Abstract
The title compound, C6H12O5, is the α-pyranose form of the reducing aldose 2-deoxy-d-arabino-hexose. The six-membered pyranose ring adopts a 4 C 1 conformation, with the anomeric hydroxy group in axial and the other substituents in equatorial positions. In the crystal, each of the four hydroxy groups acts as an intermolecular hydrogen-bond donor function, resulting in a three-dimensional hydrogen-bonded network.
Related literature
For the crystal structure of 2-deoxy-β-d-arabino-hexopyranose, see: Maluszynska et al. (1981 ▶) and for the crystal structures of α-d-glucose and α-d-mannose, see Brown et al. (1965 ▶) and Longchambon et al. (1976 ▶), respectively. For puckering parameters, see: Cremer & Pople (1975 ▶). Crystals of the title compound were obtained during the course of attemps to grow crystals of a phenylboronic acid ester of 2-deoxy-d-arabino-hexose, see: Hess & Klüfers (2011 ▶).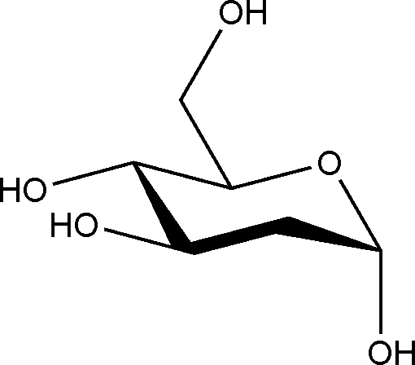
Experimental
Crystal data
C6H12O5
M r = 164.16
Orthorhombic,
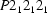
a = 4.8538 (2) Å
b = 9.5323 (4) Å
c = 15.6718 (6) Å
V = 725.12 (5) Å3
Z = 4
Mo Kα radiation
μ = 0.13 mm−1
T = 200 K
0.21 × 0.06 × 0.05 mm
Data collection
Nonius KappaCCD diffractometer
5622 measured reflections
1001 independent reflections
937 reflections with I > 2σ(I)
R int = 0.030
Refinement
R[F 2 > 2σ(F 2)] = 0.035
wR(F 2) = 0.097
S = 1.14
1001 reflections
104 parameters
H-atom parameters constrained
Δρmax = 0.43 e Å−3
Δρmin = −0.19 e Å−3
Data collection: COLLECT (Nonius, 2004 ▶); cell refinement: SCALEPACK (Otwinowski & Minor, 1997 ▶); data reduction: DENZO (Otwinowski & Minor, 1997 ▶) and SCALEPACK; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: ORTEP-3 (Farrugia, 1997 ▶) and SCHAKAL99 (Keller, 1999 ▶); software used to prepare material for publication: SHELXL97.
Supplementary Material
Crystal structure: contains datablock(s) I, global. DOI: 10.1107/S1600536811035264/kj2184sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536811035264/kj2184Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| O1—H81⋯O5i | 0.84 | 1.95 | 2.780 (2) | 171 |
| O3—H83⋯O1ii | 0.84 | 2.00 | 2.784 (2) | 155 |
| O4—H84⋯O6iii | 0.84 | 1.94 | 2.776 (3) | 174 |
| O6—H86⋯O3iv | 0.84 | 1.84 | 2.670 (2) | 170 |
Symmetry codes: (i) 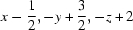 ; (ii)
; (ii) 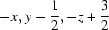 ; (iii)
; (iii) 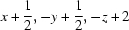 ; (iv)
; (iv) 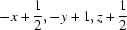 .
.
Acknowledgments
The authors thank Moritz Reichvilser for experimental support.
supplementary crystallographic information
Comment
2-Deoxy-D-arabino-hexose is the 2-deoxy derivate of both D-glucose and D-mannose. The crystals of the title compound were obtained in the course of attemps to grow crystals of a phenylboronic acid ester of 2-deoxy-D-arabino-hexose (Hess & Klüfers, 2011).
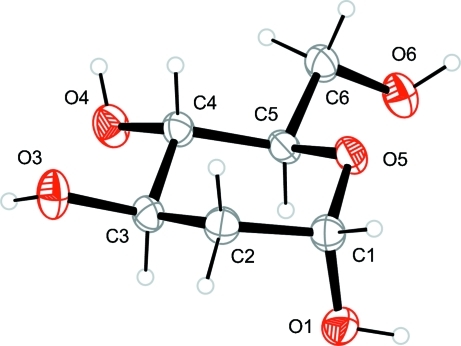
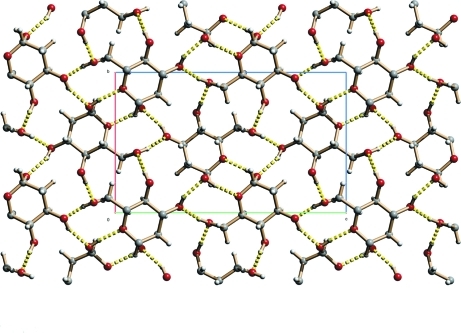
The bond lenghts and angles between the non-hydrogen atoms are normal. The pyranose ring adopts a slightly distorted 4C1 conformation, the puckering parameters (Cremer & Pople, 1975) being Q = 0.551 (2) Å and θ = 6.0 (2)° (Fig. 1). The exocyclic C6—O6 bond is orientated gauche-trans relative to the C5—O5 and C4—C5 bonds of the ring. In the crystal structure the compound forms a three-dimensional hydrogen-bonded network, where each hydroxy group acts as a donor in an intermolecular hydrogen bond to a different neighboring molecule. Acceptor functions are either the ring oxygen atom (O5) or the hydroxy oxygen atoms (O1, O3, O6). The hydrogen bond pattern is shown in Figure 2.
Experimental
2-Deoxy-D-arabino-hexose (0.164 g, 1 mmol) was dissolved in 1 ml of water and a solution of phenylboronic acid (0.122 g, 1 mmol) in 1 ml of methanol was added. The obtained solution was stirred at ambient temperature for 2 h. The solvent was then removed under reduced pressure. The remaining solid was dissolved in aceton and slowly evaporated to give colourless crystals suitable for X-ray analysis.
Refinement
Since the compound is a weak anomalous scatterer, 662 Friedel pairs were merged. The absolute structure was assigned according to the known stereochemistry of the starting material. Carbon-bound as well as oxygen-bound H atoms were placed in calculated positions (C—H 0.99 Å for CH2-groups, C—H 1.00 Å for CH-groups and O—H 0.84 Å for hydoxy groups) and were included in the refinement in the riding model approximation, with Uiso(H) set to 1.2Ueq(C) for the CH2-groups and CH-groups and 1.5Ueq(O) for the hydroxy groups.
Figures
Fig. 1.
ORTEP-representation of the asymmetric unit of the title compound, with atom labels and anisotropic displacement ellipsoids (drawn at 50% probability level) for non-H atoms.
Fig. 2.
SCHAKAL-representation of hydrogen bonds in the crystal packing of the title compound viewed along the a axis.
Crystal data
| C6H12O5 | F(000) = 352 |
| Mr = 164.16 | Dx = 1.504 (1) Mg m−3 |
| Orthorhombic, P212121 | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: P 2ac 2ab | Cell parameters from 2672 reflections |
| a = 4.8538 (2) Å | θ = 3.1–27.5° |
| b = 9.5323 (4) Å | µ = 0.13 mm−1 |
| c = 15.6718 (6) Å | T = 200 K |
| V = 725.12 (5) Å3 | Rod, colourless |
| Z = 4 | 0.21 × 0.06 × 0.05 mm |
Data collection
| Nonius KappaCCD diffractometer | 937 reflections with I > 2σ(I) |
| Radiation source: rotating anode | Rint = 0.030 |
| MONTEL, graded multilayered X-ray optics | θmax = 27.5°, θmin = 3.4° |
| CCD; rotation images; thick slices scans | h = −6→6 |
| 5622 measured reflections | k = −12→12 |
| 1001 independent reflections | l = −20→20 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.035 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.097 | H-atom parameters constrained |
| S = 1.14 | w = 1/[σ2(Fo2) + (0.0454P)2 + 0.298P] where P = (Fo2 + 2Fc2)/3 |
| 1001 reflections | (Δ/σ)max < 0.001 |
| 104 parameters | Δρmax = 0.43 e Å−3 |
| 0 restraints | Δρmin = −0.19 e Å−3 |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| O1 | −0.0906 (4) | 0.75609 (18) | 0.88687 (10) | 0.0296 (4) | |
| H81 | −0.1430 | 0.8030 | 0.9292 | 0.044* | |
| O3 | 0.3063 (4) | 0.45829 (17) | 0.72256 (9) | 0.0308 (4) | |
| H83 | 0.2179 | 0.3892 | 0.7036 | 0.046* | |
| O4 | 0.0700 (4) | 0.28748 (16) | 0.85187 (10) | 0.0292 (4) | |
| H84 | 0.1642 | 0.2202 | 0.8705 | 0.044* | |
| O5 | 0.2069 (4) | 0.61635 (15) | 0.96649 (9) | 0.0251 (4) | |
| O6 | −0.0846 (4) | 0.42652 (18) | 1.09339 (9) | 0.0305 (4) | |
| H86 | −0.0049 | 0.4550 | 1.1377 | 0.046* | |
| C1 | 0.1847 (6) | 0.7158 (2) | 0.89833 (14) | 0.0259 (5) | |
| H1 | 0.2957 | 0.8008 | 0.9132 | 0.031* | |
| C2 | 0.2911 (5) | 0.6559 (2) | 0.81511 (13) | 0.0248 (5) | |
| H2A | 0.2463 | 0.7213 | 0.7681 | 0.030* | |
| H2B | 0.4941 | 0.6473 | 0.8182 | 0.030* | |
| C3 | 0.1683 (5) | 0.5136 (2) | 0.79562 (12) | 0.0229 (5) | |
| H3 | −0.0325 | 0.5243 | 0.7828 | 0.027* | |
| C4 | 0.2049 (5) | 0.4156 (2) | 0.87086 (12) | 0.0219 (4) | |
| H4 | 0.4055 | 0.3980 | 0.8806 | 0.026* | |
| C5 | 0.0780 (5) | 0.4826 (2) | 0.95053 (12) | 0.0219 (4) | |
| H5 | −0.1235 | 0.4975 | 0.9406 | 0.026* | |
| C6 | 0.1161 (5) | 0.3944 (2) | 1.02968 (13) | 0.0263 (5) | |
| H6A | 0.1017 | 0.2939 | 1.0143 | 0.032* | |
| H6B | 0.3026 | 0.4108 | 1.0532 | 0.032* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| O1 | 0.0353 (9) | 0.0299 (8) | 0.0238 (7) | 0.0080 (8) | 0.0022 (7) | 0.0000 (7) |
| O3 | 0.0436 (10) | 0.0274 (8) | 0.0214 (7) | −0.0055 (8) | 0.0082 (8) | −0.0041 (6) |
| O4 | 0.0396 (9) | 0.0213 (7) | 0.0269 (8) | −0.0068 (8) | −0.0051 (8) | −0.0013 (6) |
| O5 | 0.0372 (9) | 0.0192 (7) | 0.0189 (6) | −0.0015 (7) | −0.0060 (7) | −0.0002 (6) |
| O6 | 0.0379 (9) | 0.0340 (9) | 0.0195 (7) | −0.0042 (8) | 0.0005 (7) | −0.0010 (6) |
| C1 | 0.0346 (12) | 0.0215 (10) | 0.0216 (9) | 0.0010 (10) | −0.0013 (10) | 0.0013 (8) |
| C2 | 0.0297 (11) | 0.0221 (10) | 0.0226 (9) | −0.0003 (10) | 0.0013 (10) | 0.0023 (8) |
| C3 | 0.0261 (11) | 0.0256 (10) | 0.0170 (8) | 0.0004 (10) | 0.0017 (8) | −0.0003 (8) |
| C4 | 0.0261 (10) | 0.0186 (10) | 0.0211 (9) | −0.0016 (9) | −0.0034 (9) | −0.0015 (7) |
| C5 | 0.0265 (10) | 0.0206 (9) | 0.0186 (9) | −0.0012 (9) | −0.0049 (9) | 0.0003 (7) |
| C6 | 0.0357 (12) | 0.0239 (10) | 0.0192 (9) | 0.0020 (10) | −0.0017 (9) | 0.0010 (8) |
Geometric parameters (Å, °)
| O1—C1 | 1.402 (3) | C2—C3 | 1.512 (3) |
| O1—H81 | 0.8400 | C2—H2A | 0.9900 |
| O3—C3 | 1.428 (2) | C2—H2B | 0.9900 |
| O3—H83 | 0.8400 | C3—C4 | 1.515 (3) |
| O4—C4 | 1.418 (3) | C3—H3 | 1.0000 |
| O4—H84 | 0.8400 | C4—C5 | 1.532 (3) |
| O5—C1 | 1.433 (3) | C4—H4 | 1.0000 |
| O5—C5 | 1.442 (3) | C5—C6 | 1.510 (3) |
| O6—C6 | 1.428 (3) | C5—H5 | 1.0000 |
| O6—H86 | 0.8400 | C6—H6A | 0.9900 |
| C1—C2 | 1.515 (3) | C6—H6B | 0.9900 |
| C1—H1 | 1.0000 | ||
| ?···? | ? | ||
| C1—O1—H81 | 109.5 | C2—C3—H3 | 109.5 |
| C3—O3—H83 | 109.5 | C4—C3—H3 | 109.5 |
| C4—O4—H84 | 109.5 | O4—C4—C3 | 108.28 (16) |
| C1—O5—C5 | 115.04 (15) | O4—C4—C5 | 110.16 (18) |
| C6—O6—H86 | 109.5 | C3—C4—C5 | 109.27 (17) |
| O1—C1—O5 | 110.39 (19) | O4—C4—H4 | 109.7 |
| O1—C1—C2 | 108.54 (19) | C3—C4—H4 | 109.7 |
| O5—C1—C2 | 111.50 (18) | C5—C4—H4 | 109.7 |
| O1—C1—H1 | 108.8 | O5—C5—C6 | 107.26 (16) |
| O5—C1—H1 | 108.8 | O5—C5—C4 | 109.60 (18) |
| C2—C1—H1 | 108.8 | C6—C5—C4 | 112.82 (17) |
| C3—C2—C1 | 112.20 (18) | O5—C5—H5 | 109.0 |
| C3—C2—H2A | 109.2 | C6—C5—H5 | 109.0 |
| C1—C2—H2A | 109.2 | C4—C5—H5 | 109.0 |
| C3—C2—H2B | 109.2 | O6—C6—C5 | 111.79 (18) |
| C1—C2—H2B | 109.2 | O6—C6—H6A | 109.3 |
| H2A—C2—H2B | 107.9 | C5—C6—H6A | 109.3 |
| O3—C3—C2 | 107.95 (17) | O6—C6—H6B | 109.3 |
| O3—C3—C4 | 109.96 (17) | C5—C6—H6B | 109.3 |
| C2—C3—C4 | 110.45 (16) | H6A—C6—H6B | 107.9 |
| O3—C3—H3 | 109.5 | ||
| C5—O5—C1—O1 | 66.3 (2) | C2—C3—C4—C5 | 56.0 (2) |
| C5—O5—C1—C2 | −54.4 (3) | C1—O5—C5—C6 | −178.49 (18) |
| O1—C1—C2—C3 | −71.6 (2) | C1—O5—C5—C4 | 58.7 (2) |
| O5—C1—C2—C3 | 50.2 (3) | O4—C4—C5—O5 | −176.95 (16) |
| C1—C2—C3—O3 | −172.65 (19) | C3—C4—C5—O5 | −58.1 (2) |
| C1—C2—C3—C4 | −52.4 (2) | O4—C4—C5—C6 | 63.6 (2) |
| O3—C3—C4—O4 | −65.0 (2) | C3—C4—C5—C6 | −177.56 (19) |
| C2—C3—C4—O4 | 175.95 (18) | O5—C5—C6—O6 | 81.9 (2) |
| O3—C3—C4—C5 | 174.99 (18) | C4—C5—C6—O6 | −157.32 (19) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| O1—H81···O5i | 0.84 | 1.95 | 2.780 (2) | 171. |
| O3—H83···O1ii | 0.84 | 2.00 | 2.784 (2) | 155. |
| O4—H84···O6iii | 0.84 | 1.94 | 2.776 (3) | 174. |
| O6—H86···O3iv | 0.84 | 1.84 | 2.670 (2) | 170. |
Symmetry codes: (i) x−1/2, −y+3/2, −z+2; (ii) −x, y−1/2, −z+3/2; (iii) x+1/2, −y+1/2, −z+2; (iv) −x+1/2, −y+1, z+1/2.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: KJ2184).
References
- Brown, G. M. & Levy, H. A. (1965). Science, 147, 1038–1039. [DOI] [PubMed]
- Cremer, D. & Pople, J. A. (1975). J. Am. Chem. Soc. 97, 1354–1358.
- Farrugia, L. J. (1997). J. Appl. Cryst. 30, 565.
- Hess, D. & Klüfers, P. (2011). Carbohydr. Res. doi:10.1016/j.carres.2011.05.031. [DOI] [PubMed]
- Keller, E. (1999). SCHAKAL99 University of Freiburg, Germany.
- Longchambon, F., Avenel, D. & Neuman, A. (1976). Acta Cryst. B32, 1822–1826.
- Maluszynska, H., Ruble, J. R. & Jeffrey, G. A. (1981). Carbohydr. Res. 97, 199–204.
- Nonius (2004). COLLECT. Nonius BV, Delft, The Netherlands.
- Otwinowski, Z. & Minor, W. (1997). Methods in Enzymology, Vol. 276, Macromolecular Crystallography, Part A, edited by C. W. Carter Jr & R. M. Sweet, pp. 307–326. New York: Academic Press.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I, global. DOI: 10.1107/S1600536811035264/kj2184sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536811035264/kj2184Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report