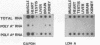Abstract
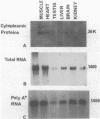
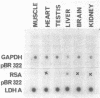
We have isolated and identified cDNA clones containing part of the coding sequence for rat glyceraldehyde-3-phosphate-dehydrogenase (GAPDH, E.C. 1.2.1.12). By using one of these clones as a probe, we have shown that: i) the abundance of GAPDH mRNA is different in various tissues of the adult rat and in good correlation with the abundance of the enzyme; ii) the transcription rates are quite similar in all tissues tested. We therefore conclude that the tissue-specific differential GAPDH gene expression is regulated by adjusting the abundance of its mRNA at the post-transcriptional level.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akusjärvi G., Persson H. Controls of RNA splicing and termination in the major late adenovirus transcription unit. Nature. 1981 Jul 30;292(5822):420–426. doi: 10.1038/292420a0. [DOI] [PubMed] [Google Scholar]
- Bantle J. A., Maxwell I. H., Hahn W. E. Specificity of oligo (dT)-cellulose chromatography in the isolation of polyadenylated RNA. Anal Biochem. 1976 May 7;72:413–427. doi: 10.1016/0003-2697(76)90549-2. [DOI] [PubMed] [Google Scholar]
- Biggin M. D., Gibson T. J., Hong G. F. Buffer gradient gels and 35S label as an aid to rapid DNA sequence determination. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3963–3965. doi: 10.1073/pnas.80.13.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Bruns G. A., Pierce P., Regina V. M., Gerald P. S. Expression of GAPDH and TPI in dog-rodent hybrids. Cytogenet Cell Genet. 1978;22(1-6):547–551. doi: 10.1159/000131021. [DOI] [PubMed] [Google Scholar]
- Domdey H., Wiebauer K., Klapthor H., Arnold H. H. Sequence analysis of the cloned mRNA coding for glyceraldehyde-3-phosphate dehydrogenase from chicken heart muscle. Eur J Biochem. 1983 Mar 1;131(1):129–135. doi: 10.1111/j.1432-1033.1983.tb07239.x. [DOI] [PubMed] [Google Scholar]
- Eriksson K., Halkka O., Lokki J., Saura A. Enzyme polymorphism in feral, outbred and inbred rats (Rattus norvegicus). Heredity (Edinb) 1976 Dec;37(3):341–349. doi: 10.1038/hdy.1976.98. [DOI] [PubMed] [Google Scholar]
- Guyette W. A., Matusik R. J., Rosen J. M. Prolactin-mediated transcriptional and post-transcriptional control of casein gene expression. Cell. 1979 Aug;17(4):1013–1023. doi: 10.1016/0092-8674(79)90340-4. [DOI] [PubMed] [Google Scholar]
- Harpold M. M., Wilson M. C., Darnell J. E., Jr Chinese hamster polyadenylated messenger ribonucleic acid: relationship to non-polyadenylated sequences and relative conservation during messenger ribonucleic acid processing. Mol Cell Biol. 1981 Feb;1(2):188–198. doi: 10.1128/mcb.1.2.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbschleb-Voogt E., Monteba-van Heuvel M., Wijnen L. M., Westerveld A., Pearson P. L., Meera Khan P. Chromosomal assignment and regional localization of CS, ENO2, GAPDH, LDHB, PEPB, and TPI in man-rodent cell hybrids. Cytogenet Cell Genet. 1978;22(1-6):482–486. doi: 10.1159/000131003. [DOI] [PubMed] [Google Scholar]
- Land H., Grez M., Hauser H., Lindenmaier W., Schütz G. 5'-Terminal sequences of eucaryotic mRNA can be cloned with high efficiency. Nucleic Acids Res. 1981 May 25;9(10):2251–2266. doi: 10.1093/nar/9.10.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMaster G. K., Samulski R. J., Stein J. L., Stein G. S. Rapid purification of covalently closed circular DNAs of bacterial plasmids and animal tumor viruses. Anal Biochem. 1980 Nov 15;109(1):47–54. doi: 10.1016/0003-2697(80)90008-1. [DOI] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Miles M. F., Hung P., Jungmann R. A. Cyclic AMP regulation of lactate dehydrogenase. Quantitation of lactate dehydrogenase M-subunit messenger RNA in isoproterenol-and N6,O2'-dibutyryl cyclic AMP-stimulated rat C6 glioma cells by hybridization analysis using a cloned cDNA probe. J Biol Chem. 1981 Dec 10;256(23):12545–12552. [PubMed] [Google Scholar]
- Milner R. J., Brow M. D., Cleveland D. W., Shinnick T. M., Sutcliffe J. G. Glyceraldehyde 3-phosphate dehydrogenase protein and mRNA are both differentially expressed in adult chickens but not chick embryos. Nucleic Acids Res. 1983 May 25;11(10):3301–3315. doi: 10.1093/nar/11.10.3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mota G., Gheţie V., Sjöquist J. Characterization of the soluble complex formed by reacting rabbit IgG with protein A of S. aureus. Immunochemistry. 1978 Sep;15(9):639–642. doi: 10.1016/0161-5890(78)90037-8. [DOI] [PubMed] [Google Scholar]
- Nadal-Ginard B. Regulation of lactate dehydrogenase levels in the mouse. J Biol Chem. 1978 Jan 10;253(1):170–177. [PubMed] [Google Scholar]
- Panabières F., Piechaczyk M., Rainer B., Dani C., Fort P., Riaad S., Marty L., Imbach J. L., Jeanteur P., Blanchard J. M. Complete nucleotide sequence of the messenger RNA coding for chicken muscle glyceraldehyde-3-phosphate dehydrogenase. Biochem Biophys Res Commun. 1984 Feb 14;118(3):767–773. doi: 10.1016/0006-291x(84)91461-x. [DOI] [PubMed] [Google Scholar]
- Rougeon F., Kourilsky P., Mach B. Insertion of a rabbit beta-globin gene sequence into an E. coli plasmid. Nucleic Acids Res. 1975 Dec;2(12):2365–2378. doi: 10.1093/nar/2.12.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHONK C. E., BOXER G. E. ENZYME PATTERNS IN HUMAN TISSUES. I. METHODS FOR THE DETERMINATION OF GLYCOLYTIC ENZYMES. Cancer Res. 1964 May;24:709–721. [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R. The use of thin acrylamide gels for DNA sequencing. FEBS Lett. 1978 Mar 1;87(1):107–110. doi: 10.1016/0014-5793(78)80145-8. [DOI] [PubMed] [Google Scholar]
- Sargent T. D., Wu J. R., Sala-Trepat J. M., Wallace R. B., Reyes A. A., Bonner J. The rat serum albumin gene: analysis of cloned sequences. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3256–3260. doi: 10.1073/pnas.76.7.3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schibler U., Hagenbüchle O., Wellauer P. K., Pittet A. C. Two promoters of different strengths control the transcription of the mouse alpha-amylase gene Amy-1a in the parotid gland and the liver. Cell. 1983 Jun;33(2):501–508. doi: 10.1016/0092-8674(83)90431-2. [DOI] [PubMed] [Google Scholar]
- Serville F., Junien C., Kaplan J. C., Gachet M., Cadoux J., Broustet A. Gene dosage effect for human triosephosphate isomerase and glyceraldehyde-3-phosphate dehydrogenase in partial trisomy 12p13 and trisomy 18p. Hum Genet. 1978 Nov 24;45(1):63–69. doi: 10.1007/BF00277574. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker J. R., Granum P. E. An absolute method for protein determination based on difference in absorbance at 235 and 280 nm. Anal Biochem. 1980 Nov 15;109(1):156–159. doi: 10.1016/0003-2697(80)90024-x. [DOI] [PubMed] [Google Scholar]
- Wilson S. H., Hill H. Z., Hoagland M. B. Physiology of rat-liver polysomes. Protein synthesis by stable polysomes. Biochem J. 1967 May;103(2):567–572. doi: 10.1042/bj1030567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiskocil R., Bensky P., Dower W., Goldberger R. F., Gordon J. I., Deeley R. G. Coordinate regulation of two estrogen-dependent genes in avian liver. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4474–4478. doi: 10.1073/pnas.77.8.4474. [DOI] [PMC free article] [PubMed] [Google Scholar]