Transcription factors c-Rel and RelA/p65 bind and activate two Rorg promoters to drive Th17 differentiation.
Abstract
The Th17 cells use the retinoid-related orphan receptor-γ (Rorg or Rorc) to specify their differentiation and lineage-specific function. However, how Rorg is switched on during Th17 differentiation is unknown. We report here that c-Rel and RelA/p65 transcription factors drive Th17 differentiation by binding to and activating two distinct Rorg promoters that control RORγT and RORγ expression, respectively. Similar to RORγT, RORγ is selectively expressed in Th17 cells and is effective in specifying the Th17 phenotype. T cells deficient in c-Rel or RelA are significantly compromised in Th17 differentiation, and c-Rel–deficient mice are defective in Th17 responses. Thus, Th17 immunity is controlled by a Rel–RORγ–RORγT axis, and strategies targeting Rel/NF-κB can be effective for controlling Th17 cell–mediated diseases.
Th17 cells are a subset of T cells that produce IL-17A, IL-17F, IL-6, and TNF (Cua et al., 2003; Langrish et al., 2005; Veldhoen and Stockinger, 2006; Ivanov et al., 2006; Weaver et al., 2006; Bettelli et al., 2006; Sutton et al., 2006). They are involved primarily in mediating inflammatory diseases and immune defense against extracellular bacteria. Th17 cells can be generated from naive precursors in the presence of TGF-β and IL-6 or IL-21 (Bettelli et al., 2006), which can be further augmented by TNF or IL-1β (inflammatory cytokines that inhibit Th1 and Th2 cell differentiation; Sutton et al., 2006; Chung et al., 2009). Developments of Th1, Th2, and regulatory T cells (T reg cells) are specified by transcription factors such as Tbet, GATA3, and Foxp3, respectively, whereas that of Th17 cells appears to depend on the retinoid-related orphan receptor (ROR) γT, RORα, and signal transducer and activator of transcription (STAT3; Ivanov et al., 2006; Yang et al., 2007, 2008).
RORγT and its isoform RORγ are encoded by a single gene called Rorg (also known as Rorc). Both isoforms use the last 9 exons (exons 3–11) of the Rorg gene, but the other exons used by them are different. As a consequence, the RORγT mRNA differs from that of RORγ in the first 100 nt, which translate into distinct N-terminal aa sequences (He et al., 1998; Villey et al., 1999). Nonetheless, both isoforms have the same DNA-binding domain and ligand-binding domain. In fact, RORγ contains all but three N-terminal aa of RORγT, which has a total of 495 aa (RORγ has 516 aa). The two isoforms differ in their expression patterns. RORγT is preferentially expressed in the thymus and differentiated Th17 cells, whereas RORγ is expressed in a variety of organ systems, including muscle, kidney, and liver, although its expression in Th17 cells has not been examined. Whether the two isoforms are generated as a result of alternative usage of different promoters or alternative splicing of a common pre-mRNA is not clear. RORγT is considered to be a lineage-specific marker of Th17 cells. It is induced during Th17 differentiation, and can directly activate genes encoding IL-17A and IL-17F. RORγT-deficient T cells are significantly compromised in their Th17 differentiation program (Ivanov et al., 2006). Whether and to what degree the RORγ isoform plays a role in Th17 differentiation is unknown.
The mammalian Rel/NF-κB family consists of five members: c-Rel, p65 (or RelA), RelB, NF-κB1 (p50/p105), and NF-κB2 (p52/p100; Beg and Baltimore, 1996; Barnes and Karin, 1997). Unlike other members that are constitutively expressed in multiple cell types, c-Rel is expressed primarily in lymphoid tissues by lymphoid and myeloid cells (Brownell et al., 1987; Simek and Rice, 1988; Wang et al., 1997; Huguet et al., 1998; Gerondakis et al., 1998). c-Rel–deficient mice do not suffer from developmental problems or infectious diseases, and c-Rel–deficient T cells are competent in survival but are significantly compromised in TCR-induced gene expression (Köntgen et al., 1995; Tumang et al., 1998; Hilliard et al., 2002; Bunting et al., 2007). We report here that c-Rel and p65 play a crucial role in activating the Rorg gene that is required for initiating Th17 differentiation.
RESULTS
T cells deficient in c-Rel or RelA/p65 are significantly compromised in Th17 differentiation and Th17 responses
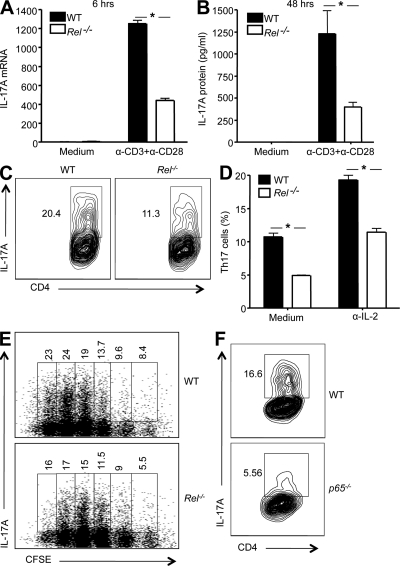
To determine the potential roles of c-Rel in Th17 immunity, we compared WT and c-Rel–deficient T cells for their Th17 responses. We found that CD4+ T cells freshly isolated from c-Rel–deficient mice produced significantly less IL-17A mRNA and protein upon activation with anti-CD3 and anti-CD28 (Fig. 1, A and B). This was associated with reduced levels of both RORγ and RORγT mRNAs as compared with WT cells (Fig. S1 A). Consistent with this result, we found that c-Rel deficiency caused a 46% reduction in the frequency of Th17 cells in the lung (1.1 ± 0.1% in WT as compared with 0.6 ± 0.05% in c-Rel KO mice), and 44% reduction in the Peyer’s patches (1.6 ± 0.1% in WT as compared with 0.9 ± 0.07% in c-Rel KO mice; P < 0.03 for both organs). Importantly, when purified naive CD4+ T cells were cultured under Th17 cell–inducing conditions, IL-17A expression was significantly reduced in the c-Rel–deficient group, leading to ∼50% reduction in Th17 cell frequencies (Fig. 1, C and D); this reduction was more dramatic (up to 80%) if anti–IL-2 was added to the culture twice and phorbol myristate acetate (PMA) and ionomycin were not used to stimulate the cells (Fig. S1 B). c-Rel deficiency not only reduced the Th17 cell frequency, but also decreased the levels of IL-17 in differentiated Th17 cells by ∼50% (Fig. S1 C). Because IL-2 represses Th17 differentiation through Stat5 (Laurence et al., 2007), the percentage of Th17 cells was higher in the presence of anti–IL-2.
Figure 1.
T cells deficient in c-Rel or p65 are significantly compromised in their IL-17 gene expression and Th17 differentiation. (A and B) CD4+ T cells were isolated from 6-wk-old WT and Rel−/− mice (n = 5), and stimulated with anti-CD3 and anti-CD28 in the presence of anti–IL-2 and anti–IFN-γ for 6 h (A), or 48 h (B). Total RNA was extracted, and IL-17 mRNA levels were determined by real-time RT-PCR (A). The cytokine concentrations in the culture supernatants were determined by ELISA (B). (C and D) Purified CD4+ naive splenic T cells from WT and Rel−/− mice (n = 5) were cultured under Th17 cell–inducing conditions, as described in Materials and methods, with or without anti–IL-2 for 3 d. Cells were then restimulated with PMA and ionomycin in the presence of GolgiStop for 4 h, stained intracellularly with antibodies to IL-17A, and analyzed by flow cytometry. In C, IL-17A expression level of cells cultured with anti–IL-2 was plotted against CD4. In D, the percentages of IL-17A-expressing cells (as shown in C) from each group were compared. Error bars indicate the SDs of the means. (E) Purified CD4+ naive splenic T cells from WT and Rel−/− mice (n = 3) were incubated with 5 µM of CFSE for 10 min. After washing, cells were cultured under the Th17 cell–inducing condition, as described in Materials and methods. 3 d later, cells were restimulated with PMA and ionomycin in the presence of GolgiStop for 4 h, stained intracellularly with antibodies to IL-17A, and analyzed by flow cytometry. Each numerical figure in the graph represents the percentage of cells with the same number of divisions in the gated area. (F) p65−/− and p65+/+ chimeric mice were generated by adoptively transferring p65−/− and p65+/+ fetal liver cells, respectively, into irradiated B6 recipients as previously described (Ouaaz et al., 2002). 6 wk later, mice were sacrificed and CD4+ naive splenic T cells isolated. Cells were cultured under Th17 cell–inducing medium for 3 d, as described in Materials and methods. They were then restimulated and tested as described in C. The number in each panel represents the percentage of gated IL-17A–producing cells in total CD4+ cells. Results are representative of three independent experiments. *, P < 0.01.
In addition to IL-6, IL-1 and IL-23 can also drive Th17 cell differentiation. The effect of c-Rel deficiency on IL-1– and IL-23–induced Th17 cell differentiation was even more dramatic, reducing the Th17 cell frequency by ∼80% (Fig. S1 D). It has been reported that IL-17+IFN-γ+ cells can be induced by IL-6, IL-23, and IL-1. Because c-Rel also regulates IFN-γ expression, we next examined whether c-Rel deficiency affected the generation of IL-17+IFN-γ+ cells. We found that the frequency of IL-17 and IFN-γ double-positive cells was markedly reduced in c-Rel–deficient cultures (Fig. S1 E). Rel−/− T cells may have reduced proliferation (Hilliard et al., 2002). To determine whether c-Rel regulates Th17 cell differentiation independent of its effect on cell proliferation, we repeated the Th17 differentiation experiment using naive CD4+ T cells labeled with CFSE, which allows direct tracking of cell divisions by flow cytometry. As expected, Rel−/− T cells have reduced proliferation (Fig. S2 A). Th17 differentiation peaked after the fourth cell division in both WT and Rel−/− groups (Fig. 1 E). Importantly, the percentage of Th17 cells generated was substantially reduced in the Rel−/− group even when cells that had undergone the same number of cell divisions were compared. Although a small population of cells that had undergone no division or less than two divisions also produced low levels of IL-17 (as indicated by a weak IL-17 signal; Fig. 1 E), they were likely uncommitted Th0-type cells, not Th17 cells, and were less affected by the c-Rel deficiency. T cell death as determined by flow cytometry was not significantly affected by the c-Rel deficiency. Rel−/− mice used in this study were >7 wk old, and did not have significantly reduced splenic CD4+ T cells as compared with WT mice. There were no significant differences in the expression of CD44 and CCR6 between WT and Rel−/− T cells (Fig. S2 B). Upon activation, a low percentage of Rel−/− T cells produced IFN-γ, but few WT or Rel−/− T cells produced IL-22 (Fig. S2 C).
Because significant numbers of Th17 cells were generated in the absence of c-Rel, we asked whether other members of the Rel/NF-κB family played a role in Th17 differentiation. We found that p65−/− CD4+ T cells had a greater defect in Th17 differentiation than Rel−/− T cells (Fig. 1 F). Up to 70% reduction in Th17 cell frequencies was observed in the p65−/− group as compared with the WT group. Using CFSE-labeled p65−/− cells, we found that p65 deficiency significantly blocked Th17 differentiation, even when cells that had undergone same numbers of divisions were compared (Fig. S3 A). Specifically, the percentages of Th17 cells were reduced from 32.2% in WT to 12.5% in p65 cultures after four divisions, and from 19.9% in WT to 11.5% in p65 cultures after three divisions (P < 0.01). To ensure that the culture condition we used was effective in driving Th17 differentiation, unlabeled cells were also cultured in a nondifferentiation medium. Few IL-17–producing cells were generated in the latter medium (Fig. S3 B). Because p65 deficiency causes embryonic lethality, fetal liver transfer was performed to generate the p65-deficient cells, as previously described (Ouaaz et al., 2002). In contrast to c-Rel and p65, p50 and RelB were dispensable because T cells deficient in them exhibited no defects in Th17 cell differentiation (unpublished data). These results indicate that c-Rel and p65 play critical roles in Th17 responses, and deficiency in one may only be partially compensated by the other.
RORγ and RORγT expression is markedly reduced in c-Rel–deficient T cells, and their reconstitution rescues the Th17 defect
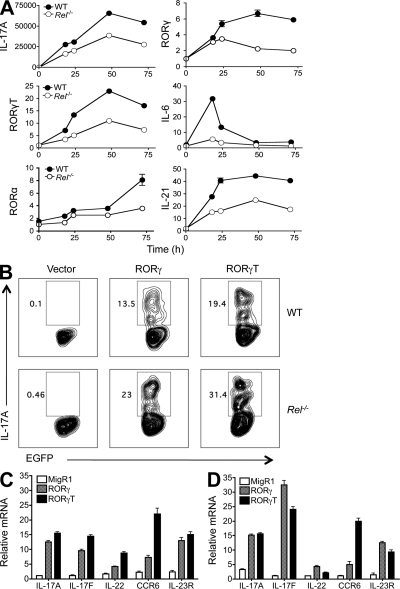
c-Rel–deficient T cells have a defect in their Th1 but not Th2 responses (Hilliard et al., 2002). To establish the degree to which Th17 response was affected in these cells, we examined the expression of seven Th17-related genes at different stages of Th17 differentiation. We found that c-Rel deficiency significantly reduced the expression of RORγT, RORγ, RORα4, IL-6, IL-17A, and IL-21, but not Stat3 mRNAs (Fig. 2 A and Fig. S4 A). As expected, RORγT and RORγ protein levels were also markedly reduced in c-Rel–deficient T cells (Fig. S4 B). Because RORγT plays a key role in Th17 differentiation, we next examined whether RORγT or RORγ reconstitution in c-Rel–deficient cells could rescue the Th17 defect. After infection of T cells with a retrovirus that carried either RORγT or RORγ cDNA, significant increases in the number of Th17 cells were observed in both WT and c-Rel–deficient groups. This was true for T cells cultured under either Th0 (Fig. 2 B) or Th17 (Fig. S4 C) cell–inducing conditions. RORγT and RORγ were both effective in driving IL-17 expression, and coexpressing them together did not appear to generate any synergistic effect (Fig. S4 C; unpublished data). The difference in the frequency of IL-17A producers between WT and c-Rel–deficient cells (Fig. 2 B) was most likely caused by the increased IL-2 production in WT cultures (Hilliard et al., 2002). Importantly, similar to the reported effect of RORγT, RORγ also drove the expression of other Th17 signature molecules such as IL-17F, CCR6, IL-22, and IL-23R (Fig. 2, C and D). Because T cell proliferation may affect Th17 cell development, we also examined whether enforced expression of RORγ or RORγT had any effect on the proliferation of Rel−/− cells. Our data showed that enforced expression of neither RORγ nor RORγT rescued the proliferation defect of Rel−/− T cells (Fig. S5, A and B).
Figure 2.
RORγ and RORγT expression is significantly reduced in c-Rel–deficient T cells, and RORγ reconstitution rescues the Th17 defect. (A) Purified naive CD4+ splenic T cells from 6-wk-old WT (n = 5) and Rel−/− (n = 5) mice were cultured under Th17 differentiation condition as described in Materials and methods. Cells were collected at different time points as indicated, and total RNA was extracted. The mRNA levels of the indicated genes were determined by real-time RT-PCR and normalized to GAPDH. Error bars indicate the SDs of the means, which are visible only if they are bigger than the diameters of the symbols. (B) Purified splenic CD4+ T cells from WT (n = 5) and Rel−/− mice (n = 5) were cultured under Th0-inducing conditions in the presence of anti–IL-4 and anti–IFN-γ for 24 h. Cells were then infected with recombinant retroviruses that carry EGFP (Vector as a control), RORγ-EGFP (RORγ), or RORγt-EGFP (RORγt) cDNA as described in Materials and methods. 4 d later, cells were restimulated with PMA and ionomycin and stained intracellularly with anti–IL-17A, and analyzed by flow cytometry. Data shown are for gated EGFP+ cells. Results are representative of two independent experiments. (C and D) Splenic CD4+ T cells were isolated from 6-wk-old WT (C) and Rel−/− (D) mice (n = 3), and infected with recombinant retroviruses that carry EGFP (vector control), RORγ-EGFP, or RORγT-EGFP cDNA, as described in B. 4 d later, EGFP+ cells were sorted, total RNA was extracted, and mRNA levels of the indicated genes were determined by real-time RT-PCR. Data shown are fold increases over vector-treated groups, and are means and SD of triplicate cultures. Results are representative of two independent experiments.
Similar to RORγT, RORγ is preferentially expressed in Th17 cells and is effective in driving Th17 differentiation
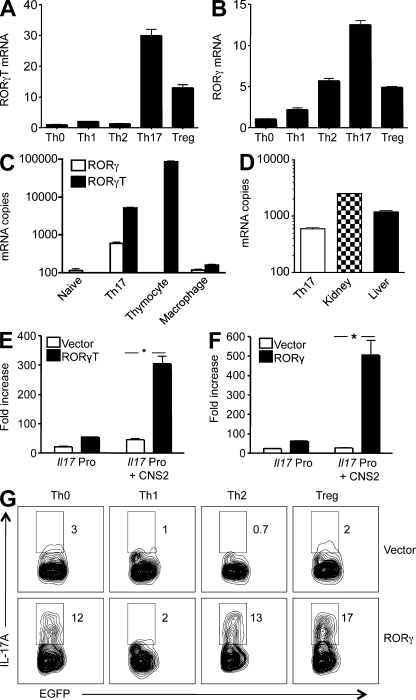
Although it is well recognized that RORγT plays a key role in specifying Th17 differentiation, whether RORγ plays a similar role is unknown. During in vitro Th cell differentiation, Th17-like cells expressed the highest levels of RORγ, in addition to RORγT (Fig. 3, A and B), although RORγT was clearly more abundant than RORγ (Fig. 3 C). T reg cell–like cells also expressed RORγ and RORγT during the differentiation stage, although their levels were much lower. Low levels of RORγ, but not RORγT, were also detected in Th1- and Th2-like cells at this stage (Fig. 3, A and B). RORγ is known to be expressed in nonhematopoietic cells, with the highest expression levels detected in the kidney and liver. We therefore compared RORγ mRNA copy numbers in Th17 cells to those in the kidney and liver. We found that the RORγ mRNA in Th17 cells was approximately one-half and one-fourth of those in the liver and kidney, respectively (Fig. 3 D). These results indicate that RORγ, in addition to RORγT, may be involved in specifying Th17 differentiation. Indeed, RORγ was at least as effective as RORγT in activating the Il17a promoter (Fig. 3, E and F). And enforced expression of RORγ through retroviral gene transfer induced Th17 differentiation even under Th0-, Th2-, and T reg cell–polarizing conditions (Fig. 3 G). Enforced expression of RORγ also decreased IL-4 levels under Th2-polarizing condition, but not IFN-γ under Th1-polarizing condition or Foxp3 under T reg cell–polarizing condition (Fig. S6 A). Because it has been reported that RORγT activates the Il17a gene by binding to its conserved noncoding sequence (CNS) 2 region (Yang et al., 2008), we performed chromatin immunoprecipitation (ChIP) analysis of this region for RORγ binding. Because the currently available anti-RORγ antibodies do not distinguish RORγ from RORγT, we infected CD4+ T cells with a retrovirus that encodes Flag-tagged RORγ, or a combination of equal amounts of RORγ-Flag and RORγT retroviruses. RORγ-Flag binding to the Il17a gene was detected in both conditions, although RORγT retrovirus infection reduced the RORγ-Flag binding suggesting competition between RORγ and RORγT (Fig. S6 B). Collectively, these data indicate that in addition to RORγT, RORγ may also be involved in driving Th17 differentiation.
Figure 3.
RORγT and RORγ activate the Il17 promoter and drive Th17 differentiation. (A and B) Purified CD4+ T cells were cultured under conditions that induce Th0, Th1, Th2, Th17, and T reg cells as described in Materials and methods. 3 d later, cells were harvested and restimulated with PMA and ionomycin for an additional 3 h. The RORγT (A) and RORγ (B) mRNA levels were determined by real-time RT-PCR, and are presented as fold increases over that of the Th0 cells. Data shown are representative of two independent experiments. (C and D) Total RNA was extracted from naive CD4+ T cells (Naive), Th17 cells, thymocytes, peritoneal macrophages, kidney, and liver of B6 mice as indicated. The mRNA copy numbers of RORγ and RORγT were determined by real-time RT-PCR using the respective cDNAs as standards. (E and F) EL4 T cells were transiently transfected with luciferase constructs of the murine Il17a promoter (Il17 Pro) or Il17a promoter linked to its CNS2 (Il17 Pro + CNS2), together with an expression vector for full-length RORγT (C) or RORγ (D), or the empty vector as indicated. After 24 h, cells were treated with PMA and ionomycin for 4 h, and the luciferase activities measured. The promoter activity is presented as fold increase over cells transfected with the empty vector. To normalize the transfection efficiency across samples, the Renilla luciferase expression vector pRLTK was used as an internal control. Data are representative of two independent experiments. *, P < 0.01. (G) Purified CD4+ T cells were cultured under conditions that induce Th0, Th1, Th2, Th17, and T reg cells for 24 h, and then infected with retroviruses that encode EGFP (Vector) or RORγ and EGFP (RORγ) as described in Materials and methods. 4 d later, cells were restimulated with PMA and ionomycin in the presence of GolgiStop for 4 h, stained intracellularly with antibodies to IL-17A, and analyzed by flow cytometry. Data shown are for gated EGFP+ cells. The experiment was repeated twice with similar results.
RORγ and RORγT expression is controlled by two different promoters in Th17 cells
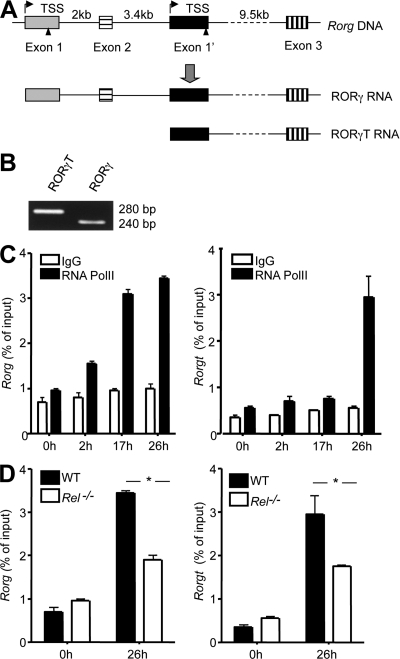
The Rorg gene encodes both RORγ and RORγT that are different in their N-terminal sequences. Whether this is a result of differential usage of different promoters or differential splicing of the same pre-mRNA in Th17 cells is not clear. To address this issue, we undertook three complementary approaches. First, we performed 5′-RACE (rapid amplification of cDNA ends) using a cap-dependent RACE kit to identify the transcription start sites (TSS) of Rorg mRNA in newly differentiated Th17 cells. This led to the discovery of two different types of ROR mRNAs in Th17 cells, with unique 5′ capped ends corresponding to two predicted TSS of the Rorg gene (Fig. 4, A and B; Fig. S7; and Fig. S8). The Rorgt TSS we identified in Th17 cells is identical to that reported for thymocytes (Xi et al., 2006), whereas the Rorg TSS we identified is the same as the one reported for hepatocytes (Medvedev et al., 1997).
Figure 4.
Identification of two Rorg promoters that control RORγ and RORγT expression in Th17 cells. (A) The translation start sites for RORγ and RORγT promoters are indicated by triangles and their TSS marked with arrows. Only 3 of the 11 Rorg exons are shown. (B) Purified CD4+ T cells from 6-wk-old WT mice (n = 3) were cultured under Th17 differentiation condition for 17 h, as described in Materials and methods. Total RNA was extracted and treated with RNase-free DNase I. 5′-RACE was performed and the PCR products of the 5′ ends of RORγ and RORγt mRNA were visualized by agarose gel electrophoresis (which were then cloned and sequenced). (C) Purified CD4+ T cells from 6-wk-old WT mice (n = 3) were cultured under Th17 differentiation conditions, as described in Materials and methods. At the indicated times, cells were fixed, and ChIP was performed using antibodies to RNA polymerase II (PolII) or control IgG. (D) Purified naive CD4+ T cells from WT (n = 3) and Rel−/− mice (n = 3) were cultured under Th17 differentiation conditions, as described in Materials and methods. At the indicated times, cells were fixed, and ChIP was performed using antibodies to PolII. Error bars indicate the SEM. Data are representative of two independent experiments (*, P < 0.05).
Second, we asked whether the RNA polymerase II (PolII) was able to bind to the two putative Rorg promoters during Th17 differentiation by ChIP. We found that although they followed different kinetics, both Rorg and Rorgt promoters associated with PolII during Th17 differentiation, especially at a later stage (Fig. 4 C). As expected, the binding was significantly reduced in Rel−/− cells (Fig. 4 D). Each Rorg promoter contains several CNS regions (Fig. S7 and Fig. S8). A TATA box and putative binding sites for several transcription factors including SP1, NFAT, AP-1, and GATA3, as well as Rel/NF-κB, are present within, or close to, the CNS-1 region. Collectively, these results indicate that differential promoter usage is likely responsible for the production of the two isoforms of the Rorg proteins.
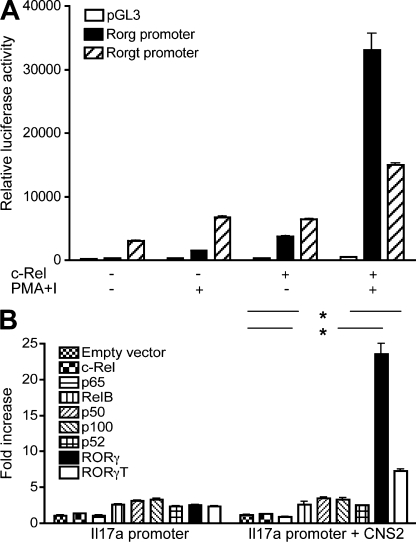
Third, we tested whether the putative Rorg promoter (nt −771 to +98; Fig. S7) and the Rorgt promoter (nt −400 to +151; Fig. S8) had any detectable promoter activity in the Th17-like EL-4 cells. We found that although the Rorgt promoter was less sensitive than the Rorg promoter to stimulation, both promoter constructs could drive luciferase expression (Fig. 5 A). In fact, even in the absence of stimulation, the Rorgt promoter had a detectable basal activity. This is reminiscent of the study by Xi et al. that Rorgt promoter is constitutively active in a thymocyte cell line (Xi et al., 2006).
Figure 5.
Rorg and Il17 promoters. (A) EL4 T cells were transfected with empty pGL3 vector, Rorg, or Rorgt promoter constructs with or without a c-Rel expression plasmid as indicated. After 24 h, cells were treated with or without 50 ng/ml PMA and 1 µM ionomycin (I) for 5 h and the luciferase activities of whole-cell lysates were analyzed as described in Materials and methods using a dual-luciferase reporter assay system. (B) EL4 cells were transiently transfected with murine Il17a core promoter or Il17a core promoter-CNS2 luciferase construct together with an expression vector for full-length RORγT, RORγ, p50, p52, c-Rel, p65, p100, or RelB, or the empty vector as indicated. After 24 h, cells were treated with PMA and ionomycin for 4 h, and the luciferase activities measured. The promoter activity is presented as fold increase over cells transfected with empty vector. To normalize the transfection efficiency across samples, the Renilla luciferase expression vector pRLTK was included as an internal control. Data are representative of two independent experiments. *, P < 0.01.
Rel activates both Rorg and Rorgt promoters, but not those of Rora4 and Il17a
Although c-Rel–deficient T cells have a defect in IL-17A and RORα expression, c-Rel does not appear to regulate their promoter activities. Thus, the Il17a promoter construct containing its CNS2 enhancer was readily activated by RORγ and RORγT, but not by any members of the NF-κB family (Fig. 5 B). Similarly, c-Rel did not activate the promoter construct of Rora4 (our unpublished data). These results indicate that the c-Rel effect on Th17 cells may be gene-specific.
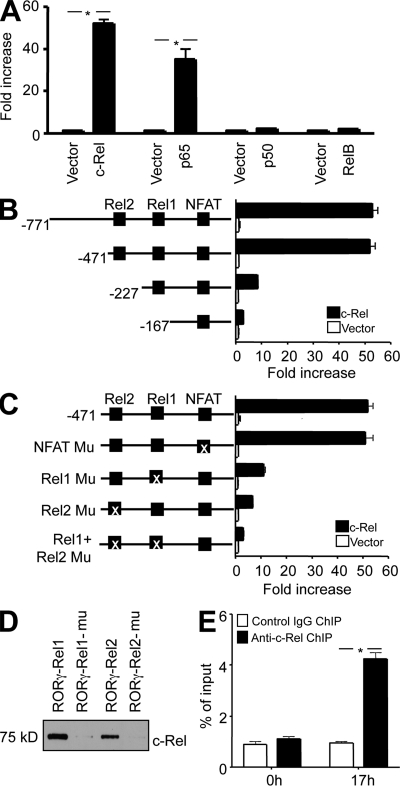
To test the theory that Rel proteins control Rorg gene expression, we examined whether they activate the Rorg promoters in luciferase-based promoter reporter assays. We found that c-Rel and p65, but not p50 or RelB, significantly activated the Rorg promoters upon coexpression (Fig. 6 A; unpublished data). p50 and RelB failed to activate the Rorg promoters even when co-transfected together (unpublished data). This indicates that the Rel/NF-κB family of transcription factors may regulate Rorg gene expression in a member-specific manner.
Figure 6.
c-Rel binds to and activates the Rorg promoter through two specific Rel sites. (A) EL4 cells were transiently transfected with murine Rorg promoter luciferase construct together with an expression vector for full-length c-Rel, p65, p50, or RelB, or the empty vector as indicated. After 24 h, cells were treated with PMA and ionomycin for 5 h, and the luciferase activities measured. The promoter activity is presented as fold increase over cells transfected with empty vector. To normalize the transfection efficiency across samples, the Renilla luciferase expression vector pRLTK was included as an internal control. (B) Deletion mutants of the Rorg promoter were analyzed in the luciferase reporter assay with or without c-Rel co-transfection. Putative binding sites for c-Rel (Rel1 and Rel2) and NFAT are indicated. (C) WT and Rel or NFAT site-mutated Rorg promoter constructs were analyzed in the luciferase reporter assay with or without c-Rel co-transfection. The Rel1 site was mutated to TGGGACTCG (−201 to −193), the Rel2 site to CTGAAGTGC (−288 to −280), and the NFAT site to TTGTCAC (−96 to −90). The “X” indicates the mutated site. (D) Nuclear extracts were prepared from EL4 cells after stimulation for 6 h with PMA and ionomycin. Biotinylated Rorg Rel oligonucleotides or their mutants were absorbed by streptavidin-agarose beads, and then added to the nuclear extracts. The amount of c-Rel proteins in the precipitates were assessed by immunoblotting with anti–c-Rel. (E) Purified CD4+ T cells from 6-wk-old WT mice (n = 3) were cultured under Th17 differentiation conditions, as described in Materials and methods. After 17 h, cells were fixed, and ChIP was performed using anti–c-Rel or control IgG. *, P < 0.01. Data are representative of three (A–D) and two (E) independent experiments, respectively.
Rel binds to both Rorg and Rorgt promoters and controls their activities through specific Rel sites
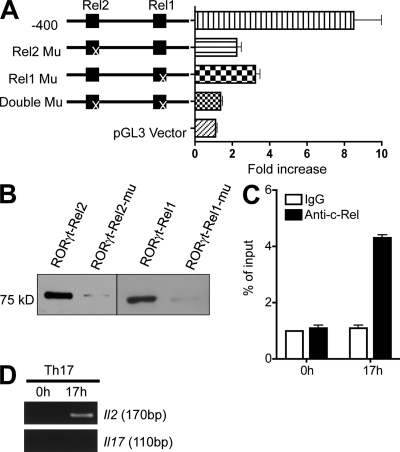
To identify the c-Rel–responsive region in the murine Rorg promoter, we performed deletional analysis of the promoter. We found that nucleotides −471 to −167 were required for the c-Rel activity (Fig. 6 B). A close examination of the nucleotide sequence in this region using the Transcription Element Search System (TESS) software revealed two putative Rel (or NF-κB) binding sites (Rel1, −201 to −193; Rel2, −288 to −280), and a putative NFAT-binding site (−96 to −90; Fig. S7). To determine whether these sites were required for c-Rel and p65 actions, we mutated them either individually or in combination. We found that both Rel1 and Rel2 sites, but not the putative NFAT site, were required for the c-Rel and p65 response (Fig. 6 C; unpublished data). Similarly, the Rorgt promoter contains a putative Rel site (Rel2, −268 to −260) and a putative Rel-NFAT–binding site (Rel1-NFAT, −166 to −156; Fig. S8). To identify the Rel-responsive site, we tested, in a luciferase reporter assay, the WT Rorgt promoter construct (−400 to +151) and its mutants (Fig. 7 A). We found that both Rel site mutations significantly reduced the Rorgt promoter activity, indicating that Rel regulates the Rorgt promoter through these sites.
Figure 7.
c-Rel binds to and activates the Rorgt promoter through two Rel sites. (A) WT and Rel site-mutated Rorgt promoter constructs and the empty vector were analyzed in a luciferase reporter assay with c-Rel co-transfection. The “X” indicates the mutated (Mu) Rel site. Data are representative of three independent experiments. (B) Nuclear extracts were prepared from EL4 cells after stimulation for 6 h with PMA and ionomycin. Biotinylated Rorgt Rel oligonucleotides or their mutants were absorbed by streptavidin-agarose beads, and then added to the nuclear extracts. The amount of c-Rel proteins in the precipitates were assessed by immunoblotting with anti–c-Rel. (C) Purified CD4+ T cells from 6-wk-old WT mice (n = 3) were cultured under Th17 differentiation condition as described in Materials and methods. After 17 h, cells were fixed, and ChIP was performed using anti–c-Rel or control IgG. Data are representative of two independent experiments. (D) Purified CD4+ T cells from 6-wk-old WT mice (n = 3) were cultured under Th17 differentiation conditions, as described in Materials and methods. After 17 h, cells were fixed, and ChIP was performed using anti–c-Rel. Data are representative of two independent experiments.
To directly test c-Rel binding to the identified Rel nucleotides of the Rorg and Rorgt promoter, we performed nucleotide pull-down analyses using both wild-type and mutant Rel nucleotides. We found that c-Rel readily bound to the nucleotides of all four Rel sites, but not their mutants (Fig. 6 D and Fig. 7 B).
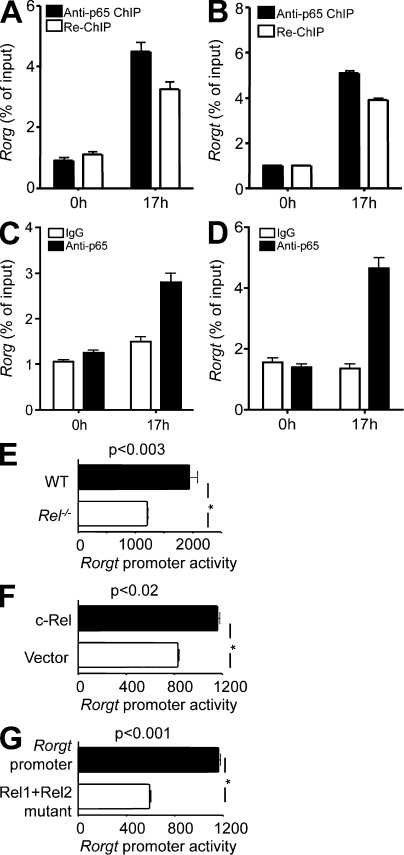
To establish whether Rel binds to the Rorg and Rorgt promoter in Th17 cells, we performed ChIP analysis. The Rorg and Rorgt DNA–protein complexes were precipitated using specific antibodies to c-Rel and p65 at different time points of Th17 differentiation. The nature of the precipitated DNA was then defined by PCR using primers specific for Rorg and Rorgt promoter. We found that in resting naive T cells, the Rorg and Rorgt promoter was devoid of c-Rel and p65. 17 h after Th17 differentiation, c-Rel and p65 were readily detected on both the Rorg and Rorgt promoter (Fig. 6 E and Fig. 7 C). In addition to Rorg and Rorgt, c-Rel also bound to Il2, but not Il17a, during Th17 differentiation (Fig. 7 D). Re-ChIP analysis showed that c-Rel and p65 colocalized in the same complex (Fig. 8, A and B). p65 was also detected on the Rorg and Rorgt promoters in Rel−/− T cells during Th17 differentiation (Fig. 8, C and D). This result may explain why significant numbers of Th17 cells were generated in the absence of c-Rel,
Figure 8.
Rel controls Rorg promoter activities in primary Th17 cells. (A and B) Purified naive CD4+ T cells from WT (n = 3) mice were cultured under Th17 differentiation conditions, as described in Materials and methods. At indicated times, cells were fixed, and ChIP was performed using anti-p65 antibody. Re-ChIP was performed with anti–c-Rel using DNA precipitated by anti-p65. (C and D) Purified naive CD4+ T cells from 6-wk-old Rel−/− mice (n = 3) were cultured under Th17 differentiation conditions, as described in Materials and methods. After 17 h, cells were fixed, and ChIP was performed using anti-p65 antibody. (E–G) Naive CD4+ T cells from WT (E–G; n = 3) and Rel−/− mice (E; n = 3) were transiently transfected with WT (E–G) and Rel sites mutated (E) murine Rorgt promoter luciferase constructs using Amaxa Nucleofector reagent. An expression vector for full-length c-Rel was also used to co-transfect cells in E. Cells were cultured under Th17 differentiation condition for 24 h, and the luciferase activities measured. To normalize the transfection efficiency across samples, the Renilla luciferase expression vector pRLTK was included as an internal control. Error bars indicate the SEM. Data are representative of two independent experiments.
These results indicate that c-Rel is required for activating the Rorg and Rorgt promoter during Th17 differentiation. To directly test this possibility, we measured the Rorgt promoter activity in differentiating Th17 cells. We found that c-Rel deficiency significantly diminished, whereas c-Rel overexpression enhanced, the Rorgt promoter activity (Fig. 8, E and F). Additionally, we found that mutating the Rel binding sites of the Rorgt promoter significantly reduced its activity in primary Th17 cells (Fig. 8 G). These results establish that c-Rel not only can, but also does, activate the Rorgt promoter in Th17 cells.
c-Rel–deficient mice have diminished autoimmune Th17 responses
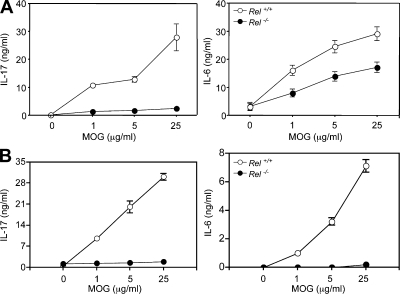
Th17 cells play crucial roles in the pathogenesis of autoimmune diseases. Because c-Rel–deficient T cells have a defect in Th17 differentiation, we asked whether Th17 responses to self-antigens were affected in c-Rel–deficient mice. As we reported, c-Rel–deficient mice were resistant to experimental autoimmune encephalomyelitis (EAE), an animal model for multiple sclerosis (Hilliard et al., 2002). To determine whether anti-self MOG (myelin oligodendrocyte glycoprotein) Th17 response was affected by the c-Rel deficiency, we immunized WT and c-Rel–deficient mice with a MOG peptide and examined the splenic T cell response to the self-peptide 2 wk later. We found that the anti-MOG Th17 response (as measured by IL-17 and IL-6 production) was significantly reduced in c-Rel–deficient mice as compared with their WT littermates (Fig. 9 A). To determine whether the in vivo effect of c-Rel on Th17 development was T cell intrinsic, we transferred WT and Rel−/− T cells into Rag1−/− mice. Mice were then immunized with MOG peptide, and the splenic T cell responses to MOG were tested 2 wk later. The results showed that Rel−/− T cells produced significantly less IL-17 as compared with WT T cells (Fig. 9 B).
Figure 9.
MOG-induced Th17 cytokine production by WT and Rel−/− splenocytes. (A) WT (Rel+/+) and c-Rel–deficient (Rel−/−) C57BL/6 mice (n = 3) were immunized s.c. with 200 µg MOG38-50 peptide in 0.1 ml PBS emulsified in an equal volume of complete Freund’s adjuvant containing 400 µg Mycobacterium tuberculosis H37RA. 14 d later, mice were sacrificed, and their splenocytes (5 × 106/ml) were cultured with or without MOG38-50 peptide as indicated. Culture supernatants were collected 40 h later and tested for cytokines by ELISA (Hilliard et al., 2002). (B) Purified T cells from WT (Rel+/+) and c-Rel–deficient (Rel−/−) C57BL/6 mice (n = 3) were transferred into Rag1−/− C57BL/6 mice via tail vein. Mice were treated and the splenic T cell responses to MOG examined as in A. Graphs show means ± SD of triplicate cultures. The differences between the two groups for all cultures with MOG are statistically significant (P < 0.01). Data are representative of two experiments.
To determine if c-Rel deficiency affects other models of autoimmunity, we crossed our c-Rel–deficient B6 mice with a NOD mouse line that carried a transgenic diabetogenic TCR called BDC2.5 (Gonzalez et al., 1997). All WT progenies developed autoimmune diabetes upon challenging with cyclophosphamide (CY) even though they were of a mixed MHC background. Remarkably, none of the c-Rel–deficient mice developed diabetes as measured by blood glucose levels (Fig. S9 A). Consistent with these results, splenocytes from c-Rel–deficient mice produced significantly lower levels of IL-17A, IL-6, IL-2, and IFN-γ than WT cells upon stimulation with anti-CD3, with or without anti-CD28 (Fig. S9 B). Flow cytometric analysis revealed that in CD4+ T cells, both Th1 and Th17 cytokines were reduced as a result of the c-Rel deficiency (our unpublished data). Collectively, these results indicate that autoreactive Th17 responses are markedly diminished in c-Rel–deficient mice.
DISCUSSION
How Th17 cells are generated remains enigmatic. Results reported here enable us to propose the following model (Fig. 5): APCs engage naive CD4+ T cells by TCR and CD28, in the presence of IL-1, IL-6, IL-23, and TGF-β; ligation of TCR, CD28, IL-1, and possibly IL-23 receptors leads to the activation of IKKβ, which phosphorylates IκBα (inhibitor of κBα), releasing c-Rel and p65. We propose that the freed c-Rel-p65 dimer migrates into the nucleus, binds to the Rorg and Rorgt promoters, and initiates Th17 differentiation, with the help of other factors such as NFAT and Stat. Thus, although the Rorg gene specifies Th17 lineage differentiation (Ivanov et al., 2006), its own expression may not be controlled by lineage-specific factors. Similar principles also apply to other lineage-specifying genes, including Foxp3, which is controlled by c-Rel. In the latter case, c-Rel forms a multifactorial complex called the enhanceosome, together with other nonlineage-specific factors, to control the on and off states of the Foxp3 gene. Although each factor in the enhanceosome is not unique, the complex as a whole is (Ruan et al., 2009). The enhanceosome operates as a “coincidence detector,” turning on the Foxp3 gene only when all the essential factors are present (Ruan et al., 2009). This concept explains how nonlineage-specific factors such as c-Rel control gene expression in a lineage-specific manner. Data presented in this work indicate that c-Rel and p65 may regulate Rorg gene expression in a similar manner, i.e., by forming an enhanceosome that in turn controls the on and off states of the Rorg gene. It is to be emphasized that the transcription factors (c-Rel and p65) we identified are the only known ones that bind to and activate Rorg promoters. Stat3 was recently shown to bind to the first intron of the Rorg gene, but not the promoter (Durant et al., 2010). The nature of other Rorg promoter-binding transcription factors remains to be established.
Unlike RORγT, which is primarily expressed in developing thymocytes and Th17 cells, RORγ is expressed in a variety of cell types (He et al., 1998; Villey et al., 1999). However, naive T cells and Th0 cells do not express RORγ. Upon Th differentiation, RORγ is most significantly up-regulated in Th17 cells and is able to specify the Th17 phenotype. Therefore, at least in T cells, RORγ shares similar expression patterns and functions with RORγT. Because we found evidence for the presence of two active Rorg promoters and two types of 5′-capped RORγ mRNAs in Th17 cells, we propose that RORγT and RORγ are generated by differential usage of promoters. It has been recognized that more than one ROR member is involved in Th17 differentiation. Thus, Th17 cells were detected in Rorg knockout cultures that lack both RORγT and RORγ (Ivanov et al., 2006), and Th17 responses could be completely blocked only in mice that lacked all three ROR members, i.e., RORγT, RORγ, and RORα (Yang et al., 2008). Therefore, no single ROR protein deficiency may completely block Th17 differentiation. One of our novel findings is that RORγ, which is not expressed in naive T cells, is selectively induced in Th17 cells by c-Rel, and controls Il17 gene expression. Although non–T cells can express RORγ, they may not express IL-17. This may be because of the fact that other T cell–associated factors, in addition to RORγT and RORγ, are required to activate the Il17 gene.
The novel discovery from this work is that c-Rel regulates Th17 response via RORγ and RORγT. The possibility that c-Rel also directly activates the Il17a gene, in addition to activating Rorg, cannot be completely ruled out, but is not supported by our data. c-Rel failed to activate the Il17a promoter, and was not recruited to it during Th17 differentiation, as we demonstrated in this study. Additionally, we searched for putative Rel/NF-κB sites between nucleotides +1 and −4,000 of the mouse Il17a locus using the Transcription Element Search System software, and identified only one putative site at −3,629 bp. However, subsequent ChIP analysis using anti–c-Rel revealed no binding of c-Rel to this site during Th17 differentiation. Therefore, there is no evidence that c-Rel may also bind to the Il17a locus. Similarly, because we showed that Stat3 expression was not affected by c-Rel deficiency, c-Rel could not regulate Th17 differentiation through Stat3.
Differentiation of autoreactive CD4+ naive T cells into Th1 or Th17 effector cells is required for the development of autoimmune diseases. Nondifferentiated autoreactive naive T cells are not pathogenic. Our observation that c-Rel–deficient mice are resistant to EAE and T1D indicates that c-Rel–mediated T cell differentiation may play a critical role in the development of autoimmune diseases (Hilliard et al., 2002; Lamhamedi-Cherradi et al., 2003). However, because c-Rel regulates multiple gene expressions in both lymphocytes and myeloid cells (Gerondakis et al., 2006; Bunting et al., 2007), the disease resistance of c-Rel–deficient mice can be caused by the defect in either lymphoid or myeloid cells, or in both. Similarly, c-Rel may regulate Th cell differentiation in vivo through two distinct mechanisms: (1) c-Rel expressed by T cells may regulate their differentiation through an autonomous mechanism, e.g., by activating the promoters of Th17-specific genes such as Rorg; (2) c-Rel expressed by myeloid cells may regulate Th cell differentiation through a T cell nonautonomous mechanism, e.g., by regulating the expression of cytokines that drive Th17 differentiation. The autonomous theory is supported by the findings that (a) purified Rel−/− T cells exhibit a significant defect in Th17 cell differentiation; (b) c-Rel directly controls the RORγ and RORγT expression; and (c) IL-1, IL-23, and TGF-β, cytokines that promote Th17 cell differentiation, activate Rel/NF-κB (Shim et al., 2005; Cho et al., 2006; Chung et al., 2009). Similarly, the nonautonomous theory is supported by the finding that the expression of IL-6 and IL-23, two cytokines that promote Th17 cell differentiation, is significantly reduced in c-Rel–deficient myeloid cells (Lamhamedi-Cherradi et al., 2003; Carmody et al., 2007). Interestingly, using cDNA microarrays that cover the whole mouse genome, Bunting et al. (2007) reported that the expression of ∼130 genes was reduced in c-Rel–deficient T cells as compared with WT cells treated with anti-CD3 and anti-CD28. These genes encode cytokines, chemokines, and cell surface molecules, as well as intracellular signal transducers and transcription factors. However, because Rorg expression is induced during Th17 differentiation, Bunting et al. (2007) did not detect it in their cells, which were not of the Th17 type. Therefore, in addition to Rorg, other c-Rel target genes could also regulate Th17 responses in a cell autonomous manner. For example, both Il2 and Foxp3 are c-Rel target genes that are known to promote T reg cell differentiation and inhibit Th17 differentiation (Isomura et al., 2009; Long et al., 2009; Ruan et al., 2009; Zheng et al., 2010). Because c-Rel–deficient T cells produce less IL-2 than WT T cells, the effect of c-Rel deficiency on Th17 differentiation could be masked if anti–IL-2 was not included in the Th17 differentiation medium (Liou et al., 1999; Visekruna et al., 2010).
In summary, we have discovered that c-Rel and p65 play critical roles in Th17 immune responses. This finding may not only help advance our understanding of the Th17 differentiation program, but also aid in developing new drugs targeting Rel and RORγ for the treatment of inflammatory diseases.
MATERIALS AND METHODS
Mice.
7–8-wk-old C57BL/6 (B6) mice that carry a Rel gene null mutation were generated as previously described, and were backcrossed to B6 mice for 12 generations before used in this study (Liou et al., 1999; Hilliard et al., 2002). p65-deficient fetal liver chimeric B6 mice were generated as previously described (Ouaaz et al., 2002). B6.NOD F1 mice were generated by crossing Rel−/− B6 and NOD BDC2.5 TCR transgenic mice (Gonzalez et al., 1997). The F1 mice were then crossed to generate Rel−/− and Rel+/+ B6.NOD BDC2.5 TCR transgenic mice. All B6.NOD F2 mice used in this study were H-2 of b and g7 and express the BDC2.5 transgenic TCR as determined by flow cytometry. All mice were housed in the University of Pennsylvania animal care facilities under pathogen-free conditions, and all procedures were preapproved by the Institutional Animal Care and Use Committee.
Induction and clinical evaluation of diabetes.
7–8-wk-old B6.NOD BDC2.5 TCR transgenic mice were injected i.p. with 200 mg/kg CY. Mice were tested every other day for blood glucose levels, and were considered diabetic if the glucose levels equaled or exceeded 250 mg/dl on two consecutive tests.
Recombinant retroviruses.
The recombinant RORγT retrovirus was a gift from D.R. Littman (New York University, New York, NY; Ivanov et al., 2006). A recombinant RORγ retrovirus was generated by inserting the murine RORγ cDNA with or without a Flag tag into the XhoI and EcoRI site of the MigR1 vector, and by transfecting 293T cells with the recombinant plasmid (Pear et al., 1998; Pui et al., 1999; Sun et al., 2008). In the recombinant viral genome, the RORγ or RORγT cDNA lies directly 5′ of an internal ribosome entry site, which is followed by a cDNA encoding enhanced GFP (EGFP).
T cell isolation and differentiation.
Naive CD4+CD25−CD44lowCD62L+ splenic T cells were isolated by FACS after staining the cells with the specific antibodies. Total CD4+ T cells were isolated using an autoMACS cell sorter after staining splenocytes with anti-CD4. Cells were cultured with 2 µg/ml soluble anti-CD28 and 2 µg/ml plate-bound anti-CD3 under conditions that induce either Th0 (50 U/ml IL-2; also known as nondifferentiation medium), Th1 (50 U/ml IL-2, 10 ng/ml IL-12, and 1 µg/ml anti–IL-4), Th2 (50 U/ml IL-2, 10 ng/ml IL-4, 1 µg/ml anti–IL-12, and 1 µg/ml anti–IFN-γ), or Th17 (2 µg/ml anti–IL-2, 1 µg/ml anti–IL-4, 1 µg/ml anti–IFN-γ, 20 ng/ml IL-6, and 5 ng/ml TGF-β1) cells. 3 d later, cells were washed and restimulated with 50 ng/ml PMA and 1 µM ionomycin in the presence of GolgiStop (1:1,500 dilution; BD) for 4–5 h, stained intracellularly with anti–IL-17A and anti–IFN-γ (eBioscience), and examined by flow cytometry.
ChIP assay.
ChIP was performed using the ChIP assay kit, per the manufacturer’s instructions (Millipore). In brief, cells were fixed with 1% formaldehyde at room temperature for 10 min and lysed in lysis buffer. DNA was then fragmented by sonication. After preclearance for 1 h at 4°C with salmon sperm DNA-saturated protein A-agarose, chromatin solutions were immunoprecipitated overnight at 4°C using 1 µg of rabbit antibodies to c-Rel, p65, RNA Polymerase II (Santa Cruz Biotechnology, Inc.), or control rabbit IgG. For the RORγ ChIP, anti-Flag, control mouse IgG, and the salmon sperm DNA-saturated protein G-agarose were used. Input and immunoprecipitated chromatins were incubated for 4 h at 65°C to reverse cross-links. After proteinase K digestion, DNA was extracted with phenol/chloroform and precipitated with ethanol. ChIP DNA was then analyzed by real-time PCR (the sequences of the primers are listed in the Supplemental materials and methods).
RT-PCR.
Total RNA was extracted using TRIzol (Invitrogen) according to the manufacturer’s instructions. Reverse transcription was performed using oligo dT primers. Quantitative real-time PCR was performed in the Applied Biosystems 7500 system using Power SYBR Green PCR Master Mix (Applied Biosystems). Relative levels of gene expression were determined using GAPDH as the control (the sequences of the primers are listed in the Supplemental materials and methods).
ELISA.
Antibodies used in ELISA were purchased from BD, including purified and biotinylated rat anti–mouse IL-2, IL-6, IFN-γ, and IL-17A. Quantitative ELISA was performed according to the manufacturer’s recommendations using paired mAbs specific for corresponding cytokines.
Western blot.
Total cell lysates were prepared by treating cells with the RIPA buffer, which contains 150 mM NaCl, 10 mM Tris, pH 7.4, 0.1% SDS, 1% Triton X-100, 1% sodium deoxycholate, 100 µM Na3VO4, 5 mM EDTA, 1 mM PMSF, and protease inhibitors. Proteins (20 µg per lane) were resolved by SDS-PAGE, transferred to a nitrocellulose membrane, and blotted with specific antibodies.
5′-RACE.
Purified CD4+ T cells from 6-wk-old WT mice were cultured under Th17 differentiation condition for 17 h. Total RNA was extracted (TRIzol) and treated with RNase-free DNase I (Promega). 5′-RACE was performed with a RNA ligase-mediated RACE (RLM-RACE) kit (Invitrogen). PCR products of 5′ end of mRNA were purified by agarose gel electrophoresis, cloned, and sequenced.
Nucleotide pull-down assay.
EL4 T cells were stimulated with 50 ng/ml PMA (Sigma-Aldrich) and 1 µM/ml ionomycin (Sigma-Aldrich) for 6 h at 37°C. The cells were resuspended in lysis buffer (20 mM Hepes, 420 mM NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 1 mM DTT, and 0.1% Nonidet P-40) with protease inhibitors, and incubated on ice for 15 min. Insoluble materials were removed by centrifugation. 100 µg lysate protein was diluted with dilution buffer (which is the same as the aforementioned lysis buffer, but without NaCl) and incubated with 10 µg of poly(deoxyinosinic-deoxycytidylic acid) (Roche) and 50 µl of streptavidin-agarose beads (Sigma-Aldrich) carrying biotinylated oligonucleotides (as described at teh end of this section) for 3 h at 4°C. The beads were washed twice with dilution buffer, resuspended in 50 µl 2X SDS sample loading buffer (Bio-Rad Laboratories), and heated to 95°C for 10 min. The eluants were resolved by SDS-PAGE. c-Rel was detected by immunoblotting with specific antibodies against c-Rel (Santa Cruz Biotechnology, Inc.). The oligonucleotides containing either the WT or mutated Rel binding sites used in this study are as follows: RORγ-Rel1-201, 5′-GGGTTATCATGTGGGATTCCAAAAAACAAAC-3′; RORγ-Rel1-201 mutant, 5′-GGGTTATCATGTGGGACTCGAAAAAACAAAC-3′; RORγ-Rel2-288, 5′-GAATTGCCCTTGTGAATTCCTGGCAAGTCTT-3′; RORγ-Rel2-288 mutant, 5′-GAATTGCCCTTCTGAAGTGCTGGCAAGTCTT-3′; RORγt-Rel1-166, 5′-TAGCTGTGGTTTTGGAATTTTCCAACGCCCCCT-3′; RORγt-Rel1-166 mutant, 5′-TAGCTGTGGTTTTAACATTGGATAACGCCCCCT-3′; RORγt-Rel2-268, 5′-GTACTCAGGAGAGGGGATTTCGAGCCTGGCCTC-3′; RORγt-Rel2-268 mutant, 5′-GTACTCAGGAGAGACGATCCCGAGCCTGGCCTC-3′.
Promoter luciferase assay.
The following DNA fragments were cloned into pGL3 luciferase reporter plasmid: the Rorg promoter (0.87 kb) and their mutants, the Rorgt promoter (0.55 kb) and their mutants, the Il17a promoter (1.1 kb) with or without the Il17a CNS2 (0.9 kb), and the Rora4 promoter (1.1 kb). Site-directed mutagenesis of Rel and NFAT binding sites was performed using the QuikChange kit (Stratagene), according to manufacturer’s instructions. DNA sequencing was used to confirm the mutated nucleotides. To test the promoter activity, T cells were transfected with the aforementioned reporter constructs together with expression plasmids carrying murine c-Rel, p65, p50, RelB, RORγ, or RORγT cDNA using Lipofectamine LTX (Invitrogen) or Amaxa Nucleofector reagents (Lonza). After 24 h, cells were treated with or without 50 ng/ml PMA and 1 µM ionomycin for 4–5 h, and the luciferase activities of whole-cell lysates were analyzed using the dual-luciferase reporter assay system (Promega). Co-transfection with the Renilla-luciferase expression vector pRL-TK (Promega) was performed in all reporter assays. For all samples, the assays were repeated at least three times and the data were normalized for transfection efficiency by dividing firefly luciferase activity by that of the Renilla luciferase.
Statistical analyses.
Glucose concentrations, real-time PCR results, and cytokine concentrations were analyzed by Student’s t test. Disease incidence was analyzed by Mann-Whitney U test.
Online supplemental material.
Fig. S1 shows reduced Th17 response of c-Rel–deficient T cells. Fig. S2 shows proliferation and phenotypes of Rel−/− T cells. Fig. S3 shows defective Th17 differentiation of p65−/− T cells. Fig. S4 shows regulation of the IL-17 gene expression. Fig. S5 shows that enforced expression of RORγ and RORγT did not rescue the proliferation defect of Rel−/− CD4 T cells. Fig. S6 shows the role of RORγ in Th cell differentiation. Fig. S7 shows sequence alignment of the human, mouse, and rat Rorg promoter regions. Fig. S8 shows sequence alignment of the human, mouse, and rat Rorgt promoter regions. Fig. S9 shows that c-Rel–deficient mice are completely resistant to type 1 diabetes. Supplemental materials and methods lists nucleotide sequences. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20110462/DC1.
Acknowledgments
The authors thank Daniel R. Littman for kindly providing the RORγT retrovirus, and Warren Pear, Ruaidhri Carmody, Scott Palmer, and Shunyou Gong for reagents and valuable discussions.
This work is supported by grants from the National Multiple Sclerosis Society (RG 4018A2/1) and National Institutes of Health (AI50059, DK070691, and AI069289).
The authors have no competing financial interests related to this work.
Footnotes
Abbreviations used:
- ChIP
- chromatin immunoprecipitation
- CNS
- conserved noncoding sequence
- EGFP
- enhanced GFP
- PMA
- phorbol myristate acetate
- RACE
- rapid amplification of cDNA ends
- RORγ
- RAR-related orphan receptor γ
- RORγt
- RAR-related orphan receptor γt
- Stat3
- signal transducer and activator of transcription 3
- TSS
- transcription start site
References
- Barnes P.J., Karin M. 1997. Nuclear factor-kappaB: a pivotal transcription factor in chronic inflammatory diseases. N. Engl. J. Med. 336:1066–1071 10.1056/NEJM199704103361506 [DOI] [PubMed] [Google Scholar]
- Beg A.A., Baltimore D. 1996. An essential role for NF-kappaB in preventing TNF-alpha-induced cell death. Science. 274:782–784 10.1126/science.274.5288.782 [DOI] [PubMed] [Google Scholar]
- Bettelli E., Carrier Y., Gao W., Korn T., Strom T.B., Oukka M., Weiner H.L., Kuchroo V.K. 2006. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 441:235–238 10.1038/nature04753 [DOI] [PubMed] [Google Scholar]
- Brownell E., Mathieson B., Young H.A., Keller J., Ihle J.N., Rice N.R. 1987. Detection of c-rel-related transcripts in mouse hematopoietic tissues, fractionated lymphocyte populations, and cell lines. Mol. Cell. Biol. 7:1304–1309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunting K., Rao S., Hardy K., Woltring D., Denyer G.S., Wang J., Gerondakis S., Shannon M.F. 2007. Genome-wide analysis of gene expression in T cells to identify targets of the NF-kappa B transcription factor c-Rel. J. Immunol. 178:7097–7109 [DOI] [PubMed] [Google Scholar]
- Carmody R.J., Ruan Q., Liou H.-C., Chen Y.H. 2007. Essential roles of c-Rel in Toll-like receptor-induced interleukin-23 p19 gene expression in dendritic cells. J. Immunol. 178:186–191 [DOI] [PubMed] [Google Scholar]
- Cho M.L., Kang J.W., Moon Y.M., Nam H.J., Jhun J.Y., Heo S.B., Jin H.T., Min S.Y., Ju J.H., Park K.S., et al. 2006. STAT3 and NF-kappaB signal pathway is required for IL-23-mediated IL-17 production in spontaneous arthritis animal model IL-1 receptor antagonist-deficient mice. J. Immunol. 176:5652–5661 [DOI] [PubMed] [Google Scholar]
- Chung Y., Chang S.H., Martinez G.J., Yang X.O., Nurieva R., Kang H.S., Ma L., Watowich S.S., Jetten A.M., Tian Q., Dong C. 2009. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity. 30:576–587 10.1016/j.immuni.2009.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cua D.J., Sherlock J., Chen Y., Murphy C.A., Joyce B., Seymour B., Lucian L., To W., Kwan S., Churakova T., et al. 2003. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 421:744–748 10.1038/nature01355 [DOI] [PubMed] [Google Scholar]
- Durant L., Watford W.T., Ramos H.L., Laurence A., Vahedi G., Wei L., Takahashi H., Sun H.W., Kanno Y., Powrie F., O’Shea J.J. 2010. Diverse targets of the transcription factor STAT3 contribute to T cell pathogenicity and homeostasis. Immunity. 32:605–615 10.1016/j.immuni.2010.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerondakis S., Grumont R., Rourke I., Grossmann M. 1998. The regulation and roles of Rel/NF-kappa B transcription factors during lymphocyte activation. Curr. Opin. Immunol. 10:353–359 10.1016/S0952-7915(98)80175-1 [DOI] [PubMed] [Google Scholar]
- Gerondakis S., Grumont R., Gugasyan R., Wong L., Isomura I., Ho W., Banerjee A. 2006. Unravelling the complexities of the NF-kappaB signalling pathway using mouse knockout and transgenic models. Oncogene. 25:6781–6799 10.1038/sj.onc.1209944 [DOI] [PubMed] [Google Scholar]
- Gonzalez A., Katz J.D., Mattei M.G., Kikutani H., Benoist C., Mathis D. 1997. Genetic control of diabetes progression. Immunity. 7:873–883 10.1016/S1074-7613(00)80405-7 [DOI] [PubMed] [Google Scholar]
- He Y.W., Deftos M.L., Ojala E.W., Bevan M.J. 1998. RORgamma t, a novel isoform of an orphan receptor, negatively regulates Fas ligand expression and IL-2 production in T cells. Immunity. 9:797–806 10.1016/S1074-7613(00)80645-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilliard B.A., Mason N., Xu L., Sun J., Lamhamedi-Cherradi S.-E., Liou H.-C., Hunter C., Chen Y.H. 2002. Critical roles of c-Rel in autoimmune inflammation and helper T cell differentiation. J. Clin. Invest. 110:843–850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huguet C., Bouali F., Enrietto P.J., Stehelin D., Vandenbunder B., Abbadie C. 1998. The avian transcription factor c-Rel is expressed in lymphocyte precursor cells and antigen-presenting cells during thymus development. Dev. Immunol. 5:247–261 10.1155/1998/58608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isomura I., Palmer S., Grumont R.J., Bunting K., Hoyne G., Wilkinson N., Banerjee A., Proietto A., Gugasyan R., Wu L., et al. 2009. c-Rel is required for the development of thymic Foxp3+ CD4 regulatory T cells. J. Exp. Med. 206:3001–3014 10.1084/jem.20091411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov I.I., McKenzie B.S., Zhou L., Tadokoro C.E., Lepelley A., Lafaille J.J., Cua D.J., Littman D.R. 2006. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 126:1121–1133 10.1016/j.cell.2006.07.035 [DOI] [PubMed] [Google Scholar]
- Köntgen F., Grumont R.J., Strasser A., Metcalf D., Li R., Tarlinton D., Gerondakis S. 1995. Mice lacking the c-rel proto-oncogene exhibit defects in lymphocyte proliferation, humoral immunity, and interleukin-2 expression. Genes Dev. 9:1965–1977 10.1101/gad.9.16.1965 [DOI] [PubMed] [Google Scholar]
- Lamhamedi-Cherradi S.E., Zheng S., Hilliard B.A., Xu L., Sun J., Alsheadat S., Liou H.C., Chen Y.H. 2003. Transcriptional regulation of type I diabetes by NF-kappa B. J. Immunol. 171:4886–4892 [DOI] [PubMed] [Google Scholar]
- Langrish C.L., Chen Y., Blumenschein W.M., Mattson J., Basham B., Sedgwick J.D., McClanahan T., Kastelein R.A., Cua D.J. 2005. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J. Exp. Med. 201:233–240 10.1084/jem.20041257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurence A., Tato C.M., Davidson T.S., Kanno Y., Chen Z., Yao Z., Blank R.B., Meylan F., Siegel R., Hennighausen L., et al. 2007. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 26:371–381 10.1016/j.immuni.2007.02.009 [DOI] [PubMed] [Google Scholar]
- Liou H.C., Jin Z., Tumang J., Andjelic S., Smith K.A., Liou M.L. 1999. c-Rel is crucial for lymphocyte proliferation but dispensable for T cell effector function. Int. Immunol. 11:361–371 10.1093/intimm/11.3.361 [DOI] [PubMed] [Google Scholar]
- Long M., Park S.G., Strickland I., Hayden M.S., Ghosh S. 2009. Nuclear factor-kappaB modulates regulatory T cell development by directly regulating expression of Foxp3 transcription factor. Immunity. 31:921–931 10.1016/j.immuni.2009.09.022 [DOI] [PubMed] [Google Scholar]
- Medvedev A., Chistokhina A., Hirose T., Jetten A.M. 1997. Genomic structure and chromosomal mapping of the nuclear orphan receptor ROR gamma (RORC) gene. Genomics. 46:93–102 10.1006/geno.1997.4980 [DOI] [PubMed] [Google Scholar]
- Ouaaz F., Arron J., Zheng Y., Choi Y., Beg A.A. 2002. Dendritic cell development and survival require distinct NF-kappaB subunits. Immunity. 16:257–270 10.1016/S1074-7613(02)00272-8 [DOI] [PubMed] [Google Scholar]
- Pear W.S., Miller J.P., Xu L., Pui J.C., Soffer B., Quackenbush R.C., Pendergast A.M., Bronson R., Aster J.C., Scott M.L., Baltimore D. 1998. Efficient and rapid induction of a chronic myelogenous leukemia-like myeloproliferative disease in mice receiving P210 bcr/abl-transduced bone marrow. Blood. 92:3780–3792 [PubMed] [Google Scholar]
- Pui J.C., Allman D., Xu L., DeRocco S., Karnell F.G., Bakkour S., Lee J.Y., Kadesch T., Hardy R.R., Aster J.C., Pear W.S. 1999. Notch1 expression in early lymphopoiesis influences B versus T lineage determination. Immunity. 11:299–308 10.1016/S1074-7613(00)80105-3 [DOI] [PubMed] [Google Scholar]
- Ruan Q., Kameswaran V., Tone Y., Li L., Liou H.C., Greene M.I., Tone M., Chen Y.H. 2009. Development of Foxp3(+) regulatory t cells is driven by the c-Rel enhanceosome. Immunity. 31:932–940 10.1016/j.immuni.2009.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim J.H., Xiao C., Paschal A.E., Bailey S.T., Rao P., Hayden M.S., Lee K.Y., Bussey C., Steckel M., Tanaka N., et al. 2005. TAK1, but not TAB1 or TAB2, plays an essential role in multiple signaling pathways in vivo. Genes Dev. 19:2668–2681 10.1101/gad.1360605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simek S., Rice N.R. 1988. Detection and characterization of the protein encoded by the chicken c-rel protooncogene. Oncogene Res. 2:103–119 [PubMed] [Google Scholar]
- Sun H., Gong S., Carmody R.J., Hilliard A., Li L., Sun J., Kong L., Xu L., Hilliard B., Hu S., et al. 2008. TIPE2, a negative regulator of innate and adaptive immunity that maintains immune homeostasis. Cell. 133:415–426 10.1016/j.cell.2008.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton C., Brereton C., Keogh B., Mills K.H., Lavelle E.C. 2006. A crucial role for interleukin (IL)-1 in the induction of IL-17–producing T cells that mediate autoimmune encephalomyelitis. J. Exp. Med. 203:1685–1691 10.1084/jem.20060285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumang J.R., Owyang A., Andjelic S., Jin Z., Hardy R.R., Liou M.L., Liou H.C. 1998. c-Rel is essential for B lymphocyte survival and cell cycle progression. Eur. J. Immunol. 28:4299–4312 [DOI] [PubMed] [Google Scholar]
- Veldhoen M., Stockinger B. 2006. TGFbeta1, a “Jack of all trades”: the link with pro-inflammatory IL-17-producing T cells. Trends Immunol. 27:358–361 10.1016/j.it.2006.06.001 [DOI] [PubMed] [Google Scholar]
- Villey I., de Chasseval R., de Villartay J.P. 1999. RORgammaT, a thymus-specific isoform of the orphan nuclear receptor RORgamma / TOR, is up-regulated by signaling through the pre-T cell receptor and binds to the TEA promoter. Eur. J. Immunol. 29:4072–4080 [DOI] [PubMed] [Google Scholar]
- Visekruna A., Huber M., Hellhund A., Bothur E., Reinhard K., Bollig N., Schmidt N., Joeris T., Lohoff M., Steinhoff U. 2010. c-Rel is crucial for the induction of Foxp3(+) regulatory CD4(+) T cells but not T(H)17 cells. Eur. J. Immunol. 40:671–676 10.1002/eji.200940260 [DOI] [PubMed] [Google Scholar]
- Wang W., Tam W.F., Hughes C.C., Rath S., Sen R. 1997. c-Rel is a target of pentoxifylline-mediated inhibition of T lymphocyte activation. Immunity. 6:165–174 10.1016/S1074-7613(00)80423-9 [DOI] [PubMed] [Google Scholar]
- Weaver C.T., Harrington L.E., Mangan P.R., Gavrieli M., Murphy K.M. 2006. Th17: an effector CD4 T cell lineage with regulatory T cell ties. Immunity. 24:677–688 10.1016/j.immuni.2006.06.002 [DOI] [PubMed] [Google Scholar]
- Xi H., Schwartz R., Engel I., Murre C., Kersh G.J. 2006. Interplay between RORgammat, Egr3, and E proteins controls proliferation in response to pre-TCR signals. Immunity. 24:813–826 10.1016/j.immuni.2006.03.023 [DOI] [PubMed] [Google Scholar]
- Yang X.O., Panopoulos A.D., Nurieva R., Chang S.H., Wang D., Watowich S.S., Dong C. 2007. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J. Biol. Chem. 282:9358–9363 10.1074/jbc.C600321200 [DOI] [PubMed] [Google Scholar]
- Yang X.O., Pappu B.P., Nurieva R., Akimzhanov A., Kang H.S., Chung Y., Ma L., Shah B., Panopoulos A.D., Schluns K.S., et al. 2008. T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity. 28:29–39 10.1016/j.immuni.2007.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y., Josefowicz S., Chaudhry A., Peng X.P., Forbush K., Rudensky A.Y. 2010. Role of conserved non-coding DNA elements in the Foxp3 gene in regulatory T-cell fate. Nature. 463:808–812 10.1038/nature08750 [DOI] [PMC free article] [PubMed] [Google Scholar]