Abstract
The asymmetric unit of the title compound, Cs2[Co(CH4O6P2)2(H2O)2], is comprised of one bidentate methylenediphosphonate ligand and one water molecule which are coordinated to the CoII atom, as well as a caesium counter-cation. The Co atom occupies a special position on a crystallographic inversion center. The caesium ion is octahedrally coordinated by six O atoms with Cs—O distances ranging from 3.119 (2) to 3.296 (2) Å. A three-dimensional network is formed through O—H⋯O hydrogen bonds.
Related literature
For related structures, see: Fleisch (1991 ▶); Neville-Webbe et al. (2002 ▶); Van der Merwe et al. (2010 ▶). For bond lengths and bond angles in related structures, see: Bao et al. (2003 ▶); Cao et al. (2007 ▶); Gong et al. (2006 ▶); Van der Merwe et al. (2009 ▶); Visser et al. (2010 ▶); Yin et al. (2003 ▶).
Experimental
Crystal data
Cs2[Co(CH4O6P2)2(H2O)2]
M r = 708.75
Triclinic,

a = 7.333 (5) Å
b = 7.412 (5) Å
c = 7.666 (5) Å
α = 74.621 (5)°
β = 83.064 (5)°
γ = 86.496 (5)°
V = 398.6 (5) Å3
Z = 1
Mo Kα radiation
μ = 5.96 mm−1
T = 293 K
0.38 × 0.07 × 0.05 mm
Data collection
Bruker APEXII CCD diffractometer
Absorption correction: multi-scan (SADABS; Bruker, 2001 ▶) T min = 0.210, T max = 0.755
4203 measured reflections
1904 independent reflections
1827 reflections with I > 2σ(I)
R int = 0.015
Refinement
R[F 2 > 2σ(F 2)] = 0.015
wR(F 2) = 0.044
S = 0.75
1904 reflections
132 parameters
11 restraints
H atoms treated by a mixture of independent and constrained refinement
Δρmax = 0.47 e Å−3
Δρmin = −0.48 e Å−3
Data collection: APEX2 (Bruker, 2005 ▶); cell refinement: SAINT-Plus (Bruker, 2004 ▶); data reduction: SAINT-Plus; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: DIAMOND (Brandenburg & Putz, 2005 ▶); software used to prepare material for publication: WinGX (Farrugia, 1999 ▶).
Supplementary Material
Crystal structure: contains datablock(s) global, I. DOI: 10.1107/S1600536811035355/jh2322sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536811035355/jh2322Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| O1—H1A⋯O4i | 0.92 (2) | 1.94 (2) | 2.860 (3) | 173 (3) |
| O5—H5B⋯O4ii | 0.85 (2) | 1.69 (3) | 2.518 (3) | 164 (7) |
Symmetry codes: (i)  ; (ii)
; (ii)  .
.
Acknowledgments
The University of the Free State and Professor A. Roodt are gratefully acknowledged for financial support.
supplementary crystallographic information
Comment
This work is part of an ongoing investigation aimed at synthesizing and characterizing new methylene diphosphonate complexes and expanding on our knowledge of the interactions of the methylene diphosphonate ligand with various metal centers. (Van der Merwe et al. (2009) & Van der Merwe et al. (2010)).
Methylene diphosphonates (O3PCH2PO3) has a diversified coordination capability with metal ions, due to the single methyl group which divides the two phosphonate groups. The formation of a stable six-membered ring comprised of M—O—P—C—P—O is favoured (Bao et al. (2003). Bisphosphonates adhere strongly to hydroxyapatite crystals and constrain their formation and dissolution (Fleisch (1991)). This physicochemical in vivo effect may result in the prevention of soft tissue calcification or even prevent normal calcification. The bis(phosphonic acid) has a high affinity for bone surfaces and it is also non-hydrolyzable (Neville-Webbe et al. (2002).
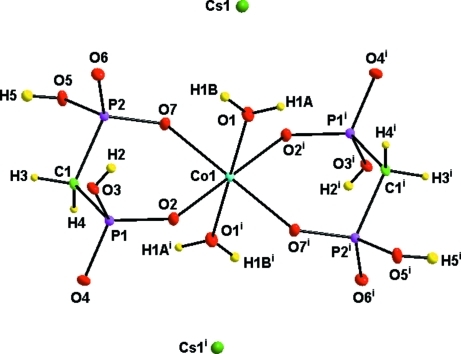
The asymmetric unit of the title compound, Cs2[Co(CH4O6P2)2(H2O)2], is comprised of one bidentate methylene diphosphonate ligand and one water molecule which are coordinated to the CoII atom, as well as a non-coordinated caesium cation. The Co atom occupies a special position on a crystallographic inversion center. The caesium ion is octahedrally coordinated to six oxygen atoms with Cs—O distances ranging from 3.119 (2) to 3.296 (2) Å. The two methylene diphosphonate ligands chelate to the central cobalt metal via four oxygen atoms (O2/O2' and O7/O7') from the phosphonate groups. This leads to the formation of two six-membered rings.
The CoII metal center has a slightly distorted octahedral geometry with O—Co—O angles ranging between 85.35 (8) ° and 94.65 (8) °. The Co—O bond lengths vary between 2.0761 (18) and 2.1272 (19) Å. These distances correspond to literature values (Bao et al. (2003); Cao et al. (2007); Gong et al. (2006); Van der Merwe et al. (2009); Visser et al. (2010); Yin et al. (2003).
A three-dimensional network is provided by O—H–O hydrogen bonds (Table 2).
Experimental
[Co(NH3)6]Cl3(0,1700 g, 0,714 mmol) was dissolved in distilled water (10 cm3) and the pH of the solution was lowered to 1.87 using hydrochloric acid. The solution was heated for 30 minutes at 313.15 K. Methylene diphosphonate (0.251 g, 1.43 mmol) was dissolved in distilled water (7 cm3) and the pH of the solution was elevated to 1.93 using caesium chloride. Both solutions were combined and the pH was adjusted to 2.04, the pink solution was heated for 3 h at 353.15 K. Pink crystals, suitable for X-ray diffraction, was obtained. (Yield: 7.2%)
Refinement
All H atoms were located from difference Fourier maps and were refined isotropically without further restraints. The highest residual electron density was located 0.88 Å from P1.
Figures
Fig. 1.
Representation of the title compound, showing the numbering scheme and displacement ellipsoids drawn at the 50% probability level. [Symmetry code: (i) 1 - x, -y, 1 - z].
Crystal data
| Cs2[Co(CH4O6P2)2(H2O)2] | Z = 1 |
| Mr = 708.75 | F(000) = 253 |
| Triclinic, P1 | Dx = 2.953 Mg m−3 |
| Hall symbol: -P 1 | Mo Kα radiation, λ = 0.71073 Å |
| a = 7.333 (5) Å | Cell parameters from 3160 reflections |
| b = 7.412 (5) Å | θ = 2.8–28.4° |
| c = 7.666 (5) Å | µ = 5.96 mm−1 |
| α = 74.621 (5)° | T = 293 K |
| β = 83.064 (5)° | Needle, pink |
| γ = 86.496 (5)° | 0.38 × 0.07 × 0.05 mm |
| V = 398.6 (5) Å3 |
Data collection
| Bruker APEXII CCD diffractometer | 1827 reflections with I > 2σ(I) |
| φ and ω scans | Rint = 0.015 |
| Absorption correction: multi-scan (SADABS; Bruker, 2001) | θmax = 28°, θmin = 3.7° |
| Tmin = 0.210, Tmax = 0.755 | h = −9→9 |
| 4203 measured reflections | k = −9→9 |
| 1904 independent reflections | l = −10→10 |
Refinement
| Refinement on F2 | 11 restraints |
| Least-squares matrix: full | H atoms treated by a mixture of independent and constrained refinement |
| R[F2 > 2σ(F2)] = 0.015 | w = 1/[σ2(Fo2) + (0.0412P)2 + 0.4899P] where P = (Fo2 + 2Fc2)/3 |
| wR(F2) = 0.044 | (Δ/σ)max = 0.002 |
| S = 0.75 | Δρmax = 0.47 e Å−3 |
| 1904 reflections | Δρmin = −0.48 e Å−3 |
| 132 parameters |
Special details
| Geometry. All s.u.'s (except the s.u. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell s.u.'s are taken into account individually in the estimation of s.u.'s in distances, angles and torsion angles; correlations between s.u.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell s.u.'s is used for estimating s.u.'s involving l.s. planes. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | Occ. (<1) | |
| Cs1 | 0.284577 (17) | 0.954858 (18) | 0.692901 (17) | 0.00965 (6) | |
| Co1 | 0.5 | 0.5 | 0.5 | 0.00595 (9) | |
| P1 | 0.26392 (7) | 0.34252 (8) | 0.22866 (8) | 0.00590 (11) | |
| P2 | 0.19616 (7) | 0.74778 (8) | 0.25147 (8) | 0.00589 (11) | |
| O1 | 0.2886 (2) | 0.4437 (2) | 0.7201 (2) | 0.0112 (3) | |
| O2 | 0.3857 (2) | 0.3210 (2) | 0.3786 (2) | 0.0088 (3) | |
| O3 | 0.0667 (2) | 0.2706 (2) | 0.3122 (2) | 0.0086 (3) | |
| O4 | 0.3336 (2) | 0.2396 (2) | 0.0861 (2) | 0.0086 (3) | |
| O5 | 0.1822 (2) | 0.9384 (2) | 0.1036 (2) | 0.0109 (3) | |
| O6 | 0.0107 (2) | 0.7058 (2) | 0.3621 (2) | 0.0102 (3) | |
| O7 | 0.3541 (2) | 0.7427 (2) | 0.3623 (2) | 0.0086 (3) | |
| C1 | 0.2397 (3) | 0.5858 (3) | 0.1121 (3) | 0.0071 (4) | |
| H1A | 0.313 (4) | 0.383 (4) | 0.837 (3) | 0.023 (9)* | |
| H1B | 0.185 (4) | 0.410 (6) | 0.704 (6) | 0.063 (15)* | |
| H2 | 0.048 (5) | 0.287 (5) | 0.417 (3) | 0.035 (10)* | |
| H3 | 0.145 (3) | 0.599 (4) | 0.033 (4) | 0.018 (8)* | |
| H4 | 0.357 (3) | 0.616 (4) | 0.046 (4) | 0.015 (8)* | |
| H5A | 0.156 (9) | 0.935 (9) | 0.002 (5) | 0.022* | 0.5 |
| H5B | 0.239 (8) | 1.030 (7) | 0.116 (9) | 0.022* | 0.5 |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Cs1 | 0.01010 (9) | 0.00910 (9) | 0.00854 (9) | 0.00056 (5) | −0.00040 (5) | −0.00068 (6) |
| Co1 | 0.00540 (19) | 0.00593 (19) | 0.0067 (2) | −0.00035 (15) | −0.00109 (15) | −0.00174 (16) |
| P1 | 0.0061 (2) | 0.0053 (2) | 0.0063 (3) | −0.00119 (19) | −0.0007 (2) | −0.0015 (2) |
| P2 | 0.0060 (2) | 0.0060 (3) | 0.0057 (3) | −0.00059 (19) | −0.0006 (2) | −0.0014 (2) |
| O1 | 0.0084 (8) | 0.0160 (9) | 0.0088 (8) | −0.0027 (7) | −0.0002 (6) | −0.0019 (7) |
| O2 | 0.0097 (7) | 0.0077 (7) | 0.0092 (8) | −0.0008 (6) | −0.0028 (6) | −0.0015 (6) |
| O3 | 0.0076 (7) | 0.0110 (8) | 0.0077 (8) | −0.0033 (6) | 0.0007 (6) | −0.0032 (6) |
| O4 | 0.0098 (7) | 0.0072 (7) | 0.0096 (8) | −0.0012 (6) | 0.0008 (6) | −0.0040 (6) |
| O5 | 0.0173 (8) | 0.0063 (7) | 0.0093 (8) | −0.0012 (6) | −0.0063 (7) | −0.0002 (6) |
| O6 | 0.0072 (7) | 0.0139 (8) | 0.0096 (8) | −0.0017 (6) | 0.0012 (6) | −0.0040 (6) |
| O7 | 0.0096 (7) | 0.0067 (7) | 0.0098 (8) | −0.0003 (6) | −0.0032 (6) | −0.0016 (6) |
| C1 | 0.0087 (9) | 0.0066 (10) | 0.0056 (10) | −0.0009 (8) | −0.0011 (8) | −0.0005 (8) |
Geometric parameters (Å, °)
| Cs1—O5i | 3.119 (3) | P2—O6 | 1.5158 (18) |
| Cs1—O3ii | 3.165 (2) | P2—O5 | 1.5663 (19) |
| Cs1—O2iii | 3.162 (2) | P2—C1 | 1.799 (2) |
| Cs1—O2iv | 3.173 (2) | O1—H1A | 0.922 (17) |
| Cs1—O6v | 3.192 (2) | O1—H1B | 0.846 (19) |
| Cs1—O4iii | 3.485 (2) | O2—Cs1iii | 3.162 (2) |
| Cs1—O7vi | 3.490 (2) | O2—Cs1vii | 3.173 (2) |
| Co1—O2iii | 2.0761 (18) | O3—Cs1ii | 3.165 (2) |
| Co1—O2 | 2.0761 (18) | O3—H2 | 0.840 (19) |
| Co1—O1iii | 2.1209 (19) | O4—Cs1iii | 3.485 (2) |
| Co1—O1 | 2.1209 (19) | O5—Cs1viii | 3.119 (3) |
| Co1—O7iii | 2.1272 (19) | O5—H5A | 0.83 (2) |
| Co1—O7 | 2.1272 (19) | O5—H5B | 0.85 (2) |
| P1—O2 | 1.5087 (18) | O6—Cs1v | 3.192 (2) |
| P1—O4 | 1.5158 (18) | O7—Cs1vi | 3.490 (2) |
| P1—O3 | 1.5732 (18) | C1—H3 | 0.963 (14) |
| P1—C1 | 1.796 (3) | C1—H4 | 0.951 (14) |
| P2—O7 | 1.5099 (18) | ||
| O5i—Cs1—O3ii | 91.51 (5) | O7—Co1—Cs1iii | 128.78 (6) |
| O5i—Cs1—O2iii | 113.53 (4) | O2iii—Co1—Cs1vi | 45.80 (6) |
| O3ii—Cs1—O2iii | 103.25 (7) | O2—Co1—Cs1vi | 134.20 (6) |
| O5i—Cs1—O2iv | 125.59 (5) | O1iii—Co1—Cs1vi | 60.18 (5) |
| O3ii—Cs1—O2iv | 120.31 (5) | O1—Co1—Cs1vi | 119.82 (5) |
| O2iii—Cs1—O2iv | 101.31 (5) | O7iii—Co1—Cs1vi | 125.13 (6) |
| O5i—Cs1—O6v | 82.95 (5) | O7—Co1—Cs1vi | 54.87 (6) |
| O3ii—Cs1—O6v | 81.36 (7) | Cs1iii—Co1—Cs1vi | 123.10 (4) |
| O2iii—Cs1—O6v | 162.47 (4) | O2iii—Co1—Cs1vii | 134.20 (6) |
| O2iv—Cs1—O6v | 62.52 (5) | O2—Co1—Cs1vii | 45.80 (6) |
| O5i—Cs1—O4iii | 73.40 (5) | O1iii—Co1—Cs1vii | 119.82 (5) |
| O3ii—Cs1—O4iii | 124.24 (6) | O1—Co1—Cs1vii | 60.18 (5) |
| O2iii—Cs1—O4iii | 44.75 (5) | O7iii—Co1—Cs1vii | 54.87 (6) |
| O2iv—Cs1—O4iii | 111.44 (5) | O7—Co1—Cs1vii | 125.13 (6) |
| O6v—Cs1—O4iii | 144.68 (4) | Cs1iii—Co1—Cs1vii | 56.90 (4) |
| O5i—Cs1—O7vi | 94.14 (4) | Cs1vi—Co1—Cs1vii | 180 |
| O3ii—Cs1—O7vi | 170.61 (4) | O2—P1—O4 | 114.76 (10) |
| O2iii—Cs1—O7vi | 81.34 (7) | O2—P1—O3 | 109.86 (10) |
| O2iv—Cs1—O7vi | 50.37 (5) | O4—P1—O3 | 107.73 (9) |
| O6v—Cs1—O7vi | 91.89 (7) | O2—P1—C1 | 109.55 (10) |
| O4iii—Cs1—O7vi | 64.74 (6) | O4—P1—C1 | 107.05 (11) |
| O5i—Cs1—C1i | 44.75 (6) | O3—P1—C1 | 107.62 (10) |
| O3ii—Cs1—C1i | 72.92 (5) | O2—P1—Cs1iii | 51.16 (7) |
| O2iii—Cs1—C1i | 78.47 (6) | O4—P1—Cs1iii | 63.66 (7) |
| O2iv—Cs1—C1i | 165.97 (5) | O3—P1—Cs1iii | 124.40 (8) |
| O6v—Cs1—C1i | 118.91 (5) | C1—P1—Cs1iii | 127.77 (8) |
| O4iii—Cs1—C1i | 58.52 (5) | O2—P1—Cs1vii | 49.06 (7) |
| O7vi—Cs1—C1i | 116.25 (5) | O4—P1—Cs1vii | 103.57 (8) |
| O5i—Cs1—O1iv | 74.68 (4) | O3—P1—Cs1vii | 68.88 (7) |
| O3ii—Cs1—O1iv | 126.32 (5) | C1—P1—Cs1vii | 148.54 (8) |
| O2iii—Cs1—O1iv | 130.04 (5) | Cs1iii—P1—Cs1vii | 61.70 (2) |
| O2iv—Cs1—O1iv | 50.97 (5) | O7—P2—O6 | 114.83 (11) |
| O6v—Cs1—O1iv | 46.06 (5) | O7—P2—O5 | 111.69 (10) |
| O4iii—Cs1—O1iv | 101.41 (4) | O6—P2—O5 | 109.44 (10) |
| O7vi—Cs1—O1iv | 48.72 (4) | O7—P2—C1 | 110.57 (10) |
| C1i—Cs1—O1iv | 118.60 (6) | O6—P2—C1 | 108.09 (10) |
| O5i—Cs1—O3iv | 125.42 (5) | O5—P2—C1 | 101.32 (11) |
| O3ii—Cs1—O3iv | 78.65 (6) | O7—P2—Cs1v | 124.05 (8) |
| O2iii—Cs1—O3iv | 121.02 (5) | O5—P2—Cs1v | 65.52 (7) |
| O2iv—Cs1—O3iv | 42.33 (5) | C1—P2—Cs1v | 125.05 (8) |
| O6v—Cs1—O3iv | 42.63 (4) | O7—P2—Cs1viii | 118.54 (7) |
| O4iii—Cs1—O3iv | 152.49 (4) | O6—P2—Cs1viii | 125.77 (7) |
| O7vi—Cs1—O3iv | 91.97 (5) | C1—P2—Cs1viii | 61.03 (8) |
| C1i—Cs1—O3iv | 148.87 (5) | Cs1v—P2—Cs1viii | 94.916 (19) |
| O1iv—Cs1—O3iv | 69.55 (5) | Co1—O1—Cs1vii | 89.88 (6) |
| O5i—Cs1—O5v | 55.64 (5) | Co1—O1—H1A | 122 (2) |
| O3ii—Cs1—O5v | 56.50 (6) | Cs1vii—O1—H1A | 81 (2) |
| O2iii—Cs1—O5v | 153.15 (4) | Co1—O1—H1B | 120 (3) |
| O2iv—Cs1—O5v | 104.38 (5) | Cs1vii—O1—H1B | 66 (3) |
| O6v—Cs1—O5v | 41.87 (4) | H1A—O1—H1B | 108 (3) |
| O4iii—Cs1—O5v | 128.47 (5) | P1—O2—Co1 | 135.98 (10) |
| O7vi—Cs1—O5v | 121.54 (5) | P1—O2—Cs1iii | 107.03 (9) |
| C1i—Cs1—O5v | 78.50 (5) | Co1—O2—Cs1iii | 103.97 (7) |
| O1iv—Cs1—O5v | 73.92 (4) | P1—O2—Cs1vii | 109.88 (8) |
| O3iv—Cs1—O5v | 75.37 (5) | Co1—O2—Cs1vii | 106.22 (8) |
| O5i—Cs1—P1iii | 94.28 (4) | Cs1iii—O2—Cs1vii | 78.69 (5) |
| O3ii—Cs1—P1iii | 115.59 (6) | P1—O3—Cs1ii | 150.94 (9) |
| O2iii—Cs1—P1iii | 21.82 (3) | P1—O3—Cs1vii | 87.66 (8) |
| O2iv—Cs1—P1iii | 106.97 (4) | Cs1ii—O3—Cs1vii | 101.35 (6) |
| O6v—Cs1—P1iii | 162.96 (3) | P1—O3—H2 | 108 (3) |
| O4iii—Cs1—P1iii | 22.94 (3) | Cs1ii—O3—H2 | 101 (3) |
| O7vi—Cs1—P1iii | 71.49 (6) | Cs1vii—O3—H2 | 59 (3) |
| C1i—Cs1—P1iii | 67.80 (4) | P1—O4—Cs1iii | 93.39 (8) |
| O1iv—Cs1—P1iii | 116.98 (4) | P2—O5—Cs1viii | 120.07 (9) |
| O3iv—Cs1—P1iii | 138.50 (3) | P2—O5—Cs1v | 91.93 (8) |
| O5v—Cs1—P1iii | 145.78 (4) | Cs1viii—O5—Cs1v | 124.36 (5) |
| O2iii—Co1—O2 | 180 | P2—O5—H5A | 118 (5) |
| O2iii—Co1—O1iii | 90.76 (8) | Cs1v—O5—H5A | 99 (5) |
| O2—Co1—O1iii | 89.24 (8) | P2—O5—H5B | 117 (5) |
| O2iii—Co1—O1 | 89.24 (8) | Cs1viii—O5—H5B | 103 (5) |
| O2—Co1—O1 | 90.76 (8) | Cs1v—O5—H5B | 100 (5) |
| O1iii—Co1—O1 | 180 | H5A—O5—H5B | 121 (6) |
| O2iii—Co1—O7iii | 94.65 (8) | P2—O6—Cs1v | 115.52 (9) |
| O2—Co1—O7iii | 85.35 (8) | P2—O7—Co1 | 126.62 (10) |
| O1iii—Co1—O7iii | 91.47 (7) | P2—O7—Cs1vi | 131.62 (9) |
| O1—Co1—O7iii | 88.53 (7) | Co1—O7—Cs1vi | 95.23 (7) |
| O2iii—Co1—O7 | 85.35 (8) | P1—C1—P2 | 116.79 (13) |
| O2—Co1—O7 | 94.65 (8) | P1—C1—Cs1viii | 149.47 (11) |
| O1iii—Co1—O7 | 88.53 (7) | P2—C1—Cs1viii | 93.21 (10) |
| O1—Co1—O7 | 91.47 (7) | P1—C1—H3 | 108.1 (19) |
| O7iii—Co1—O7 | 180.00 (9) | P2—C1—H3 | 110.1 (19) |
| O2iii—Co1—Cs1iii | 132.78 (6) | Cs1viii—C1—H3 | 62.8 (19) |
| O2—Co1—Cs1iii | 47.22 (6) | P1—C1—H4 | 104.3 (19) |
| O1iii—Co1—Cs1iii | 63.15 (5) | P2—C1—H4 | 105.3 (19) |
| O1—Co1—Cs1iii | 116.85 (5) | Cs1viii—C1—H4 | 59.3 (18) |
| O7iii—Co1—Cs1iii | 51.22 (6) | H3—C1—H4 | 112 (3) |
| O2iii—Co1—O1—Cs1vii | −144.21 (6) | C1—P2—O5—Cs1viii | −8.70 (12) |
| O2—Co1—O1—Cs1vii | 35.79 (6) | Cs1v—P2—O5—Cs1viii | −132.22 (9) |
| O7iii—Co1—O1—Cs1vii | −49.55 (6) | O7—P2—O5—Cs1v | −118.75 (9) |
| O7—Co1—O1—Cs1vii | 130.45 (6) | O6—P2—O5—Cs1v | 9.54 (9) |
| Cs1iii—Co1—O1—Cs1vii | −5.22 (5) | C1—P2—O5—Cs1v | 123.53 (8) |
| Cs1vi—Co1—O1—Cs1vii | 180 | Cs1viii—P2—O5—Cs1v | 132.22 (9) |
| O4—P1—O2—Co1 | 130.04 (13) | O7—P2—O6—Cs1v | 114.18 (10) |
| O3—P1—O2—Co1 | −108.42 (14) | O5—P2—O6—Cs1v | −12.34 (11) |
| C1—P1—O2—Co1 | 9.60 (17) | C1—P2—O6—Cs1v | −121.88 (10) |
| Cs1iii—P1—O2—Co1 | 132.87 (17) | Cs1viii—P2—O6—Cs1v | −55.00 (10) |
| Cs1vii—P1—O2—Co1 | −143.29 (18) | O6—P2—O7—Co1 | 80.65 (14) |
| O4—P1—O2—Cs1iii | −2.83 (11) | O5—P2—O7—Co1 | −154.00 (11) |
| O3—P1—O2—Cs1iii | 118.71 (9) | C1—P2—O7—Co1 | −41.97 (15) |
| C1—P1—O2—Cs1iii | −123.27 (9) | Cs1v—P2—O7—Co1 | 131.64 (9) |
| Cs1vii—P1—O2—Cs1iii | 83.84 (8) | Cs1viii—P2—O7—Co1 | −109.34 (10) |
| O4—P1—O2—Cs1vii | −86.67 (11) | O6—P2—O7—Cs1vi | −135.06 (11) |
| O3—P1—O2—Cs1vii | 34.87 (11) | O5—P2—O7—Cs1vi | −9.70 (14) |
| C1—P1—O2—Cs1vii | 152.89 (9) | C1—P2—O7—Cs1vi | 102.32 (13) |
| Cs1iii—P1—O2—Cs1vii | −83.84 (8) | Cs1v—P2—O7—Cs1vi | −84.06 (12) |
| O1iii—Co1—O2—P1 | −80.87 (15) | Cs1viii—P2—O7—Cs1vi | 34.96 (13) |
| O1—Co1—O2—P1 | 99.13 (15) | O2iii—Co1—O7—P2 | −168.93 (12) |
| O7iii—Co1—O2—P1 | −172.41 (15) | O2—Co1—O7—P2 | 11.07 (12) |
| O7—Co1—O2—P1 | 7.59 (15) | O1iii—Co1—O7—P2 | 100.19 (13) |
| Cs1iii—Co1—O2—P1 | −133.77 (17) | O1—Co1—O7—P2 | −79.81 (13) |
| Cs1vi—Co1—O2—P1 | −35.83 (17) | Cs1iii—Co1—O7—P2 | 47.08 (14) |
| Cs1vii—Co1—O2—P1 | 144.17 (17) | Cs1vi—Co1—O7—P2 | 154.02 (14) |
| O1iii—Co1—O2—Cs1iii | 52.90 (7) | Cs1vii—Co1—O7—P2 | −25.98 (14) |
| O1—Co1—O2—Cs1iii | −127.10 (7) | O2iii—Co1—O7—Cs1vi | 37.05 (6) |
| O7iii—Co1—O2—Cs1iii | −38.64 (6) | O2—Co1—O7—Cs1vi | −142.95 (6) |
| O7—Co1—O2—Cs1iii | 141.36 (6) | O1iii—Co1—O7—Cs1vi | −53.82 (6) |
| Cs1vi—Co1—O2—Cs1iii | 97.94 (6) | O1—Co1—O7—Cs1vi | 126.18 (6) |
| Cs1vii—Co1—O2—Cs1iii | −82.06 (6) | Cs1iii—Co1—O7—Cs1vi | −106.94 (4) |
| O1iii—Co1—O2—Cs1vii | 134.96 (7) | Cs1vii—Co1—O7—Cs1vi | 180 |
| O1—Co1—O2—Cs1vii | −45.04 (7) | O2—P1—C1—P2 | −44.78 (16) |
| O7iii—Co1—O2—Cs1vii | 43.42 (6) | O4—P1—C1—P2 | −169.80 (12) |
| O7—Co1—O2—Cs1vii | −136.58 (6) | O3—P1—C1—P2 | 74.63 (14) |
| Cs1iii—Co1—O2—Cs1vii | 82.06 (6) | Cs1iii—P1—C1—P2 | −100.25 (12) |
| Cs1vi—Co1—O2—Cs1vii | 180 | Cs1vii—P1—C1—P2 | −3.5 (2) |
| O2—P1—O3—Cs1ii | −136.98 (17) | O2—P1—C1—Cs1viii | 123.58 (19) |
| O4—P1—O3—Cs1ii | −11.3 (2) | O4—P1—C1—Cs1viii | −1.4 (2) |
| C1—P1—O3—Cs1ii | 103.8 (2) | O3—P1—C1—Cs1viii | −117.01 (19) |
| Cs1iii—P1—O3—Cs1ii | −81.1 (2) | Cs1iii—P1—C1—Cs1viii | 68.1 (2) |
| Cs1vii—P1—O3—Cs1ii | −109.40 (19) | Cs1vii—P1—C1—Cs1viii | 164.84 (9) |
| O2—P1—O3—Cs1vii | −27.58 (9) | O7—P2—C1—P1 | 62.05 (15) |
| O4—P1—O3—Cs1vii | 98.08 (9) | O6—P2—C1—P1 | −64.42 (15) |
| C1—P1—O3—Cs1vii | −146.80 (8) | O5—P2—C1—P1 | −179.41 (12) |
| Cs1iii—P1—O3—Cs1vii | 28.30 (7) | Cs1v—P2—C1—P1 | −111.48 (11) |
| O2—P1—O4—Cs1iii | 2.46 (9) | Cs1viii—P2—C1—P1 | 174.11 (15) |
| O3—P1—O4—Cs1iii | −120.24 (8) | O7—P2—C1—Cs1viii | −112.06 (8) |
| C1—P1—O4—Cs1iii | 124.27 (8) | O6—P2—C1—Cs1viii | 121.47 (8) |
| Cs1vii—P1—O4—Cs1iii | −48.42 (5) | O5—P2—C1—Cs1viii | 6.48 (9) |
| O7—P2—O5—Cs1viii | 109.03 (10) | Cs1v—P2—C1—Cs1viii | 74.41 (7) |
| O6—P2—O5—Cs1viii | −122.68 (10) |
Symmetry codes: (i) x, y, z+1; (ii) −x, −y+1, −z+1; (iii) −x+1, −y+1, −z+1; (iv) x, y+1, z; (v) −x, −y+2, −z+1; (vi) −x+1, −y+2, −z+1; (vii) x, y−1, z; (viii) x, y, z−1.
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| O1—H1A···O4i | 0.92 (2) | 1.94 (2) | 2.860 (3) | 173 (3) |
| O5—H5B···O4iv | 0.85 (2) | 1.69 (3) | 2.518 (3) | 164 (7) |
Symmetry codes: (i) x, y, z+1; (iv) x, y+1, z.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: JH2322).
References
- Bao, S., Zheng, L., Liu, Y., Xu, W. & Feng, S. (2003). Inorg. Chem. 42, 5037–5039. [DOI] [PubMed]
- Brandenburg, K. & Putz, H. (2005). DIAMOND Crystal Impact GbR, Bonn, Germany.
- Bruker (2001). SADABS Bruker AXS Inc., Madison, Wisconsin, USA.
- Bruker (2004). SAINT-Plus Bruker AXS Inc., Madison, Wisconsin, USA.
- Bruker (2005). APEX2 Bruker AXS Inc., Madison, Wisconsin, USA.
- Cao, D., Li, Y. & Zheng, L. (2007). Inorg. Chem. 46, 7571–7578. [DOI] [PubMed]
- Farrugia, L. J. (1999). J. Appl. Cryst. 32, 837–838.
- Fleisch, H. (1991). Drugs, 42, 919–944. [DOI] [PubMed]
- Gong, Y., Tang, W., Hou, W., Zha, Z. & Hu, C. (2006). Inorg. Chem. 45, 4987–4995. [DOI] [PubMed]
- Neville-Webbe, H. L., Holen, I. & Coleman, R. E. (2002). Cancer Treat. Rev. 28, 305–319. [DOI] [PubMed]
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Van der Merwe, K. A., Visser, H. G. & Venter, J. A. (2009). Acta Cryst. E65, m1394. [DOI] [PMC free article] [PubMed]
- Van der Merwe, K., Visser, H. G. & Venter, J. A. (2010). Acta Cryst. E66, m1011–m1012. [DOI] [PMC free article] [PubMed]
- Visser, H. G., Venter, J. A. & Van der Merwe, K. A. (2010). Acta Cryst. E66, m159. [DOI] [PMC free article] [PubMed]
- Yin, P., Gao, S., Zheng, L. & Xin, X. (2003). Chem. Mater. 15, 3233–3236.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) global, I. DOI: 10.1107/S1600536811035355/jh2322sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536811035355/jh2322Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report