Abstract
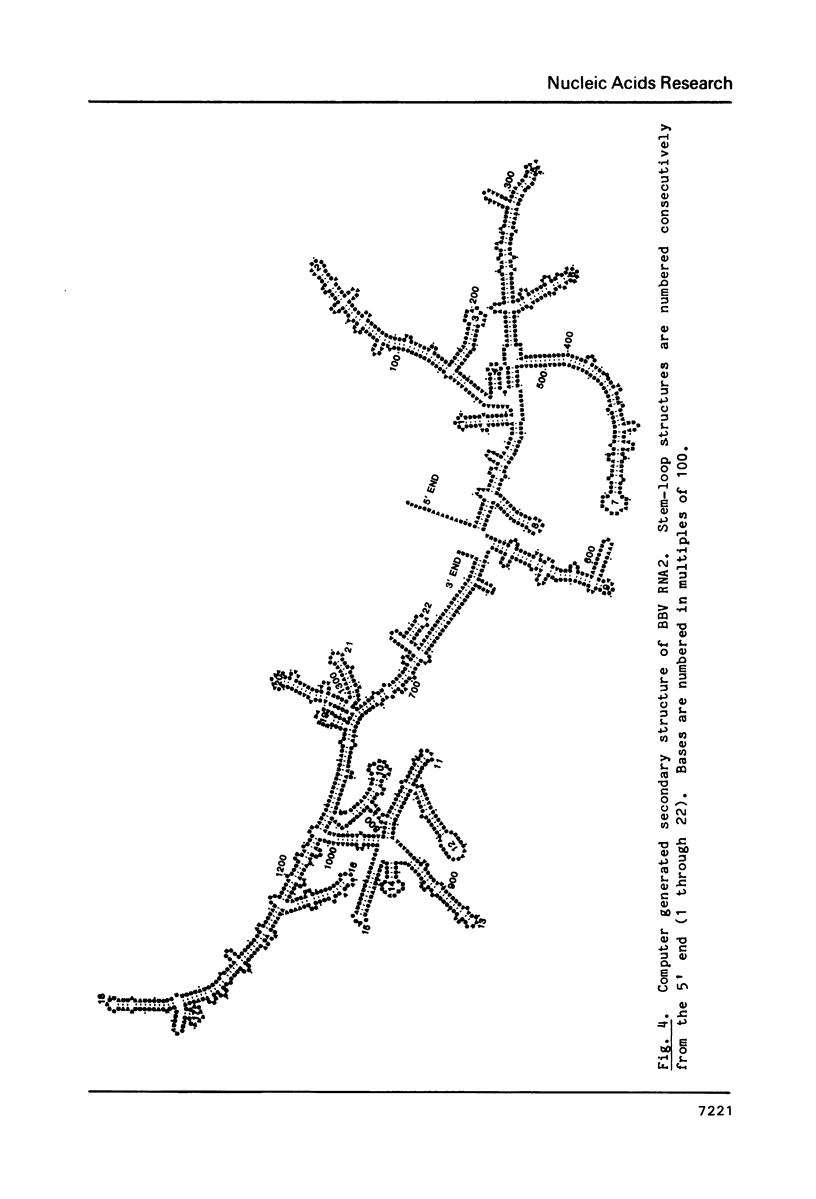
The nucleotide sequence of black beetle virion (BBV) RNA2 has been determined. RNA2 is 1399 b long. Its 5' terminus is capped. Its 3' terminus has an unidentified moiety that renders the RNA resistent to polyadenylation and ligation. The first AUG codon at base 23 is followed by an open reading frame for a protein 407 amino acids long, the predicted size of coat protein precursor. A second open reading frame for a putative protein 72 amino acid residues long begins at base 1110. No other large open reading frames exist. The 5' half of the RNA can be folded into a long, imperfect hairpin of high predicted stability. The 3' half of the RNA can fold into a complex set of multiply bifurcated stem and loop regions.
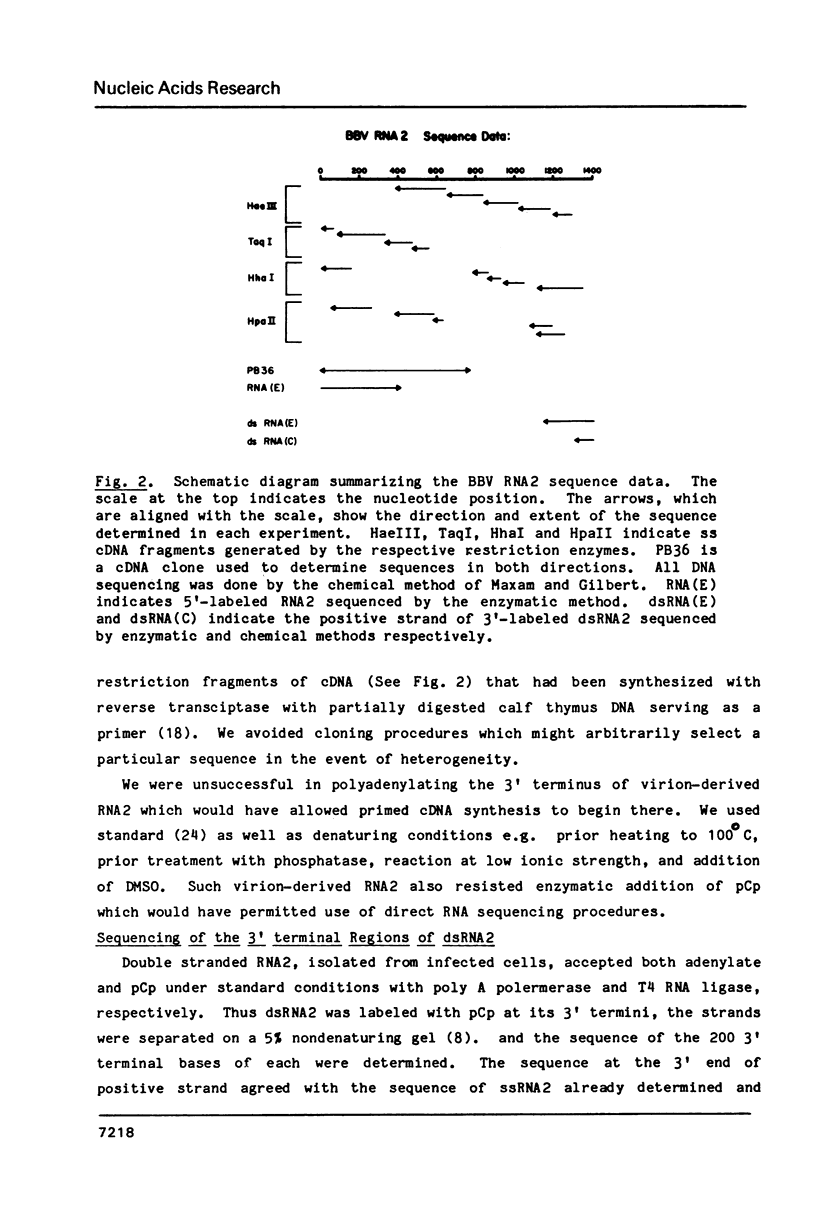
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahlquist P., Dasgupta R., Kaesberg P. Near identity of 3- RNA secondary structure in bromoviruses and cucumber mosaic virus. Cell. 1981 Jan;23(1):183–189. doi: 10.1016/0092-8674(81)90283-x. [DOI] [PubMed] [Google Scholar]
- Ahlquist P., Luckow V., Kaesberg P. Complete nucleotide sequence of brome mosaic virus RNA3. J Mol Biol. 1981 Nov 25;153(1):23–38. doi: 10.1016/0022-2836(81)90524-6. [DOI] [PubMed] [Google Scholar]
- Baltimore D. Purification and properties of poliovirus double-stranded ribonucleic acid. J Mol Biol. 1966 Jul;18(3):421–428. doi: 10.1016/s0022-2836(66)80034-7. [DOI] [PubMed] [Google Scholar]
- Dasgupta R., Kaesberg P. Complete nucleotide sequences of the coat protein messenger RNAs of brome mosaic virus and cowpea chlorotic mottle virus. Nucleic Acids Res. 1982 Jan 22;10(2):703–713. doi: 10.1093/nar/10.2.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta R., Kaesberg P. Sequence of an oligonucleotide derived from the 3' end of each of the four brome mosaic viral RNAs. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4900–4904. doi: 10.1073/pnas.74.11.4900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devos R., van Emmelo J., Seurinck-Opsomer C., Gillis E., Fiers W. Addition by ATP: RNA adenylyltransferase from Escherichia coli of 3'-linked oligo(A) to bacteriophage Qbeta RNA and its effect on RNA replication. Biochim Biophys Acta. 1976 Oct 18;447(3):319–327. doi: 10.1016/0005-2787(76)90055-1. [DOI] [PubMed] [Google Scholar]
- Donis-Keller H. Phy M: an RNase activity specific for U and A residues useful in RNA sequence analysis. Nucleic Acids Res. 1980 Jul 25;8(14):3133–3142. doi: 10.1093/nar/8.14.3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efstratiadis A., Vournakis J. N., Donis-Keller H., Chaconas G., Dougall D. K., Kafatos F. C. End labeling of enzymatically decapped mRNA. Nucleic Acids Res. 1977 Dec;4(12):4165–4174. doi: 10.1093/nar/4.12.4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- England T. E., Uhlenbeck O. C. 3'-terminal labelling of RNA with T4 RNA ligase. Nature. 1978 Oct 12;275(5680):560–561. doi: 10.1038/275560a0. [DOI] [PubMed] [Google Scholar]
- Friesen P. D., Rueckert R. R. Early and late functions in a bipartite RNA virus: evidence for translational control by competition between viral mRNAs. J Virol. 1984 Jan;49(1):116–124. doi: 10.1128/jvi.49.1.116-124.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher T. M., Friesen P. D., Rueckert R. R. Autonomous replication and expression of RNA 1 from black beetle virus. J Virol. 1983 May;46(2):481–489. doi: 10.1128/jvi.46.2.481-489.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarino L. A., Hruby D. E., Ball L. A., Kaesberg P. Translation of black beetle virus RNA and heterologous viral RNAs in cell-free lysates derived from Drosophila melanogaster. J Virol. 1981 Jan;37(1):500–505. doi: 10.1128/jvi.37.1.500-505.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemper B. Inactivation of parathyroid hormone mRNA by treatment with periodate and aniline. Nature. 1976 Jul 22;262(5566):321–323. doi: 10.1038/262321a0. [DOI] [PubMed] [Google Scholar]
- Kozak M. Possible role of flanking nucleotides in recognition of the AUG initiator codon by eukaryotic ribosomes. Nucleic Acids Res. 1981 Oct 24;9(20):5233–5252. doi: 10.1093/nar/9.20.5233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longworth J. F., Carey G. P. A small RNA virus with a divided genome from Heteronychus arator (F.) [Coleoperai Scarabaeidae]. J Gen Virol. 1976 Oct;33(1):31–40. doi: 10.1099/0022-1317-33-1-31. [DOI] [PubMed] [Google Scholar]
- Longworth J. F. Small isometric viruses of invertebrates. Adv Virus Res. 1978;23:103–157. doi: 10.1016/s0065-3527(08)60099-8. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Moss B. Utilization of the guanylyltransferase and methyltransferases of vaccinia virus to modify and identify the 5'-terminals of heterologous RNA species. Biochem Biophys Res Commun. 1977 Jan 24;74(2):374–383. doi: 10.1016/0006-291x(77)90314-x. [DOI] [PubMed] [Google Scholar]
- Peattie D. A. Direct chemical method for sequencing RNA. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1760–1764. doi: 10.1073/pnas.76.4.1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice C. M., Strauss J. H. Nucleotide sequence of the 26S mRNA of Sindbis virus and deduced sequence of the encoded virus structural proteins. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2062–2066. doi: 10.1073/pnas.78.4.2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossmann M. G., Abad-Zapatero C., Hermodson M. A., Erickson J. W. Subunit interactions in southern bean mosaic virus. J Mol Biol. 1983 May 5;166(1):37–73. doi: 10.1016/s0022-2836(83)80049-7. [DOI] [PubMed] [Google Scholar]
- Sanger F., Brownlee G. G., Barrell B. G. A two-dimensional fractionation procedure for radioactive nucleotides. J Mol Biol. 1965 Sep;13(2):373–398. doi: 10.1016/s0022-2836(65)80104-8. [DOI] [PubMed] [Google Scholar]
- Taylor J. M., Illmensee R., Summers J. Efficeint transcription of RNA into DNA by avian sarcoma virus polymerase. Biochim Biophys Acta. 1976 Sep 6;442(3):324–330. doi: 10.1016/0005-2787(76)90307-5. [DOI] [PubMed] [Google Scholar]
- Tremaine J. H., Ronald W. P., Agrawal H. O. Some tryptic peptides of bromovirus proteins. Virology. 1977 Dec;83(2):404–412. doi: 10.1016/0042-6822(77)90185-4. [DOI] [PubMed] [Google Scholar]
- Zuker M., Stiegler P. Optimal computer folding of large RNA sequences using thermodynamics and auxiliary information. Nucleic Acids Res. 1981 Jan 10;9(1):133–148. doi: 10.1093/nar/9.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]



