible than RAG2−/− mice to Mtb. (a) Bacterial load (CFU) in the lungs and spleen of WT, IFN-γR−/−, and RAG2−/− mice on day 23 after Mtb infection (n = 5 mice/group). Error bars, SEM. *, P < 0.05. (b) Survival of IFN-γR−/− and RAG2−/− mice after Mtb infection (n = 5 mice/ group). (c) Survival of RAG2−/− and RAG2/IFN-γR−/− mice after Mtb infection (n = 5 mice/ group). (d) Representative lung sections stained with hematoxylin and eosin from the mice represented in a. Two representative lesions are shown for each mouse genotype to demonstrate the size range. (e) Neutrophils (CD11b+Gr1hi) from the lungs of WT (top) or IFN-γR−/− (bottom) mice 4 wk after Mtb infection. Numbers represent percentage of gated cells. (f) Frequency and absolute number of neutrophils (CD11b+Gr1hi) in the lungs of WT and IFN-γR−/− mice (n = 3 mice/group). Error bars, SEM. *, P < 0.05. Data in a and d were from the same experiment. The three-way comparison was performed once; however, pairwise comparisons have been repeated multiple times with similar results. All other data are representative of two independent experiments. Survival analyses were analyzed by the log-rank test; a was analyzed by one way ANOVA; and f was analyzed by Student’s t test.
To distinguish between these possibilities, we measured the contribution of IFN-γ to host resistance in the absence of T cells. RAG2/IFN-γR−/− mice were significantly more susceptible than RAG2−/− mice after Mtb infection (Fig. 1 c). Thus, in the absence of T cells, innate sources of IFN-γ are crucial for host resistance (Feng et al., 2006). Next, lungs from infected IFN-γR−/− and RAG2−/− mice were examined to assess the contribution of IFN-γ to pulmonary pathology. Although WT mice had discrete lesions containing myeloid and lymphoid infiltrates, IFN-γR−/− and RAG2−/− mice had larger lesions (Fig. 1 d). As the infection progressed, the lesions in IFN-γR−/− mice became larger, infiltrated with polymorphonuclear neutrophils (PMNs), and more necrotic (Fig. S1). To confirm that neutrophil infiltrates were more abundant in the lungs of Mtb-infected IFN-γR−/− mice, we enumerated neutrophils (CD11b+Gr-1Hi; Fig. 1 e). More neutrophils were found in the lungs of Mtb-infected IFN-γR−/− mice than in WT mice (Fig. 1 f). This difference was not apparent in the lungs of uninfected mice (unpublished data). These results are similar to published data that shows an important role for IFN-γ in limiting pulmonary inflammation and neutrophil recruitment to the lung (Pearl et al., 2001; Feng et al., 2006; Desvignes and Ernst, 2009). These data raised the possibility that in the absence of IFN-γ signaling, the immune response is dysregulated and generates an inflammatory response that leads to worse pulmonary pathology that is detrimental to the host, as measured by increased mortality.
Antigen-specific CD4+ T cell responses in the absence of IFN-γ signaling
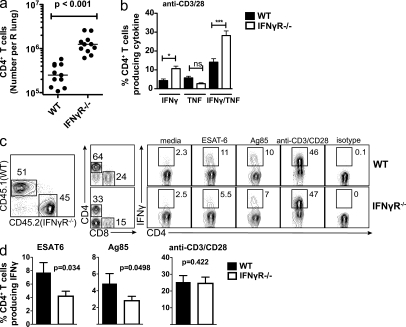
In uninfected mice, the numbers of CD4+ T cells in the lung and spleen were similar in IFN-γR−/− and C57BL/6 mice (unpublished data). However, after Mtb infection, fivefold more CD4+ T cells were found in the lungs of IFN-γR−/− mice compared with C57BL/6 mice (P < 0.0001; Fig. 2 a). Cytokine production by CD4+ T cells was measured after in vitro stimulation with the ESAT61-15 epitope or anti-CD3/CD28 antibodies. More pulmonary CD4+ T cells from IFN-γR−/− mice produced IFN-γ, alone or in combination with TNF, after ESAT61-15 or polyclonal stimulation compared with B6 mice (Fig. 2 b). The greater T cell accumulation and their propensity to produce cytokines could be antigen driven, secondary to their increased pulmonary bacterial burden in the lungs of IFN-γR−/− mice. To test this possibility, we made mixed BM chimeric mice.
Figure 2.
Antigen-specific CD4+ T cells are elicited in the absence of IFN-γ signaling. (a) Absolute number of CD4+ T cells per lung from WT or IFN-γR−/− mice 4–5 wk after Mtb infection. Data represents 11 mice/condition from three independent experiments. Horizontal bars, median. (b) Frequency of WT or IFN-γR−/− CD4+ T cells producing only IFN-γ, only TNF, or both IFN-γ and TNF after stimulation with anti-CD3/CD28, 3.5 wk after Mtb infection. Data are representative of two independent experiments. Error bars, SEM. *, P < 0.05; ***, P < 0.001. (c) Flow cytometric analysis of mixed WT and IFN-γR−/− BM chimeric mice. Lung lymphocytes were gated by size and CD45.1 (WT) or CD45.2 (IFN-γR−/−) cells identified. CD4+ T cells were gated and their production of IFN-γ after stimulation in vitro was determined. (d) Intracellular production of IFN-γ by WT or IFN-γR−/− CD4+ T cells from mixed BM chimera as indicated in c. Error bars, SEM. Data represents the analysis of 16 mice/condition from four independent experiments. Statistical analysis was performed using a Student’s t test.
BM from CD45.2+ IFN-γR−/− mice and congenic WT CD45.1 mice was mixed and used to make chimeras in lethally irradiated RAG2−/− or B6 recipient mice. These two approaches yielded similar data and the results are combined in Figs. 2 and 3. By gating on cells expressing CD45.2 (IFN-γR−/−) or CD45.1 (WT), we assessed how IFN-γR expression affects T cell immunity in an environment in which all cells are exposed to the same amount of antigen, bacteria, and inflammatory cytokines. IFN-γR−/− CD4+ T cells in the lungs of Mtb-infected chimeric mice produced slightly less IFN-γ after ESAT61-15 and Ag85B241-256 stimulation compared with WT CD4+ T cells (Fig. 2, c and d). In contrast, IFN-γR−/− and WT CD4+ T cells produced the same amount of IFN-γ after anti-CD3/28 stimulation (Fig. 2, c and d). We conclude that there is little or no intrinsic difference in the generation of activated IFN-γR−/− CD4+ T cells during Mtb infection.
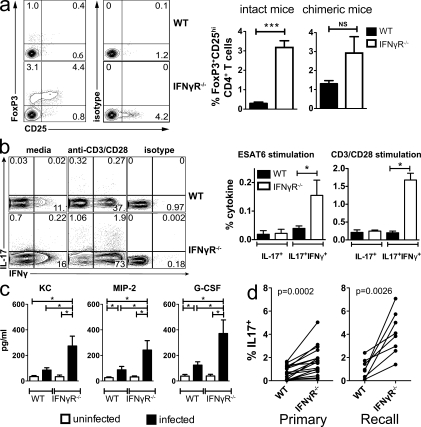
Figure 3.
IFN-γ signaling inhibits IL-17A production by CD4+ T cells during Mtb infection. (a) T reg cells in the lung 4 wk after Mtb infection of WT and IFN-γR−/− mice were detected by flow cytometry based on coexpression of CD25 and intracellular FoxP3 (left). T reg cells were enumerated in the lungs of WT and IFN-γR−/− mice (intact, middle) and WT/IFN-γR−/− chimeric mice (right). Each experiment analyzed five mice/group and statistical testing was performed using a Student’s t test. Error bars, SEM. ***, P < 0.001; NS, not significant. (b) Representative flow cytometric analysis (left) and analysis (right) of intracellular IL-17A production by lung CD4+ T cells from WT or IFN-γR−/− mice 4 wk after Mtb (n = 4 mice/group). Data were analyzed using a Student’s t test. Error bars, SEM. *, P < 0.05. (c) Levels of the chemokines KC, MIP-2, and G-CSF were measured in cell-free supernatants of lung homogenates prepared from WT and IFN-γR−/− mice (n = 5 mice/group) 4 wk after Mtb infection. Data was analyzed using a Student’s t test. Error bars, SEM. *, P < 0.05. (d) IL-17A production by lung CD4+ T cells from lungs of mixed WT/IFN-γR−/− BM chimeric mice as described in Fig. 2 c. Mice were analyzed 4–5 wk after Mtb infection (n = 13 mice/condition) or recall response 23–29 d after challenge of memory immune (n = 8 mice/condition). The data are pooled from three independent experiments and analyzed using a paired Student’s t test.
IFN-γ signaling inhibits generation of Th17 cells during Mtb infection
As IFN-γ negatively regulates the generation of Th2, Th17, and regulatory T (T reg) cells, we became interested in whether these other CD4+ T cell subsets were more abundant after Mtb infection of IFN-γR−/− mice. Few IL-4–producing CD4+ cells were detected in the lung, but there were no differences between infected WT and IFN-γR−/− mice as previously demonstrated (not depicted; Flynn et al., 1993). T reg cells were detected in greater frequency in IFN-γR−/− than WT mice; however, in WT/IFN-γR−/− mixed BM chimeric mice (described in the previous section), T reg cells from the two genotypes were detected with similar frequency, indicating that IFN-γ signaling may not affect T reg cell generation (Fig. 3 a). Both WT and IFN-γR−/− mice had similar low frequencies of lung CD4+ T cells producing IL-17 after ESAT61-15 stimulation in vitro. However, more CD4+ T cells producing both IL-17 and IFN-γ were detected in the lungs of infected IFN-γR−/− mice (P < 0.05; Fig. 3 b). Similarly, more pulmonary CD4+ T cells from IFN-γR−/− mice produced both IL-17 and IFN-γ after anti-CD3/28 polyclonal stimulation (P < 0.05; Fig. 3 b). These IL-17 and IFN-γ double-producing cells are similar to Th17(B) CD4+ T cells described by Geiger et al. (2009), Napolitani et al. (2009), and Zielinski et al. (2011) which are IL-1β dependent.
Among the known functions of IL-17 are the recruitment and activation of granulocytes. IL-17 recruits neutrophils to inflammatory sites by stimulating the production of cytokines and chemoattractants including G-CSF, MIP-2, and KC (Ye et al., 2001). The increased IL-17 production in the lungs of Mtb-infected IFN-γR−/− mice is predicted to stimulate secretion of neutrophil chemokines and increase the trafficking of neutrophils to the lung. Therefore, we measured KC, MIP-2, and G-CSF in lung homogenates (Fig. 3 c). All three were expressed at higher levels in the lungs of infected IFN-γR−/− mice than in WT mice. These data provide further evidence that in the absence of IFN-γ signaling, IL-17 production is increased in the lungs of infected mice and alters the nature of the pulmonary immune response.
To verify that the difference in IL-17 production was not a result of differences in the bacterial load, we analyzed IL-17 production by T cells from CD45.2 (IFN-γR−/−) and CD45.1 (WT) mixed BM chimeras. In these mice, more IL-17 was produced by IFN-γR−/− than WT CD4+ T cells after stimulation with anti-CD3/CD28 (Fig. 3 d, primary). These differences were evident despite similar IFN-γ production (Fig. 2 and not depicted). Infected chimeric mice were treated with antibiotics to generate memory-immune mice. Mtb challenge of memory-immune mice also led to an increase in IL-17 production by IFN-γR−/− CD4+ T cells (Fig. 3 d, recall). Thus, cell-intrinsic factors led to greater IL-17 production by IFN-γR−/− CD4+ T cells, indicating that IFN-γ signaling in CD4+ T cells inhibits IL-17 production during Mtb infection.
IFN-γ from CD4+ T cells is not required for control of Mtb but suppresses pulmonary inflammation
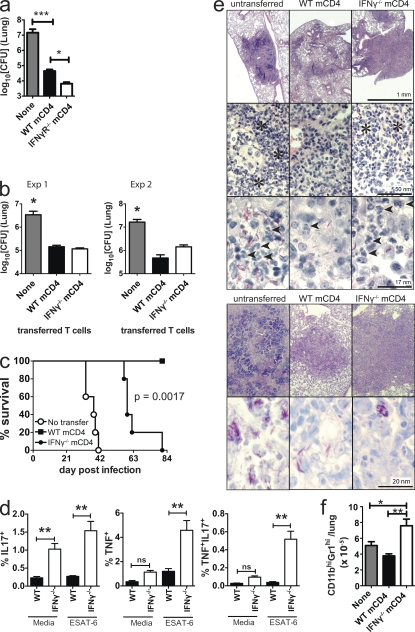
The previous experiments showed that in the absence of IFN-γ signaling, a T cell response is generated, and antigen-specific T cells are recruited to the lung. We next determined whether IFN-γR−/− or IFN-γ−/− CD4+ T cells are protective. CD4+ T cells sufficiently pure and free of bacterial contamination could not be obtained from infected IFN-γR−/− or IFN-γ−/− mice. To circumvent this problem, we purified T cells from WT, IFN-γR−/−, and IFN-γ−/− memory-immune mice. IFN-γR expression by memory CD4+ T cells is not required to adoptively transfer protection (Fig. 4 a). More importantly, in two independent transfer experiments, IFN-γ−/− and WT memory CD4+ T cells similarly protected RAG2−/− mice from virulent Mtb challenge (Fig. 4 b). In addition to limiting bacterial growth in the lungs of RAG2−/− mice, IFN-γ−/− memory CD4+ T cells also significantly prolonged the survival of Mtb-infected RAG2−/− mice (median survival time, 38 vs. 59 d, P < 0.0017; Fig. 4 c).
Figure 4.
IFN-γ is required by CD4+ T cells for suppressing pulmonary inflammation. (a) Lung bacterial burden in recipient RAG2−/− mice 4 wk after Mtb infection and adoptive transfer of no cells, WT, or IFN-γR−/− memory CD4+ T cells. This data was analyzed by one-way ANOVA and is representative of two independent experiments, each with n = 5 mice/group. Error bars, SEM. ***, P < 0.001; *, P < 0.05. (b) Pulmonary bacterial burden 3–4 wk after Mtb infection of RAG2−/− recipient mice that received nothing, WT, or IFN-γ−/− memory CD4+ T cells. Two independent experiments are shown (n = 5 mice/group) analyzed by one-way ANOVA. Error bars, SEM. *, P < 0.05. (c) Survival of RAG2−/− mice that received nothing, WT, or IFN-γ−/− memory CD4+ T cells at the time of Mtb infection (n = 5 mice/group). The figure is representative of two independent experiments analyzed by log-rank test. (d) Cytokine production by WT or IFN-γ−/− memory CD4+ T cells. RAG2−/− mice that received nothing, WT, or IFN-γ−/− memory CD4+ T cells and were infected with Mtb are shown. Lung cells were isolated after 23 d and analyzed after culture alone or stimulation with ESAT61-15. The frequency of CD4+ T cells making IL17A only, TNF only, or both IL17A and TNF was determined (n = 5 mice/condition, from a, Exp1). Error bars, SEM. **, P < 0.01. (e) Lung histology and AFB staining of RAG2−/− mice transferred with nothing, WT, or IFN-γ−/− memory CD4+ T cells (from Exp 1, top; and Exp 2, bottom). Asterisks, neutrophil infiltrates; arrow heads, neutrophils. (f) Neutrophils in the lungs of RAG2−/− mice that received nothing (NoTx), WT, or IFN-γ−/− memory CD4+ T cells (from a, Exp1). Data was analyzed by one-way ANOVA. Error bars, SEM. *, P < 0.05, **, P < 0.01.
Using this same adoptive transfer model, we determined whether IFN-γ affects IL-17 production by CD4+ T cells. An increased proportion of IFN-γ−/− memory CD4+ T cells produced IL-17 in the absence of any stimulation (Fig. 4 d). Also, more IFN-γ−/− memory CD4+ T cells produced TNF, or TNF and IL-17, after ESAT6 stimulation in vitro (Fig. 4 d). These data show that IFN-γ signaling inhibits T cell production of IL-17 during Mtb infection. This alteration is unlikely to be secondary to differences in antigen load because the two experimental groups had similar lung CFU (Fig. 4 b, Exp 1).
We next examined the lung histopathology of RAG2−/− mice that received no cells, WT, or IFN-γ−/− memory CD4+ T cells. The lung lesions were considerably larger in mice receiving IFN-γ−/− memory CD4+ T cells compared with those that received WT memory CD4+ T cells, despite a similar bacterial burden (Fig. 4 e). In addition to the larger lesion size, numerous neutrophils accumulated in the lungs of RAG2−/− recipients reconstituted with IFN-γ−/− memory CD4+ T cells after Mtb challenge. In contrast, few neutrophilic infiltrate were found after transfer of WT memory CD4+ T cells (Fig. 4 e). The absolute number of lung neutrophils in mice receiving IFN-γ−/− memory CD4+ T cells was nearly twice that of mice receiving WT memory CD4+ T cells (Fig. 4 f). Finally, the AFB stains confirmed that the transfer of T cells, whether producing IFN-γ or not, was able to control the infection (Fig. 4 e). These data indicate that IFN-γ has a role in reducing tissue inflammation and preventing the accumulation of neutrophils independently of its antibacterial function.
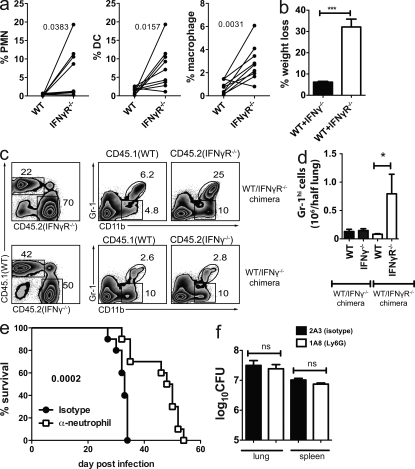
Neutrophil depletion enhances the resistance of IFN-γR−/− mice
As IL-17 is associated with neutrophil recruitment, we next used the WT/IFN-γR−/− mixed BM chimeras to test the hypothesis that IFN-γR signaling inhibits the neutrophil accumulation in the lung. Neutrophils lacking IFN-γR preferentially accumulated in the lungs during infection as did DC and macrophages (Fig. 5 a). To confirm that IFN-γ inhibits neutrophil accumulation, WT/IFN-γR−/− and WT/IFN-γ−/− mixed BM chimeras were made. We predicted that IFN-γ would act in trans as a soluble factor to suppress the neutrophil accumulation in the lung of infected mice. Major differences were observed between WT/IFN-γR−/− and WT/IFN-γ−/− chimeras as the WT/IFN-γR−/− chimeras became clinically ill within 4 wk of infection, as indicated by significant weight loss (Fig. 5 b). We observed that IFN-γR inhibited neutrophil accumulation in the lung (Fig. 5 c, top). Greater numbers of neutrophils lacking the IFN-γR were found in the lung, a difference that was apparent only after infection (Fig. 5 d and not depicted). In contrast, both WT and IFN-γ−/− neutrophils were found in the lungs of WT/IFN-γ−/− chimeras at a similarly low frequency (Fig. 5 c, bottom). These data show that IFN-γ acts directly on neutrophils to inhibit their accumulation in the lung during Mtb infection.
Figure 5.
Neutrophil accumulation is inhibited by IFN-γ and is detrimental to outcome. (a) The percentage of CD45.1 (WT) or CD45.2 (IFN-γR−/−) cells that are neutrophils (PMN), DC, or macrophages in the lungs of mixed BM chimeric mice 4 wk after Mtb infection (n = 9 mice/condition pooled for two independent experiments). (b) Weight loss of [WT+IFN-γR−/−] or [WT+IFN-γ−/−] mixed BM chimeric mice, 4 wk after Mtb infection (n = 5 mice/ group). Error bars, SEM. ***, P < 0.001 by Student’s t test. (c) Flow cytometric analysis of neutrophils in the lungs of mixed [WT+IFN-γR−/−] or [WT+IFN-γ−/−] BM chimeric mice. Numbers indicate the percentage of gated cells. (d) The absolute neutrophil number in the lungs of mixed BM chimeric mice. Error bars, SEM. *, P < 0.05 by Student’s t test. (e) Survival of Mtb-infected IFN-γR−/− mice depleted of neutrophils. The neutrophil-specific antibody anti-Ly6G (clone 1A8) or the isotype control (clone 2A3) was administered to mice and their survival monitored (n = 10 mice/group). Data are analyzed by the log-rank test and are representative of two independent experiments. (f) Bacterial burden in the lungs and spleens of IFN-γR−/− mice treated with 1A8 (neutrophil depleting) or 2A3 (isotype control) monoclonal antibodies, 4 wk after Mtb infection. Data were analyzed by one-way ANOVA and is representative of two independent experiments, each with n = 5 mice/group. Error bars, SEM.
The finding that IFN-γ directly inhibits neutrophil accumulation, and the association between neutrophils and lung inflammation, led us to hypothesize that neutrophil influx into the lung adversely affects the outcome of tuberculosis. To test this possibility, neutrophils were depleted from infected IFN-γR−/− mice using the Ly-6G–specific mAb 1A8. Infected IFN-γR−/− mice lived significantly longer after treatment with 1A8 compared with the control mAb (Fig. 5 e). Lung and spleen CFU were measured 4 wk after infection. Depletion of neutrophils did not lead to better bacterial control at this time point (Fig. 5 f). These data indicate that IFN-γ plays an important role in preventing the accumulation of neutrophils in the lung during infection, a cell type which can contribute to tissue pathology and impair the survival of Mtb-infected mice.
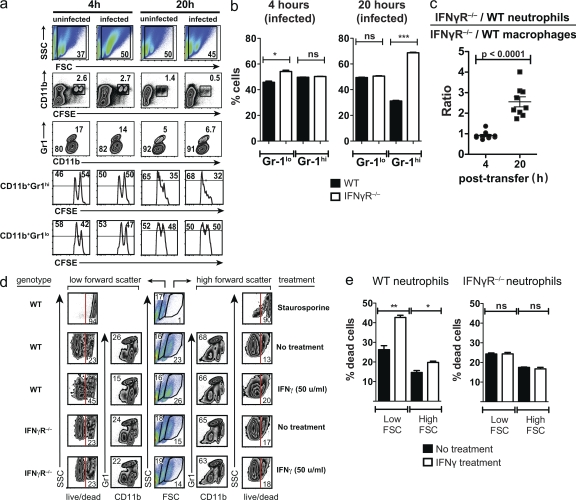
IFN-γ affects neutrophil accumulation in the lung and their survival
A direct role for IFN-γR in preventing the accumulation of neutrophils in the lungs of Mtb-infected mice suggested that IFN-γ affects their recruitment or survival. To test these possibilities, elicited peritoneal neutrophils from WT or IFN-γR−/− mice were differentially labeled with CFSE and transferred into uninfected or Mtb-infected WT mice. 4 h after transfer, the ratio of IFN-γR−/− to WT neutrophils (CD11b+Gr-1Hi) was ∼1:1 (Fig. 6, a and b). As an internal control, the ratio of IFN-γR−/− to WT macrophages/monocytes (CD11b+Gr-1lo) was also determined and found to be ∼1:1. By 20 h, there was a dramatic change and IFN-γR−/− neutrophils outnumbered WT cells by a ratio of 2:1 (Fig. 6 b). In contrast, the ratio of IFN-γR−/− to WT macrophages/monocytes was still 1:1. This effect was independent of whether the recipient mice were infected or not (Fig. 6 a). Thus, IFN-γ signaling through the IFN-γR prevents the accumulation of neutrophils without affecting the recruitment of macrophages/monocytes (Fig. 6 c).
Figure 6.
IFN-γ regulates survival of neutrophils in vitro and in vivo. (a) In vivo survival of WT or IFN-γR−/− neutrophils. Thioglycollate-elicited peritoneal exudate cells from WT (CFSEhi) or IFN-γR−/− (CFSElo) mice were mixed 1:1 and injected into uninfected or Mtb-infected mice. Cells were isolated from the lungs of recipient mice 4 and 20 h after transfer. After gating the cells for size (first row), the CD11b+CFSE+ cells were identified (second row). The CFSE+ (transferred cells) were regated (third row) to display CD11b+Gr-1+ (neutrophils, top gate) and CD11b+Gr-1lo (monocyte/macrophages, bottom gate) populations. The relative frequency of WT (CFSEhi) or IFN-γR−/− (CFSElo) cells in the CD11b+Gr-1+ (neutrophils, fourth row) and CD11b+Gr-1lo (monocyte/macrophages, fifth row) populations was determined. (b) The relative frequency of surviving WT and IFN-γR−/− monocyte/macrophages (CD11b+Gr-1lo) and neutrophils (CD11b+Gr-1+) at 4 and 20 h after cell transfer. The data are compiled from three mice/condition and are representative of two experiments. (c) Relative ratio of [IFN-γR−/−/WT neutrophils]/[IFN-γR−/−/WT monocyte/macrophages] 4 or 20 h after cell transfer. Results for uninfected and infected mice were pooled from two experiments. Horizontal bars, mean ± SEM. (d) In vitro survival of WT or IFN-γR−/− neutrophils. Peritoneal exudate cells from WT or IFN-γR−/− mice were isolated 4 h after thioglycollate injection. The cells were cultured in the absence or presence of IFN-γ (as indicated in the figure). After 20 h, the cultures were analyzed by flow cytometry and cells with a low (left) or high (right) forward scatter were gated and analyzed separately (middle). Each population was analyzed for the expression of CD11b and Gr-1, and CD11b+Gr-1Hi cells (neutrophils) were analyzed for cell death using a live/dead stain. The numbers indicated the percentage of gated cells. Staurosporine was used to induce cell death as a control. (e) The percentage of dead cells after in vitro treatment with or without IFN-γ. Statistical analysis was done by Student’s t test. Error bars, SEM. *, P < 0.05; **, P < 0.01. The assay was done in triplicate and the data are representative data of two experiments.
Next, we cultured neutrophils in the absence or presence of IFN-γ in vitro. After 4 h of culture, there was little difference between the experimental groups (unpublished data). After 20 h, there was a reduction in the viability of all neutrophils and IFN-γ appeared to accelerate their death (Fig. 6, d and e). As a control, IFN-γR−/− neutrophils were cultured with IFN-γ. As predicted, IFN-γ had no effect on the survival of IFN-γR−/− neutrophils. These results show that IFN-γ, particularly in the lungs of Mtb-infected mice, impairs the accumulation and the survival of PMN. This inhibitory action of IFN-γ, independent of its antibacterial action, may limit tissue pathology during tuberculosis.
DISCUSSION
In the absence of IFN-γ or the IFN-γ receptor, mice succumb rapidly after infection with Mtb (Cooper et al., 1993; Flynn et al., 1993). The increased susceptibility of people with congenital and acquired deficiencies of IFN-γ shows that IFN-γ is a key effector molecule for human resistance to Mtb (Jouanguy et al., 1999). Based on this genetic data and other experimental data, IFN-γ has been assigned a central and pivotal role in controlling bacteria and promoting host immunity to tuberculosis. However, IFN-γ is not sufficient to mediate host resistance to Mtb and multiple cell-mediated interactions and soluble mediators are required to control bacterial growth (Flynn et al., 1995; Woodworth et al., 2008b; Lázár-Molnár et al., 2010; Mayer-Barber et al., 2010). Conversely, IFN-γ is a powerful immunomodulatory cytokine that regulates thousands of genes (Ehrt et al., 2001). It is naive to ascribe its importance for host resistance solely to its capacity to induce one or two antimicrobial effector molecules.
We find that IFN-γ is required neither for priming of Mtb-specific CD4+ T cells nor for the recruitment of CD4+ T cells to the lung. Furthermore, memory CD4+ T cells generated in the absence of IFN-γ retain their ability to protect mice against Mtb challenge. As shown in this paper and by others, CD4+ T cells can control bacterial growth both in vivo and in vitro independently of IFN-γ (Cowley and Elkins, 2003; Gallegos et al., 2011). Cowley and Elkins (2003) showed that although IFN-γ−/− mice were susceptible to infection, BCG-primed IFN-γ−/− memory mice were able to resist Mtb challenge. The resistance of IFN-γ−/− memory mice still required CD4+ T cell immunity because CD4+ T cell depletion during rechallenge led to the rapid demise of the IFN-γ−/− mice. We have extended these studies by demonstrating that adoptive transfer of WT and IFN-γ−/− memory CD4+ T cells into RAG2−/− mice similarly controlled bacterial growth for 4 wk after infection with Mtb.
However, in the absence of IFN-γ other detrimental changes develop. Although IFN-γ was formerly considered to be important along with IL-12 in generating Th1 cells (Szabo et al., 2003), recent studies in various infection models, including Listeria, Chlamydia, and Leishmania, find that IFN-γ is not essential for generating Th1 cells (Swihart et al., 1995; Haring and Harty, 2006; Gondek et al., 2009). As described, we find that Th1 cells are elicited during Mtb infection in the absence of IFN-γ signaling. In absence of IFN-γ, the generation of Th2 may be disinhibited; however, we did not detect more IL-4 production by CD4+ T cells from Mtb-infected IFN-γR−/− mice. Similarly, although T reg cells appeared to be more frequent in IFN-γR−/− mice, our analysis of chimeric mice suggests that the increase in T reg cell number is predominantly driven by the higher bacterial burden and is not mediated directly by the IFN-γR. Finally, we examined whether the generation of Th17 cells is increased in the absence of IFN-γ.
We find CD4+ T cells from Mtb-infected IFN-γR−/− mice are predisposed to making IL-17. More IL-17 was produced by IFN-γ−/− memory CD4+ T cells and by IFN-γR−/− CD4+ T cells from Mtb-infected WT/IFN-γR−/− mixed BM chimeric mice. Together, these data establish an inhibitory role for IFN-γ in the production of IL-17 by CD4+ T cells independent of the bacterial burden. Th17 cells can provide protection against Mtb. Th17 cells differentiated in vitro using BCG antigens protect RAG2−/− mice against Mtb challenge (Masters et al., 2010; Wozniak et al., 2010). IL-17 is required for protection against Mtb infection in vaccination models and has a role in granuloma formation (Khader et al., 2007). The initial protection mediated by IFN-γ−/− CD4+ T cells may result from an increase in IL-17 and neutrophils (Feng et al., 2006). However, although Th17 cells may be important early in the absence of IFN-γ signaling, the dysregulated production of IL-17 ultimately has a detrimental effect on the host.
The suppression of inflammation by IFN-γ may be one reason why WT memory CD4+ T cells are superior to IFN-γ−/− memory CD4+ T cells at transferring long-term protection. Although adoptively transferred IFN-γ−/− memory CD4+ T cells restrict Mtb growth, recipient mice developed larger areas of lung inflammation compared with mice that received IFN-γ–producing CD4+ T cells. Similar to IFN-γR−/− mice, the lung lesions in RAG2−/− mice that received IFN-γ−/− memory CD4+ T cells contained more neutrophils and early necrotic foci. Under these conditions, more CD4+ T cells produce IL-17, which acts to recruit neutrophils. Although we did not prove that IL-17 recruited neutrophils to the lung, it is reasonable to conclude that it contributes to neutrophil accumulation (Lindén et al., 2005). Repeated vaccination with BCG increases IL-17 and is associated with lung inflammation (Cruz et al., 2010). Under these conditions, treatment of vaccinated mice with anti–IL-17 reduces neutrophil accumulation in the lung (Cruz et al., 2010). IL-17 has also been associated with liver fibrosis in humans (Wang et al., 2011). IL-17–expressing cells are associated with the fibrotic lining, which produces TGF-β, IL-6, and IL-1, all known stimulators of IL-17 production (Wang et al., 2011). Similar findings were reported in the mouse model of idiopathic pulmonary fibrosis where IL-17 and TGF-β were associated with fibrosis (Wilson et al., 2010). Fibrosis is required for repairing and remodeling damaged tissues; however, unregulated fibrosis can lead to organ failure and death. We noted increased fibrosis in the pulmonary lesions of IFN-γR−/− mice, which correlates with their greater production of IL-17. Although IFN-γ may directly inhibit fibrosis (Blanton et al., 2005; Hu and Ivashkiv, 2009), both neutrophils and IL-17, which are increased in Mtb-infected mice with defective IFN-γ signaling, are associated with fibrosis.
In the presence of intact IFN-γ signaling, large numbers of neutrophils do not accumulate in the lungs of Mtb-infected B6 mice. We find that IFN-γ inhibits neutrophil accumulation by two mechanisms. First, by inhibiting IL-17 production, IFN-γ limits the production of an important signal for neutrophil recruitment. IL-17 is known to stimulate the production of CXC chemokines neutrophil and G-CSF, an important neutrophil hematopoietic factor. Indeed, we observed increased levels of G-CSF, MIP-2, and KC in the lungs of Mtb-infected IFN-γR−/− mice. Second, our mixed BM chimeric experiments showed that IFN-γ signaling has a direct effect on neutrophils and prevents their accumulation in the lung independently of bacterial load. IFN-γ could affect neutrophil recruitment to the lung or their survival. After adoptive transfer of IFN-γR−/− and WT neutrophils into uninfected or Mtb-infected mice, IFN-γR−/− neutrophils persisted longer in the lung than WT cells. Furthermore, IFN-γ appears to accelerate neutrophil death in vitro. Although IFN-γ has been shown to delay apoptosis of human neutrophils via the STAT1 and STAT3 pathway in vitro (Sakamoto et al., 2005), IFN-γ and IL-6 can have a proapoptotic effect on mouse neutrophils recruited after sterile inflammation to the peritoneum (McLoughlin et al., 2003). The short-lived nature of neutrophils and their propensity to die by apoptosis serve to limit unintended tissue damage. IFN-γ can also affect the net accumulation of neutrophils by enhancing macrophage phagocytosis of apoptotic neutrophils (Fernandez-Boyanapalli et al., 2010). However, if apoptotic neutrophils are not cleared by macrophages, their death by secondary necrosis would lead to the release of potentially harmful mediators, including active elastase, MMPs, degradative enzymes, and reactive oxygen species, all products which injure other cells (Silva et al., 2008). Modulation of neutrophil recruitment by IFN-γ can occur via the modulation of adhesion molecules and chemokines. IFN-γ suppresses expression of E- and P-selectin on endothelial cells and these molecules are important for neutrophil trafficking into inflamed tissue (Melrose et al., 1998; Sarraj et al., 2006). Finally, IFN-γ directly down-regulates KC and MIP-2 production by macrophages (Ohmori and Hamilton, 1994). Thus, inappropriate neutrophil accumulation in the lungs may to lead to more severe tissue inflammation and damage (Silva et al., 2008; Kennedy and DeLeo, 2009).
To test our hypothesis that that in the absence of IFN-γ, dysregulated immunity leads to excessive pulmonary inflammation and is detrimental to the outcome of infection, we depleted neutrophils after Mtb infection of IFN-γR−/− mice. Neutrophil depletion significantly prolonged the survival of IFN-γR−/− mice, confirming that neutrophils have a detrimental effect on host resistance to Mtb.
In summary, IFN-γ is known to play an immunomodulatory role during chronic inflammation. Although it is initially proinflammatory, it also acts to inhibit inflammation (Hu and Ivashkiv, 2009). We considered the role of IFN-γ in the context of its modulation of innate and adaptive immunity during chronic infection and inflammation caused by Mtb. In addition to its important role in activating antibacterial activity in macrophages, IFN-γ has an essential function in limiting lung inflammation independent of its antibacterial activity. In this study, we find that in the absence of IFN-γ signaling more neutrophils accumulate in the lungs of Mtb-infected mice. Our results using mixed BM chimeric mice show that IFN-γ signaling directly inhibits neutrophil accumulation in the lung. We envision that if T cell immunity fails for any reason, or IFN-γ responsiveness is reduced, increased neutrophil recruitment to the lung and causes immunopathology, compounding the loss of bacterial control and worsening the outcome of infection. In the absence of IFN-γ, the morbidity of the infected host may be as much a result of the development of detrimental inflammation as the loss of antimicrobial activity. This antiinflammatory action of IFN-γ is likely to be relevant to other chronic infection such as leishmaniasis and leprosy, which are also associated with continuous IFN-γ production and inflammation.
MATERIALS AND METHODS
Mice.
Age-matched female mice of the following strains were obtained from The Jackson Laboratory: B6 (C57BL6/J, stock #0664), congenic CD45.1 (B6.SJL-Ptprca Pepcb/BoyJ, stock #2014), IFN-γR1−/− (B6.129S7-Ifngrtm1Agt/J, stock #3288) or IFN-γ−/− (B6.129S7-Ifngtm1Ts/J, stock #2287), and RAG2−/− (B6.129S7-Rag1tm1Mom/J, stock #2216). RAG2/IFN-γR1−/− mice were generated by crossing RAG2−/− and IFN-γR1−/− mice. After infection, mice were housed in an Animal Biosafety Level 3 laboratory under specific pathogen-free condition and were used in a protocol approved by the institution (Dana-Farber Cancer Institute, Boston, MA).
Mixed BM chimeric mice.
Recipient WT B6 mice (CD45.2+) or RAG2−/− (CD45.2+) mice were irradiated with doses of 600 rad and 400 rad at 2-h intervals. Within 6 h of the last dose, the recipient mice were reconstituted by IV injection with 2 × 105 total BM cells consisting of a 1:1 mixture of BM obtained from WT (CD45.1+) and IFN-γR1−/− (CD45.2+) or WT (CD45.1+) and IFN-γ−/− (CD45.2+) mice. Mice were given Baytryl (150 mg/ml sulfamethoxazole and 30 mg/ml trimethoprim) in drinking water for the first 4 wk of reconstitution. Chimeras were used 8 wk after BM transplantation. Before infection, reconstitution by donor-derived leukocytes in the lung, spleen, and blood was confirmed in representative mice.
Bacteria and in vivo infections.
Mice were infected with Mtb (Erdman strain) by aerosol exposure using a nose-only exposure system as described previously (Jayaraman et al., 2010).
Generation of memory-immune mice.
2 wk after M. tuberculosis infection, mice were treated with isoniazid and rifabutin (each at 100 mg/liter) for at least 6 wk as previously described (Kamath et al., 2006). Lungs from representative mice were used to confirm clearance of bacteria.
Tissue histopathology.
Lung tissues were fixed in 10% zinc formalin and embedded in paraffin. Sections 5 µm thick were stained with hematoxylin and eosin, Mason’s trichrome, or stained for acid-fast bacilli. Images were obtained using a microscope (DMLB; Leica) and a digital camera (DFC420; Leica). The images were adjusted and assembled in Photoshop (Adobe).
Isolation of cells.
Single-cell suspensions were prepared from spleens and lungs of infected mice as previously described (Woodworth et al., 2008b).
Detection of cytokines in lung homogenates.
Infected or naive lungs were perfused with 10 ml of sterile PBS followed by digestion with collagenase as mentioned in the previous section. Collagenase-treated cells were passed through a 70-µm cell strainer in a total volume of 4 ml PBS 1% FCS according to the protocol of Mayer-Barber et al. (2010). After centrifugation, 100 µl of the cell-free lung suspensions was analyzed by ELISA for KC, MIP-2, and G-CSF (all from PeproTech). Absorbance was recorded at 450 nm using an ELISA microplate reader (VersaMac; Molecular Devices)
Flow cytometry.
Single cell suspensions from spleen and lung, or macrophages, were stained using mAbs specific for mouse cell surface markers conjugated to FITC, PE, PerCP, allophycocyanin, PE-Cy7, or allophycocyanin-Cy7 (eBioscience and BioLegend) at a concentration of 5 µg/ml in 2% FBS-PBS for 20 min at 4°C. Before staining with specific mAb, the Fc receptors were blocked using anti-FcR (BioLegend) at 5 µg/ml for 10 min at 4°C. The following mAbs were used: CD3 (145.2C11), CD4 (GK1.5), CD8 (53–6.7), CD11b (M1/70), CD11c (HL3), Gr-1 (RB6-8C5), CD19 (MB19-1), CD45.1 (A20), CD45.2 (104), CD25 (PC61.5), and FoxP3 (FJK-16s). Staining for T reg cells was performed using the mouse regulatory T cell staining kit #2 (eBioscience). Single-cell events were gated by forward scatter area versus height and side scatter for size and granularity. Cells were analyzed using a FACSCanto (BD) and FlowJo analysis software (Tree Star).
Intracellular cytokine staining).
Total lung or spleen cells from infected mice were cultured at 106 cells/150 µl/well in complete media at 37°C with or without 10 µM peptide or 1 µg/ml anti-CD3/CD28. Peptides used in this study were Ag85A(241–256) (QDAYNAAGGHNAVFNF) and ESAT-6(1–15) (EQQWNFAGIEAAASA; Woodworth et al., 2008a). After 1 h, 50 µl brefeldin A (25 µg/ml; Sigma-Aldrich) was added and cells were cultured for an additional 4 h. After activation, the cells were washed and incubated with Fc block solution (BioLegend) for 10 min, followed by staining with mAbs directed against CD45.1, CD45.2, CD4, and CD8. Cells were then washed and fixed with 1% paraformaldehyde for 1 h. Fixed cells were permeabilized for 30 min at room temperature in 0.5% saponin and followed by staining with mAbs directed against IFN-γ (XMG1.2), TNF (MP6-XT22), IL-17A (TC11-18H10.1), IL-4(11B11), or an appropriate isotype control for 30 min at 4°C in the same permeabilization buffer. The cells were then analyzed as described in the previous sections.
T cell adoptive transfer.
Splenic memory CD4+ T cells were from mice infected for a total of 8–9 wk that had been treated with antibiotics during the last 6–7 wk. CD4+ T cells were purified using negative selection followed by CD4 positive selection using a MACS Pro Separator (Miltenyi Biotech). Cell purity was consistently ≥95% CD4+, with <0.05% CD8+ cells. 5 × 106 cells were injected IV into recipient mice. Within 24 h of transfer, recipient mice were infected with low dose aerosolized Mtb.
1A8-mediated neutrophil depletion.
In vivo depletion of neutrophils from infected IFN-γR1−/− mice was achieved by administering 0.2 mg anti-Ly6G mAb (clone 1A8; BioXcell) or an isotype control (clone 2A3; BioXcell) starting 10 d after infection. Mice were dosed every other day for 11 d.
In vivo neutrophil death assay.
Peritoneal exudate cells (PEC)s were obtained from WT and IFN-γR1−/− mice 24 h after IP injection of 3% thioglycollate. WT and IFN-γR1−/− PEC were labeled with 5 and 1.5 µM CFSE, respectively. The labeled cells were mixed at a ratio of 1:1 and a total of 10 × 106 cells were injected IV into uninfected or Mtb-infected WT mice. Lung cells were stained with antibodies to CD11b and Gr1 and analyzed by flow cytometry 4 and 20 h after transfer. The following ratio of ratios was calculated at two time points: CD11b+Gr-1HiCFSElo[IFN-γR−/−]/CD11b+Gr-1HCFSEhi[WT] versus CD11b+Gr-1loCFSElo[IFN-γR−/−]/CD11b+Gr-1HiCFSEhi[WT].
In vitro neutrophil death assay.
PECs were obtained from WT mice 4 h after IP injection of 3% thioglycollate. Cells were cultured in a 96-well plate at a concentration of 106 cells/well in a volume of 200 µl for 4 or 20 h with or without varying IFN-γ. At each time point, cells were harvested and stained with antibodies to CD11b, Gr1, and a live/dead stain (Invitrogen).
Statistics.
CFU data were log10 transformed before analysis. The Prism software program (GraphPad Software) was used to perform Student’s t tests and one-way ANOVA and Bonferroni’s multiple comparison post-test. A p-value of <0.05 was considered significant. The log-rank (Mantel-Cox) test was used for statistical analysis for survival experiments.
Online supplemental material.
Fig. S1 shows examples of advance lesions in from the lungs of IFN-γR−/− and WT mice after infection with Mtb. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20110919/DC1.
Acknowledgments
We’d like to thank Connie Martin, Claudio Alves Nunes, Alissa Rothchild, and Lester Kobzik for their advice, and Danielle Desjardins and Sarah Beladi for their expert technical assistance.
This work was supported by the National Institutes of Health grant R01 AI067731 to S.M. Behar.
The authors have no conflicting financial interests.
B. Nandi and S.M. Behar conceived of and designed the experiments, analyzed the data, and wrote the paper. B. Nandi did the experiments.
Footnotes
Abbreviations used:
- BCG
- Bacillus Calmette-Guerin
- Mtb
- Mycobacterium tuberculosis
- PEC
- peritoneal exudate cell
- PMN
- polymorphonuclear neutrophil
References
- Aly S., Wagner K., Keller C., Malm S., Malzan A., Brandau S., Bange F.-C., Ehlers S. 2006. Oxygen status of lung granulomas in Mycobacterium tuberculosis-infected mice. J. Pathol. 210:298–305 10.1002/path.2055 [DOI] [PubMed] [Google Scholar]
- Berry M.P.R., Graham C.M., McNab F.W., Xu Z., Bloch S.A.A., Oni T., Wilkinson K.A., Banchereau R., Skinner J., Wilkinson R.J., et al. 2010. An interferon-inducible neutrophil-driven blood transcriptional signature in human tuberculosis. Nature. 466:973–977 10.1038/nature09247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanton R.E., Salam E.A., Ehsan A., King C.H., Goddard K.A. 2005. Schistosomal hepatic fibrosis and the interferon gamma receptor: a linkage analysis using single-nucleotide polymorphic markers. Eur. J. Hum. Genet. 13:660–668 10.1038/sj.ejhg.5201388 [DOI] [PubMed] [Google Scholar]
- Cooper A.M., Dalton D.K., Stewart T.A., Griffin J.P., Russell D.G., Orme I.M. 1993. Disseminated tuberculosis in interferon γ gene-disrupted mice. J. Exp. Med. 178:2243–2247 10.1084/jem.178.6.2243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper A.M., Adams L.B., Dalton D.K., Appelberg R., Ehlers S. 2002. IFN-gamma and NO in mycobacterial disease: new jobs for old hands. Trends Microbiol. 10:221–226 10.1016/S0966-842X(02)02344-2 [DOI] [PubMed] [Google Scholar]
- Cowley S.C., Elkins K.L. 2003. CD4+ T cells mediate IFN-gamma-independent control of Mycobacterium tuberculosis infection both in vitro and in vivo. J. Immunol. 171:4689–4699 [DOI] [PubMed] [Google Scholar]
- Cruz A., Khader S.A., Torrado E., Fraga A., Pearl J.E., Pedrosa J., Cooper A.M., Castro A.G. 2006. Cutting edge: IFN-gamma regulates the induction and expansion of IL-17-producing CD4 T cells during mycobacterial infection. J. Immunol. 177:1416–1420 [DOI] [PubMed] [Google Scholar]
- Cruz A., Fraga A.G., Fountain J.J., Rangel-Moreno J., Torrado E., Saraiva M., Pereira D.R., Randall T.D., Pedrosa J., Cooper A.M., Castro A.G. 2010. Pathological role of interleukin 17 in mice subjected to repeated BCG vaccination after infection with Mycobacterium tuberculosis. J. Exp. Med. 207:1609–1616 10.1084/jem.20100265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton D.K., Haynes L., Chu C.Q., Swain S.L., Wittmer S. 2000. Interferon γ eliminates responding CD4 T cells during mycobacterial infection by inducing apoptosis of activated CD4 T cells. J. Exp. Med. 192:117–122 10.1084/jem.192.1.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desvignes L., Ernst J.D. 2009. Interferon-gamma-responsive nonhematopoietic cells regulate the immune response to Mycobacterium tuberculosis. Immunity. 31:974–985 10.1016/j.immuni.2009.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorhoi A., Desel C., Yeremeev V., Pradl L., Brinkmann V., Mollenkopf H.-J., Hanke K., Gross O., Ruland J., Kaufmann S.H. 2010. The adaptor molecule CARD9 is essential for tuberculosis control. J. Exp. Med. 207:777–792 10.1084/jem.20090067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrt S., Schnappinger D., Bekiranov S., Drenkow J., Shi S., Gingeras T.R., Gaasterland T., Schoolnik G., Nathan C. 2001. Reprogramming of the macrophage transcriptome in response to interferon-γ and Mycobacterium tuberculosis: signaling roles of nitric oxide synthase-2 and phagocyte oxidase. J. Exp. Med. 194:1123–1140 10.1084/jem.194.8.1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eruslanov E.B., Lyadova I.V., Kondratieva T.K., Majorov K.B., Scheglov I.V., Orlova M.O., Apt A.S. 2005. Neutrophil responses to Mycobacterium tuberculosis infection in genetically susceptible and resistant mice. Infect. Immun. 73:1744–1753 10.1128/IAI.73.3.1744-1753.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eum S.-Y., Kong J.-H., Hong M.-S., Lee Y.-J., Kim J.-H., Hwang S.-H., Cho S.-N., Via L.E., Barry C.E., III 2010. Neutrophils are the predominant infected phagocytic cells in the airways of patients with active pulmonary TB. Chest. 137:122–128 10.1378/chest.09-0903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng C.G., Kaviratne M., Rothfuchs A.G., Cheever A., Hieny S., Young H.A., Wynn T.A., Sher A. 2006. NK cell-derived IFN-gamma differentially regulates innate resistance and neutrophil response in T cell-deficient hosts infected with Mycobacterium tuberculosis. J. Immunol. 177:7086–7093 [DOI] [PubMed] [Google Scholar]
- Fernandez-Boyanapalli R., McPhillips K.A., Frasch S.C., Janssen W.J., Dinauer M.C., Riches D.W., Henson P.M., Byrne A., Bratton D.L. 2010. Impaired phagocytosis of apoptotic cells by macrophages in chronic granulomatous disease is reversed by IFN-γ in a nitric oxide-dependent manner. J. Immunol. 185:4030–4041 10.4049/jimmunol.1001778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn J.L., Chan J., Triebold K.J., Dalton D.K., Stewart T.A., Bloom B.R. 1993. An essential role for interferon γ in resistance to Mycobacterium tuberculosis infection. J. Exp. Med. 178:2249–2254 10.1084/jem.178.6.2249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn J.L., Goldstein M.M., Chan J., Triebold K.J., Pfeffer K., Lowenstein C.J., Schreiber R., Mak T.W., Bloom B.R. 1995. Tumor necrosis factor-alpha is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity. 2:561–572 10.1016/1074-7613(95)90001-2 [DOI] [PubMed] [Google Scholar]
- Gallegos A.M., van Heijst J.W., Samstein M., Su X., Pamer E.G., Glickman M.S. 2011. A gamma interferon independent mechanism of CD4 T cell mediated control of M. tuberculosis infection in vivo. PLoS Pathog. 7:e1002052 10.1371/journal.ppat.1002052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger R., Duhen T., Lanzavecchia A., Sallusto F. 2009. Human naive and memory CD4+ T cell repertoires specific for naturally processed antigens analyzed using libraries of amplified T cells. J. Exp. Med. 206:1525–1534 10.1084/jem.20090504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gondek D.C., Roan N.R., Starnbach M.N. 2009. T cell responses in the absence of IFN-gamma exacerbate uterine infection with Chlamydia trachomatis. J. Immunol. 183:1313–1319 10.4049/jimmunol.0900295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haring J.S., Harty J.T. 2006. Aberrant contraction of antigen-specific CD4 T cells after infection in the absence of gamma interferon or its receptor. Infect. Immun. 74:6252–6263 10.1128/IAI.00847-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X., Ivashkiv L.B. 2009. Cross-regulation of signaling pathways by interferon-gamma: implications for immune responses and autoimmune diseases. Immunity. 31:539–550 10.1016/j.immuni.2009.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaraman P., Sada-Ovalle I., Beladi S., Anderson A.C., Dardalhon V., Hotta C., Kuchroo V.K., Behar S.M. 2010. Tim3 binding to galectin-9 stimulates antimicrobial immunity. J. Exp. Med. 207:2343–2354 10.1084/jem.20100687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouanguy E., Altare F., Lamhamedi S., Revy P., Emile J.F., Newport M., Levin M., Blanche S., Seboun E., Fischer A., Casanova J.L. 1996. Interferon-gamma-receptor deficiency in an infant with fatal bacille Calmette-Guérin infection. N. Engl. J. Med. 335:1956–1961 10.1056/NEJM199612263352604 [DOI] [PubMed] [Google Scholar]
- Jouanguy E., Lamhamedi-Cherradi S., Altare F., Fondanèche M.C., Tuerlinckx D., Blanche S., Emile J.F., Gaillard J.L., Schreiber R., Levin M., et al. 1997. Partial interferon-gamma receptor 1 deficiency in a child with tuberculoid bacillus Calmette-Guérin infection and a sibling with clinical tuberculosis. J. Clin. Invest. 100:2658–2664 10.1172/JCI119810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouanguy E., Döffinger R., Dupuis S., Pallier A., Altare F., Casanova J.L. 1999. IL-12 and IFN-gamma in host defense against mycobacteria and salmonella in mice and men. Curr. Opin. Immunol. 11:346–351 10.1016/S0952-7915(99)80055-7 [DOI] [PubMed] [Google Scholar]
- Kamath A., Woodworth J.S.M., Behar S.M. 2006. Antigen-specific CD8+ T cells and the development of central memory during Mycobacterium tuberculosis infection. J. Immunol. 177:6361–6369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller C., Hoffmann R., Lang R., Brandau S., Hermann C., Ehlers S. 2006. Genetically determined susceptibility to tuberculosis in mice causally involves accelerated and enhanced recruitment of granulocytes. Infect. Immun. 74:4295–4309 10.1128/IAI.00057-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy A.D., DeLeo F.R. 2009. Neutrophil apoptosis and the resolution of infection. Immunol. Res. 43:25–61 10.1007/s12026-008-8049-6 [DOI] [PubMed] [Google Scholar]
- Khader S.A., Bell G.K., Pearl J.E., Fountain J.J., Rangel-Moreno J., Cilley G.E., Shen F., Eaton S.M., Gaffen S.L., Swain S.L., et al. 2007. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat. Immunol. 8:369–377 10.1038/ni1449 [DOI] [PubMed] [Google Scholar]
- Kondratieva E., Logunova N., Majorov K., Averbakh M., Apt A. 2010. Host genetics in granuloma formation: human-like lung pathology in mice with reciprocal genetic susceptibility to M. tuberculosis and M. avium. PLoS ONE. 5:e10515 10.1371/journal.pone.0010515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lázár-Molnár E., Chen B., Sweeney K.A., Wang E.J., Liu W., Lin J., Porcelli S.A., Almo S.C., Nathenson S.G., Jacobs W.R., Jr 2010. Programmed death-1 (PD-1)-deficient mice are extraordinarily sensitive to tuberculosis. Proc. Natl. Acad. Sci. USA. 107:13402–13407 10.1073/pnas.1007394107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindén A., Laan M., Anderson G.P. 2005. Neutrophils, interleukin-17A and lung disease. Eur. Respir. J. 25:159–172 10.1183/09031936.04.00032904 [DOI] [PubMed] [Google Scholar]
- MacMicking J.D., North R.J., LaCourse R., Mudgett J.S., Shah S.K., Nathan C.F. 1997. Identification of nitric oxide synthase as a protective locus against tuberculosis. Proc. Natl. Acad. Sci. USA. 94:5243–5248 10.1073/pnas.94.10.5243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacMicking J.D., Taylor G.A., McKinney J.D. 2003. Immune control of tuberculosis by IFN-gamma-inducible LRG-47. Science. 302:654–659 10.1126/science.1088063 [DOI] [PubMed] [Google Scholar]
- Madariaga L., Amurrio C., Martín G., García-Cebrian F., Bicandi J., Lardelli P., Suarez M.D., Cisterna R. 1998. Detection of anti-interferon-gamma autoantibodies in subjects infected by Mycobacterium tuberculosis. Int. J. Tuberc. Lung Dis. 2:62–68 [PubMed] [Google Scholar]
- Masters S.L., Mielke L.A., Cornish A.L., Sutton C.E., O’Donnell J., Cengia L.H., Roberts A.W., Wicks I.P., Mills K.H., Croker B.A. 2010. Regulation of interleukin-1beta by interferon-gamma is species specific, limited by suppressor of cytokine signalling 1 and influences interleukin-17 production. EMBO Rep. 11:640–646 10.1038/embor.2010.93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer-Barber K.D., Barber D.L., Shenderov K., White S.D., Wilson M.S., Cheever A., Kugler D., Hieny S., Caspar P., Núñez G., et al. 2010. Caspase-1 independent IL-1beta production is critical for host resistance to mycobacterium tuberculosis and does not require TLR signaling in vivo. J. Immunol. 184:3326–3330 10.4049/jimmunol.0904189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLoughlin R.M., Witowski J., Robson R.L., Wilkinson T.S., Hurst S.M., Williams A.S., Williams J.D., Rose-John S., Jones S.A., Topley N. 2003. Interplay between IFN-gamma and IL-6 signaling governs neutrophil trafficking and apoptosis during acute inflammation. J. Clin. Invest. 112:598–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melrose J., Tsurushita N., Liu G., Berg E.L. 1998. IFN-gamma inhibits activation-induced expression of E- and P-selectin on endothelial cells. J. Immunol. 161:2457–2464 [PubMed] [Google Scholar]
- Minguela A., Pastor S., Mi W., Richardson J.A., Ward E.S. 2007. Feedback regulation of murine autoimmunity via dominant anti-inflammatory effects of interferon gamma. J. Immunol. 178:134–144 [DOI] [PubMed] [Google Scholar]
- Mogues T., Goodrich M.E., Ryan L., LaCourse R., North R.J. 2001. The relative importance of T cell subsets in immunity and immunopathology of airborne Mycobacterium tuberculosis infection in mice. J. Exp. Med. 193:271–280 10.1084/jem.193.3.271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napolitani G., Acosta-Rodriguez E.V., Lanzavecchia A., Sallusto F. 2009. Prostaglandin E2 enhances Th17 responses via modulation of IL-17 and IFN-gamma production by memory CD4+ T cells. Eur. J. Immunol. 39:1301–1312 10.1002/eji.200838969 [DOI] [PubMed] [Google Scholar]
- Ohmori Y., Hamilton T.A. 1994. IFN-gamma selectively inhibits lipopolysaccharide-inducible JE/monocyte chemoattractant protein-1 and KC/GRO/melanoma growth-stimulating activity gene expression in mouse peritoneal macrophages. J. Immunol. 153:2204–2212 [PubMed] [Google Scholar]
- Pan H., Yan B.-S., Rojas M., Shebzukhov Y.V., Zhou H., Kobzik L., Higgins D.E., Daly M.J., Bloom B.R., Kramnik I. 2005. Ipr1 gene mediates innate immunity to tuberculosis. Nature. 434:767–772 10.1038/nature03419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastor S., Minguela A., Mi W., Ward E.S. 2009. Autoantigen immunization at different sites reveals a role for anti-inflammatory effects of IFN-gamma in regulating susceptibility to experimental autoimmune encephalomyelitis. J. Immunol. 182:5268–5275 10.4049/jimmunol.0800681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearl J.E., Saunders B., Ehlers S., Orme I.M., Cooper A.M. 2001. Inflammation and lymphocyte activation during mycobacterial infection in the interferon-gamma-deficient mouse. Cell. Immunol. 211:43–50 10.1006/cimm.2001.1819 [DOI] [PubMed] [Google Scholar]
- Pichugin A.V., Yan B.-S., Sloutsky A., Kobzik L., Kramnik I. 2009. Dominant role of the sst1 locus in pathogenesis of necrotizing lung granulomas during chronic tuberculosis infection and reactivation in genetically resistant hosts. Am. J. Pathol. 174:2190–2201 10.2353/ajpath.2009.081075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatino J.J., Jr, Shires J., Altman J.D., Ford M.L., Evavold B.D. 2008. Loss of IFN-gamma enables the expansion of autoreactive CD4+ T cells to induce experimental autoimmune encephalomyelitis by a nonencephalitogenic myelin variant antigen. J. Immunol. 180:4451–4457 [DOI] [PubMed] [Google Scholar]
- Sakamoto E., Hato F., Kato T., Sakamoto C., Akahori M., Hino M., Kitagawa S. 2005. Type I and type II interferons delay human neutrophil apoptosis via activation of STAT3 and up-regulation of cellular inhibitor of apoptosis 2. J. Leukoc. Biol. 78:301–309 10.1189/jlb.1104690 [DOI] [PubMed] [Google Scholar]
- Sarraj B., Ludányi K., Glant T.T., Finnegan A., Mikecz K. 2006. Expression of CD44 and L-selectin in the innate immune system is required for severe joint inflammation in the proteoglycan-induced murine model of rheumatoid arthritis. J. Immunol. 177:1932–1940 [DOI] [PubMed] [Google Scholar]
- Silva M.T., do Vale A., dos Santos N.M. 2008. Secondary necrosis in multicellular animals: an outcome of apoptosis with pathogenic implications. Apoptosis. 13:463–482 10.1007/s10495-008-0187-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland J.S., Jeffries D.J., Donkor S., Walther B., Hill P.C., Adetifa I.M.O., Adegbola R.A., Ota M.O.C. 2009. High granulocyte/lymphocyte ratio and paucity of NKT cells defines TB disease in a TB-endemic setting. Tuberculosis (Edinb.). 89:398–404 10.1016/j.tube.2009.07.004 [DOI] [PubMed] [Google Scholar]
- Swihart K., Fruth U., Messmer N., Hug K., Behin R., Huang S., Del Giudice G., Aguet M., Louis J.A. 1995. Mice from a genetically resistant background lacking the interferon gamma receptor are susceptible to infection with Leishmania major but mount a polarized T helper cell 1-type CD4+ T cell response. J. Exp. Med. 181:961–971 10.1084/jem.181.3.961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo S.J., Sullivan B.M., Peng S.L., Glimcher L.H. 2003. Molecular mechanisms regulating Th1 immune responses. Annu. Rev. Immunol. 21:713–758 10.1146/annurev.immunol.21.120601.140942 [DOI] [PubMed] [Google Scholar]
- Ulrichs T., Kaufmann S.H. 2006. New insights into the function of granulomas in human tuberculosis. J. Pathol. 208:261–269 10.1002/path.1906 [DOI] [PubMed] [Google Scholar]
- Wang L., Chen S., Xu K. 2011. IL-17 expression is correlated with hepatitis B‑related liver diseases and fibrosis. Int. J. Mol. Med. 27:385–392 [DOI] [PubMed] [Google Scholar]
- Wilson M.S., Madala S.K., Ramalingam T.R., Gochuico B.R., Rosas I.O., Cheever A.W., Wynn T.A. 2010. Bleomycin and IL-1β-mediated pulmonary fibrosis is IL-17A dependent. J. Exp. Med. 207:535–552 10.1084/jem.20092121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodworth J.S., Fortune S.M., Behar S.M. 2008a. Bacterial protein secretion is required for priming of CD8+ T cells specific for the Mycobacterium tuberculosis antigen CFP10. Infect. Immun. 76:4199–4205 10.1128/IAI.00307-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodworth J.S., Wu Y., Behar S.M. 2008b. Mycobacterium tuberculosis-specific CD8+ T cells require perforin to kill target cells and provide protection in vivo. J. Immunol. 181:8595–8603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak T.M., Saunders B.M., Ryan A.A., Britton W.J. 2010. Mycobacterium bovis BCG-specific Th17 cells confer partial protection against Mycobacterium tuberculosis infection in the absence of gamma interferon. Infect. Immun. 78:4187–4194 10.1128/IAI.01392-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye P., Rodriguez F.H., Kanaly S., Stocking K.L., Schurr J., Schwarzenberger P., Oliver P., Huang W., Zhang P., Zhang J., et al. 2001. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J. Exp. Med. 194:519–527 10.1084/jem.194.4.519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielinski C.E., Corti D., Mele F., Pinto D., Lanzavecchia A., Sallusto F. 2011. Dissecting the human immunologic memory for pathogens. Immunol. Rev. 240:40–51 10.1111/j.1600-065X.2010.01000.x [DOI] [PubMed] [Google Scholar]