Abstract
The existence in higher plants of an additional β-oxidation system in mitochondria, besides the well-characterized peroxisomal system, is often considered controversial. Unequivocal demonstration of β-oxidation activity in mitochondria should rely on identification of the enzymes specific to mitochondrial β-oxidation. Acyl-coenzyme A dehydrogenase (ACAD) (EC 1.3.99.2,3) activity was detected in purified mitochondria from maize (Zea mays L.) root tips and from embryonic axes of early-germinating sunflower (Helianthus annuus L.) seeds, using as the enzyme assay the reduction of 2,6-dichlorophenolindophenol, with phenazine methosulfate as the intermediate electron carrier. Subcellular fractionation showed that this ACAD activity was associated with mitochondrial fractions. Comparison of ACAD activity in mitochondria and acyl-coenzyme A oxidase activity in peroxisomes showed differences of substrate specificities. Embryonic axes of sunflower seeds were used as starting material for the purification of ACADs. Two distinct ACADs, with medium-chain and long-chain substrate specificities, respectively, were separated by their chromatographic behavior, which was similar to that of mammalian ACADs. The characterization of these ACADs is discussed in relation to the identification of expressed sequenced tags corresponding to ACADs in cDNA sequence analysis projects and with the potential roles of mitochondrial β-oxidation in higher plants.
All of the tissues of higher plants, even nonfatty and nonsenescent, appear to possess the capacity for fatty acid β-oxidation (Gerhardt, 1983, 1985; Kindl, 1987). This catabolism in higher plants proceeds primarily by peroxisomal β-oxidation (Cooper and Beevers, 1969a, 1969b; Gerhardt, 1985), in contrast with mammalian tissues where β-oxidation takes place in both peroxisomes and mitochondria. Thus, the existence in higher plants of an additional mitochondrial β-oxidation system is often considered controversial (Gerhardt et al., 1995; Hoppe and Theimer, 1997). It is well established that the massive degradation of fatty acids during early growth of fatty seeds proceeds through glyoxysomal β-oxidation (Cooper and Beevers, 1969a, 1969b; Hoppe and Theimer, 1997). However, studies of the catabolism of branched-chain amino acids, in which the isobutyryl-CoA, 2-methyl-butyryl-CoA, and isovaleryl-CoA catabolites of Val, Ile, and Leu, respectively, undergo β-oxidation, have led Gerbling and Gerhardt (1989) to hypothesize the existence of extra-peroxisomal β-oxidation for Leu and Val degradation. The localization of β-methyl-crotonyl-CoA carboxylase, which catalyzes a subsequent step of Leu catabolism, in mitochondria of sycamore cells (Aubert et al., 1996) may be an indication of the mitochondrial location of this extra-peroxisomal β-oxidation. Furthermore, mitochondria from pea cotyledons (Wood et al., 1986) were shown to contain at least some of the enzymes of β-oxidation. In the case of enoyl-CoA hydratase, an isoenzyme immunologically distinct from the peroxisomal enzyme was partially purified from mitochondria (Miernyk et al., 1991). However, the existence of enzymes that are known to be specific to mitochondrial β-oxidation in mammalian tissues remains to be fully demonstrated in higher plants (Hoppe and Theimer, 1997).
We obtained direct evidence of acetyl-CoA production from substrate fatty acids by mitochondria purified from carbohydrate-starved maize (Zea mays L.) root tips (Dieuaide et al., 1993). The inhibition of this acetyl-CoA production by respiratory-chain inhibitors further showed that, like in mammalian cells, mitochondrial β-oxidation in higher plants was dependent on the respiratory chain. In this case, mitochondrial β-oxidation, in contrast with peroxisomal activity, was found to be strictly dependent on carbohydrate starvation (Dieuaide et al., 1993). However, Gerhardt et al. (1995) showed that pea cotyledon mitochondria could catalyze the formation of acid-soluble [14C] products from [1-14C]palmitoyl-l-carnitine. This activity was significant and showed inhibition by cyanide, thus indicating a limited level of mitochondrial fatty acid β-oxidation in nonfatty and nonstarved plant tissues. Moreover, Salon (1988) has shown that the oxidation of hexanoate in early-germinating embryos of lettuce (Salon et al., 1988) was inhibited by mercaptopropionate, which is an inhibitor of mitochondrial β-oxidation in mammals (Sabbagh et al., 1985). Early-germinating embryos of sunflower (Helianthus annuus L.) seeds appeared to have a similar fatty acid metabolism (Salon, 1988).
The first step of β-oxidation consists of the desaturation of acyl-CoA to 2-trans-enoyl-CoA. In animal tissues this is catalyzed by mitochondrial ACAD, transferring electrons to an electron-transferring flavoprotein, which feeds reducing equivalents to the respiratory chain (Engel, 1992), and by peroxisomal ACOX, the flavin moiety of which is reoxidized directly by O2 (Osmundsen et al., 1991). Inhibition of mitochondrial β-oxidation by respiratory-chain inhibitors (Dieuaide et al., 1993; Gerhardt et al., 1995) thus strongly suggested that higher-plant mitochondria possessed ACAD activity, which was identified in mitochondria from carbohydrate-starved maize root tips (Dieuaide et al., 1993). The peroxisomal ACOX activity of higher plants was described more than 25 years ago (Cooper and Beevers, 1969a, 1969b). Long-chain ACOX from cucumber (Kirsch et al., 1986) and pumpkin (Hayashi et al., 1998) have been characterized, and we showed that higher plants also possess distinct short-chain and medium-chain ACOX (Hooks et al., 1996), which appear to be differentially expressed, depending on developmental and metabolic status (Eccleston et al., 1995; Hooks et al., 1995). In animal systems the ACOX- and ACAD-catalyzed steps exert strong control on the discrimination of substrates and on the overall flux of β-oxidation (Aoyama et al., 1994a, 1994b). Furthermore, in mammalian tissues the comparison of ACOX (Vanhove et al., 1993b) and ACAD (Nagao and Tanaka, 1992) has greatly clarified the respective functions of peroxisomal and mitochondrial β-oxidation. This is why the systematic study of higher-plant ACOX and ACAD is likely to provide new information concerning the functions of peroxisomal β-oxidation and to determine the functions of mitochondrial β-oxidation.
Here we present the characterization of the substrate specificity of ACAD activity in purified mitochondria and in partially purified preparations of ACAD from maize root tips and from embryos of early-germinating sunflower seeds. The existence of true ACAD activities that are distinct from the well-described ACOX activity is thus confirmed. Furthermore, the typical activities that have been described in animal cells, with straight short-chain, medium-chain, and long-chain substrates, and with branched-chain substrates, such as isovaleryl-CoA, are shown to exist in higher plants. The partial purification of distinct ACAD of the medium-chain and long-chain types (Ikeda et al., 1983, 1985) further demonstrates that these ACAD activities are due to a family of enzymes.
MATERIALS AND METHODS
Plant Material
Germination of maize (Zea mays L. cv DEA, Pioneer France Maïs, France) seeds was carried out at 25°C in the dark for 3 d between sheets of Whatman 3MM chromatography paper soaked in the mineral nutrient medium described by Saglio and Pradet (1980). Three-millimeter-long tips of seminal roots were excised and either immediately used for the preparation of organelles or incubated for carbohydrate starvation treatment. In this latter case, the excised root tips were incubated at 25°C in the mineral nutrient medium supplemented with 1% (v/v) of the antibiotic and antimycotic mixture A7292 from Sigma and 0.1 m Mes-KOH, pH 6.0. A gas mixture containing 50% (v/v) O2 and 50% (v/v) N2 was continuously bubbled through the incubation medium to maintain a partial O2 pressure above 35 kPa, which is the critical O2 pressure for maize roots in aqueous solutions (Saglio et al., 1984). Sunflower (Helianthus annuus L. cv Frankasol) seeds were obtained from the Centre Technique Interprofessionnel des Oléagineux Métropolitains (Paris, France). Seeds were soaked in sterile distilled water for 6 h at 25°C in the dark. At this stage the seminal root did not emerge from the testa. Embryonic axes were excised from early-germinating seeds and either immediately used for organelle separation or frozen and stored at −80°C until protein extraction.
Preparation of Organellar Fractions from Maize Root Tips and from Embryonic Axes of Early-Germinating Sunflower Seeds
The isolation of low- and high-density mitochondria from freshly excised maize root tips or from maize root tips that had been subjected to 48 h of carbohydrate starvation treatment was carried out by differential and Percoll (Pharmacia) gradient isopyknic centrifugations as previously described (Couée et al., 1992). In the present work only the mitochondria of high density, which are more differentiated, with many cristae and a dense matrix, were used. Mitochondrial preparations from nonstarved and carbohydrate-starved tissues were resuspended at final protein concentrations of 6 ± 2 and 14 ± 3 mg mL−1, respectively (means ± se for at least five separate experiments), in 0.1 mL of 10 mm KH2PO4-KOH buffer, pH 7.2, containing 1 mm sodium EDTA, 300 mm mannitol, and 0.1% (w/v) fatty acid-free BSA. The latency of matrix enzyme markers was 95%, thus showing the integrity of the purified mitochondria. Peroxisome-enriched fractions were obtained by centrifugation of a crude cellular extract on a one-step (35% and 60%, w/w) Suc gradient, as described by Dieuaide et al. (1992). Both peroxisomes and mitochondria were enriched in this preparation, with a 2.5-fold enrichment factor. Separation of organelles from the embryonic axes of early-germinating sunflower seeds was carried out by a modification of the protocol of Attucci et al. (1991). Differential centrifugations consisted of a low-speed centrifugation at 120g for 15 min, which was followed by a medium-speed centrifugation at 12,000g for 20 min, thus yielding a crude organellar preparation. The main modification consisted of using a 20% (v/v), instead of 12% (Attucci et al., 1991), Percoll gradient for the subsequent isopyknic centrifugation of this crude organellar fraction. The resulting mitochondrial and peroxisomal preparations were diluted in 10 mm KH2PO4-KOH buffer, pH 7.2, containing 1 mm sodium EDTA, 700 mm sorbitol, and 1% (w/v) fatty acid-free BSA, and then centrifuged at 12,000g for 20 min to eliminate Percoll. The pellets were finally resuspended in 0.1 mL of the same medium.
Analysis of Proteins
Protein was determined by the method of Bradford (1976) using γ-globulin (Calbiochem) as the standard. SDS-PAGE analysis of protein was carried out in an electrophoresis unit (Mighty Small II, Hoefer, San Francisco, CA), essentially as described by O'Farrell (1975).
Enzyme Activities
All enzyme activities were assayed spectrophotometrically at 30°C, unless otherwise specified, according to previously published methods. All assays were first performed on blanks containing all of the constituents of the assay except the substrate, which was added to initiate the reaction. Activities were linear with respect to time for at least 2 min and were proportional to the amounts of sample protein added to the assay. The activities of fumarase (EC 4.2.1.2) and catalase (EC 1.11.1.6) were assayed as described by Hill and Bradshaw (1969) and Aebi (1987), respectively. The activity of Glc-6-P dehydrogenase-6-phosphogluconate dehydrogenase was assayed as described by Brouquisse et al. (1991). ACOX (EC 1.3.3.6) was assayed as described by Gerhardt (1987). ACAD (EC 1.3.99.2,3) activity was assayed in terms of the reduction of DCPIP as an electron acceptor and PMS as an intermediate electron carrier (Izai et al., 1992). The decrease in A600 was followed in a reaction mixture containing 50 mm Hepes-KOH buffer, pH 8.0, 1 mm KCN, 1 mm salicylhydroxamic acid, 50 μm FAD, 100 μg mL−1 DCPIP, 100 μg mL−1 PMS, the enzyme sample, and 50 μm acyl-CoA substrate in a final volume of 1.1 mL. The reaction was started by the addition of the acyl-CoA substrate. Blanks in the absence of enzyme sample were carried out to assess the rate of reduction by contaminating CoA-SH. This activity was assayed at 25°C to minimize the background rate. Purified ACOX from maize plantlets showed no apparent activity of DCPIP reduction (Hooks et al., 1996), thus confirming the specificity of the assay. The activity of acyl-CoA thioesterase (EC 3.1.2.2) was assayed in the presence of 0.15 mm 5,5′-dithiobis-(2-nitrobenzoate) under the same conditions, except that DCPIP and PMS were omitted, by following the appearance of 2-nitro-5-thiobenzoate at 412 nm. The extinction coefficient of 2-nitro-5-thiobenzoate was taken to be 14,150 m−1 cm−1 (Riddles et al., 1983). This activity was also assayed at 25°C, which served as a control of the PMS-DCPIP assay.
Partial Purification of ACAD
Proteins from carbohydrate-starved maize root tips, maize whole seminal roots, and embryonic axes or cotyledons from early-germinating sunflower seeds were extracted by homogenization in a Waring blender in 10 mm KH2PO4-KOH buffer, pH 7.5, containing 0.1% (w/v) Triton X-100, 0.2% (w/v) polyvinylpolypyrrolidone, 5 mm Cys, 0.5 mm EDTA, and 0.1 mm PMSF. Particulate material was eliminated by squeezing the sample through cheesecloth and subsequent centrifugation at 5,000g for 30 min. Sunflower extracts were further centrifuged at 5,000g for 10 min to remove the superficial lipid layer. For purification, these crude extracts were sequentially fractionated at different saturations of ammonium sulfate. Fractionation was performed by adding ammonium sulfate over a period of 30 min to ice-cold protein extracts at a concentration of approximately 10 mg mL−1. Solutions were stirred for another 30 min and then centrifuged at 12,000g for 15 min. The different precipitated fractions were then assayed for ACAD activity. The 40% to 60% ammonium sulfate fraction from embryonic axes of early-germinating sunflower seeds was purified further according to the method of Ikeda et al. (1983), which was developed for the separation of the different ACAD from rat liver.
After a thorough dialysis against 10 mm KH2PO4-KOH buffer, pH 8.0, containing 0.5 mm EDTA and 10% (w/v) glycerol, the whole 40% to 60% ammonium sulfate fraction was applied to a DEAE-Sepharose CL-6B column (2 × 30 cm, Pharmacia), which had been equilibrated with 200 mm KH2PO4-KOH buffer, pH 7.0, and then with 10 mm KH2PO4-KOH buffer, pH 8.0, containing 0.5 mm EDTA and 10% (w/v) glycerol. Elution was carried out at 0.75 mL min−1 with a linear gradient of NaCl from 0 to 600 mm in 10 mm KH2PO4-KOH buffer, pH 8.0, containing 0.5 mm EDTA and 10% (w/v) glycerol.
The fractions containing ACAD activity were concentrated by osmotic dehydration against Suc and dialyzed against 10 mm KH2PO4-KOH buffer, pH 7.0, containing 10% (w/v) glycerol. The resulting preparation was applied to a hydroxylapatite BIO-GEL HT column (1 × 15 cm, Bio-Rad), which had been equilibrated with 200 mm KH2PO4-KOH buffer, pH 7.0, and then with 10 mm KH2PO4-KOH buffer, pH 7, containing 10% (w/v) glycerol. Elution was carried out at 0.75 mL min−1 with a linear gradient of phosphate from 10 to 500 mm, pH 7.0, in the presence of 10% (w/v) glycerol. All chromatographic steps were carried out at 0°C to 4°C and driven by an Econo System (Bio-Rad).
RESULTS
Characterization of ACAD Activities Associated with Mitochondria in Maize Root Tips
The purification of mitochondria from maize root tips yields two main populations of mitochondria, low density and high density, corresponding to the meristematic and differentiating regions of the tip, respectively (Couée et al., 1992). Low-density mitochondria are not present in carbohydrate-starved maize root tips and show poor integrity of the outer membrane and low respiration rates with weak respiratory controls (Couée et al., 1992). Furthermore, these low-density mitochondria showed low levels of ACAD activity (data not shown). Therefore, for the present study only the high-density mitochondria, which are more differentiated, with many cristae and a dense matrix, were used for the characterization of ACAD activity. The ratios of ACAD-specific activities in purified high-density mitochondria to that in the crude extract were 13 (±1, se) and 4.5 (±0.5, se) during purification from nonstarved and carbohydrate-starved maize root tips, respectively. These enrichment factors were therefore identical to those of typical enzyme markers of mitochondria such as fumarase and NAD-specific isocitrate dehydrogenase (Couée et al., 1992). In contrast, the activities of the peroxisomal enzyme markers catalase, urate oxidase, and ACOX were decreased 7- to 30-fold in the course of the purification from the crude organellar extract to Percoll-purified mitochondria (Dieuaide et al., 1993). The removal of peroxisomal enzyme markers during this purification was comparable with that obtained for highly purified mitochondria from potato tuber (Neuburger et al., 1982).
We have already shown (Dieuaide et al., 1993) that DCPIP-reducing activity in purified maize root tip mitochondria was not due to acyl-CoA thioesterase activities. Furthermore, the dye-reduction assay with the C5 acyl-CoA valeryl-CoA gave no detectable activity whether with crude protein extracts or with purified mitochondria, whereas the acyl-CoA thioesterase activity with the same substrate using the 5,5′-dithiobis-(2-nitrobenzoate) assay was significant, thus indicating that the dye-reduction system was specific to ACAD activity. Therefore, it was clear that ACAD activity in maize root tips was associated with high-density mitochondria.
The levels of ACAD activity in purified mitochondria were compared with the yields of mitochondrial protein and mitochondrial enzyme markers (Couée et al., 1992) to estimate the levels of ACAD activity in the high-density mitochondrial pool of maize root tips prior to and after carbohydrate starvation. Table I shows that carbohydrate starvation resulted in an increase of ACAD activity associated with high-density mitochondria of maize root tips. Carbohydrate starvation was previously shown to result in a 5- to 10-fold increase of ACOX activities in the peroxisomal pool of maize root tips (Dieuaide et al., 1993; Hooks et al., 1995). Table II shows the levels of ACOX and ACAD activities in peroxisomes and mitochondria from carbohydrate-starved maize root tips. The absence of ACOX activity in purified mitochondria (Dieuaide et al., 1993) implied that ACOX activity in partially purified peroxisomes was not due to contaminating mitochondrial enzymes. The main feature of ACOX substrate specificities in peroxisomes was the significantly lower level of activity with isobutyryl-CoA and isovaleryl-CoA relative to the activity with straight-chain substrates. The partial purification of peroxisomes also implied that ACOX-specific activities were underestimated. Activities with butyryl-CoA, hexanoyl-CoA, and octanoyl-CoA substrates were therefore genuinely higher for peroxisomal ACOX than for mitochondrial ACAD. In contrast, the main feature of ACAD substrate specificities in mitochondria was the significant level of activity with isobutyryl-CoA and isovaleryl-CoA relative to that with straight-chain substrates.
Table I.
Effects of carbohydrate starvation on total ACAD activity in maize root tip high-density mitochondria
| Substrate | ACAD Activity in the High-Density
Mitochondria of 1000 Root Tips
|
|
|---|---|---|
| Nonstarved | Carbohydrate starved | |
| 50 μm | nmol min−1 | |
| Butyryl-CoA | 7 ± 1 | 10 ± 2 |
| Octanoyl-CoA | 8 ± 2 | 16 ± 8 |
| Palmitoyl-CoA | 8 ± 2 | 20 ± 4 |
ACAD activity was measured as described in Methods. High-density mitochondria were purified from nonstarved or 48-h carbohydrate-starved maize root tips, as previously described (Couée et al., 1992). The size of the high-density mitochondrial pool in 1000 root tips was estimated from the yields of mitochondrial protein and mitochondrial enzyme markers (Couée et al., 1992). Results are the means ± se of at least three experiments.
Table II.
Substrate specificity of ACOX and ACAD activities in peroxisomes and high-density mitochondria from carbohydrate-starved maize root tips
| Substrate | ACOX Activity in Peroxisomes | ACAD Activity in High-Density Mitochondria |
|---|---|---|
| 50 μm | nmol min−1 mg−1 | |
| Butyryl-CoA | 5.7 ± 0.5 | 2.5 ± 0.4 |
| Isobutyryl-CoA | 0.60 ± 0.05 | 2.3 ± 0.6 |
| Isovaleryl-CoA | NDa | 1.2 ± 0.3 |
| Hexanoyl-CoA | 8.0 ± 0.7 | 4 ± 1 |
| Octanoyl-CoA | 7.1 ± 0.3 | 4 ± 2 |
| Palmitoyl-CoA | 3.5 ± 0.3 | 5 ± 1 |
ACOX and ACAD activities were measured as described in Methods. Partially purified peroxisomes and purified high-density mitochondria were isolated from 48-h carbohydrate-starved maize root tips, as previously described (Couée et al., 1992; Dieuaide et al., 1993). Results are the means ± se of at least three experiments.
ND, Not detected.
Characterization of ACAD Activities Associated with Mitochondria in Embryonic Axes of Early-Germinating Sunflower Seeds
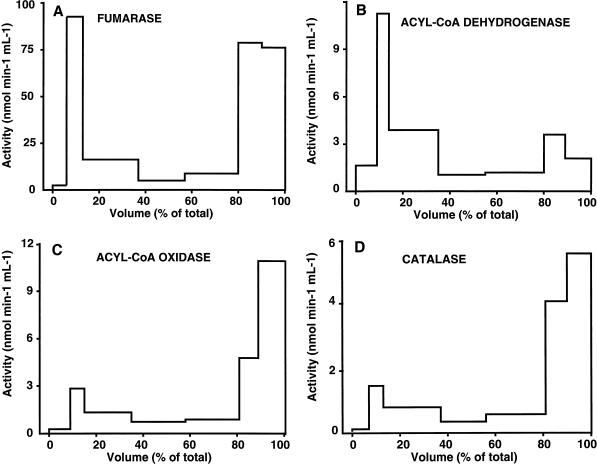
Attucci et al. (1991) had shown that functional mitochondria could be isolated from dry embryonic axes of sunflower seeds. Differential centrifugation of organellar extracts from embryonic axes of 6-h-germinating sunflower seeds yielded a medium-speed pellet showing enrichment in both mitochondrial and peroxisomal enzyme markers. There was no detectable activity of the cytosolic and plastid enzyme marker Glc-6-P dehydrogenase-6-phosphogluconate dehydrogenase (data not shown), thus indicating that this pellet was free of cytosolic and plastid contaminants. Furthermore, the medium-speed pellet did not contain any detectable acyl-CoA thioesterase activity. This organellar preparation was separated by isopyknic centrifugation on a 20% (v/v) Percoll gradient. Separation of mitochondria from 6-h-germinating sunflower seeds thus necessitated higher densities of Percoll for isopyknic centrifugation, which was in line with the differentiation and densification of mitochondria during imbibition (Attucci et al., 1991). Figure 1 shows that the mitochondrial enzyme marker fumarase and the peroxisomal enzyme markers catalase and ACOX showed distinct patterns of distribution along the Percoll gradient. Catalase and ACOX activities were recovered mainly in the high-density fractions of the gradient, which was in accordance with the high density of intact peroxisomes (Schwitzguebel and Siegenthaler, 1984). In contrast, fumarase activity was recovered in two peaks, in the low-density and in the high-density fractions. The distribution of palmitoyl-CoA-dependent ACAD activity was clearly different from that of ACOX and was identical to that of fumarase activity in the upper fractions of the gradient. However, its low level in the high-density fractions of the gradient contrasted with the significant level of fumarase.
Figure 1.
Subcellular localization of palmitoyl-CoA-dependent ACAD activity in embryonic axes from early-germinating sunflower seeds. Crude mitochondria from embryonic axes of early-germinating sunflower seeds were further separated by isopyknic centrifugation on a 20% Percoll gradient. Fractions of 2 mL were collected and assayed for the activity of mitochondrial fumarase (A), palmitoyl-CoA-dependent ACAD (B), peroxisomal ACOX (C), and peroxisomal catalase (D). The scale of volumes from 0% to 100% ranges from the top to the bottom of the Percoll gradient. Separation of organelles and enzyme activity measurements were carried out as described in Methods.
The fractions corresponding to 5% to 25% and 85% to 95% volume (Fig. 1) were diluted as described in Methods to obtain mitochondrial and peroxisomal preparations, respectively, from embryonic axes of early- germinating sunflower seeds. The mitochondrial preparation was essentially free of other organelles as previously described by Attucci et al. (1991) and showed a latency of 95% with mitochondrial enzyme markers such as NAD-specific isocitrate dehydrogenase. However, it showed variable levels of contamination with ACOX activity ranging from 0 to 2 nmol min−1 mg−1. Isopyknic centrifugation of the mitochondrial preparation on a 12% (v/v) Percoll gradient resulted in the separation of catalase and ACOX activities in the upper part of the gradient and of ACAD and fumarase activities in the lower part of the gradient, thus showing that these ACOX and catalase activities were due to the presence of soluble contaminants rather than to contaminating peroxisomes. The peroxisomal preparation was contaminated with mitochondria, in accordance with the results of Attucci et al. (1991).
Table III shows the levels of ACOX and ACAD activities in these peroxisomal and mitochondrial preparations. The absence of ACOX activity in mitochondria that had been purified by two successive isopyknic centrifugations, as described above, implied that ACOX activity in partially purified peroxisomes was not due to contaminating mitochondrial enzymes. The main feature of ACOX substrate specificities in peroxisomes was the significantly lower level of activity with isobutyryl-CoA and isovaleryl-CoA relative to the activity with straight-chain substrates, as was shown to be the case for maize root tip peroxisomes (Table II). The partial purification of peroxisomes also implied that ACOX-specific activities were underestimated. Activities with butyryl-CoA, octanoyl-CoA, decanoyl-CoA, and palmitoyl-CoA substrates were therefore genuinely higher for peroxisomal ACOX than for mitochondrial ACAD. In contrast, the main feature of ACAD substrate specificities in mitochondria was the significant level of activity with isobutyryl-CoA relative to that with straight-chain substrates. However, the reason why isovalerylCoA-dependent activities were low or undetected remains unclear.
Table III.
Substrate specificity of ACOX and ACAD activities in peroxisomes and mitochondria from embryonic axes of earlygerminating sunflower seeds
| Substrate | ACOX Activity in Peroxisomes | ACAD Activity in Mitochondria |
|---|---|---|
| 50 μm | nmol min−1 mg−1 | |
| Butyryl-CoA | 18 ± 1 | 11 ± 2 |
| Isobutyryl-CoA | NDa | 14 ± 3 |
| Isovaleryl-CoA | 0.70 ± 0.07 | ND |
| Octanoyl-CoA | 10 ± 1 | 7 ± 1 |
| Decanoyl-CoA | 16 ± 2 | 5 ± 1 |
| Palmitoyl-CoA | 16 ± 1 | 10 ± 3 |
| Stearoyl-CoA | 3.4 ± 0.3 | 6 ± 1 |
ACOX and ACAD activities were measured as described in Methods. Partially purified peroxisomes and purified mitochondria were isolated from embryonic axes of 6-h-soaked sunflower seeds, as described in Methods and in Results. Results are the means ± se of at least three experiments.
ND, Not detected.
Partial Purification and Characterization of Medium-Chain and Long-Chain ACAD Activities from Embryonic Axes of Early-Germinating Sunflower Seeds
Ikeda et al. (1983) and Ikeda and Tanaka (1983a, 1983b) described in detail the optimal strategy for separation and purification of all of the different ACAD from rat liver. This strategy was therefore attempted for the purification of distinct higher-plant ACAD. A number of maize and sunflower tissues were tested as starting material for purification. Table IV shows that embryonic axes and cotyledons from early-germinating sunflower seeds as well as carbohydrate-starved maize root tips showed high levels of palmitoyl-CoA-dependent ACAD activity in crude protein extracts. However, protein extracts of sunflower embryonic axes, in contrast with extracts of sunflower cotyledons or carbohydrate-starved maize root tips, did not present any detectable acyl-CoA thioesterase activity.
Table IV.
Total palmitoyl-CoA-dependent ACAD activity in crude protein extracts from maize and sunflower tissues
| Tissue | Enzyme Activity
|
|
|---|---|---|
| ACAD | Acyl-CoA thioesterase | |
| nmol min−1 g−1 fresh wt | ||
| Maize plantlets | ||
| Whole seminal root | 4 ± 1 | 12 ± 2 |
| Carbohydrate-starved root tip | 32 ± 8 | 100 ± 10 |
| Early-germinating sunflower seeds | ||
| Embryonic axes | 30 ± 10 | NDa |
| Cotyledons | 25 ± 5 | 100 ± 10 |
ACAD and acyl-CoA thioesterase activities were measured as described in Methods in the presence of 50 μm palmitoyl-CoA. Maize and sunflower tissues were obtained and protein extraction was carried out as described in Methods. Results are the means ± range or ± se from two or three experiments.
ND, Not detected.
Fifty to one hundred grams fresh weight of embryonic axes from early-germinating sunflower seeds was therefore used as starting material for purification. Ammonium sulfate fractionation at 0% to 40%, 40% to 60%, and 60% to 80% saturation resulted in the separation of ACAD activities (assayed with butyryl-CoA, octanoyl-CoA, decanoyl-CoA, lauroyl-CoA, myristoyl-CoA, palmitoyl-CoA, and stearoyl-CoA) exclusively in the precipitated fraction of 40% to 60% saturation, in accordance with the recovery of mammalian ACAD activities in the precipitated fraction of 35% to 80% saturation (Ikeda et al., 1983). However, isobutyryl-CoA-dependent ACAD activity (Table III) was not recovered in any of the ammonium sulfate fractions.
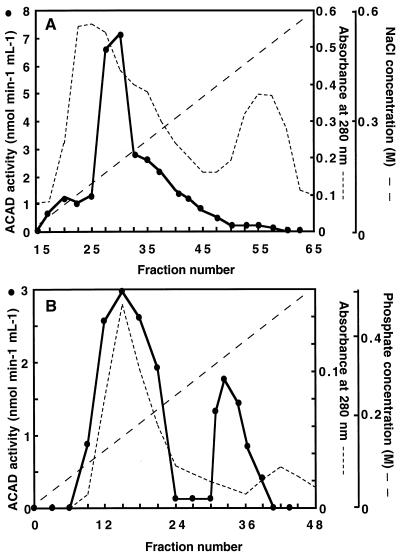
The ACAD-specific activity of this 40% to 60% saturation fraction was 1.5 nmol min−1 mg−1 and 1.1 nmol min−1 mg−1 with palmitoyl-CoA and myristoyl-CoA, respectively, as the substrates. After the sample was dialyzed extensively against 10 mm KH2PO4-KOH buffer, pH 8.0, containing 0.5 mm EDTA and 10% (w/v) glycerol, the 40% to 60% fraction was applied to a DEAE-Sepharose column as described in Methods. Approximately 80% of total palmitoyl-CoA-dehydrogenating activity using the dye-reduction assay was retained on the column. Elution with a 0 to 0.6 m gradient of NaCl yielded a large peak of palmitoyl-CoA-dehydrogenating activity (Fig. 2A). Fractions 25 to 48 were pooled, concentrated by dehydration against Suc, and extensively dialyzed against 10 mm KH2PO4-KOH buffer, pH 7.0, containing 10% (w/v) glycerol. The resulting fraction was applied to a hydroxylapatite column. Nearly 100% of palmitoyl-CoA-dehydrogenating activity was retained on the column. Figure 2B shows that elution with increasing concentrations of phosphate from 0 to 0.5 m resulted in the resolution of two distinct peaks of palmitoyl-CoA-dehydrogenating activity eluting at 0.25 and 0.4 m phosphate. Fractions 15 and 32, which showed highest activity, had specific activities, with palmitoyl-CoA as the substrate, of 20 and 140 nmol min−1 mg−1, respectively, which corresponded to apparent purification factors of 13- and 100-fold relative to the 40% to 60% ammonium sulfate fraction.
Figure 2.
Separation of distinct ACAD by column chromatography on DEAE-Sepharose (A) and hydroxylapatite-HT (B). Proteins from embryonic axes of early-germinating sunflower seeds were fractionated by ammonium sulfate precipitation as described in Methods. Column chromatography and ACAD activity measurements with 50 μm palmitoyl-CoA as the substrate were carried out as described in Methods. A, The resuspended 40% to 60% ammonium sulfate fraction was dialyzed and then loaded onto a DEAE-Sepharose column. Bound proteins were eluted by a linear NaCl gradient from 0 to 0.6 m. B, The pooled fractions from nos. 25 to 48 were concentrated and dialyzed before application to a hydroxylapatite-HT column. Bound proteins were eluted by a linear gradient of phosphate from 0 to 0.5 m. The two peaks of active fractions were pooled separately to give ACAD1 and ACAD2 preparations.
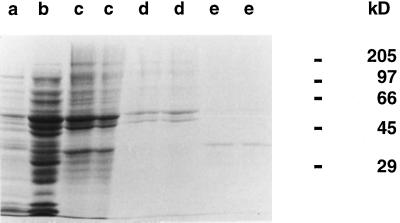
The two peaks, ACAD1 and ACAD2, were separately pooled and analyzed for their protein composition, which is shown in Figure 3. The ACAD1 preparation contained two major proteins with molecular masses of 50 and 60 kD, whereas the ACAD2 preparation consisted of one major protein of 40 kD. These two ACAD preparations were also assayed for their ACAD activity with a range of straight-chain acyl-CoA substrates. ACAD1 and ACAD2 preparations drove the dye-reduction assay in a concentration-dependent way (data not shown). Table V shows that there were clear differences of substrate specificity between ACAD1 and ACAD2. Whereas ACAD1 showed no or little activity with butyryl-CoA and hexanoyl-CoA, it was significantly active with straight-chain acyl-CoA substrates from C8 to C18 with specific activities corresponding to an apparent purification factor of 13- to 30-fold relative to the 40% to 60% ammonium sulfate fraction. In contrast, ACAD2 showed significant activity with butyryl-CoA and hexanoyl-CoA but no or little activity with long-chain acyl-CoA substrates such as palmitoyl-CoA and stearoyl-CoA. ACAD2 showed maximum activity with decanoyl-CoA and myristoyl-CoA, with a specific activity of 850 nmol min−1 mg−1 for this latter substrate, which corresponded to an apparent purification factor of 770-fold relative to the 40% to 60% ammonium sulfate fraction. Neither ACAD1 nor ACAD2 showed activity with isobutyryl-CoA or isovaleryl-CoA. Finally, the dependency on FAD was tested. Thus, ACAD1 and ACAD2, when assayed with the substrates giving highest activity, showed a 2- to 3-fold decrease of activity in the absence of FAD, which is in line with previous results on mammalian ACAD (Ikeda et al., 1985). Attempts to purify ACAD1 and ACAD2 to homogeneity by affinity chromatography on palmitoyl-CoA-agarose or Cibacron blue 3GA (Ikeda et al., 1983) were unsuccessful as a result of instability of enzyme activity during these chromatographic steps.
Figure 3.
SDS-PAGE analysis of protein fractions in the course of partial purification of ACAD from embryonic axes of early-germinating sunflower seeds. The different protein fractions were obtained as described in Methods and in the legend of Figure 2. Aliquots of the 0% to 40% (lane a, 100 μg of protein) and 40% to 60% (lane b, 100 μg of protein) ammonium sulfate fractions, of the pooled fractions from the DEAE-Sepharose step (lanes c, 100 μg of protein), and of ACAD1 (lanes d, 50 μg of protein) and ACAD2 (lanes e, 12.5 μg of protein) preparations were separated by SDS-PAGE. Proteins were visualized by Coomassie blue staining. The migration of molecular mass markers is given on the right.
Table V.
Chain-length substrate specificity of ACAD activities in partially purified ACAD preparations from embryonic axes of early germinating sunflower seeds
| Substrate | ACAD1 | ACAD2 |
|---|---|---|
| 50 μm | % | |
| Butyryl-CoA | NDa | 78 |
| Hexanoyl-CoA | 39 | 59 |
| Octanoyl-CoA | 63 | 75 |
| Decanoyl-CoA | 100 | 98 |
| Myristoyl-CoA | 84 | 100 |
| Palmitoyl-CoA | 100 | 16 |
| Stearoyl-CoA | 62 | ND |
ACAD was purified as described in Methods. Partially purified ACAD1 and ACAD2 preparations were obtained as described in the legend for Figure 2. ACAD activities in ACAD1 and ACAD2, which were measured as described in “Materials and Methods,” are given as activities relative to palmitoyl-CoA-dependent (20 nmol min−1 mg−1) and myristoyl-CoA-dependent (850 nmol min−1 mg−1) specific activities, respectively.
ND, Not detected.
DISCUSSION
Previous (Dieuaide et al., 1993) and present data show that the PMS-DCPIP dye-reduction assay is functional with protein preparations from higher plants and specific to ACAD activity. Thus, ACOX enzymes do not drive this assay, as shown by subcellular fractionation experiments and more definitely by the inability of purified ACOX to show any apparent ACAD activity (Hooks et al., 1996). However, one must bear in mind that at least some ACAD enzymes, such as the human short-chain ACAD, can show ACOX activity (Vanhove et al., 1993a), but it did not seem to be the case for ACAD activities in maize root or sunflower seed mitochondria (Dieuaide et al., 1993; this work). Unexpectedly, acyl-CoA thioesterase activity did not seem to be able to drive the PMS-DCPIP dye-reduction assay, whether in purified mitochondria or in protein extracts from maize (Dieuaide et al., 1993; this work). The PMS-DCPIP dye-reduction assay was therefore found to be useful to measure ACAD activity in organelle purification or protein purification from higher plants.
Subcellular localization studies in maize and sunflower showed that ACAD activity was associated with mitochondria and distinct from peroxisomal ACOX enzymes, which is in line with the mitochondrial localization of ACAD enzymes in mammals and their implication in mitochondrial β-oxidation (Schulz, 1991). However, the differences of distribution between fumarase activity and palmitoyl-CoA-dependent ACAD activity in mitochondria from embryonic axes of early-germinating sunflower seeds (Fig. 1) also indicated that this ACAD activity was associated with a particular subset of mitochondria. This was in agreement with the heterogeneity of mitochondrial subpopulations during seed imbibition (Attucci et al., 1991) and would further suggest that the different mitochondrial subpopulations may show metabolic specialization. In both maize and sunflower differences of substrate specificities were observed between ACOX activities of peroxisomes and ACAD activities of mitochondria. Thus, isobutyryl-CoA and isovaleryl-CoA generally gave significant activities with mitochondrial ACAD and low, or undetectable, activities with peroxisomal ACOX, which would be in line with the possible extra-peroxisomal location of catabolism of Leu and Val (Gerbling and Gerhardt, 1989).
Unfortunately, extraction of ACAD enzymes from sunflower embryonic axes in the presence of FAD, EDTA, PMSF, Cys, and Triton X-100 resulted in the loss of isobutyryl-CoA-dependent ACAD activity. Further purification resulted in the separation of two distinct ACAD1 and ACAD2 preparations, which were active with straight-chain acyl-CoA substrates. ACAD2 was the best purified preparation, with a purification factor of 770-fold for myristoyl-CoA-dependent activity, and its main 40-kD protein was close to the 40- to 45-kD range of subunit size of mammalian ACAD (Tanaka et al., 1990). Chromatographic behavior and substrate specificities of ACAD1 and ACAD2 were similar to those of long-chain ACAD and medium-chain ACAD, respectively (Ikeda et al., 1983). However, apparent substrate specificities were somewhat different. Mammalian long-chain ACAD shows no activity with short-chain acyl-CoAs, whereas ACAD1 showed some activity with hexanoyl-CoA, and mammalian medium-chain ACAD shows no activity with myristoyl-CoA, whereas ACAD2 showed significant activity with this C14 substrate.
The present biochemical data thus show the existence of distinct ACAD in embryonic axes of sunflower seeds. The existence of distinct ACAD enzymes would be in line with the existence of an acyl-CoA dehydrogenase gene family in higher plants. The rice and Arabidopsis EST databases (http://www.ncbi.nlm.nih.gov) contain a number of cDNA clones with high homologies with ACADs. Thus, interrogation of dbEST (Boguski et al., 1993) with the protein sequence of rat mitochondrial long-chain ACAD (Tanaka et al., 1990) through TBLASTN (Altschul et al., 1990) resulted in the identification of rice EST no. D24729 (K. Yamamoto and T. Sasaki, unpublished data). After complete sequencing of this clone (I. Couée, unpublished data), the resulting sequence gave its best BLASTX homology score (373) with mammalian mitochondrial isovaleryl-CoA dehydrogenase. This sequence and the derived protein sequence were also used to interrogate further dbEST through BLASTN and TBLASTN. This interrogation resulted in the identification of EST no. AA231888 from oat (A.E. VanDeynze, M.E. Sorrells, W.D. Park, N.M. Ayres, H. Fu, S.W. Cartinhour, and S.R. McCouch, unpublished data) and EST nos. H77217 and AA650785 from Arabidopsis, which correspond to EST no. U72505 (F. Grellet, P. Gaubier, H.-J. Wu, M. Laudie, C. Berger, and M. Delseny, unpublished data). Whereas EST no. AA231888 from oat gave its best BLASTX homology score (193) with a putative isovaleryl-CoA dehydrogenase from Caenorhabditis elegans, EST no. U72505 from Arabidopsis gave its best BLASTX homology score (605) with glutaryl-CoA dehydrogenase from the hyperthermophilic, strictly anaerobic, sulfate-reducing (Aalen et al., 1997) archaeon Archaeoglobus fulgidus. Thus, all of these clones show the best homologies with ACADs involved in the metabolism of amino acids, which would also be in line with the possible extra-peroxisomal catabolism of Leu and Val (Gerbling and Gerhardt, 1989) and with the activity measurements of Tables II and III. It must also be noted that only the clones from rice and oat show best homologies with the sequences of well-characterized mitochondrial enzymes from eukaryotic organisms.
Thus, data from metabolic studies (Dieuaide et al., 1993), enzyme purification (this work), and large-scale sequencing projects (Newman et al., 1994) reveal the existence of ACAD in higher plants. The identification of ESTs and the cloning of the corresponding full-length cDNAs should greatly facilitate the precise characterization of higher-plant ACAD. Knowledge of their substrate specificities will provide direct insight into the physiological functions of these enzymes, as to whether they are involved in massive degradation of quantitatively important compounds, such as fatty acids or branched-chain amino acids, or in the synthesis or removal of specialized molecules, such as hormones or growth regulators. Thus, for a number of β-oxidation pathways, where the substrates would be specialized molecules such as 12-oxophytodienoate (Mueller, 1997) or cinnamate (Klessig and Malamy, 1994), the enzymes involved are not yet known. For instance, conventional β-oxidation of the C18 precursor to jasmonate would involve 15 steps, including the initial activation of the carboxyl group and the final release of jasmonate by acyl-CoA thioesterase activity (Mueller, 1997). The compartment in which these transformations take place is not known (Mueller, 1997; Parchmann et al., 1997). It would obviously be of great interest to determine whether the enzymes involved are mitochondrial or peroxisomal.
ACKNOWLEDGMENT
We thank Dr. Ian A. Graham for critical reading of the manuscript.
Abbreviations:
- ACAD
acyl-CoA dehydrogenase
- ACOX
acyl-CoA oxidase
- DCPIP
2,6-dichlorophenolindophenol
- EST
expressed sequence tag
- PMS
phenazine methosulfate
Footnotes
This work was partly funded by the Aquitaine (France) Regional Council.
LITERATURE CITED
- Aalen N, Steen IH, Birkeland NK, Lien T. Purification and properties of an extremely thermostable NADP+-specific glutamate dehydrogenase from Archaeoglobus fulgidus. Arch Microbiol. 1997;168:536–539. doi: 10.1007/s002030050533. [DOI] [PubMed] [Google Scholar]
- Aebi HE. Catalase. In: Bergmeyer HU, Bergmeyer J, Grassl M, editors. Methods of Enzymatic Analysis, Vol 3. Weinheim, Germany: VCH; 1987. pp. 273–286. [Google Scholar]
- Altschul SF, Gish W, Miller W, Myers EW, Lipman D. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Aoyama T, Souri M, Kamijo T, Ushikubo S, Hashimoto T. Peroxisomal acyl-coenzyme A oxidase is a rate-limiting enzyme in a very-long-chain fatty acid β-oxidation system. Biochem Biophys Res Commun. 1994a;201:1541–1547. doi: 10.1006/bbrc.1994.1879. [DOI] [PubMed] [Google Scholar]
- Aoyama T, Ueno I, Kamijo T, Hashimoto T. Rat very-long-chain acyl-CoA dehydrogenase, a novel mitochondrial acyl-CoA dehydrogenase gene product, is a rate-limiting enzyme in long-chain fatty acid β-oxidation system. J Biol Chem. 1994b;269:19088–19094. [PubMed] [Google Scholar]
- Attucci S, Carde J-P, Raymond P, Saint-Gès V, Spiteri A, Pradet A. Oxidative phosphorylation by mitochondria extracted from dry sunflower seeds. Plant Physiol. 1991;95:390–398. doi: 10.1104/pp.95.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubert S, Alban C, Bligny R, Douce R. Induction of β-methyl-crotonyl-coenzyme A carboxylase in higher plant cells during carbohydrate starvation: evidence for a role of MCCase in leucine catabolism. FEBS Lett. 1996;383:175–180. doi: 10.1016/0014-5793(96)00244-x. [DOI] [PubMed] [Google Scholar]
- Boguski MS, Lowe TMJ, Tolstoshev SH. dbEST: database for “expressed sequence tags.”. Nat Genet. 1993;4:332–333. doi: 10.1038/ng0893-332. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brouquisse R, James F, Raymond P, Pradet A. Study of glucose starvation in excised maize root tips. Plant Physiol. 1991;96:619–626. doi: 10.1104/pp.96.2.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper TG, Beevers H. Mitochondria and glyoxysomes from castor bean endosperm. J Biol Chem. 1969a;244:3507–3513. [PubMed] [Google Scholar]
- Cooper TG, Beevers H. β-Oxidation in glyoxysomes from castor bean endosperm. J Biol Chem. 1969b;244:3514–3520. [PubMed] [Google Scholar]
- Couée I, Jan M, Carde J-P, Brouquisse R, Raymond P, Pradet A. Effects of glucose starvation on mitochondrial subpopulations in the meristematic and submeristematic regions of maize root. Plant Physiol. 1992;98:411–421. doi: 10.1104/pp.100.4.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieuaide M, Brouquisse R, Pradet A, Raymond P. Increased fatty acid β-oxidation after glucose starvation in maize root tips. Plant Physiol. 1992;99:595–600. doi: 10.1104/pp.99.2.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieuaide M, Couée I, Pradet A, Raymond P. Effects of glucose starvation on the oxidation of fatty acids by maize root tip mitochondria and peroxisomes: evidence for mitochondrial fatty acid β-oxidation and acyl-CoA dehydrogenase activity in a higher plant. Biochem J. 1993;296:199–207. doi: 10.1042/bj2960199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccleston VS, Cranmer AM, Voelker TA, Ohlrogge JB. Medium-chain fatty acid biosynthesis and utilization in Brassica napus plants expressing lauroyl-acyl carrier protein thioesterase. Planta. 1995;198:46–53. [Google Scholar]
- Engel PC. Acyl-coenzyme A dehydrogenases. In: Müller F, editor. Chemistry and Biochemistry of Flavoenzymes, Vol 3. Boca Raton, FL: CRC Press; 1992. pp. 597–655. [Google Scholar]
- Gerbling H, Gerhardt B. Peroxisomal degradation of branched-chain 2-oxo-acids. Plant Physiol. 1989;91:1387–1392. doi: 10.1104/pp.91.4.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhardt B. Localization of β-oxidation enzymes in peroxisomes isolated from nonfatty plant tissues. Planta. 1983;159:238–246. doi: 10.1007/BF00397531. [DOI] [PubMed] [Google Scholar]
- Gerhardt B. Substrate specificity of peroxisomal acyl-CoA oxidase. Phytochemistry. 1985;24:351–352. [Google Scholar]
- Gerhardt B. Peroxisomes and fatty acid degradation. Methods Enzymol. 1987;148:516–525. [Google Scholar]
- Gerhardt B, Fischer K, Maier U. Effect of palmitoylcarnitine on mitochondrial activities. Planta. 1995;196:720–726. [Google Scholar]
- Hayashi H, De Bellis L, Yamaguchi K, Kato A, Hayashi M, Nishimura M. Molecular characterization of a glyoxysomal long chain acyl-CoA oxidase that is synthesized as a precursor of higher molecular mass in pumpkin. J Biol Chem. 1998;273:8301–8307. doi: 10.1074/jbc.273.14.8301. [DOI] [PubMed] [Google Scholar]
- Hill RL, Bradshaw RA. Fumarase. Methods Enzymol. 1969;13:91–99. [Google Scholar]
- Hooks MA, Bode K, Couée I. Regulation of acyl-CoA oxidases in maize seedlings. Phytochemistry. 1995;40:657–660. [Google Scholar]
- Hooks MA, Bode K, Couée I. Higher-plant medium- and short-chain acyl-CoA oxidases: identification, purification and characterization of two novel enzymes of eukaryotic peroxisomal β-oxidation. Biochem J. 1996;320:607–614. doi: 10.1042/bj3200607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppe A, Theimer RR. Enzymes for lipolysis and fatty acid metabolism in different organelle fractions from rape seed cotyledons. Planta. 1997;202:227–234. [Google Scholar]
- Ikeda Y, Dabrowski C, Tanaka K. Separation and properties of five distinct acyl-CoA dehydrogenases from rat liver mitochondria. J Biol Chem. 1983;25:1066–1076. [PubMed] [Google Scholar]
- Ikeda Y, Okamura-Ikeda K, Tanaka K. Purification and characterization of short-chain, medium-chain, and long-chain acyl-CoA dehydrogenases from rat liver mitochondria. J Biol Chem. 1985;260:1311–1325. [PubMed] [Google Scholar]
- Ikeda Y, Tanaka K. Purification and characterization of isovaleryl coenzyme A dehydrogenase from rat liver mitochondria. J Biol Chem. 1983a;258:1077–1085. [PubMed] [Google Scholar]
- Ikeda Y, Tanaka K. Purification and characterization of 2-methyl-branched chain acyl coenzyme A dehydrogenase, an enzyme involved in the isoleucine and valine metabolism, from rat liver mitochondria. J Biol Chem. 1983b;258:9477–9487. [PubMed] [Google Scholar]
- Izai K, Uchida Y, Orii T, Yamamoto S, Hashimoto T. Novel fatty acid β-oxidation enzymes in rat liver mitochondria. J Biol Chem. 1992;267:1027–1033. [PubMed] [Google Scholar]
- Kindl H. β-Oxidation of fatty acids by specific organelles. In: Stumpf PK, Conn EE, editors. The Biochemistry of Plants, Vol 9. London: Academic Press; 1987. pp. 31–52. [Google Scholar]
- Kirsch T, Löffler H-G, Kindl H. Plant acyl-CoA oxidase. J Biol Chem. 1986;261:8570–8575. [PubMed] [Google Scholar]
- Klessig DF, Malamy J. The salicylic acid signal in plants. Plant Mol Biol. 1994;26:1439–1458. doi: 10.1007/BF00016484. [DOI] [PubMed] [Google Scholar]
- Miernyk JA, Thomas DR, Wood C. Partial purification and characterization of the mitochondrial and peroxisomal isoenzymes of enoyl-coenzyme A hydratase from germinating pea seedlings. Plant Physiol. 1991;95:564–569. doi: 10.1104/pp.95.2.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller MJ. Enzymes involved in jasmonic acid biosynthesis. Physiol Plant. 1997;100:653–663. [Google Scholar]
- Nagao M, Tanaka K. FAD-dependent regulation of transcription, translation, post-translational processing, and post-processing stability of various mitochondrial acyl-CoA dehydrogenases and of electron transfer flavoprotein and the site of holoenzyme formation. J Biol Chem. 1992;267:17925–17932. [PubMed] [Google Scholar]
- Neuburger M, Journet E-P, Bligny R, Carde J-P, Douce R. Purification of plant mitochondria by isopycnic centrifugation in density gradients of Percoll. Arch Biochem Biophys. 1982;217:312–323. doi: 10.1016/0003-9861(82)90507-0. [DOI] [PubMed] [Google Scholar]
- Newman T, deBruijn FJ, Green P, Keegstra K, Kende H, McIntosh L, Ohlrogge J, Raikhel N, Somerville S, Thomashow M and others. Genes galore. A summary of methods for accessing results from large-scale partial sequencing of anonymous Arabidopsis cDNA clones. Plant Physiol. 1994;106:1241–1255. doi: 10.1104/pp.106.4.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell PH. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975;250:4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Osmundsen H, Bremer J, Pedersen JI. Metabolic aspects of peroxisomal β-oxidation. Biochim Biophys Acta. 1991;1085:141–158. doi: 10.1016/0005-2760(91)90089-z. [DOI] [PubMed] [Google Scholar]
- Parchmann S, Gundlach H, Mueller MJ. Induction of 12-oxo-phytodienoic acid in wounded plants and elicited plant cell cultures. Plant Physiol. 1997;115:1057–1064. doi: 10.1104/pp.115.3.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddles PW, Blakeley RL, Zerner B. Reassessment of Ellman's reagent. Methods Enzymol. 1983;91:49–60. doi: 10.1016/s0076-6879(83)91010-8. [DOI] [PubMed] [Google Scholar]
- Sabbagh E, Cuebas D, Schulz H. 3-Mercaptopropionic acid, a potent inhibitor of fatty acid oxidation in rat heart mitochondria. J Biol Chem. 1985;260:7337–7342. [PubMed] [Google Scholar]
- Saglio P, Pradet A. Soluble sugars, respiration, and energy charge during aging of excised maize root tips. Plant Physiol. 1980;66:516–519. doi: 10.1104/pp.66.3.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saglio P, Rancillac M, Bruzeau F, Pradet A. Critical oxygen pressure for growth and respiration of excised and intact roots. Plant Physiol. 1984;76:151–154. doi: 10.1104/pp.76.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salon C (1988) Quantification des flux de carbone respiratoire dans les embryons de laitue en germination. Thèse d'Université, Sciences de la Vie, Bordeaux II, France
- Salon C, Raymond P, Pradet A. Quantification of carbon fluxes through the tricarboxylic acid cycle in early germinating lettuce embryos. J Biol Chem. 1988;263:12278–12287. [PubMed] [Google Scholar]
- Schulz H. Beta oxidation of fatty acids. Biochim Biophys Acta. 1991;1081:109–120. doi: 10.1016/0005-2760(91)90015-a. [DOI] [PubMed] [Google Scholar]
- Schwitzguebel J-P, Siegenthaler P-A. Purification of peroxisomes and mitochondria from spinach leaf by Percoll gradient centrifugation. Plant Physiol. 1984;75:670–674. doi: 10.1104/pp.75.3.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K, Matsubara Y, Indo Y, Naito E, Kraus J, Ozasa H. The acyl-CoA dehydrogenase family: homology and divergence of primary sequence of four acyl-CoA dehydrogenases, and consideration of their functional significance. In: Tanaka K, Coates PM, editors. Fatty Acid Oxidation: Clinical, Biochemical, and Molecular Aspects. Inc., New York: Alan R. Liss; 1990. pp. 577–598. [PubMed] [Google Scholar]
- Vanhove G, Van Veldhoven PP, Eyssen HJ, Mannaerts GP. Mitochondrial short-chain acyl-CoA dehydrogenase of human liver and kidney can function as an oxidase. Biochem J. 1993a;292:23–30. doi: 10.1042/bj2920023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhove GF, Van Veldhoven PP, Fransen M, Denis S, Eyssen HJ, Wanders RJA, Mannaerts GP. The CoA esters of 2-methyl branched chain fatty acids and of the bile acid intermediates di- and trihydroxycoprostanic acids are oxidised by one single peroxisomal branched chain acyl-CoA oxidase in human liver and kidney. J Biol Chem. 1993b;268:10335–10344. [PubMed] [Google Scholar]
- Wood C, Burgess N, Thomas DR. The dual location of β-oxidation enzymes in germinating pea cotyledons. Planta. 1986;167:54–57. doi: 10.1007/BF00446368. [DOI] [PubMed] [Google Scholar]