Abstract
As a first step in our studies of functionally rearranged K genes of man we cloned the germline JK-CK region from placenta DNA employing a mouse JK clone as hybridization probe. Subclones of the human JK-CK region were then used to characterize and clone the rearranged K genes of the lymphoid cell lines Walker and Daudi. The Walker cell line contains one rearranged and one germline K allele (K+,KO; ref. 1). Only one K gene was found in Daudi cells (K+). Restriction mapping and DNA sequencing showed, that the rearranged K genes from both cell lines are closely related. These features make the two cell lines particularly suitable for studies on the chromatin structure of K light chain genes. The 5' flanks of the two genes (388 bp) are identical while there is a 12% divergence between the VK gene segments themselves. This situation may reflect somatic mutation processes and/or gene conversion like events.
Full text
PDF



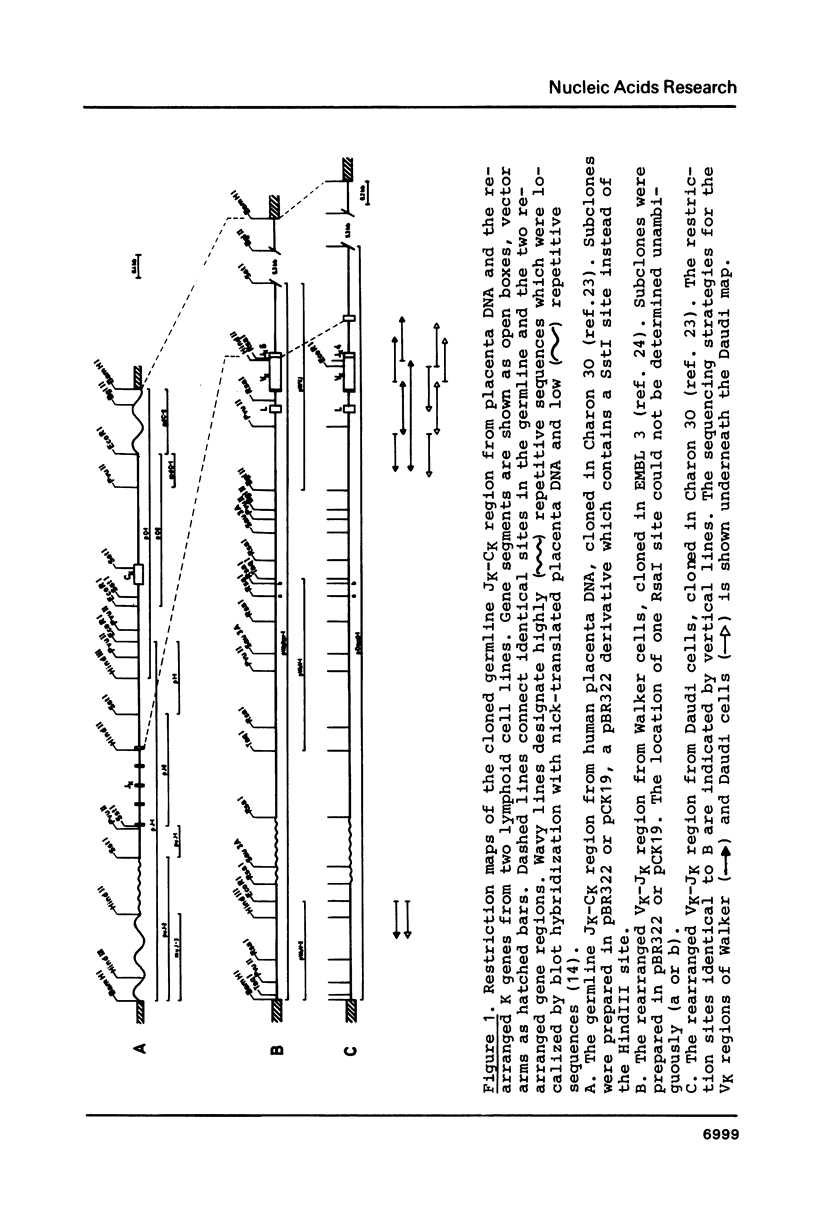
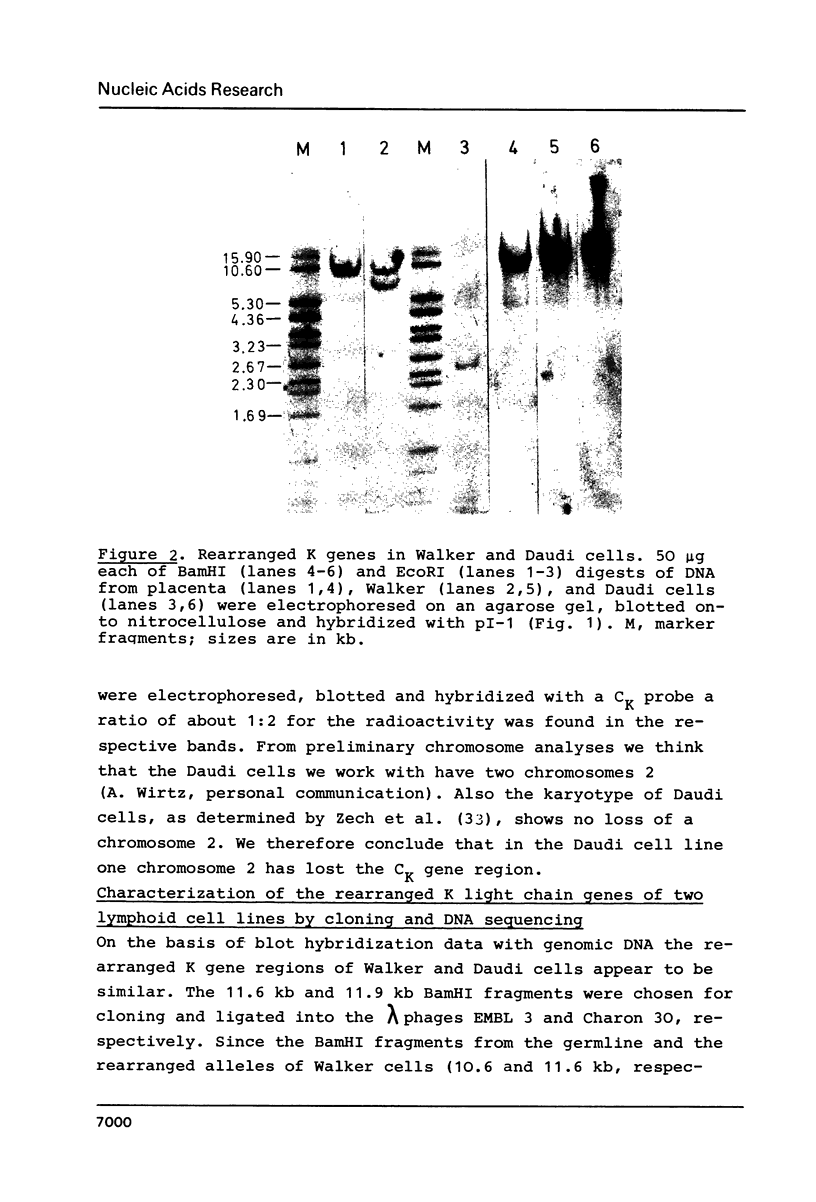






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baltimore D. Somatic mutation gains its place among the generators of diversity. Cell. 1981 Nov;26(3 Pt 1):295–296. doi: 10.1016/0092-8674(81)90196-3. [DOI] [PubMed] [Google Scholar]
- Bentley D. L. Most kappa immunoglobulin mRNA in human lymphocytes is homologous to a small family of germ-line V genes. Nature. 1984 Jan 5;307(5946):77–80. doi: 10.1038/307077a0. [DOI] [PubMed] [Google Scholar]
- Bentley D. L., Rabbitts T. H. Evolution of immunoglobulin V genes: evidence indicating that recently duplicated human V kappa sequences have diverged by gene conversion. Cell. 1983 Jan;32(1):181–189. doi: 10.1016/0092-8674(83)90508-1. [DOI] [PubMed] [Google Scholar]
- Bentley D. L., Rabbitts T. H. Human V kappa immunoglobulin gene number: implications for the origin of antibody diversity. Cell. 1981 Jun;24(3):613–623. doi: 10.1016/0092-8674(81)90088-x. [DOI] [PubMed] [Google Scholar]
- Bentley D. L., Rabbitts T. H. Human immunoglobulin variable region genes--DNA sequences of two V kappa genes and a pseudogene. Nature. 1980 Dec 25;288(5792):730–733. doi: 10.1038/288730a0. [DOI] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Blin N., Stafford D. W. A general method for isolation of high molecular weight DNA from eukaryotes. Nucleic Acids Res. 1976 Sep;3(9):2303–2308. doi: 10.1093/nar/3.9.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cory S., Tyler B. M., Adams J. M. Sets of immunoglobulin V kappa genes homologous to ten cloned V kappa sequences: implications for the number of germline V kappa genes. J Mol Appl Genet. 1981;1(2):103–116. [PubMed] [Google Scholar]
- Falkner F. G., Zachau H. G. Correct transcription of an immunoglobulin kappa gene requires an upstream fragment containing conserved sequence elements. Nature. 1984 Jul 5;310(5972):71–74. doi: 10.1038/310071a0. [DOI] [PubMed] [Google Scholar]
- Frischauf A. M., Lehrach H., Poustka A., Murray N. Lambda replacement vectors carrying polylinker sequences. J Mol Biol. 1983 Nov 15;170(4):827–842. doi: 10.1016/s0022-2836(83)80190-9. [DOI] [PubMed] [Google Scholar]
- Gearhart P. J., Bogenhagen D. F. Clusters of point mutations are found exclusively around rearranged antibody variable genes. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3439–3443. doi: 10.1073/pnas.80.11.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hieter P. A., Maizel J. V., Jr, Leder P. Evolution of human immunoglobulin kappa J region genes. J Biol Chem. 1982 Feb 10;257(3):1516–1522. [PubMed] [Google Scholar]
- Hieter P. A., Max E. E., Seidman J. G., Maizel J. V., Jr, Leder P. Cloned human and mouse kappa immunoglobulin constant and J region genes conserve homology in functional segments. Cell. 1980 Nov;22(1 Pt 1):197–207. doi: 10.1016/0092-8674(80)90168-3. [DOI] [PubMed] [Google Scholar]
- Honjo T. Immunoglobulin genes. Annu Rev Immunol. 1983;1:499–528. doi: 10.1146/annurev.iy.01.040183.002435. [DOI] [PubMed] [Google Scholar]
- Jaenichen H. R., Pech M., Lindenmaier W., Wildgruber N., Zachau H. G. Composite human VK genes and a model of their evolution. Nucleic Acids Res. 1984 Jul 11;12(13):5249–5263. doi: 10.1093/nar/12.13.5249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klobeck H. G., Solomon A., Zachau H. G. Contribution of human V kappa II germ-line genes to light-chain diversity. Nature. 1984 May 3;309(5963):73–76. doi: 10.1038/309073a0. [DOI] [PubMed] [Google Scholar]
- Loening U. E. The fractionation of high-molecular-weight ribonucleic acid by polyacrylamide-gel electrophoresis. Biochem J. 1967 Jan;102(1):251–257. doi: 10.1042/bj1020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Morton H. J. A survey of commercially available tissue culture media. In Vitro. 1970 Sep-Oct;6(2):89–108. doi: 10.1007/BF02616112. [DOI] [PubMed] [Google Scholar]
- Neumaier P. S., Zachau H. G. Nucleotide sequence of a region downstream of the mouse CK immunoglobulin gene. Nucleic Acids Res. 1983 Jun 11;11(11):3631–3636. doi: 10.1093/nar/11.11.3631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrander J., Kempe T., Messing J. Construction of improved M13 vectors using oligodeoxynucleotide-directed mutagenesis. Gene. 1983 Dec;26(1):101–106. doi: 10.1016/0378-1119(83)90040-9. [DOI] [PubMed] [Google Scholar]
- Pech M., Höchtl J., Schnell H., Zachau H. G. Differences between germ-line and rearranged immunoglobulin V kappa coding sequences suggest a localized mutation mechanism. Nature. 1981 Jun 25;291(5817):668–670. doi: 10.1038/291668a0. [DOI] [PubMed] [Google Scholar]
- Pech M., Jaenichen H. R., Pohlenz H. D., Neumaier P. S., Klobeck H. G., Zachau H. G. Organization and evolution of a gene cluster for human immunoglobulin variable regions of the kappa type. J Mol Biol. 1984 Jun 25;176(2):189–204. doi: 10.1016/0022-2836(84)90420-0. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rimm D. L., Horness D., Kucera J., Blattner F. R. Construction of coliphage lambda Charon vectors with BamHI cloning sites. Gene. 1980 Dec;12(3-4):301–309. doi: 10.1016/0378-1119(80)90113-4. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Scalenghe F., Turco E., Edström J. E., Pirrotta V., Melli M. Microdissection and cloning of DNA from a specific region of Drosophila melanogaster polytene chromosomes. Chromosoma. 1981;82(2):205–216. doi: 10.1007/BF00286105. [DOI] [PubMed] [Google Scholar]
- Schnell H., Steinmetz M., Zachau H. G., Schechter I. An unusual translocation of immunoglobulin gene segments in variants of the mouse myeloma MPC11. Nature. 1980 Jul 10;286(5769):170–173. doi: 10.1038/286170a0. [DOI] [PubMed] [Google Scholar]
- Sherman L. A., Vitiello A., Klinman N. R. T-cell and B-cell responses to viral antigens at the clonal level. Annu Rev Immunol. 1983;1:63–86. doi: 10.1146/annurev.iy.01.040183.000431. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Tonegawa S. Somatic generation of antibody diversity. Nature. 1983 Apr 14;302(5909):575–581. doi: 10.1038/302575a0. [DOI] [PubMed] [Google Scholar]
- Valbuena O., Marcu K. B., Weigert M., Perry R. P. Multiplicity of germline genes specifying a group of related mouse kappa chains with implications for the generation of immunoglobulin diversity. Nature. 1978 Dec 21;276(5690):780–784. doi: 10.1038/276780a0. [DOI] [PubMed] [Google Scholar]
- Weischet W. O., Glotov B. O., Schnell H., Zachau H. G. Differences in the nuclease sensitivity between the two alleles of the immunoglobulin kappa light chain genes in mouse liver and myeloma nuclei. Nucleic Acids Res. 1982 Jun 25;10(12):3627–3645. doi: 10.1093/nar/10.12.3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K. R., Alberts B. M., Benzinger R., Lawhorne L., Treiber G. Rapid bacteriophage sedimentation in the presence of polyethylene glycol and its application to large-scale virus purification. Virology. 1970 Mar;40(3):734–744. doi: 10.1016/0042-6822(70)90218-7. [DOI] [PubMed] [Google Scholar]
- Zech L., Haglund U., Nilsson K., Klein G. Characteristic chromosomal abnormalities in biopsies and lymphoid-cell lines from patients with Burkitt and non-Burkitt lymphomas. Int J Cancer. 1976 Jan 15;17(1):47–56. doi: 10.1002/ijc.2910170108. [DOI] [PubMed] [Google Scholar]