Abstract
Chronic myeloid leukemia (CML) is induced by the oncogenic BCR-ABL1 tyrosine kinase and can be effectively treated for many years with tyrosine kinase inhibitors (TKIs). However, unless CML patients receive life-long TKI treatment, leukemia will eventually recur; this is attributed to the failure of TKI treatment to eradicate leukemia-initiating cells (LICs). Recent work demonstrated that FoxO factors are critical for maintenance of CML-initiating cells; however, the mechanism of FoxO-dependent leukemia initiation remained elusive. Here, we identified the BCL6 protooncogene as a critical effector downstream of FoxO in self-renewal signaling of CML-initiating cells. BCL6 represses Arf and p53 in CML cells and is required for colony formation and initiation of leukemia. Importantly, peptide inhibition of BCL6 in human CML cells compromises colony formation and leukemia initiation in transplant recipients and selectively eradicates CD34+ CD38− LICs in patient-derived CML samples. These findings suggest that pharmacological inhibition of BCL6 may represent a novel strategy to eradicate LICs in CML. Clinical validation of this concept could limit the duration of TKI treatment in CML patients, which is currently life-long, and substantially decrease the risk of blast crisis transformation.
Chronic myeloid leukemia (CML), first identified in 1845 (Bennett, 1845; Virchow, 1845), is characterized by the Philadelphia chromosome encoding the oncogenic BCR-ABL1 tyrosine kinase (Rowley, 1973; de Klein et al., 1982). CML develops from a hematopoietic stem cell and consequently displays multilineage differentiation potential (Calabretta and Perrotti, 2004). If not efficiently treated, CML follows a triphasic clinical course with an initial indolent chronic phase (CP; 5–15 yr), followed by an intermediate accelerated phase and, eventually, a blast crisis of myeloid, B lymphoid, or biphenotypic myeloid/lymphoid lineage (Calabretta and Perrotti, 2004). Whereas CML can be effectively treated with tyrosine kinase inhibitors (TKIs; e.g., Imatinib) for many years in the CP (Druker et al., 2006), CML blast crisis is invariably multidrug-resistant and fatal within weeks or months (Druker et al., 2001). The majority of patients in lymphoid blast crisis acquire secondary genetic lesions, some of which are introduced by aberrant activity of the AID mutator enzyme (Klemm et al., 2009). During blast crisis progression, mutations of the CDKN2A (ARF), MYC, RB1, AML1, TP53, and RAS genes are frequently acquired (Melo and Barnes, 2007), and in the majority of CML blast crisis cases, mutations within the BCR-ABL1 kinase domain encode resistance against TKI treatment (Shah et al., 2002).
The development of Imatinib mesylate, a selective BCR-ABL1 kinase inhibitor, achieved an overall survival of 95% over a 5-yr period for CML patients in CP (Druker et al., 2006). Despite its clinical success, Imatinib fails to eradicate CML entirely (Corbin et al., 2011), and in virtually all cases residual leukemia-initiating cells (LICs) persist (Kantarjian et al., 2009). Despite having low numbers, LICs have the capacity to reinitiate leukemia, which is typically the case upon discontinuation of TKI treatment (Rousselot et al., 2007). Previous works showed that classical pathways of self-renewal signal transduction in normal stem cell populations (e.g., WNT/β-catenin; Sonic hedgehog) are also required for self-renewal signaling in CML-LIC (Zhao et al., 2007; Zhao et al., 2009).
A recent study demonstrated that FoxO factors are critical for maintenance of LICs in CML (Naka et al., 2010). FoxO activity is negatively regulated by BCR-ABL1–AKT signaling and positively regulated by TKI treatment (e.g., Imatinib; Fernández de Mattos et al., 2004) and Pten (Trotman et al., 2006; Fig. S1). For this reason, the identification of FoxO as a critical factor for the maintenance of LICs in CML is of particular interest, as it provides a direct explanation for how CML-initiating cells persist despite long-term TKI treatment. The mechanisms through which FoxO3A mediates self-renewal and maintenance of CML-initiating cells, however, remain unclear.
In this study, we identified the BCL6 transcription factor downstream of FoxO as a critical effector molecule for protection and maintenance of leukemia-initiating cells in CML. BCL6 was first identified as a protooncogene in diffuse large B cell lymphoma, which is characterized by a high frequency of BCL6-IGH translocations (Ye et al., 1995). BCL6 is required for affinity maturation of mature B cells in germinal centers (Dent et al., 1997; Ye et al., 1997), a process that critically depends on BCL6-mediated transcriptional repression of p53 (Phan and Dalla-Favera, 2004). More recently, we demonstrated that BCL6 is also critical for pre–B cell survival (Duy et al., 2010). Moreover, BCR-ABL1–transformed pre–B cell acute lymphoblastic leukemia (Ph+ ALL) cells respond to TKI treatment by up-regulation of BCL6. TKI-induced up-regulation of BCL6 enables Ph+ ALL cells to survive TKI treatment (Duy et al., 2011). This study is focused on myeloid lineage CML and widens the emerging role of BCL6 in BCR-ABL1–driven leukemias.
RESULTS AND DISCUSSION
CML cells up-regulate BCL6 in response to TKI treatment
To study genes potentially contributing to the maintenance of CML cells exposed to TKI treatment, CML cells were incubated in the presence or absence of the TKI Imatinib and subjected to gene expression analysis. Because Stat5 represents a central mediator of BCR-ABL1 signaling, we studied a BCR-ABL1 leukemia mouse model in the context of inducible deletion of Stat5 (Fig. S2). This analysis showed that many TKI-induced gene expression changes, including BCL6, are in fact Stat5-dependent (Fig. 1 A). TKI-induced gene expression changes that occurred in a Stat5-independent manner involved multiple erythroid lineage transcripts, including hemoglobins (HBA1, HBB, HBD, and HBE1), erythroid surface antigens (CD36 and GYPA), Beatty’s protein (elliptocytosis; EPB41), and δ-aminolevulinate synthase (ALAS2; Fig. 1 A). TKI-induced up-regulation of BCL6 in human CML cells was confirmed in vitro at the mRNA and protein levels (Fig. 1 B and D), and other gene expression changes were confirmed by quantitative RT-PCR (Fig. S3). In agreement with in vitro observations, BCL6 is strongly up-regulated in CML cells from patients who were treated with TKI.
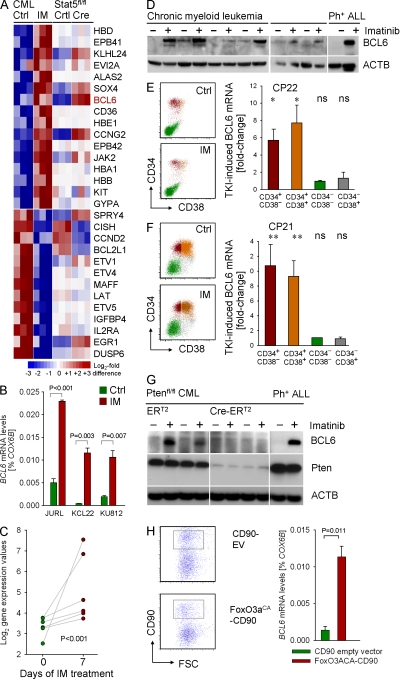
Figure 1.
Regulation of BCL6 expression in CML cells. (A) To identify TKI-regulated genes in human CML cells, three human CML cell lines (KCL22, KU812, and JURL) were treated with (IM) or without (Ctrl) 1 μmol/liter Imatinib for 16 h and studied in an Affymetrix GeneChip analysis. Genes were sorted based on gene expression differences between TKI-treated and untreated CML cells. Likewise, BCR-ABL1–transformed leukemia cells from Stat5fl/fl bone marrow were transduced with Cre or an empty vector control. Gene expression values for Stat5fl/fl (Ctrl) and Stat5-deleted (Cre) leukemia cells are indicated for the genes identified in TKI-treated cells. (B) Affymetrix GeneChip data for BCL6 was validated by quantitative RT-PCR on three cases of human CML (with or without 1 μmol/liter Imatinib overnight). (C) BCL6 gene expression values for CML cells from six patients before and after 7 d of Imatinib-treatment are shown (meta-analysis of data from Bruennert et al. [2009]). (D) Human CML cells were treated with TKI (10 µM for 24 h) and BCL6 expression was evaluated by Western blotting using β-actin as a loading control. (E and F) Leukapheresis samples from two patients with CML-CP (CP21 and CP22) were sorted into four subpopulations based on CD34 and CD38 surface expression as depicted in the flow cytometry dot plots. Subpopulations were incubated overnight in the presence and absence of 10 µmol/liter Imatinib and then subjected to quantitative RT-PCR for BCL6 mRNA levels using COX6B as a reference gene. For each subpopulation, fold-induction of BCL6 mRNA levels are shown (triplicate measurements were performed; *, P < 0.05; **, P < 0.01). (G) Ptenfl/fl mouse CML cells were transduced with 4-OHT–inducible Cre-ERT2 or ERT2 empty vectors. BCL6 protein levels are shown after treatment with 4-OHT in the presence or absence of Imatinib. Pten deletion in Cre-ERT2–transduced cells was verified by Western blot. (H) Human CML cells (KCL22) were transduced with a retroviral vector encoding a constitutive active form of FoxO3A (FoxO3aCA CD90) or an empty control vector (CD90 EV). CD90+ cells were sorted (sort gate indicated) and studied for BCL6 mRNA levels by quantitative RT-PCR using COX6B as a reference gene. Mean values of three experiments ± SD and p-value are indicated.
The ability to up-regulate BCL6 upon TKI treatment is restricted to CD34+ CML cells
CML cells were isolated via leukapheresis from two patients in CP, and then sorted for CD34+ CD38− and CD34+ CD38+ multilineage progenitors and more mature CD34− CD38− and CD34− CD38+ transient amplifying cells. Treatment with Imatinib for 12 h resulted in strong up-regulation of BCL6 mRNA levels in the CD34+ CML cells, but not in CD34− CML cells, regardless of CD38 expression (Fig. 1, E and F). These findings indicate that the ability to up-regulate BCL6 in response to TKI treatment is restricted to cells within the pool of CD34+ cells with multilineage potential.
BCL6 is a downstream effector molecule of FoxO factors
Previous work implicated FoxO factors as positive regulator of BCL6 (Fernández de Mattos et al., 2004). In agreement with this study, we found that FoxO activity is required for TKI-induced BCL6 expression in CML cells (Fig. 1 G). Although AKT-mediated phosphorylation downstream of the BCR-ABL1 kinase results in global inactivation of FoxO factors (Tran et al., 2002; Fig. S1), the Pten phosphatase is required for FoxO activation. Here, we demonstrate that conditional deletion of Pten abrogates the ability of CML-like cells to up-regulate BCL6 in response to TKI treatment (Fig. 1 G). In fact, overexpression of a constitutively active FoxO3A mutant was sufficient to induce an ∼10-fold increase of BCL6 mRNA levels in human CML cells (Fig. 1 H). The finding of FoxO3A as upstream regulator of BCL6 is of particular importance, given that FoxO3A was recently identified as a requirement for the maintenance of CML-initiating cells (Naka et al., 2010).
BCL6 is required for a basic level of Imatinib-resistance in CML cells
We then tested the significance of TKI-induced BCL6 in a genetic loss-of-function experiment. To this end, BCL6+/+ and BCL6−/− bone marrow hematopoietic progenitor cells were transformed with p210 BCR-ABL1 according to a classical model for CML in mice (Pear et al., 1998; Li et al., 1999). The transformation efficiency of BCL6+/+ and BCL6−/− hematopoietic progenitor cells was undistinguishable and, in both cases, after ∼1 wk a growth factor–independent CML-like leukemia developed (Fig. S4; n = 3). Because BCR-ABL1 can give rise to both B cell lineage and myeloid lineage leukemia (i.e., CML), a myeloid-specific transformation protocol was used in all experiments (Li et al., 1999). Myeloid lineage identity of the BCR-ABL1–transformed cells was routinely verified by flow cytometry. The phenotype of CML-like cells was characterized in depth when the CML-like leukemia model for BCL6+/+ and BCL6−/− hematopoietic progenitor cells was established (Fig. S5 and Fig. S6).
Because the pool of CD34+ cells with multilineage potential represents the main source of BCL6 expression in human CML (Fig. 1, E and F), we studied the effect of BCL6 deficiency in the equivalent population (Lin− Sca-1+ c-Kit+ [LSK]) in our CML-like mouse model. Given that BCL6 was strongly up-regulated in response to Imatinib-treatment of CML cells (Fig. 1), we tested whether BCL6 regulates sensitivity of CML cells to Imatinib. Mouse LSK cells in CML-like leukemia are highly refractory to Imatinib, and Imatinib concentrations of >3 μmol/liter are required to induce cell death in LSK+ CML cells. BCL6-deficient CML LSK cells, however, were sensitive to Imatinib even at concentrations <0.5 μmol/liter (Fig. 2 A). Comparing the response of CML-like cells to Imatinib treatment (10 μmol/liter), BCL6+/+ CML-like cells were significantly less sensitive compared with BCL6−/− CML-like cells (mean viability 71.8 ± 2.7% compared with 31.4 ± 1.6% viable cells; P < 0.0003; Fig. 2 B). We next studied whether inducible reconstitution of BCL6 rescues Imatinib-resistance in BCL6−/− CML-like cells. To this end, BCL6−/− CML-like cells were transduced with a 4-hydroxy-tamoxifen (4-OHT)–inducible BCL6-ER fusion molecule, which is activated within minutes after 4-OHT addition (Shaffer et al., 2000). Although an ER empty vector control had no effect on the survival of leukemia cells, inducible BCL6 activation conferred a strong survival advantage as reflected by a 30-fold increase of BCL6-ER–transduced cells (Fig. 2, C and D).
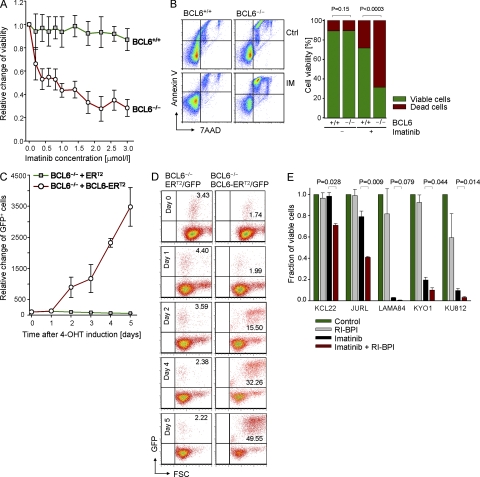
Figure 2.
BCL6 is required for a basic level of Imatinib-resistance in CML cells. (A) BCL6−/− and BCL6+/+ CML-like cells were treated with Imatinib at various concentrations for 3 d, and cell metabolism was measured in a Resazurin assay. Mean values ± SD of three experiments are depicted. (B) BCL6+/+ and BCL6−/− CML cells were treated with Imatinib as in A, and apoptosis was assessed by flow cytometry analysis of 7AAD and annexin V. Mean values ± SD of three experiments are depicted. (C) BCL6−/− CML-like cells were transduced with 4-OHT–inducible BCL6 (BCL6-ERT2/GFP) or an ERT2/GFP empty vector control. BCL6−/− CML-like cells were then treated with 1 μmol/liter Imatinib for the times indicated and in the presence of 4-OHT–mediated induction of BCL6-ERT2 or ERT2. Percentage of GFP+ cells were measured by flow cytometry as an indication of a BCL6-ERT2– or ERT2-mediated survival advantage. A time course of mean values ± SD of three experiments is depicted in C, and examples of the flow cytometry plots are shown in D. (E) Human CML cell lines (KCL22, JURL, LAMA84, KYO1, and KU812) were incubated in the presence or absence of 1 µmol/liter Imatinib, 5 µmol/liter RI-BPI, or a combination of both for 3 d. Viability was measured by flow cytometry. Mean values, SD, and p-values from three experiments are indicated.
Pharmacological inhibition of BCL6 sensitizes CML cells to TKI treatment
Because the aforementioned genetic experiments indicate a critical role for BCL6 in Imatinib resistance of CML cells, we next tested if the implicated synthetic lethality could be targeted pharmacologically using a combination of Imatinib and a BCL6-peptide inhibitor in human CML cells. To this end, human CML cells were incubated in the presence or absence of Imatinib, a novel retro-inverso BCL6 peptide inhibitor (RI-BPI; Cerchietti et al., 2009), or a combination of both (Fig. 2 E). Although RI-BPI alone did not significantly affect CML cell viability, it strongly enhanced the effect of Imatinib, except for one case, in which nearly all cells underwent apoptosis upon Imatinib treatment alone (Fig. 2 E).
BCL6 is required for the maintenance of LSK cells in a mouse model for CML
When mouse LSK cells were transformed with BCR-ABL1 in the presence of IL-3, IL-6 and SCF, the vast majority of BCL6+/+ CML-like cells retain an LSK-phenotype. In contrast, the LSK population of the BCL6−/− CML-like cells rapidly undergoes apoptosis. Within 2 wk, the LSK population, which is thought to comprise the pool of LICs was reduced from ∼15 to <1% in BCL6−/− CML (Fig. 3, A and B). As opposed to LSK cells, the non-LSK population consistently lacks the ability to initiate leukemia in serial transplantation experiments (Hu et al., 2006; Neering et al., 2007; Ito et al., 2008; Zhao et al., 2009). Besides loss of the LSK phenotype, an Affymetrix GeneChip expression analysis revealed loss of expression of multiple other stem cell–related molecules in BCL6−/− CML-like cells (e.g., Egr1, Ptgs1, Slamf1/CD150, Gfi1, Rora, Abcg2; Fig. S7).
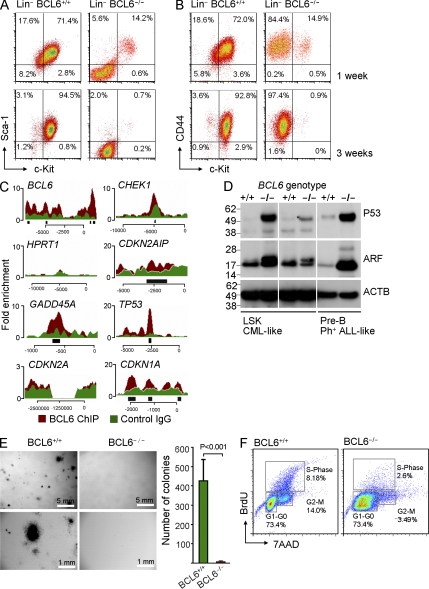
Figure 3.
BCL6 is required for the maintenance of LSK+ cells in CML. (A and B) BCR-ABL1 transformed CML-like cells from BCL6+/+ and BCL6−/− bone marrow at 1 and 3 wk after transduction. Cells were gated on Lin− phenotype and surface expression of Sca-1 and c-Kit (LSK; A) and CD44 and c-kit (B) is shown (n = 3). (C) Human CML cells (JURL cell line) were subjected to one round of ChIP-seq analysis for a genome-wide mapping analysis of recruitment of the BCL6 transcription factor. Overlays of input (green) and BCL6 ChIP (red) are shown for BCL6 (positive control; binding to its own promoter), HPRT (negative control), DNA damage response and cell cycle checkpoint genes including CHEK, CDKN2AIP, TP53, CDKN1A (p21), GADD45A, and CDKN2A (Arf). Peaks of significant enrichment of BCL6 in promoter regions relative to input were identified by ChIPSeeqer (black bars). (D) BCR-ABL1–transformed CML-like cells from BCL6+/+ and BCL6−/− bone marrow were analyzed by Western blot for Arf and p53 protein levels using β-actin as loading control (two experiments are shown). (E) 100,000 BCL6+/+ and BCL6−/− CML-like cells were plated in semisolid methylcellulose agar and colonies were counted after 22 d. Chart shows mean values ± SD and p-value of 5 experiments. (F) Cell cycle analysis of BCL6+/+ and BCL6−/− CML cells was performed studying BrdU incorporation in combination with 7AAD staining. Annotations indicate distribution of CML-like cells to G0/1, S, and G2/M phases of the cell cycle. One representative experiment of three is shown.
In the absence of BCL6 function, LICs in CML are poised to undergo apoptosis
These changes may occur for various reasons, such as enhanced differentiation, reduced self-renewal, or selective apoptosis/depletion of BCL6−/− LICs. To test these possibilities, we sorted viable LICs (LSK+ phenotype) and transient amplifying cells (LSK− phenotype) from freshly generated BCL6+/+ and BCL6−/− CML-like leukemia. At this time, BCL6−/− CML-like leukemia still had a high frequency of LSK+ cells. For each population, between 100,000 and 1 million cells were sorted with >98% viable cells. After 20 h of incubation, cell counts moderately increased for transient amplifying cells from both BCL6+/+ and BCL6−/− CML-like leukemia. Also counts for LSK+ LICs from BCL6+/+ CML-like leukemia increased, whereas counts for LSK+ LICs from BCL6−/− CML-like leukemia significantly dropped (Fig. S8). Selective reduction of LSK+ LIC counts from BCL6−/− CML-like leukemia is consistent with LIC depletion observed in Fig. 3, A and B. Strikingly, flow cytometry revealed that the majority of LSK+ LICs from BCL6−/− CML-like leukemia was preapoptotic after 20 h. These findings show that BCL6 represents a critical factor for LIC survival in CML. In the absence of BCL6 function, LICs are poised to undergo apoptosis.
BCL6 directly represses p53 and MYC in human CML cells
To identify transcriptional targets of BCL6 in human CML cells, we performed a genome-wide mapping analysis of BCL6 recruitment using ChIP-seq. Because BCL6 is known to function as its own repressor (Mendez et al., 2008), we studied recruitment of BCL6 to the BCL6 and HPRT promoters as positive and negative controls, respectively (Fig. 3 C). Several molecules in the DNA damage/checkpoint signaling pathway, including CHEK1, CARF (CDKN2AIP), p53, GADD45A, and p21 (CDKN1A) exhibit robust recruitment of BCL6 (Fig. 3 C). ChIP-seq was not informative for ARF, because the JURL CML cells studied carry biallelic deletions at 9p21, including the CDKN2A locus (Fig. 3 C). CARF (CDKN2AIP), which is required for protein stability of ARF (CDKN2A; Hasan et al., 2002), and p53 are both direct transcriptional targets of BCL6 (Fig. 3 C). Therefore, we tested whether the ARF/p53 pathway is deregulated in BCL6−/− CML-like cells. Indeed, protein levels of both ARF and p53 were increased in the absence of BCL6 (Fig. 3 D). Hence, BCL6 may curb excessive expression of ARF/p53 in CML-like cells.
BCL6 is required for self-renewal of LICs in CML
Because components of the Arf/p53 pathway are mediators of cellular senescence and negatively regulate self-renewal in normal hematopoiesis and leukemia (Oguro et al., 2006), we next tested whether the unrestrained expression of Arf/p53 in the absence of BCL6 interferes with CML cell self-renewal. To this end, BCL6+/+ and BCL6−/− LSK cells from CML-like leukemia were plated in semisolid agar and colonies were counted 22 d later. Immediately before plating, viability of cells was verified by flow cytometry (>90%). Strikingly, in the absence of BCL6, LSK cells from CML-like leukemia nearly entirely lack the ability to form colonies, and on most plates not a single colony was detected (Fig. 3 E). In the absence of BCL6, colony formation was reduced by >300-fold, which demonstrates a critical role of BCL6 in self-renewal of CML-initiating cells. Unlike for LICs in CML, BCL6 was dispensable for colony formation in normal LSK. Although normal BCL6−/− LSK cells had a slightly lower colony count compared with BCL6+/+ LSK cells, colony formation was not compromised in the absence of BCL6 (Fig. S9). Likewise, a recent study demonstrated that normal LSK cells from BCL6-deficient mice have multilineage potential similar to their BCL6+/+ counterparts (Broxmeyer et al., 2007). More detailed experiments to address a potentially unrecognized function of BCL6 in normal LSK cells are currently under way.
Because Arf/p53 signaling also affects cell cycle check points, we studied the consequences of BCL6-deficiency on cell cycle regulation in CML-like cells (Fig. 3 F). This analysis revealed a striking anomaly in BCL6−/− CML-like cells, which exhibit a large subpopulation with “mitotic crisis” phenotype (Fig. 3 F; Li and Dang, 1999). BrdU incorporation showed arrest in G1/S-phase with incomplete DNA replication. Consistent with the high level of DNA damage stress and Arf/p53 activation, LSK+ BCL6−/− CML cells do not survive over longer periods of time. As shown in Fig. 3 (A and B), >95% of LSK+ BCL6−/− CML cells die within 3 wk of leukemic transformation. In cell culture experiments, we observed that BCL6−/− CML-like cells occasionally ceased to propagate and the entire population underwent cell cycle arrest. In the context of the “mitotic crisis” phenotype (Fig. 3 F), we hypothesized that BCL6−/− CML-like cells may undergo replicative senescence owing to telomere shortening after multiple cell divisions. Measurement of telomere lengths in BCL6+/+ and BCL6−/− CML-like cells by flow FISH (Fig. S10), however, showed that telomere lengths were similar in BCL6+/+ (31.4 ± 1.1 kb) and BCL6−/− CML-like cells (35.5 ± 0.9 kb).
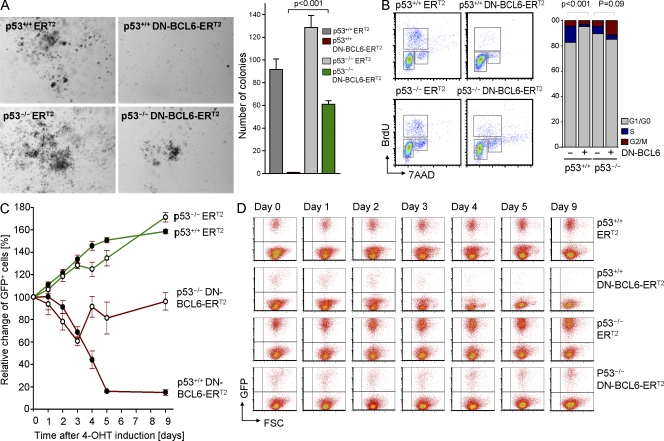
BCL6-mediated transcriptional repression of p53 enables self-renewal of LICs in CML
Because BCL6 functions as a transcriptional repressor of p53 in human and mouse CML cells (Fig. 3, C and D), we hypothesized that BCL6-mediated repression of p53 represents a key element in BCL6-dependent self-renewal signaling. To test this hypothesis, we transduced p53+/+ and p53−/− CML-like cells with a 4-OHT–inducible dominant-negative mutant of BCL6 (DN-BCL6-ERT2; Shaffer et al., 2000). This form competes with wild-type BCL6 for DNA binding, but lacks the BCL6-BTB (Bric-à-Brac, Tramtrack, and Broad complex) domain and thus lacks the ability to function as transcriptional repressor. 4-OHT–mediated induction of DN-BCL6-ERT2 nearly completely suppressed colony formation in p53+/+ CML-like cells, whereas colony formation in p53−/− CML-like cells was only slightly reduced (Fig. 4 A). Likewise, induction of DN-BCL6-ERT2 induced G1/0 cell cycle arrest in p53+/+, but not p53−/− CML-like cells (Fig. 4 B). In contrast to p53−/− CML-like cells, 4-OHT–mediated induction of DN-BCL6-ERT2 resulted in a drastic growth disadvantage in p53+/+ CML-like cells (reduction of transduced cells to <20% within 5 d; Fig. 4, C and D). These findings suggest that BCL6-mediated repression of p53 is not only required for the initiation of CML colonies (Fig. 4 A), but also for the proliferation and survival of CML LICs (Fig. 4, B and D).
Figure 4.
BCL6-mediated transcriptional repression of p53 enables colony formation and proliferation of CML cells. p53+/+ and p53−/− CML-like cells were transduced with a 4-OHT–inducible dominant-negative BCL6 (DN-BCL6-ERT2) or an empty vector control and 100,000 cells each were plated in semisolid agar (A). Photomicrographs of methylcellulose plates and statistical analysis are shown. (B) p53+/+ and p53−/− CML-like cells transduced with a 4-OHT–inducible dominant-negative BCL6 (DN-BCL6-ERT2) or an empty vector control (ERT2) and cell cycle was analyzed by flow cytometry (BrdU and 7AAD staining). FACS plots and statistical analysis are shown. (C and D) p53+/+ and p53−/− hematopoietic progenitor cells were transformed with BCR-ABL1 to generate CML-like leukemia and then transduced with DN-BCL6-ERT2 or a GFP empty control vector. CML-like cells expressing GFP, were incubated in vitro and relative changes of GFP+ percentages were plotted against time (days) after 4-OHT–mediated induction (C). In D, one example of flow cytometry measurements over 9 d is shown. Mean values of three experiments ± SD are indicated.
BCL6 function represents an absolute requirement for leukemia initiation in CML
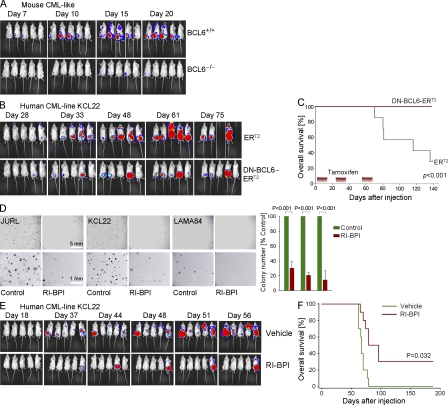
To formally test the requirement of BCL6 for leukemia initiation in vivo, we performed a classical SCID leukemia-initiating cell (SL-IC) assay (Bonnet and Dick, 1997). 500,000 luciferase-labeled BCL6+/+ and BCL6−/− CML-like leukemia cells (Lin− Sca-1+ c-Kit+ CD13+; Fig. S5) were injected intrafemorally into NOD/SCID mice, and leukemia initiation and expansion was monitored by bioimaging (Fig. 5 A). Intrafemoral injection was chosen to focus on leukemia initiation and to exclude potential defects in bone marrow homing and engraftment of BCL6−/− CML-like cells as confounding variables (Krause et al., 2006). Although BCL6+/+ LSK cells from CML-like leukemia rapidly initiated leukemia, bioimaging revealed persistence of BCL6−/− LSK cells at the site of injection, but no overt leukemia initiation (Fig. 5 A). Our intention was to perform serial transplantation experiments to assess leukemia initiation potential of BCL6−/− CML-like leukemia in secondary or tertiary transplants. Because BCL6−/− LSK cells failed to initiate leukemia in primary transplant recipients, serial transplantation was not possible. Consistent with our colony formation assays, the defect of leukemia-development from BCL6−/− CML-like cells reflects failure to maintain leukemia-initiating cells, as exemplified by the progressive loss of the LSK+ population in BCL6−/− CML-like leukemia (Fig. 3, A, B, and E).
Figure 5.
BCL6 is required for self-renewal and leukemia initiation in CML. (A) BCL6−/− and BCL6+/+ CML-like cells were labeled with firefly luciferase and 500,000 cells were injected into sublethally irradiated NOD/SCID mice (7 mice per group; two independent experiments). Engraftment and leukemic growth was measured by luciferase bioimaging at the times indicated. (B and C) Human CML cells (KCL22) were transduced with a 4-hydroxy-tamoxifen (4-OHT)-inducible dominant-negative mutant of BCL6 (DN-BCL6-ERT2; Shaffer et al., 2000) or an ERT2 empty vector control (B). Human CML cells were labeled with lentiviral firefly luciferase and 3 × 106 cells were injected into sublethally irradiated NOD/SCID mice (7 mice per group). NOD/SCID recipients were treated 30 times (3 sets of 10 injections) with tamoxifen (20 mg/kg) after leukemia cell were injected (second set of treatment was performed after 34 d, third set of treatment was performed after 55 d). Leukemia burden (B) and overall survival (Kaplan-Meier analysis; C) are shown (two independent experiments). (D) 10,000 cells from three CML cases were plated in semisolid methylcellulose agar with or without RI-BPI (5 µmol/liter). Colonies were counted after 10 d. The chart shows mean values ± SD and p-value of five experiments. (E) 3 × 106 human CML cells (KCL22) labeled with firefly luciferase were treated with or without 5 µmol/liter RI-BPI ex vivo and injected into sublethally irradiated NOD/SCID mice. Overall survival, engraftment, and leukemia progression were monitored by Kaplan-Meier analysis (E) and luciferase bioimaging (F), respectively. Two separate injections and treatments were performed and 10 mice per group were studied.
Inducible activation of a dominant-negative BCL6 mutant suppresses CML leukemogenesis in vivo
We next tested whether loss-of-function of BCL6 also affects human CML cells. To this end, human CML cells (KCL22 cell line) were transduced with DN-BCL6-ERT2 or the ERT2 empty vector control, labeled with lentiviral firefly luciferase, and injected into sublethally irradiated NOD/SCID mice (Fig. 5 B). After visible engraftment of CML, NOD/SCID mice were treated with intraperitoneal injections of 4-OHT over 3 intervals of 10 d (20 mg/kg 4-OHT/per day). Consistent with our in vitro observation, inducible inhibition of BCL6 compromised leukemia initiation in vivo (Fig. 5 B) and significantly prolonged overall survival of xenografted NOD/SCID mice (Fig. 5 C). We conclude that BCL6 function is critical for initiation of human CML in vivo.
BCL6 is a pharmacological target for eradication of leukemia-initiating cells in CML
We next tested whether pharmacological inhibition of BCL6 (RI-BPI) can interfere with leukemia initiation in human CML. We performed a colony formation assay with human CML cells that were plated on semisolid agar plates with vehicle or 5 μmol/liter RI-BPI. Consistent with our observation that BCL6−/− CML-like cells nearly entirely lacked the ability to form colonies (Fig. 3 E), RI-BPI–mediated inhibition of BCL6 reduced colony numbers by three- to eightfold compared with untreated cells in a BCL6+/+ context (Fig. 5 D). More importantly, RI-BPI also interfered with leukemia initiation of human CML cells in vivo. Upon treatment with RI-BPI, xenografted human CML cells (KCL22 cell line) failed to initiate leukemia in transplant recipients. Treatment of human CML cells with RI-BPI increased overall survival of recipient mice and latency of leukemia. 3 of 10 mice that were xenografted with human CML cells (KCL22 cell line) in the RI-BPI group were sacrificed after 180 d (Fig. 5, E and F) and had no indication of leukemia (no CML cells detectable in bone marrow and spleen), compared with no such cases in the untreated controls.
RI-BPI selectively eradicates CD34+ CD38− LICs in patient-derived CML samples
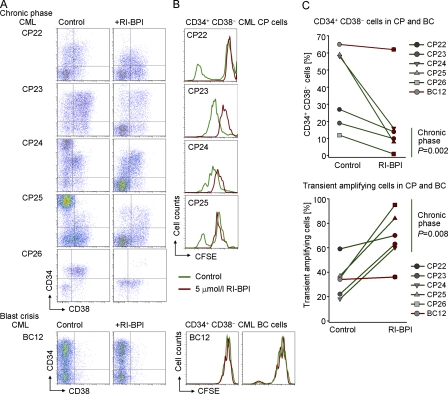
To study the effect of acute BCL6 inactivation in patient-derived CML cells, we incubated CML cells from 5 patients in CP (CP22-CP26; Table S1) and 1 patient in blast crisis (BC12; Table S1) in 5 μmol/liter RI-BPI or Vehicle for 2 h. After this incubation period, RI-BPI was washed out and cells were cultured in the presence of 100 ng/ml SCF, 100 ng/ml G-CSF, 20 ng/ml FLT3, 20 ng/ml IL-3, and 20 ng/ml IL-6. In one set of experiments, cells were stained with CFSE to track cell divisions over time. Flow cytometry revealed that RI-BPI–treated CML-CP samples selectively lost CD34+ CD38− LICs (Fig. 6, A and B), whereas CD34− subpopulations remained intact (Fig. 6 C). Likewise, RI-BPI caused cell cycle arrest in CD34+ CD38− LICs (Fig. 6 B), whereas other subpopulations continued to divide as measured by CFSE dye dilution. In one case of blast crisis CML, RI-BPI neither affected the CD34+ CD38− LIC population nor induced cell cycle arrest in CD34+ CD38− LICs. These findings are in agreement with cell cycle deregulation of BCL6−/− CML-like cells and suggest that acute inhibition of BCL6 function can commit CD34+ CD38− LICs to eradication in CML-CP, but not in CML-BC (Fig. 6 C).
Figure 6.
BCL6-inhibition results in depletion and cell cycle arrest of CD34+ CD38− CML cells. Patient-derived leukapheresis samples from 5 patients with CML in CP and 1 patient in myeloid blast crisis CML were incubated in the presence of RI-BPI or vehicle for 2 h, and then washed and incubated with cytokines (100 ng/ml SCF, 10 ng/ml G-CSF, 20 ng/ml FLT3, 20 ng/ml IL-3, and 20 ng/ml IL-6). (A) Cells were stained with CD34 and CD38 cell surface antibodies and analyzed by FACS. (B) Cells were stained with CFSE to track cell divisions over time. The analysis was gated on CD34+ CD38− LICs. (C) Synopsis of the effect of 2 h RI-BPI treatment on CD34+ CD38− LICs (top) and CD34− transient amplifying CML cells (bottom) in 5 cases of CML-CP (green lines) and 1 case of CML-BC (red line).
Concluding remarks
A recent work identified a critical role for FoxO factors in the maintenance of leukemia-initiating cells in CML (Naka et al., 2010). In this study, we demonstrate that BCL6 functions as key effector molecule downstream of FoxO and prevents CML LIC depletion by transcriptional repression of Arf/p53. Although Imatinib successfully induces cell death in transient amplifying CML cells, it fails to eradicate leukemia-initiating cells in CML. In fact, both FoxO activity (Naka et al., 2010) and BCL6 expression levels (Fig. 1) are increased by Imatinib, which provides a rationale for selective survival of leukemia-initiating cells in CML during long-term Imatinib therapy. FoxO factors are critical for LIC maintenance in CML-CP, but not CML-BC (Naka et al., 2010), and BCL6 inhibition leads to the eradication of LICs in CML-CP, but not CML-BC (Fig. 6, A–C). Consistent with these findings, both FoxO3A and BCL6 expression levels are tightly correlated during progression of CML-CP toward CML-BC (Fig. S11). We propose that pharmacological inhibition of BCL6 breaks the quiescence program of LICs in CML and renders them vulnerable to drug treatment, e.g., through derepression of MYC (Fig. S12) and reactivation of the Arf/p53 pathway (Fig. 3 D and Fig. S1). Targeting cellular quiescence for selective leukemia stem cell eradication was recently proposed based on observations on IFN-α–, G-CSF–, or As2O3-mediated activation (“awakening”) of dormant LICs (Essers and Trumpp, 2010; Trumpp et al., 2010).
Here, we show that pharmacological inhibition of BCL6 leads to LIC apoptosis (Fig. S8) and effectively prevents leukemia initiation from xenografted human CML cells in vivo. Based on these findings, we propose a dual targeting strategy, in which tyrosine kinase inhibitors (e.g., Imatinib) to target the transient amplifying pool of CML cells are coupled with BCL6 inhibition that will target quiescent LICs. Pharmacological options include the BCL6 peptide inhibitors used here (Cerchietti et al., 2009) or newly developed small molecule inhibitors against BCL6 (Cerchietti et al., 2010). Pharmacological inhibition of BCL6, thus, represents a fundamentally novel strategy to eradicate LICs in CML. Clinical validation of this concept could limit the duration of TKI treatment in CML patients, which is currently life-long, and potentially decrease the risk of blast crisis transformation.
MATERIALS AND METHODS
Patient samples, human cells and cell lines.
Patient samples (Table S1) were provided from the German CML Study Group (in compliance with the internal review board of the University of Southern California Health Sciences Campus, the University of California San Francisco, the Oregon Health and Science University, and the Universität Heidelberg Klinikum Mannheim). In total, 26 cases of CP CML and 12 cases of CML blast crisis were studied, and the characteristics of these patients are summarized in Table S1 (CP1-CP26 and BC1-BC12). The human CML cell lines EM2, JURL-MK1, KCL22, KU812, KYO, LAMA84, MEG1, and MOLM6 were obtained from the German Collection of Microorganisms and Cell Cultures, Braunschweig, Germany. Human leukemia cells were maintained in RPMI-1640 (Invitrogen) with GlutaMAX containing 20% fetal bovine serum, 100 IU/ml penicillin, and 100 µg/ml streptomycin at 37°C in a humidified incubator with 5% CO2.
CML-like leukemia model and transplantation experiments.
For all retroviral transductions with BCR-ABL1, the myeloid-restricted protocol described in Li et al. (1999) was used, which results in CML-like disease. In brief, bone marrow cells were cultured either in Iscove’s modified Dulbecco’s medium (IMDM; Invitrogen) with GlutaMAX containing 20% fetal bovine serum, 100 IU/ml penicillin, 100 µg/ml streptomycin, 50 µM 2-mercaptoethanol, 10 ng/ml recombinant mouse IL-3, 25 ng/ml recombinant mouse IL-6, and 50 ng/ml recombinant mouse SCF (PeproTech) or retrovirally transformed by BCR-ABL1. Lineage identity of CML-like leukemia cells was authenticated by flow cytometry and quantitative RT-PCR (Fig. S5) and microarray analysis (Fig. S6). Mouse CML-like cells and human CML cells were then labeled with lentiviral firefly luciferase (D.B. Kohn, University of California Los Angeles, Los Angeles, CA; Table S2), selected based on antibiotic resistance (puromycin), and injected either via intrafemoral or intravenous tail vein injection into sublethally irradiated (300 cGy) NOD/SCID recipient mice. Engraftment was monitored using luciferase bioimaging (VIS 100 bioluminescence/optical imaging system; Xenogen). D-Luciferin (Xenogen) dissolved in PBS was injected intraperitoneally at a dose of 2.5 mg per mouse 15 min before measuring the light emission.
Retroviral and lentiviral transduction.
Retroviral constructs encoding BCR-ABL1 (R. Van Etten, Tufts University, Boston, MA), FoxO3A (D.A. Fruman, University of California Irvine, Irvine, CA), dominant-negative BCL6 (DN-BCL6-ERT2), inducible activation of BCL6 (BCL6-ERT2; A.L. Shaffer and L.M. Staudt, National Cancer Institute, Bethesda, MD), Cre-GFP, and empty controls, as well as lentiviral vectors encoding firefly luciferase, were used in transduction experiments as described previously (Duy et al., 2010). A detailed list of vectors and transduction conditions is given in Table S2.
Quantitative RT-PCR.
Total RNA from cells was extracted using RNeasy isolation kit from QIAGEN. cDNA was generated using a poly(dT) oligonucleotide and the SuperScript III Reverse transcription (Invitrogen). Quantitative real-time PCR was performed with the SYBRGreenER mix (Invitrogen) and the ABI7900HT real-time PCR system (Applied Biosystems) according to standard PCR conditions. Primers for quantitative RT-PCR are listed in Table S3.
BCL6−/−, Ptenfl/fl, P53fl/fl, and Stat5a/bfl/fl mice.
Bone marrow from BCL6−/− (R. Dalla-Favera, Columbia University, New York, NY; Ye et al., 1997; Table S5) and Stat5abfl/fl mice (L. Hennighausen, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD; Liu et al., 1997; Table S5) was harvested and hematopoietic progenitor cells were propagated as described above. Deletion of Stat5a/bfl/fl, P53fl/fl, and Ptenfl/fl was induced by retroviral transduction with Cre-GFP (using a GFP empty vector control). Cells were cultured at 37°C in a humidified incubator with 5% CO2. All mouse experiments were subject to institutional approval by Children’s Hospital Los Angeles IACUC.
Western blot analysis.
Cells were lysed in CelLytic buffer (Sigma-Aldrich) supplemented with 1% protease inhibitor cocktail (Thermo Fisher Scientific). 25 µg of protein mixture per sample were separated on NuPAGE (Invitrogen) 4–12% Bis-Tris gradient gels and transferred on PVDF membranes (Immobilion; Millipore). For the detection of mouse and human proteins by Western blot, primary antibodies were used together with the WesternBreeze immunodetection system (Invitrogen). The following antibodies were used: human BCL6 (clones D8 and N3; Santa Cruz Biotechnology, Inc.), mouse BCL6 (rabbit polyclonal; Cell Signaling Technology), Arf (4C6/4; Cell Signaling Technology), p53 (1C12; Cell Signaling Technology), and pan-specific anti-phosphotyrosine (4G10; Millipore). Antibodies against β-actin were used as a loading control (H4; Santa Cruz Biotechnology, Inc.).
Flow cytometry.
Antibodies against mouse CD44 (IM7), c-kit (2B8), and respective isotype controls were purchased from BD. Anti–mouse Sca-1 antibody (clone 177228) was obtained from R&D Systems. For apoptosis analyses, annexin V, propidium iodide, and 7-aminoactinomycin D (7AAD) were used (BD). Antibodies against human CD34 (563), CD38 (HITZ), and CD90 (OX-7), as well as respective isotype controls, were purchased from BD.
Colony-forming assay.
The methylcellulose colony-forming assays were performed with 100,000 normal LSK cells, and BCR-ABL1–transformed mouse CML-like cells or 10,000 human CML cells. Cells were resuspended in mouse MethoCult medium (StemCell Technologies) and cultured on 3-cm diam dishes, with an extra water supply dish to prevent evaporation. After 7–22 d, colony numbers were counted.
Hoechst 33342 staining.
Human CML cells were labeled with Hoechst 33342 according to the protocol from Goodell et al. (1996). In brief, cells were suspended in DME (2% FBS and 10 mmol/liter Hepes buffer) and Hoechst 33342 dye was added (5 µg/ml, 90 min at 37°C). After the incubation with Hoechst 33342, cells were centrifuged and resuspended in cold Hanks’ balanced saline solution (2% FBS, 10 mM Hepes buffer, room temperature). Propidium iodide was added to the cells before analysis.
CFSE staining.
Primary human CML cells were labeled with CFSE (Invitrogen) according to the manufacturer’s protocol. Cells were resuspended in prewarmed PBS (0.1% BSA) and incubated with 0.5 µM CFSE for 10 min at 37°C. Subsequently, 5 volumes of ice-cold PBS were added and cells were incubated for 5 min on ice. After the incubation, cells were washed twice with cold PBS and then cultured in IMDM, 20% BIT, 100 IU/ml penicillin, 100 µg/ml streptomycin, 25 µmol/liter β-mercaptoethanol, 100 ng/ml SCF, 100 ng/ml G-CSF, 20 ng/ml FLT3, 20 ng/ml IL-3, and 20 ng/ml IL-6) for a maximum of 5 d. CFSE levels were measured by flow cytometry together with staining for CD34 and CD38 surface marker expression.
Affymetrix GeneChip analysis.
Biotinylated cRNA was generated and fragmented according to the Affymetrix protocol and hybridized to either a U133A 2.0 human, 430 mouse, or Mouse Gene 1.0 ST microarrays (Affymetrix). After scanning (GeneChip Scanner 3000 7G; Affymetrix), the generated data files were imported to BRB Array Tool and processed using the robust multi-array average algorithm. To determine relative signal intensities, the ratio of intensity for each sample in a probe set was calculated by normalizing to the mean value of grouped samples. Microarray data are available from the Gene Expression Omnibus (GEO) under accession nos. GSE24814 (STAT5-deletion; BCR-ABL1–transformed prep cells), GSE24813 (BCL6+/+ and BCL6−/− BCR-ABL1–transformed CML-like cells), GSE20987 (BCL6+/+ and BCL6−/− BCR-ABL1–transformed pre–B ALL cells), and GSE24493 (Imatinib treated and nontreated human CML cell lines).
Online supplemental material.
Supplementary information for this study includes information on CML patient samples (Table S1), retroviral and lentiviral vectors (Table S2), oligonucleotides used (Table S3), telomere length measurement protocol (Table S4), and an overview of genetic mouse mutants studied (Table S5). Fig. S1 presents a schematic of BCL6-dependent LIC-survival signaling in CML. Validation of gene expression changes were performed in Figs. S2 and S3. Fig. S4 shows transformation efficiency of BCL6+/+ and BCL6−/−hematopoietic progenitor cells. Phenotypic authentication of CML-like leukemia by flow cytometry and RT-PCR and microarray analysis is demonstrated in Figs. S5 and S6, respectively. Fig. S7 describes differential gene expression and side population phenotypes in BCL6+/+ and BCL6−/− CML-like cells. Fig. S8 shows increased propensity to apoptosis of BCL6−/− CML-like cells. Fig. S9 shows colony formation assays for normal BCL6+/+ and BCL6−/− hematopoietic progenitor cells. Fig. S10 summarizes results from telomere length measurements. Fig. S11 shows a meta-analysis of gene expression values of FoxO3a and BCL6. Fig. S12 shows recruitment of BCL6 to cell cycle regulators and the effects of BCL6-activation on cell cycle progression. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20110304/DC1.
Acknowledgments
We would like to thank Riccardo Dalla-Favera for sharing BCL6−/− mice generated in his laboratory and wild-type controls with us, Lothar Hennighausen for Stat5fl/fl mice, David A. Fruman for sharing his FoxO3A reagents with us, and Arthur L. Shaffer and Louis M. Staudt for sharing their inducible BCL6 constructs. We would like to thank Michael R. Lieber and Michael Kahn (Los Angeles, CA) for critical discussions and for CML leukapheresis samples.
This work is supported by grants from the National Institutes of Health/NCI through R01CA137060 (to M. Müschen), R01CA139032 (to M. Müschen), R01CA157644 (to M. Müschen) and R21CA152497 (to M. Müschen), Translational Research Program grants from the Leukemia and Lymphoma Society (grants 6132-09 and 6097-10), a Leukemia and Lymphoma Society SCOR (grant 7005-11; B.J. Druker), the William Laurence and Blanche Hughes Foundation and a Stand Up To Cancer-American Association for Cancer Research Innovative Research Grant (IRG00909, to M. Müschen), and the California Institute for Regenerative Medicine (CIRM; TR2-01816 to M. Müschen). A.M. Melnick and M. Müschen are Scholars of the Leukemia and Lymphoma Society.
The authors have no conflicting financial interests.
Footnotes
Abbreviations used:
- 4-OHT
- 4-hydroxy-tamoxifen
- 7AAD
- 7-aminoactinomycin D
- CMP
- chronic myeloid leukemia
- CP
- chronic phase
- LIC
- leukemia-initiating cell
- LSK
- Lin− Sca-1+ c-Kit+
- RI-BPI
- retro-inverso BCL6 peptide inhibitor
- TKI
- tyrosine kinase inhibitor
References
- Bennett J.H. 1845. Case of hypertrophy of the spleen and liver, in which death took place from suppuration of the blood. Edinburgh Med. Surg. J. 64:413–423 [Google Scholar]
- Bonnet D., Dick J.E. 1997. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat. Med. 3:730–737 10.1038/nm0797-730 [DOI] [PubMed] [Google Scholar]
- Broxmeyer H.E., Sehra S., Cooper S., Toney L.M., Kusam S., Aloor J.J., Marchal C.C., Dinauer M.C., Dent A.L. 2007. Aberrant regulation of hematopoiesis by T cells in BAZF-deficient mice. Mol. Cell. Biol. 27:5275–5285 10.1128/MCB.01967-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruennert D., Czibere A., Bruns I., Kronenwett R., Gattermann N., Haas R., Neumann F. 2009. Early in vivo changes of the transcriptome in Philadelphia chromosome-positive CD34+ cells from patients with chronic myelogenous leukaemia following Imatinib therapy. Leukemia. 23:983–985 10.1038/leu.2008.337 [DOI] [PubMed] [Google Scholar]
- Calabretta B., Perrotti D. 2004. The biology of CML blast crisis. Blood. 103:4010–4022 [DOI] [PubMed] [Google Scholar]
- Cerchietti L.C., Yang S.N., Shaknovich R., Hatzi K., Polo J.M., Chadburn A., Dowdy S.F., Melnick A. 2009. A peptomimetic inhibitor of BCL6 with potent antilymphoma effects in vitro and in vivo. Blood. 113:3397–3405 10.1182/blood-2008-07-168773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerchietti L.C., Ghetu A.F., Zhu X., Da Silva G.F., Zhong S., Matthews M., Bunting K.L., Polo J.M., Farès C., Arrowsmith C.H., et al. 2010. A small-molecule inhibitor of BCL6 kills DLBCL cells in vitro and in vivo. Cancer Cell. 17:400–411 10.1016/j.ccr.2009.12.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbin A.S., Agarwal A., Loriaux M., Cortes J., Deininger M.W., Druker B.J. 2011. Human chronic myeloid leukemia stem cells are insensitive to Imatinib despite inhibition of BCR-ABL activity. J. Clin. Invest. 121:396–409 10.1172/JCI35721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Klein A., van Kessel A.G., Grosveld G., Bartram C.R., Hagemeijer A., Bootsma D., Spurr N.K., Heisterkamp N., Groffen J., Stephenson J.R. 1982. A cellular oncogene is translocated to the Philadelphia chromosome in chronic myelocytic leukaemia. Nature. 300:765–767 10.1038/300765a0 [DOI] [PubMed] [Google Scholar]
- Dent A.L., Shaffer A.L., Yu X., Allman D., Staudt L.M. 1997. Control of inflammation, cytokine expression, and germinal center formation by BCL-6. Science. 276:589–592 10.1126/science.276.5312.589 [DOI] [PubMed] [Google Scholar]
- Druker B.J., Sawyers C.L., Kantarjian H., Resta D.J., Reese S.F., Ford J.M., Capdeville R., Talpaz M. 2001. Activity of a specific inhibitor of the BCR-ABL tyrosine kinase in the blast crisis of chronic myeloid leukemia and acute lymphoblastic leukemia with the Philadelphia chromosome. N. Engl. J. Med. 344:1038–1042 10.1056/NEJM200104053441402 [DOI] [PubMed] [Google Scholar]
- Druker B.J., Guilhot F., O’Brien S.G., Gathmann I., Kantarjian H., Gattermann N., Deininger M.W., Silver R.T., Goldman J.M., Stone R.M., et al. ; IRIS Investigators 2006. Five-year follow-up of patients receiving Imatinib for chronic myeloid leukemia. N. Engl. J. Med. 355:2408–2417 10.1056/NEJMoa062867 [DOI] [PubMed] [Google Scholar]
- Duy C., Yu J.J., Nahar R., Swaminathan S., Kweon S.M., Polo J.M., Valls E., Klemm L., Shojaee S., Cerchietti L., et al. 2010. BCL6 is critical for the development of a diverse primary B cell repertoire. J. Exp. Med. 207:1209–1221 10.1084/jem.20091299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duy C., Hurtz C., Shojaee S., Cerchietti L., Geng H., Swaminathan S., Klemm L., Kweon S.M., Nahar R., Braig M., et al. 2011. BCL6 enables Ph+ acute lymphoblastic leukaemia cells to survive BCR-ABL1 kinase inhibition. Nature. 473:384–388 10.1038/nature09883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essers M.A., Trumpp A. 2010. Targeting leukemic stem cells by breaking their dormancy. Mol. Oncol. 4:443–450 10.1016/j.molonc.2010.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández de Mattos S., Essafi A., Soeiro I., Pietersen A.M., Birkenkamp K.U., Edwards C.S., Martino A., Nelson B.H., Francis J.M., Jones M.C., et al. 2004. FoxO3a and BCR-ABL regulate cyclin D2 transcription through a STAT5/BCL6-dependent mechanism. Mol. Cell. Biol. 24:10058–10071 10.1128/MCB.24.22.10058-10071.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodell M.A., Brose K., Paradis G., Conner A.S., Mulligan R.C. 1996. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J. Exp. Med. 183:1797–1806 10.1084/jem.183.4.1797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan M.K., Yaguchi T., Sugihara T., Kumar P.K., Taira K., Reddel R.R., Kaul S.C., Wadhwa R. 2002. CARF is a novel protein that cooperates with mouse p19ARF (human p14ARF) in activating p53. J. Biol. Chem. 277:37765–37770 10.1074/jbc.M204177200 [DOI] [PubMed] [Google Scholar]
- Hu Y., Swerdlow S., Duffy T.M., Weinmann R., Lee F.Y., Li S. 2006. Targeting multiple kinase pathways in leukemic progenitors and stem cells is essential for improved treatment of Ph+ leukemia in mice. Proc. Natl. Acad. Sci. USA. 103:16870–16875 10.1073/pnas.0606509103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K., Bernardi R., Morotti A., Matsuoka S., Saglio G., Ikeda Y., Rosenblatt J., Avigan D.E., Teruya-Feldstein J., Pandolfi P.P. 2008. PML targeting eradicates quiescent leukaemia-initiating cells. Nature. 453:1072–1078 10.1038/nature07016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantarjian H.M., Shan J., Jones D., O’Brien S., Rios M.B., Jabbour E., Cortes J. 2009. Significance of increasing levels of minimal residual disease in patients with Philadelphia chromosome-positive chronic myelogenous leukemia in complete cytogenetic response. J. Clin. Oncol. 27:3659–3663 10.1200/JCO.2008.18.6999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemm L., Duy C., Iacobucci I., Kuchen S., von Levetzow G., Feldhahn N., Henke N., Li Z., Hoffmann T.K., Kim Y.M., et al. 2009. The B cell mutator AID promotes B lymphoid blast crisis and drug resistance in chronic myeloid leukemia. Cancer Cell. 16:232–245 10.1016/j.ccr.2009.07.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause D.S., Lazarides K., von Andrian U.H., Van Etten R.A. 2006. Requirement for CD44 in homing and engraftment of BCR-ABL-expressing leukemic stem cells. Nat. Med. 12:1175–1180 10.1038/nm1489 [DOI] [PubMed] [Google Scholar]
- Li Q., Dang C.V. 1999. c-Myc overexpression uncouples DNA replication from mitosis. Mol. Cell. Biol. 19:5339–5351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Ilaria R.L., Jr, Million R.P., Daley G.Q., Van Etten R.A. 1999. The P190, P210, and P230 forms of the BCR/ABL oncogene induce a similar chronic myeloid leukemia-like syndrome in mice but have different lymphoid leukemogenic activity. J. Exp. Med. 189:1399–1412 10.1084/jem.189.9.1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Robinson G.W., Wagner K.U., Garrett L., Wynshaw-Boris A., Hennighausen L. 1997. Stat5a is mandatory for adult mammary gland development and lactogenesis. Genes Dev. 11:179–186 10.1101/gad.11.2.179 [DOI] [PubMed] [Google Scholar]
- Melo J.V., Barnes D.J. 2007. Chronic myeloid leukaemia as a model of disease evolution in human cancer. Nat. Rev. Cancer. 7:441–453 10.1038/nrc2147 [DOI] [PubMed] [Google Scholar]
- Mendez L.M., Polo J.M., Yu J.J., Krupski M., Ding B.B., Melnick A., Ye B.H. 2008. CtBP is an essential corepressor for BCL6 autoregulation. Mol. Cell. Biol. 28:2175–2186 10.1128/MCB.01400-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naka K., Hoshii T., Muraguchi T., Tadokoro Y., Ooshio T., Kondo Y., Nakao S., Motoyama N., Hirao A. 2010. TGF-beta-FOXO signalling maintains leukaemia-initiating cells in chronic myeloid leukaemia. Nature. 463:676–680 10.1038/nature08734 [DOI] [PubMed] [Google Scholar]
- Neering S.J., Bushnell T., Sozer S., Ashton J., Rossi R.M., Wang P.Y., Bell D.R., Heinrich D., Bottaro A., Jordan C.T. 2007. Leukemia stem cells in a genetically defined murine model of blast-crisis CML. Blood. 110:2578–2585 10.1182/blood-2007-02-073031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oguro H., Iwama A., Morita Y., Kamijo T., van Lohuizen M., Nakauchi H. 2006. Differential impact of Ink4a and Arf on hematopoietic stem cells and their bone marrow microenvironment in Bmi1-deficient mice. J. Exp. Med. 203:2247–2253 10.1084/jem.20052477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pear W.S., Miller J.P., Xu L., Pui J.C., Soffer B., Quackenbush R.C., Pendergast A.M., Bronson R., Aster J.C., Scott M.L., Baltimore D. 1998. Efficient and rapid induction of a chronic myelogenous leukemia-like myeloproliferative disease in mice receiving P210 bcr/abl-transduced bone marrow. Blood. 92:3780–3792 [PubMed] [Google Scholar]
- Phan R.T., Dalla-Favera R. 2004. The BCL6 proto-oncogene suppresses p53 expression in germinal-centre B cells. Nature. 432:635–639 10.1038/nature03147 [DOI] [PubMed] [Google Scholar]
- Radich J.P., Dai H., Mao M., Oehler V., Schelter J., Druker B.J., Sawyers C., Shah N., Stock W., Willman C.L., et al. 2006. Gene expression changes associated with progression and response in chronic myeloid leukemia. Proc. Natl. Acad. Sci. USA. 103:2794–2799 10.1073/pnas.0510423103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousselot P., Huguet F., Rea D., Legros L., Cayuela J.M., Maarek O., Blanchet O., Marit G., Gluckman E., Reiffers J., et al. 2007. Imatinib mesylate discontinuation in patients with chronic myelogenous leukemia in complete molecular remission for more than 2 years. Blood. 109:58–60 10.1182/blood-2006-03-011239 [DOI] [PubMed] [Google Scholar]
- Rowley J.D. 1973. Letter: A new consistent chromosomal abnormality in chronic myelogenous leukaemia identified by quinacrine fluorescence and Giemsa staining. Nature. 243:290–293 10.1038/243290a0 [DOI] [PubMed] [Google Scholar]
- Shaffer A.L., Yu X., He Y., Boldrick J., Chan E.P., Staudt L.M. 2000. BCL-6 represses genes that function in lymphocyte differentiation, inflammation, and cell cycle control. Immunity. 13:199–212 10.1016/S1074-7613(00)00020-0 [DOI] [PubMed] [Google Scholar]
- Shah N.P., Nicoll J.M., Nagar B., Gorre M.E., Paquette R.L., Kuriyan J., Sawyers C.L. 2002. Multiple BCR-ABL kinase domain mutations confer polyclonal resistance to the tyrosine kinase inhibitor Imatinib (STI571) in chronic phase and blast crisis chronic myeloid leukemia. Cancer Cell. 2:117–125 10.1016/S1535-6108(02)00096-X [DOI] [PubMed] [Google Scholar]
- Tran H., Brunet A., Grenier J.M., Datta S.R., Fornace A.J., Jr, DiStefano P.S., Chiang L.W., Greenberg M.E. 2002. DNA repair pathway stimulated by the forkhead transcription factor FOXO3a through the Gadd45 protein. Science. 296:530–534 10.1126/science.1068712 [DOI] [PubMed] [Google Scholar]
- Trotman L.C., Alimonti A., Scaglioni P.P., Koutcher J.A., Cordon-Cardo C., Pandolfi P.P. 2006. Identification of a tumour suppressor network opposing nuclear Akt function. Nature. 441:523–527 10.1038/nature04809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trumpp A., Essers M., Wilson A. 2010. Awakening dormant haematopoietic stem cells. Nat. Rev. Immunol. 10:201–209 10.1038/nri2726 [DOI] [PubMed] [Google Scholar]
- Virchow R. 1845. Weisses Blut. Frorieps.Notizen. 36:151–156 [Google Scholar]
- Ye B.H., Chaganti S., Chang C.C., Niu H., Corradini P., Chaganti R.S., Dalla-Favera R. 1995. Chromosomal translocations cause deregulated BCL6 expression by promoter substitution in B cell lymphoma. EMBO J. 14:6209–6217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye B.H., Cattoretti G., Shen Q., Zhang J., Hawe N., de Waard R., Leung C., Nouri-Shirazi M., Orazi A., Chaganti R.S., et al. 1997. The BCL-6 proto-oncogene controls germinal-centre formation and Th2-type inflammation. Nat. Genet. 16:161–170 10.1038/ng0697-161 [DOI] [PubMed] [Google Scholar]
- Zhao C., Blum J., Chen A., Kwon H.Y., Jung S.H., Cook J.M., Lagoo A., Reya T. 2007. Loss of beta-catenin impairs the renewal of normal and CML stem cells in vivo. Cancer Cell. 12:528–541 10.1016/j.ccr.2007.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C., Chen A., Jamieson C.H., Fereshteh M., Abrahamsson A., Blum J., Kwon H.Y., Kim J., Chute J.P., Rizzieri D., et al. 2009. Hedgehog signalling is essential for maintenance of cancer stem cells in myeloid leukaemia. Nature. 458:776–779 10.1038/nature07737 [DOI] [PMC free article] [PubMed] [Google Scholar]