Abstract
In cucumber (Cucumis sativus), high lipoxygenase-1 (LOX-1) activity has been detected in the soluble fraction prepared from cotyledons of germinating seeds, and the involvement of this enzyme in lipid turnover has been suggested (K. Matsui, M. Irie, T. Kajiwara, A. Hatanaka [1992] Plant Sci 85: 23–32; I. Fuessner, C. Wasternack, H. Kindl, H. Kühn [1995] Proc Natl Acad Sci USA 92: 11849–11853). In this study we have investigated the expression of the gene lox-1, corresponding to the LOX-1 enzyme. LOX-1 expression is highly coordinated with that of a typical glyoxysomal enzyme, isocitrate lyase, during the postgerminative stage of cotyledon development. In contrast, although icl transcripts accumulated in tissue during in vitro senescence, no accumulation of lox-1 mRNA could be observed, suggesting that lox-1 plays a specialized role in fat mobilization. LOX-1 is also known to be a major lipid body protein. The partial peptide sequences of purified LOX-1 and lipid body LOX-1 entirely coincided with that deduced from the lox-1 cDNA sequence. The data strongly suggest that LOX-1 and lipid body LOX-1 are derived from a single gene and that LOX-1 can exist both in the cytosol and on the lipid bodies. We constructed an in vitro oxygenation system to address the mechanism of this dual localization and to investigate the action of LOX-1 on lipids in the lipid bodies. LOX-1 cannot act on the lipids in intact lipid bodies, although degradation of lipid body proteins, either during seedling growth or by treatment with trypsin, allows lipid bodies to become susceptible to LOX-1. We discuss the role of LOX-1 in fat mobilization and its mechanism of action.
LOXs (linoleate: oxygen oxidoreductase, EC 1.13.11.12) are widely distributed in plants and animals. They catalyze the addition of molecular oxygen to fatty acids containing at least one (Z),(Z)-1,4-pentadiene motif to give the corresponding hydroperoxides. In animals LOXs use arachidonic acid as a substrate and are involved in the formation of various regulatory compounds such as leukotrienes and lipoxines (Samuelsson et al., 1987). In plants LOXs oxygenate linoleic and linolenic acids at their 9 and 13 positions to give 9- and 13- hydroperoxy fatty acids, respectively. It is known that many isozymes of LOX occur in various parts of plants, and each is thought to play a different role in such processes as development and response to wounding. It has been widely accepted that plant LOXs have various physiological roles that are indispensable for plant life (Siedow, 1991).
In oilseed plants such as cucumber (Cucumis sativus) and sunflower, the storage lipids, which exist mostly as triacylglycerols, are tightly packed in subcellular organelles called lipid bodies (Huang, 1992). These spherical organelles are covered with a phospholipid monolayer and extrinsic proteins. Lipid bodies are formed when triacylglycerols are synthesized on the rough ER during seed development. Oleosin, a “coat” protein surrounding the triacylglycerol droplets, contributes to the stabilization of lipid bodies during dehydration and seed dormancy (Napier et al., 1996). However, once imbibition occurs and germination is triggered, triacylglycerols are degraded to act as a carbon and energy source for the developing seedling (Beevers, 1979). Acyl moieties of the stored lipids are transported to glyoxysomes, where they are degraded by the β-oxidation system to form acetyl-CoA. Resulting products are eventually converted to oxaloacetate through isocitrate and malate intermediates. This process operates via the glyoxylate cycle, a route involving enzymes of the glyoxysomes.
The glyoxysomal enzymes ICL and MS have been well characterized (Trelease et al., 1971; Weir et al., 1980). It is known that the genes encoding these enzymes are highly expressed within several days after germination in cucumber seedlings (Reynolds and Smith, 1995). It has also been reported that LOX activity increases during the early stages of seed germination in various plants (for review, see Roshal, 1996). Previously, we had found that LOX activity increased in the soluble fraction prepared from cucumber cotyledons after germination (Matsui et al., 1992). The developmental changes in LOX activity during postgerminative growth of the cotyledons resemble changes in the level of glyoxysomal enzymes. Accumulation of the enzyme in cucumber cotyledons reaches levels as high as 2% to 5% of total protein. LOX can oxygenate fatty acid moieties esterified in neutral lipids, as well as free fatty acids (Matsui and Kajiwara, 1995). These observations suggest that the enzyme is involved in lipid mobilization; if this is the case, expression of the gene corresponding to LOX must be highly coordinated with that of the glyoxysomal genes. In the present study we investigated the expression of the lox-1 gene in cucumber during the postgerminative growth of the cotyledons.
Feussner and Kindl (1992) reported that during the early stages of seed germination LOX is the main lipid body protein in cucumber cotyledons. Furthermore, by elucidating the structures of oxygenated lipids in cucumber cotyledons, Feussner et al. (1997) proposed that the lipid body LOX is involved in lipid mobilization. Considering these separate data on LOXs found both in the soluble fraction and on lipid bodies, we postulate that two different isozymes of LOX-1 may have almost the same physiological role or that one isozyme may have distinct localizations. To address this question, we isolated both of the LOXs and compared their primary structures. We also constructed an in vitro oxidation system using purified LOX and lipid bodies isolated from cotyledons at various stages of growth. Our investigation revealed how LOX on lipid bodies acts on storage lipids.
MATERIALS AND METHODS
Plant Material
Cucumber (Cucumis sativus L. cv Suyo) seeds were soaked for 12 h in tap water and sown on moistened paper towels. The seeds were grown at 25°C in the dark or under a 16-h light/8-h dark photoperiod. For in vitro senescence, the cotyledons of 10-d-old seedlings were removed from their hypocotyls, placed abaxial side up on a stack of moistened paper towels, and incubated in the dark at 25°C.
Fatty Acid Composition
We extracted total lipids from cucumber cotyledons using the method of Bligh and Dyer (1959). The crude lipids were separated by TLC (Kieselgel 60, Merck, Darmstadt, Germany) using diisobutylketone:acetic acid:water (40:25:4, v/v) as the developing solvent. Regions corresponding to neutral lipids were scraped off, extracted with ether, mixed with heptadecanoic acid as an internal standard, and then trans-esterified with 5% HCl in methanol by heating at 100°C for 3 h. We used a gas chromatograph (model GC-6A, Shimadzu, Columbia, MD) equipped with a 2% Silar 5CP column (3 mm i.d. × 3 m height; Restek, Bellefonte, PA) to quantitatively analyze the fatty acid methyl esters thus obtained. The column temperature was 180°C to 210°C (2°C/min). The respective ester was detected with a flame-ionization detector.
Purification of LOX
LOX was purified from cucumber cotyledons 5 DAG essentially as described previously (Matsui et al., 1993) but with minor modifications. We homogenized the cucumber cotyledons with cold acetone and extracted LOX from the acetone powder and fractionated it with ammonium sulfate. After dialysis, the enzyme solution was fractionated with a DEAE column (2.2 × 20.0 cm; Cellulofine A-500, Seikagaku, Tokyo) and then with a Butyl-Toyopearl 650M column (1.75 × 15.5 cm; Toyo Corp., Tokyo). The purified enzyme was stored at −20°C until use. In some experiments, we used LOX expressed in Escherichia coli cells. E. coli Y1090 (ΔpMc9) cells transformed with pTrc99A (Pharmacia) and containing a cucumber cotyledon LOX cDNA (lox-1) were grown at 30°C for 4 h in 2 L of Luria-Bertani broth, supplemented with 50 μg mL−1 ampicillin. Isopropyl-1-thio-β-d-galactoside was then added at a final concentration of 0.5 mm, and the culture was incubated at 30°C for an additional 6 h. The cells were harvested by centrifugation at 6,000 rpm for 10 min, resuspended in 80 mL of lysis buffer (50 mm Tris-Cl, pH 7.5, 1 mm EDTA, and 0.1 mm NaCl), and repelleted by centrifugation at 6,000 rpm. The washed cells were resuspended in 120 mL of lysis buffer containing 0.13 mm PMSF. Lysozyme was added at a concentration of 0.1 mg mL−1, and the solution was incubated on ice for 20 min. Finally, the cells were disrupted by a French press (AMINCO, Spectronic Instruments, Rochester, NY). The cell homogenate was centrifuged at 10,000 rpm for 10 min to give a crude extract. The extract was fractionated with ammonium sulfate, dialyzed, and column purified with a DEAE column.
We determined LOX activity polarographically with a Clark-type oxygen electrode (Yellow Springs Instruments, Yellow Springs, OH) at 25°C (Matsui and Kajiwara, 1995). With free fatty acid as the substrate, 0.1 m sodium phosphate, pH 6.3, was the buffer; whereas with triglycerides or a lipid body suspension as the substrate, 0.1 m Tris-Cl, pH 8.5, was the buffer. The activity (1 kat) is defined as the quantity of the enzyme catalyzing the consumption of 1 mol O2 s−1 at 25°C.
In Situ Digestion of LOX
We separated the purified cucumber LOX with SDS-PAGE and then transferred it electrophoretically to a PVDF membrane (Millipore). The membrane was briefly stained using Ponceau S, and the portion containing LOX was cut out. Fifty milliliters of 0.5% PVP 40 (Sigma) in 0.1 m acetic acid was added to the membrane strip and shaken at 37°C for 30 min. After the membrane was washed, it was equilibrated with 50 mL of 0.1 m Tris-Cl, pH 9.0. The protein on the membrane was digested with lysil endopeptidase (Wako Pure Chemicals, Tokyo) and then separated with reversed-phase HPLC. We separated the peptide fragments with an HPLC system equipped with a C18 column (4.6 mm i.d. × 250 mm; CosmoSIL, Nakalai Tesque, Kyoto, Japan). Peptide fragments were eluted with a linear gradient from 10% acetonitrile and 0.1% trifluoroacetic acid in water to 50% acetonitrile and 0.1% trifluoroacetic acid in water for 70 min. The flow rate was 0.5 mL min−1. We used a UV detector (model L-4000, Hitachi, Tokyo) for detection at 215 nm. The separated peptides were dried under nitrogen at 60°C, dissolved in 10 μL of formic acid and 20 μL of distilled water, and then applied to a protein sequencer (model PSQ-1, Hitachi, Tokyo).
Preparation of Lipid Bodies
Cucumber cotyledons (100 pairs) excised from seedlings grown for 1 to 6 d were homogenized with 10 mL of homogenization buffer (0.15 m Tris-Cl, pH 7.5, 15% Suc, 10 mm KCl, 1.5 mm EDTA, 0.1 mm MgSO4, 5 mm dithioerythritol, and 10 mm sodium ascorbate) in a mortar with a pestle using sea sand. The homogenate was centrifuged at 5,000 rpm for 5 min at 4°C. The resulting supernatant was centrifuged at 40,000 rpm for 1 h at 4°C in a swinging bucket rotor. The lipid layer that formed on the upper surface was carefully collected with a spatula and resuspended in 2 mL of 0.1 m NaHCO3. This suspension was centrifuged again at 40,000 rpm for 1 h at 4°C, and the lipid layer was collected and resuspended in 2 mL of the homogenization buffer. The suspension of lipid bodies was stored at −20°C until further use.
The lipid body suspension was incubated for 2 h at 25°C in 60 mL of a 0.1 m Tris-Cl buffer, pH 8.0, containing various concentrations of trypsin (Sigma). We then added PMSF at a final concentration of 0.1 mm to inactivate the trypsin. Part of the reaction mixture was heated immediately at 100°C for 3 min in an SDS sample buffer for SDS-PAGE analysis. We used the remaining solution immediately as a substrate for LOX.
RESULTS
Developmental Changes in Cucumber Cotyledon Fats
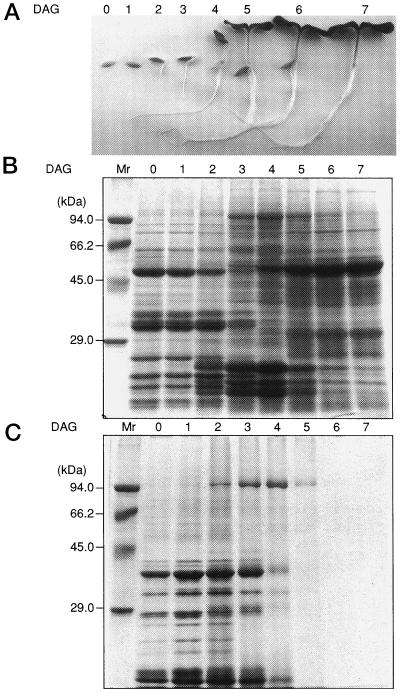
As shown in Table I, linoleic acids are the main fatty acid component in stored fats, making up as much as 65% of total fats in the cotyledons of dry cucumber seeds. The amount of total fats started to decrease from 2 DAG. The decrease was rapid, and by 6 DAG only 3% of the initial fats could be detected. This indicates that under these growth conditions turnover of the stored fats occurred rapidly from 3 to 6 DAG. The rapid degradation of the stored fats started from the time that radicles appeared and lasted until the cotyledons fully expanded (Fig. 1A). During this period, protein composition in the cotyledons also changed rapidly (Fig. 1B). Degradation of seed storage proteins preserved in dry seeds started to appear from 3 DAG (Yamaguchi et al., 1996).
Table I.
Fatty acid composition in the neutral lipid fraction of cucumber cotyledons
| DAG | Fatty Acid
Composition
|
||||||
|---|---|---|---|---|---|---|---|
| 14:0 | 16:0 | 18:0 | 18:1 | 18:2 | 18:3 | Total | |
| nmol/cotyledon pair | |||||||
| 0 | 303 (1.2)* | 3473 (13.4) | 1948 (7.5) | 3351 (12.9) | 16,760 (64.6) | 103 (0.4) | 25,938 |
| 1 | 277 (1.1) | 3346 (13.6) | 1849 (7.5) | 3294 (13.4) | 15,730 (64.0) | 87 (0.4) | 24,583 |
| 2 | 224 (0.9) | 3021 (12.8) | 1904 (8.1) | 3077 (13.1) | 14,960 (63.5) | 361 (1.5) | 23,547 |
| 3 | 120 (0.8) | 2182 (14.1) | 1372 (8.9) | 2139 (13.8) | 9,280 (63.5) | 396 (2.6) | 15,488 |
| 4 | 180 (2.1) | 1143 (13.2) | 42 (0.5) | 1229 (14.1) | 5,571 (59.9) | 524 (6.0) | 8,689 |
| 5 | 187 (7.8) | 282 (11.9) | 241 (10.1) | 284 (11.9) | 1,126 (64.1) | 261 (10.9) | 2,380 |
| 6 | 219 (27.3) | 120 (14.9) | 66 (8.2) | 52 (6.5) | 231 (47.3) | 116 (14.4) | 803 |
| 7 | 216 (44.7) | 48 (9.8) | 23 (4.7) | 20 (4.2) | 93 (19.3) | 83 (17.2) | 483 |
| 8 | 173 (45.5) | 52 (13.6) | 22 (5.7) | 10 (2.6) | 51 (13.6) | 72 (18.9) | 379 |
Values in parentheses are the percentages of total neutral lipids.
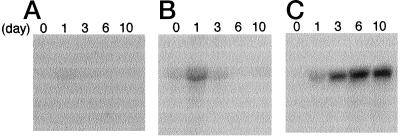
Figure 1.
Developmental changes in morphology (A), protein profiles of total soluble protein (B), and lipid body fraction (C), during early germinating stages of cucumber grown under a 14-h light/10-h dark photoperiod. For total soluble protein, proteins equivalent to 0.025 cotyledon pairs were loaded in each lane, and for lipid body fractions 0.25 cotyledon pairs were used.
When the seed storage proteins had almost disappeared, the accumulation of the large subunit of Rubisco became evident, clearly representing a transition of the metabolism of cucumber cotyledons from heterotrophic growth, which is dependent on energy sources preserved in dry seeds, to photoautotrophic growth, which is dependent on photosynthetic carbon assimilation. During this transition, a protein of about 96 kD appeared. Immunological staining of the protein indicated that it is LOX (Matsui et al., 1992). At d 4 LOX accounted for as much as 2% to 5% of total soluble proteins. Most LOX activity could be found in the soluble fraction of the cotyledon homogenate. This developmental time course of LOX accumulation in the soluble fraction is almost identical to that of the enzymatic activity (Matsui et al., 1992).
LOX activity can also be detected in the microsomal membrane fraction, as reported by Fuessner and Kindl (1994); however, this is equivalent to less than 1% of the activity in the soluble fraction. From the specific activity of the LOX purified from the soluble fraction of cucumber cotyledons at d 5 (Matsui et al., 1993), the amount of LOX in the cotyledons was calculated to be about 5% of the total protein. This is almost equivalent to the amount estimated by SDS-PAGE analysis. High expression of LOX in cucumber cotyledons was observed when the drastic metabolic change was occurring, i.e. when the stored fats rapidly degraded. Tight correlation between the developmental changes of LOX and the turnover of stored fats suggests that LOX takes part in fat degradation.
Detection of LOX Genes in Cucumber
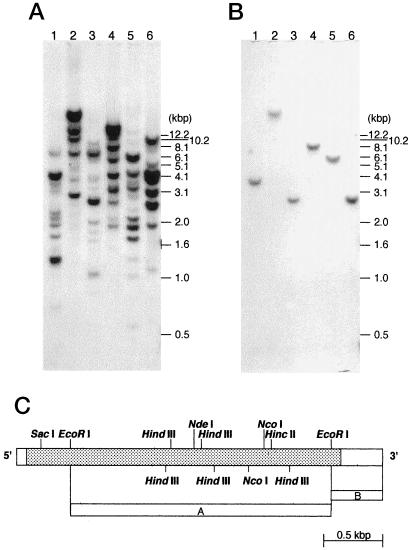
It is well known that during fat mobilization the activities of glyoxysomal enzymes such as ICL and MS are enhanced to drive the glyoxylate cycle (Beevers, 1979). If cucumber cotyledon LOXs are also involved in fat mobilization, then their expression should correlate with that of the glyoxysomal enzymes. Most plants have several isozymes of LOX (Siedow, 1991), and it is difficult to discriminate each isozyme using only an activity assay. To overcome this limitation, it was necessary to investigate LOX expression with a gene-specific detection system. Figure 2A shows the results of Southern analysis, in which a probe covering the coding region of cucumber cotyledon LOX cDNA, lox-1 (accession no. U25058, Matsui et al., 1995) produced a complicated picture of the LOX gene family. Matsui et al. (1998) reported the occurrence of a LOX gene other than lox-1, whose expression is specific to cucumber roots. However, the complicated Southern-blot pattern cannot be explained even if these two genes are taken into account. There must be more than two LOX genes in cucumber. In contrast, a lox-1 probe derived from the 3′-noncoding region detected only one band on the blot, which indicates that the probe detected specifically the lox-1 sequence (Fig. 2B).
Figure 2.
Genomic Southern analysis. About 10 μg of genomic DNA from cucumber was digested with HincII (lane 1), XbaI (lane 2), NdeI (lane 3), NcoI (lane 4), HindIII (lane 5), and EcoRI (lane 6) and then separated on a 0.6% agarose gel. As a probe, an EcoRI fragment of lox-1 (A) or an EcoRI 3′-end fragment (B) was used. A schematic diagram of lox-1 and templates used for the preparation of probes are shown in C.
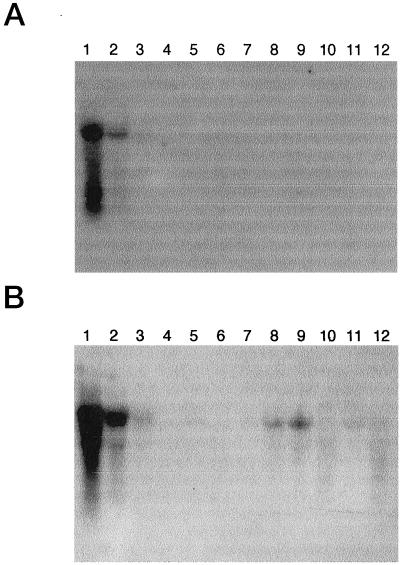
With the 3′ probe, northern analysis revealed that lox-1 is specifically expressed in cotyledons and hypocotyls in the seedlings (Fig. 3A). Matsui et al. (1988) reported that the properties of LOX activity in the hypocotyls are almost the same as those in the cotyledons. With the internal probe, expression of LOX in the roots and in reproductive organs such as buds, flowers, and immature fruits could be detected in addition to the cotyledons and hypocotyls (Fig. 3B). Expression of LOX genes in pea carpels (Rodriguez-Concepcion and Beltran, 1995) and in Arabidopsis inflorescences (Bell and Mullet, 1993) has been reported, and their involvement in a reproductive process has been proposed. Expression of LOX in fruits has been reported with various plants, including tomatoes (Ferrie et al., 1994) and bell peppers (Matsui et al., 1997). LOX activity has also been detected in cucumber fruits, and it appeared to be involved in the formation of volatile compounds such as (2E)-nonenal or n-hexanal; the former is known to be an essential determinant of cucumber flavor (Galliard et al., 1976).
Figure 3.
Expression of LOX mRNA in cucumber tissues. Blots were hybridized with a lox-1 3′ probe (A) or a lox-1 internal probe (B). Nucleic acids were loaded at 10 μg/lane. Lanes 1, Cotyledons of 4-d-old seedlings; lanes 2, hypocotyls of 4-d-old seedlings; lanes 3, roots of 4-d-old seedlings; lanes 4, mature leaves; lanes 5, stems; lanes 6, tendrils; lanes 7, mature roots; lanes 8, buds; lanes 9, male flowers; lanes 10, female flowers; lanes 11, immature fruits less than 3 cm in length; and lanes 12, immature fruits 4 to 8 cm in length.
Expression of the lox-1 Gene
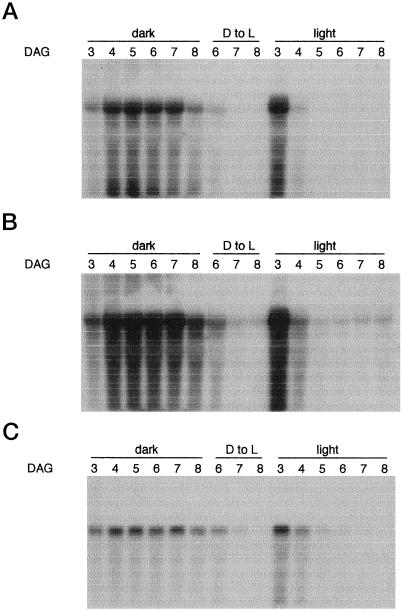
We investigated the expression of the lox-1 gene in cotyledons during seed germination in the light and in the dark. For comparison, the pattern of expression of the icl gene (Reynolds and Smith, 1995) was also investigated. The results (Fig. 4, A and C) show that the levels of lox-1 and icl mRNA increased and decreased in parallel. In the light, accumulation of lox-1 and icl mRNAs was already evident by d 3. Both rapidly declined to undetectable amounts by d 5. This rapid decline occurred concomitantly with the greening of the cotyledons, as photosynthesis became established. Figure 1A shows the accumulation of the large subunit of Rubisco. In the dark, rapid increases in the amounts of both mRNAs lasted until d 4 and remained at a high level until d 7. Both declined slowly and were still detectable at d 8. When the dark-grown seedlings were transferred to the light, the accumulated mRNAs rapidly disappeared. It is notable that expression of the lox-1 and icl genes were strictly coordinated. This may mean that they share at least one similar cis-acting DNA sequence that regulates their transcription. When we used the internal probe for the detection of LOX mRNAs in the cucumber cotyledons, we observed almost the same pattern as with the 3′ probe (Fig. 4B). However, careful examination of the blot shows that low but significant expression of the LOX gene(s) occurred in green cotyledons even after d 5, when lox-1 mRNA was undetectable (Fig. 4B). This was also observed after the transfer of the dark-grown seedlings to the light. Degradation of lox-1 mRNA was evident in northern analyses, especially when the internal probe was used. Because distinct bands can be seen with the icl probe, such degradation might account for the instability of lox mRNA. Further experiments are needed to confirm this.
Figure 4.
Accumulation of LOX and icl transcripts during early germinating stages of cucumber cotyledons in the light or dark. Cucumber seeds were germinated on vermiculite and then grown under a 14-h light/10-h dark photoperiod or in complete darkness. A portion of cucumber seedlings grown for 5 d in the dark was transferred to the 14-h light/10-h dark photoperiod. Twenty micrograms of total RNA isolated from each group of cotyledons was separated with a denaturing gel, transferred to a nylon membrane, and sequentially probed with the lox-1 3′ probe (A), the lox-1 internal probe (B), and the icl probe (C). A and B, Some degradation of lox-1 mRNA is evident. Because distinct bands can be seen with the icl probe (as in C), such degradation does not reflect a problem in RNA isolation. D to L, Dark to light transition period.
Expression of the LOX Gene during in Vitro Senescence
McLaughlin and Smith (1994) reported that the glyoxylate-cycle enzymes ICL and MS accumulate when cotyledons are detached from cucumber plants and are kept in the dark. The induction of these glyoxysomal enzymes occurs before degradation of chloroplast lipids, suggesting that the induction is not related to the turnover of lipids. Although the functions of these two enzymes in the detached cotyledons have not yet been elucidated, their expression is thought to be regulated by the depletion of a product of Suc metabolism resulting from the absence of photosynthesis (McLaughlin and Smith, 1994). To reveal whether lox-1 expression correlates with that of icl during in vitro senescence, we analyzed the amounts of lox-1 and icl transcripts in cotyledons detached from d-10 cucumber plants kept in the dark (Fig. 5). As reported, icl transcripts started to accumulate from d 1 after excision and continued to increase up to 10 d after the treatment. No induction of lox-1 expression was observed with the lox-1 3′ probe. On the other hand, the internal probe revealed the accumulation of LOX transcripts in the cotyledons 1 d after excision; although thereafter they disappeared. Bell and Mullet (1993) reported the induction of LOX gene expression in Arabidopsis upon wounding. The accumulation of LOX transcripts detected with the internal probe may represent such a wound-inducible LOX gene.
Figure 5.
Accumulation of LOX and icl transcripts during in vitro senescence of cucumber cotyledons. Cotyledons were excised from cucumber seedlings grown for 10 d under a 14-h light/10-h dark photoperiod and then kept in the dark for the number of days indicated. Total RNA (20 μg) isolated from the cotyledons was loaded in each lane, separated with a denaturing gel, transferred to a nylon membrane, and then sequentially probed with the lox-1 3′ probe (A), the lox-1 internal probe (B), and the icl probe (C).
Sequence Comparison of LOXs Purified from Soluble and Lipid Body Fractions
The observations presented above suggest that the lox-1 gene product takes part in fat mobilization during the early stages of cucumber seed germination. Enzymatic analysis of LOX-1 indicated that the enzyme can act on acyl groups esterified in neutral lipids (Matsui and Kajiwara, 1995). This property led us to suggest that LOX-1 exerts its function on lipid bodies. On the other hand, Feussner and Kindl (1992) reported that LOX is the main lipid body protein in cucumber during the period of triacylglyceride mobilization. Recently, these same investigators reported the primary sequence of the lipid body LOX gene cslblox (accession no. X92890, Hohne et al., 1996). The nucleotide sequences of lox-1 and cslblox share almost complete identity, even within the noncoding regions. When the two sequences (note that U25058 was recently updated) are compared, only three differences can be found at the nucleotide level (excluding the extreme 3′ end). To determine whether the two types of LOX are isozymes encoded by different genes or are the same molecule encoded by a single gene, we isolated both the soluble and the lipid-body-associated LOXs and determined their internal amino acid sequences. As shown in Figure 6, all of the sequences determined with the soluble LOX or the lipid body LOX are completely identical to the amino acid sequence deduced from lox-1. This indicates that the soluble LOX and the lipid body LOX may be derived from the same gene, even though their intracellular localization differs.
Figure 6.
Identity between amino acid sequences determined with soluble LOX and lipid body LOX purified from cucumber cotyledons. The amino acid sequence deduced from the lox-1 cDNA sequence (accession no. U25058) is shown. The sequences determined with peptides derived from soluble LOX are underlined; those from lipid body LOX are double underlined. Three differences can be found between lox-1 and cslblox (accession no. X92890) at the nucleotide level. Two of the three differences produce one amino acid substitution (Ser to Ala), as shown at position 491, although the amino acid sequence around Ser-491, namely IELUSLPHP, is highly conserved within plant LOXs and no such substitution can be found. The other difference is silent, and we do not know whether the difference is caused by a sequencing artifact or by the different varieties used as the gene source.
In Vitro Oxygenation of Lipid Bodies by LOX-1
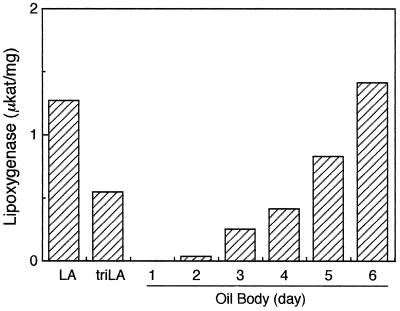
LOX-1 can oxidize fatty acids esterified in neutral lipids (Matsui and Kajiwara, 1995). Recently, Fuessner et al. (1997) reported almost the same observation with LOX isolated from lipid bodies. However, it remains unclear whether LOX-1 actually acts on lipids in lipid bodies. An in vitro oxygenation system was constructed to investigate whether fats in lipid bodies can actually be substrates for LOX-1. When lipid bodies alone were incubated under the assay conditions used here, little oxygen consumption was observed, although binding of LOX to lipid bodies was evident. When lipid bodies prepared from 1-d-old cotyledons were used as substrates for LOX-1, no activity was found (Fig. 7). However, LOX-1 did oxidize the fat constituents when lipid bodies from the d-2 or later cotyledons were used. As the cotyledons grew, LOX-1 activity on lipid bodies increased. As shown in Figure 1C, proteins associated with lipid bodies, such as oleosins, started to degrade at d 2 and almost completely disappeared by d 5. Fatty acid composition of lipids in lipid bodies was almost always independent of the stage of the cotyledons (data not shown). These results indicate that LOX-1 can act on fats sorted into lipid bodies only after lipid body proteins such as oleosins are degraded. The same tendency was also observed with LOX-1 expressed in E. coli. Association of LOX-1 with lipid bodies starts to take place at the same time as the degradation of the other lipid body proteins.
Figure 7.
Oxygenation by LOX-1 of lipids in lipid bodies isolated at different times after germination. Activity of LOX-1 on lipid bodies was determined polarographically. Lipid bodies corresponding to 8.3 μm equivalents of linoleic acid were added to the reaction mixture (1.75 mL total). For comparison, activities with linoleic acid and trilinolein are also shown.
Trypsin Digestion of the Proteins Coating Lipid Bodies
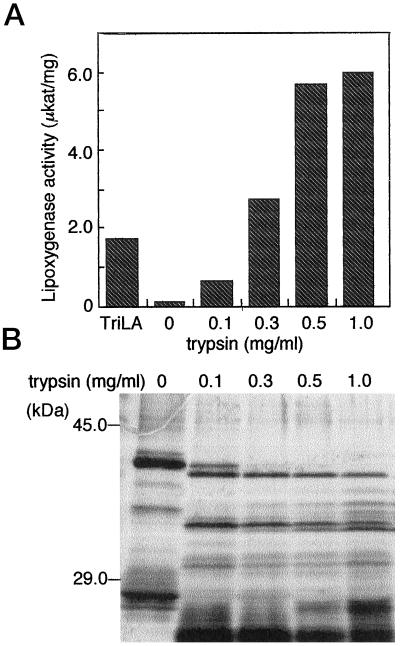
Lipid body proteins such as oleosins are known to stabilize the morphology of lipid bodies and to keep them intact during the desiccation of seeds (Huang, 1992). The results shown above suggest that lipid body proteins disturb the association of LOX-1 with these organelles and thus prevent their oxygenation. To confirm this hypothesis, lipid body proteins were digested using trypsin. As shown in Figure 8A, LOX-1 was only slightly active on the lipid bodies prepared from d-2 cotyledons that had their coating proteins mostly intact. After partial trypsin digestion, however, LOX-1 was able to oxidize fats in lipid bodies. The higher the trypsin concentration, the higher the LOX activity. With a trypsin concentration greater than 0.3 mg mL−1, activity was much higher than that observed when trilinolein emulsion was used as a substrate. Protein profiles of trypsin-treated lipid bodies are shown in Figure 8B. Treatment with low amounts of trypsin resulted in the formation of distinct bands of degraded lipid body proteins. It has been reported that oleosins are composed of three domains (Huang, 1992). The fragments remaining even after treatment with trypsin might represent the subunit protected by fats. This indicates that removing the protein moieties covering the surface of oil bodies may allow LOX-1 access to the lipids.
Figure 8.
Activity of LOX-1 on the lipids in lipid bodies treated with trypsin. A, Lipid bodies isolated from 2-d-old cotyledons were treated with a given amount of trypsin and then provided as a substrate for LOX-1. B, Degradation of the lipid body proteins.
DISCUSSION
Plants, especially oilseed plants such as cucumber, use seed storage lipids to provide energy for their postgerminative growth. The process by which this occurs had been thought to be the following: (a) acyl moieties of neutral lipids stored in lipid bodies are transported to glyoxysomes and converted to acyl-CoAs there; (b) the acyl-CoAs are then oxidized by the β-oxidation system in glyoxysomes; (c) the acetyl-CoAs formed through β-oxidation go into the glyoxylate cycle in glyoxysomes; and, finally, (d) the carbon source is transconverted into Glc via gluconeogenesis (Beevers, 1979). However, the first committed step of the turnover of the lipids has not yet been well established. It has been assumed that lipid mobilization is initiated by the liberation of free fatty acids from storage lipids, but a lipase activity thought to be responsible for fatty acid liberation from lipid bodies has not yet been detected in cucumber (Fuessner et al., 1997). How the fats stored in lipid bodies are hydrolyzed to form acyl-CoA and how they are delivered to glyoxysomes remains an enigma.
We have detected high LOX activity in germinating cucumber cotyledons and have isolated a cDNA clone (lox-1) corresponding to the activity (Matsui et al., 1992, 1995). Independently, Fuessner and Kindl (1992) found that LOX is the main lipid body protein in cucumber cotyledons. The primary sequence of the corresponding cDNA clone,cslblox, has been published (Hohne et al., 1996). In the present study we present evidence that these two LOXs are synthesized from the same gene, lox-1. We have shown that (a) the primary sequences of lox-1 and cslblox are almost entirely identical, even within the 3′- and 5′-noncoding regions; and (b) partial amino acid sequences of the LOXs purified from both the soluble and lipid body fractions of cucumber cotyledons are completely identical to the sequence deduced from lox-1. This indicates that LOX-1 can exist in the cytosol as a soluble protein and simultaneously on the lipid body surface as a membrane-associated protein.
Previously, we showed that LOX-1 binds to the lipid/water interface irreversibly (Matsui and Kajiwara, 1995). LOX-1 has an approximately 40-amino acid extension in its primary sequence that cannot be found in other plant LOXs. This N-terminal extension shows no homology to any known signal sequences. A prediction of its secondary structure indicates that it forms an amphipathic α-helix (data not shown). Such a conformation is also known for the C-terminal region of oleosins, and its amphipathicity is believed to allow the α-helix to interact with the surface of oil bodies (Huang, 1992). The remainder of LOX-1 is highly homologous to the other plant LOXs, most of which are soluble proteins. In this context, it can be assumed that LOX-1 usually exists as a soluble protein, but once it finds a lipid/water interface with specific physical properties, LOX-1 associates with it through its N-terminal extension. Such an interface must be specifically formed by the lipid body surface after degradation of the lipid body proteins.
This study demonstrates that LOX-1 can act on the neutral lipids in lipid bodies only after lipid body proteins such as oleosins are degraded. LOX-1 can oxygenate lipids upon its irreversible binding to the lipid/water interface (Matsui and Kajiwara, 1995), which means that it can associate with the lipid body surface. The surface of a lipid body is thought to be entirely covered with oleosins (Huang, 1992), and therefore LOX-1 cannot find sites to associate with on intact lipid bodies of cucumber cotyledons when fat mobilization has not yet started. As the degradation of oleosins proceeds, the naked surface of lipid bodies, composed of a half-membrane of phospholipids (and probably partly of neutral lipids) would be exposed to the cytosol. As a result of this exposure, LOX-1 can bind to the lipid bodies. We provide strong support for this theory, showing that in vitro degradation of the lipid body proteins with trypsin made lipid bodies accessible to LOX-1.
Oleosins stabilize lipid bodies not only by covering their surface but also by providing steric hindrance and electronegative repulsion. In a germinating sesame seed, Tzen et al. (1997) observed oil bodies that are larger than those found in a dry seed. Coalescence of lipid bodies is also observed after trypsin digestion of oleosins. It is conceivable that the physical state of the surface of coalesced lipid bodies is highly modified. LOX-1 might recognize such a specific interface and associate with it. Unexpectedly, the incubation of lipid bodies alone exerted little oxygen consumption, even if LOXs attached to their surfaces. Inactivation of LOX might occur during the preparation of lipid bodies. Many examples of the inactivation of LOX after incubation with its substrate have been reported (Matsui et al., 1998, and refs. therein).
Feussner et al. (1995, 1997) analyzed products formed from the neutral lipids in lipid bodies and suggested a role for LOX in lipid mobilization during seed germination of cucumber and other plants. Lipid mobilization is a highly organized system involving β-oxidation, the glyoxylate cycle, and gluconeogenesis and takes place at a distinct time during the postgerminative growth of seedlings. Therefore, the expression of lox-1 must be coordinated with other genes involved in lipid mobilization if LOX-1 is actually involved in this process. Regulation of the enzymes in the glyoxylate cycle, e.g. ICL and MS, has been intensely investigated (Trelease et al., 1971; Weir et al., 1980). When the dry seeds germinate, their enzyme activities increase from very low amounts to peak levels after a few days of growth. After reaching their highest level of activity, they decrease rapidly, concomitant with seedling exposure to light and the establishment of photosynthesis. As shown in this study, expression of lox-1 is highly coordinated with icl in cucumber cotyledons in the postgerminative stage. This suggests that lox-1 and icl share a cis-acting element for the germination response (De Bellis et al., 1997). Kim and Smith (1994) previously argued that the germination response was probably not mediated by sugars, but the rapid disappearance of the lox-1 and icl mRNAs in the greening cotyledons is now thought to be caused by the enhanced flux of some kind of sugar metabolite (Graham et al., 1994). In this context, both genes again might share a cis-acting element that suppresses their expression in response to a carbon catabolite. Jang and Sheen (1997) have reported that the sugar-repression-signaling pathways require hexokinase-mediated sugar sensing in yeast and plants. On the other hand, such a coordination between lox-1 and icl was not observed during in vitro senescence of the cotyledons. During in vitro senescence the flux of sugar metabolites is thought to be lowered through the lack of photosynthesis, which causes expression of icl in cucumber (McLaughlin and Smith, 1994). Most likely, lox-1 does not have the cis-acting element responsive to the sugar-activation pathway.
Kim and Smith (1994) have reported an expression profile similar to that of lox-1 for the microbody NAD-malate dehydrogenase gene in cucumber. Most investigators believe this enzyme is involved in the glyoxylate cycle. Like icl, the corresponding gene, mdh, showed a typical germination response; however, expression of the mdh gene was not activated by incubating detached green cotyledons in the dark; nor was it affected by exogenous Suc in the incubation medium. It should be noted that during the early stages of in vitro senescence lipid turnover was not initiated, and an alternative role for ICL during this period is expected (McLaughlin and Smith, 1994). The synthesis of ICL is neither tissue specific nor confined to the fat-storing tissues of germinating oilseeds; rather, the physiological or metabolic state of the tissue may control the expression of this gene. Lox-1 gene expression, in contrast, is confined to the fat-storing tissues of germinating oilseeds. To summarize, the expression of lox-1 is under strict developmental regulation that is coordinated with that of glyoxysomal genes, but this coordination is evident only in cotyledons undergoing postgerminative growth. This encourages us to speculate that LOX-1 plays an important role in lipid mobilization.
In conclusion, the following scheme can be proposed for the first committed step of fat mobilization: in cucumber cotyledons undergoing postgerminative growth, processing of lipid body proteins proceeds first. At the same time, lox-1 is expressed in coordination with the expression of the glyoxysomal enzymes. LOX-1 thus formed starts to associate with lipid bodies. The absorbed LOX-1 oxygenates phospholipids and neutral lipids in lipid bodies to form oxidized lipids (Feussner et al., 1997). The oxidized lipids may be hydrolyzed to form oxidized fatty acids by a still-unknown lipase. About two-thirds of the acyl moieties in neutral lipids consist of linoleic acids; therefore, this action would leave essentially monoacylglycerols and diacylglycerols containing saturated and monounsaturated fatty acids. These are not substrates for LOX-1; however, accumulation of these partially hydrolyzed lipids would lower the integrity of lipid bodies further, which might make these lipids much more susceptible to catabolism.
Currently, the most important missing part of this hypothesis is the supporting evidence for a hydroperoxide-specific lipase. Such lipase activity has been detected in rat liver (Kambayashi et al., 1997) and in several plants (Stahl et al., 1995). Another question also arises concerning LOX-1 levels and localization. A rough calculation indicates that even in cotyledons that are intensively degrading the stored lipids about 90% of LOX-1 remains in the soluble fraction and only 10% is associated with lipid bodies. This is probably due to the limited number of attachment sites that are accessible to LOX-1. LOX-1 in the soluble fraction does not seem to oxygenate the storage lipids in lipid bodies; therefore, it may have another role in the cytosol, such as transporting lipid constituents to glyoxysomes.
ACKNOWLEDGMENTS
The authors wish to thank Dr. Jack Wilkinson for his critical reading of the manuscript and Dr. Steven M. Smith for providing icl cDNA of cucumber.
Abbreviations:
- DAG
days after germination
- ICL
isocitrate lyase
- LOX
lipoxygenase
- MS
malate synthase
- X:Y
a fatty acyl group containing X carbon atoms and Y cis double bonds
LITERATURE CITED
- Beevers H. Microbodies in higher plants. Annu Rev Plant Physiol. 1979;30:159–193. [Google Scholar]
- Bell E, Mullet JE. Characterization of an Arabidopsislipoxygenase gene responsive to methyl jasmonate and wounding. Plant Physiol. 1993;103:1133–1137. doi: 10.1104/pp.103.4.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- De Bellis L, Ismail I, Reynolds SJ, Barrett MD, Smith SM. Distinct cis-acting sequences are required for the germination and sugar responses of the cucumber isocitrate lyase gene. Gene. 1997;197:375–378. doi: 10.1016/s0378-1119(97)00286-2. [DOI] [PubMed] [Google Scholar]
- Ferrie BJ, Beaudoin N, Burkhart W, Bowsher CG, Rothstein SJ. The cloning of two tomato lipoxygenase genes and their differential expression during fruit ripening. Plant Physiol. 1994;106:109–118. doi: 10.1104/pp.106.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuessner I, Balkenhohl TJ, Porzel A, Kuehn H, Wasternack C. Structural elucidation of oxygenated storage lipids in cucumber cotyledons. J Biol Chem. 1997;272:21. doi: 10.1074/jbc.272.34.21635. ,636–21,641. [DOI] [PubMed] [Google Scholar]
- Feussner I, Kindl H. A lipoxygenase is the main lipid body protein in cucumber and soybean cotyledons during the stage of triglyceride mobilization. FEBS Lett. 1992;298:223–225. doi: 10.1016/0014-5793(92)80062-l. [DOI] [PubMed] [Google Scholar]
- Feussner I, Kindl H. Particulate and soluble lipoxygenase isoenzymes. Comparison of molecular and enzymatic properties. Planta. 1994;194:22–28. [Google Scholar]
- Fuessner I, Wasternack C, Kindl H, Kühn H. Lipoxygenase-catalyzed oxygenation of storage lipids is implicated in lipid mobilization during germination. Proc Natl Acad Sci USA. 1995;92:11. doi: 10.1073/pnas.92.25.11849. ,849–11,853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galliard T, Phillips DR, Reynolds J. The formation of cis-3-nonenal, trans-2-nonenal and hexanal from linoleic acid hydroperoxide isomers by a hydroperoxide cleavage enzyme system in cucumber (Cucumis sativus) fruits. Biochim Biophys Acta. 1976;441:181–192. doi: 10.1016/0005-2760(76)90161-2. [DOI] [PubMed] [Google Scholar]
- Graham IA, Denby KJ, Leaver CJ. Carbon catabolite repression regulates glyoxylate cycle gene expression in cucumber. Plant Cell. 1994;6:761–772. doi: 10.1105/tpc.6.5.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoehne M, Nellen A, Schwennesen K, Kindl H. Lipid body lipoxygenase characterized by protein fragmentation, cDNA sequence and very early expression of the enzyme during germination of cucumber seeds. Eur J Biochem. 1996;241:6–11. doi: 10.1111/j.1432-1033.1996.0006t.x. [DOI] [PubMed] [Google Scholar]
- Huang AHC. Oil bodies and oleosins in seeds. Annu Rev Plant Physiol Plant Mol Biol. 1992;43:177–200. [Google Scholar]
- Jang JC, Sheen J. Sugar sensing in higher plants. Trends Plant Sci. 1997;2:208–214. [Google Scholar]
- Kambayashi Y, Yamashita S, Niki E, Yamamoto Y. Oxidation of rat liver phospholipids: comparison of pathways in homogeneous solution, in liposomal suspension and in whole tissue homogenates. J Biochem. 1997;121:425–431. doi: 10.1093/oxfordjournals.jbchem.a021606. [DOI] [PubMed] [Google Scholar]
- Kim D-J, Smith SM. Expression of a single gene encoding microbody NAD-malate dehydrogenase during glyoxysome and peroxisome development in cucumber. Plant Mol Biol. 1994;26:1833–1841. doi: 10.1007/BF00019496. [DOI] [PubMed] [Google Scholar]
- Matsui K, Irie M, Kajiwara T, Hatanaka A. Developmental changes in lipoxygenase activity in cotyledons of cucumber seedlings. Plant Sci. 1992;85:23–32. [Google Scholar]
- Matsui K, Irie M, Kajiwara T, Kakuno T, Hatanaka A. Rapid degradation of cucumber cotyledon lipoxygenase. Phytochemistry. 1993;32:1387–1391. doi: 10.1016/0031-9422(93)85143-f. [DOI] [PubMed] [Google Scholar]
- Matsui K, Kajiwara T. Cucumber cotyledon lipoxygenase oxygenizes trilinolein at the lipid/water interface. Lipids. 1995;30:733–738. doi: 10.1007/BF02537800. [DOI] [PubMed] [Google Scholar]
- Matsui K, Kajiwara T, Hayashi K, Hatanaka A. Tissue specific heterogeneity of lipoxygenase in cucumber seedlings. Agric Biol Chem. 1988;52:3219–3221. [Google Scholar]
- Matsui K, Nishioka M, Ikeyoshi M, Matsumura Y, Mori T, Kajiwara T. Cucumber root lipoxygenase can act on acyl groups in phosphatidylcholine. Biochim Biophys Acta. 1998;1390:8–20. doi: 10.1016/s0005-2760(97)00159-8. [DOI] [PubMed] [Google Scholar]
- Matsui K, Shibata Y, Tateba H, Hatanaka A, Kajiwara A. Changes of lipoxygenase and fatty acid hydroperoxide lyase activities in bell pepper fruits during maturation. Biosci Biotechnol Biochem. 1997;61:199–201. doi: 10.1271/bbb.61.199. [DOI] [PubMed] [Google Scholar]
- Matsui K, Tsuru E, Kajiwara T, Hase T. Nucleotide sequence of a cucumber cotyledon lipoxygenase cDNA (accession no. U25058) (PGR 95-044) Plant Physiol. 1995;109:337. [Google Scholar]
- McLaughlin JC, Smith SM. Metabolic regulation of glyoxylate-cycle enzyme synthesis in detached cucumber cotyledons and protoplasts. Planta. 1994;195:22–28. [Google Scholar]
- Napier JA, Stobart AK, Shewry PR. The structure and biogenesis of plant oil bodies: the role of the ER membrane and the oleosin class of proteins. Plant Mol Biol. 1996;31:945–956. doi: 10.1007/BF00040714. [DOI] [PubMed] [Google Scholar]
- Reynolds SJ, Smith SM. The isocitrate lyase gene of cucumber: isolation, characterization and expression in cotyledons following seed germination. Plant Mol Biol. 1995;27:487–497. doi: 10.1007/BF00019316. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Concepcion M, Beltran JP. Repression of the pea lipoxygenase gene loxgis associated with carpel development. Plant Mol Biol. 1995;27:887–899. doi: 10.1007/BF00037017. [DOI] [PubMed] [Google Scholar]
- Roshal S. Lipoxygenase in plants: their role in development and stress response. Z Naturforsch. 1996;51:123–138. doi: 10.1515/znc-1996-3-401. [DOI] [PubMed] [Google Scholar]
- Samuelsson B, Dahlen SE, Lindgren JA, Rouzer CA, Serhan CN. Leukotrienes and lipoxins: structures, biosynthesis, and biological effects. Science. 1987;237:1171–1176. doi: 10.1126/science.2820055. [DOI] [PubMed] [Google Scholar]
- Siedow JN. Plant lipoxygenase: structure and function. Annu Rev Plant Physiol. 1991;42:145–188. [Google Scholar]
- Stahl U, Banas A, Stymne S. Plant microsomal phospholipid acyl hydrolases have selectivities for uncommon fatty acids. Plant Physiol. 1995;107:953–962. doi: 10.1104/pp.107.3.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trelease RN, Becker WM, Grubber PJ, Newcomb EH. Microbodies (glyoxysomes and peroxisomes) in cucumber cotyledons. Plant Physiol. 1971;48:461–475. doi: 10.1104/pp.48.4.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzen JTC, Peng C, Cheng D, Chen ECF, Chiu JMH. A new method for seed oil body purification and examination of oil body integrity following germination. J Biochem. 1997;121:762–768. doi: 10.1093/oxfordjournals.jbchem.a021651. [DOI] [PubMed] [Google Scholar]
- Weir EM, Riezman H, Grienbenberger JM, Bechker WM, Leaver CJ. Regulation of glyoxysomal enzymes during germination of cucumber. Eur J Biochem. 1980;112:469–477. [PubMed] [Google Scholar]
- Yamaguhci Y, Sugimoto T, Sueyoshi K, Oji Y. Emergence of proteases in germinating cucumber cotyledons and their roles in the two-step degradation of storage protein. Plant Cell Physiol. 1996;37:279–284. doi: 10.1093/oxfordjournals.pcp.a028943. [DOI] [PubMed] [Google Scholar]