Abstract
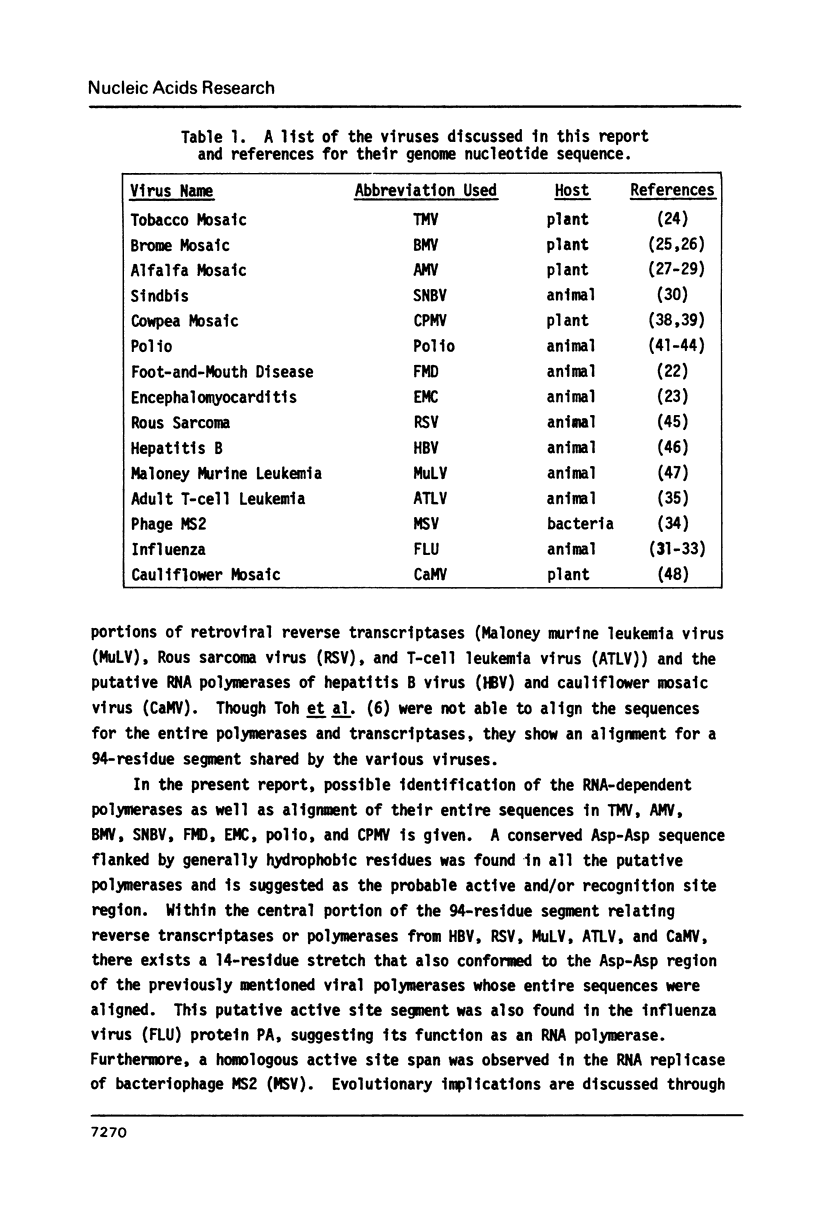
Possible alignments for portions of the genomic codons in eight different plant and animal viruses are presented: tobacco mosaic, brome mosaic, alfalfa mosaic, sindbis, foot-and-mouth disease, polio, encephalomyocarditis, and cowpea mosaic viruses. Since in one of the viruses (polio) the aligned sequence has been identified as an RNA-dependent polymerase, this would imply the identification of the polymerases in the other viruses. A conserved fourteen-residue segment consisting of an Asp-Asp sequence flanked by hydrophobic residues has also been found in retroviral reverse transcriptases, a bacteriophage, influenza virus, cauliflower mosaic virus and hepatitis B virus, suggesting this span as a possible active site or nucleic acid recognition region for the polymerases. Evolutionary implications are discussed.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahlquist P., Dasgupta R., Kaesberg P. Nucleotide sequence of the brome mosaic virus genome and its implications for viral replication. J Mol Biol. 1984 Feb 5;172(4):369–383. doi: 10.1016/s0022-2836(84)80012-1. [DOI] [PubMed] [Google Scholar]
- Ahlquist P., Luckow V., Kaesberg P. Complete nucleotide sequence of brome mosaic virus RNA3. J Mol Biol. 1981 Nov 25;153(1):23–38. doi: 10.1016/0022-2836(81)90524-6. [DOI] [PubMed] [Google Scholar]
- Argos P., Garavito R. M., Eventoff W., Rossmann M. G., Brändén C. I. Similarities in active center geometries of zinc-containing enzymes, proteases and dehydrogenases. J Mol Biol. 1978 Dec 5;126(2):141–158. doi: 10.1016/0022-2836(78)90356-x. [DOI] [PubMed] [Google Scholar]
- Argos P., Hanei M., Wilson J. M., Kelley W. N. A possible nucleotide-binding domain in the tertiary fold of phosphoribosyltransferases. J Biol Chem. 1983 May 25;258(10):6450–6457. [PubMed] [Google Scholar]
- Argos P., Palau J. Amino acid distribution in protein secondary structures. Int J Pept Protein Res. 1982 Apr;19(4):380–393. doi: 10.1111/j.1399-3011.1982.tb02619.x. [DOI] [PubMed] [Google Scholar]
- Barker R. F., Jarvis N. P., Thompson D. V., Loesch-Fries L. S., Hall T. C. Complete nucleotide sequence of alfalfa mosaic virus RNA3. Nucleic Acids Res. 1983 May 11;11(9):2881–2891. doi: 10.1093/nar/11.9.2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop D. H., Jones K. L., Huddleston J. A., Brownlee G. G. Influenza A virus evolution: complete sequences of influenza A/NT/60/68 RNA segment 3 and its predicted acidic P polypeptide compared with those of influenza A/PR/8/34. Virology. 1982 Jul 30;120(2):481–489. doi: 10.1016/0042-6822(82)90049-6. [DOI] [PubMed] [Google Scholar]
- Carroll A. R., Rowlands D. J., Clarke B. E. The complete nucleotide sequence of the RNA coding for the primary translation product of foot and mouth disease virus. Nucleic Acids Res. 1984 Mar 12;12(5):2461–2472. doi: 10.1093/nar/12.5.2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Conformational parameters for amino acids in helical, beta-sheet, and random coil regions calculated from proteins. Biochemistry. 1974 Jan 15;13(2):211–222. doi: 10.1021/bi00699a001. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of protein conformation. Biochemistry. 1974 Jan 15;13(2):222–245. doi: 10.1021/bi00699a002. [DOI] [PubMed] [Google Scholar]
- Cornelissen B. J., Brederode F. T., Moormann R. J., Bol J. F. Complete nucleotide sequence of alfalfa mosaic virus RNA 1. Nucleic Acids Res. 1983 Mar 11;11(5):1253–1265. doi: 10.1093/nar/11.5.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelissen B. J., Brederode F. T., Veeneman G. H., van Boom J. H., Bol J. F. Complete nucleotide sequence of alfalfa mosaic virus RNA 2. Nucleic Acids Res. 1983 May 25;11(10):3019–3025. doi: 10.1093/nar/11.10.3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creighton T. E. Experimental studies of protein folding and unfolding. Prog Biophys Mol Biol. 1978;33(3):231–297. doi: 10.1016/0079-6107(79)90030-0. [DOI] [PubMed] [Google Scholar]
- Fields S., Winter G. Nucleotide sequences of influenza virus segments 1 and 3 reveal mosaic structure of a small viral RNA segment. Cell. 1982 Feb;28(2):303–313. doi: 10.1016/0092-8674(82)90348-8. [DOI] [PubMed] [Google Scholar]
- Fiers W., Contreras R., Duerinck F., Haegeman G., Iserentant D., Merregaert J., Min Jou W., Molemans F., Raeymaekers A., Van den Berghe A. Complete nucleotide sequence of bacteriophage MS2 RNA: primary and secondary structure of the replicase gene. Nature. 1976 Apr 8;260(5551):500–507. doi: 10.1038/260500a0. [DOI] [PubMed] [Google Scholar]
- Flanegan J. B., Baltimore D. Poliovirus-specific primer-dependent RNA polymerase able to copy poly(A). Proc Natl Acad Sci U S A. 1977 Sep;74(9):3677–3680. doi: 10.1073/pnas.74.9.3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franssen H., Leunissen J., Goldbach R., Lomonossoff G., Zimmern D. Homologous sequences in non-structural proteins from cowpea mosaic virus and picornaviruses. EMBO J. 1984 Apr;3(4):855–861. doi: 10.1002/j.1460-2075.1984.tb01896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franssen H., Moerman M., Rezelman G., Goldbach R. Evidence That the 32,000-Dalton Protein Encoded by Bottom-Component RNA of Cowpea Mosaic Virus is a Proteolytic Processing Enzyme. J Virol. 1984 Apr;50(1):183–190. doi: 10.1128/jvi.50.1.183-190.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner R. C., Howarth A. J., Hahn P., Brown-Luedi M., Shepherd R. J., Messing J. The complete nucleotide sequence of an infectious clone of cauliflower mosaic virus by M13mp7 shotgun sequencing. Nucleic Acids Res. 1981 Jun 25;9(12):2871–2888. doi: 10.1093/nar/9.12.2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goelet P., Lomonossoff G. P., Butler P. J., Akam M. E., Gait M. J., Karn J. Nucleotide sequence of tobacco mosaic virus RNA. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5818–5822. doi: 10.1073/pnas.79.19.5818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanecak R., Semler B. L., Anderson C. W., Wimmer E. Proteolytic processing of poliovirus polypeptides: antibodies to polypeptide P3-7c inhibit cleavage at glutamine-glycine pairs. Proc Natl Acad Sci U S A. 1982 Jul;79(13):3973–3977. doi: 10.1073/pnas.79.13.3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D. D. Amino acid properties and side-chain orientation in proteins: a cross correlation appraoch. J Theor Biol. 1975 Mar;50(1):167–183. doi: 10.1016/0022-5193(75)90031-4. [DOI] [PubMed] [Google Scholar]
- Keim P., Heinrikson R. L., Fitch W. M. An examination of the expected degree of sequence similarity that might arise in proteins that have converged to similar conformational states. The impact of such expectations on the search for homology between the structurally similar domains of rhodanese. J Mol Biol. 1981 Sep 5;151(1):179–197. doi: 10.1016/0022-2836(81)90227-8. [DOI] [PubMed] [Google Scholar]
- Kitamura N., Semler B. L., Rothberg P. G., Larsen G. R., Adler C. J., Dorner A. J., Emini E. A., Hanecak R., Lee J. J., van der Werf S. Primary structure, gene organization and polypeptide expression of poliovirus RNA. Nature. 1981 Jun 18;291(5816):547–553. doi: 10.1038/291547a0. [DOI] [PubMed] [Google Scholar]
- Manavalan P., Ponnuswamy P. K. Hydrophobic character of amino acid residues in globular proteins. Nature. 1978 Oct 19;275(5681):673–674. doi: 10.1038/275673a0. [DOI] [PubMed] [Google Scholar]
- Nomoto A., Omata T., Toyoda H., Kuge S., Horie H., Kataoka Y., Genba Y., Nakano Y., Imura N. Complete nucleotide sequence of the attenuated poliovirus Sabin 1 strain genome. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5793–5797. doi: 10.1073/pnas.79.19.5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozaki Y., Tanford C. The solubility of amino acids and two glycine peptides in aqueous ethanol and dioxane solutions. Establishment of a hydrophobicity scale. J Biol Chem. 1971 Apr 10;246(7):2211–2217. [PubMed] [Google Scholar]
- Ono Y., Onda H., Sasada R., Igarashi K., Sugino Y., Nishioka K. The complete nucleotide sequences of the cloned hepatitis B virus DNA; subtype adr and adw. Nucleic Acids Res. 1983 Mar 25;11(6):1747–1757. doi: 10.1093/nar/11.6.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palau J., Argos P., Puigdomenech P. Protein secondary structure. Studies on the limits of prediction accuracy. Int J Pept Protein Res. 1982 Apr;19(4):394–401. [PubMed] [Google Scholar]
- Racaniello V. R., Baltimore D. Molecular cloning of poliovirus cDNA and determination of the complete nucleotide sequence of the viral genome. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4887–4891. doi: 10.1073/pnas.78.8.4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossmann M. G., Argos P. The taxonomy of binding sites in proteins. Mol Cell Biochem. 1978 Nov 16;21(3):161–182. doi: 10.1007/BF00240135. [DOI] [PubMed] [Google Scholar]
- Rueckert R. R., Wimmer E. Systematic nomenclature of picornavirus proteins. J Virol. 1984 Jun;50(3):957–959. doi: 10.1128/jvi.50.3.957-959.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz D. E., Tizard R., Gilbert W. Nucleotide sequence of Rous sarcoma virus. Cell. 1983 Mar;32(3):853–869. doi: 10.1016/0092-8674(83)90071-5. [DOI] [PubMed] [Google Scholar]
- Seiki M., Hattori S., Hirayama Y., Yoshida M. Human adult T-cell leukemia virus: complete nucleotide sequence of the provirus genome integrated in leukemia cell DNA. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3618–3622. doi: 10.1073/pnas.80.12.3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semler B. L., Hanecak R., Anderson C. W., Wimmer E. Cleavage sites in the polypeptide precursors of poliovirus protein P2-X. Virology. 1981 Oct 30;114(2):589–594. doi: 10.1016/0042-6822(81)90242-7. [DOI] [PubMed] [Google Scholar]
- Shinnick T. M., Lerner R. A., Sutcliffe J. G. Nucleotide sequence of Moloney murine leukaemia virus. Nature. 1981 Oct 15;293(5833):543–548. doi: 10.1038/293543a0. [DOI] [PubMed] [Google Scholar]
- Stanway G., Cann A. J., Hauptmann R., Hughes P., Clarke L. D., Mountford R. C., Minor P. D., Schild G. C., Almond J. W. The nucleotide sequence of poliovirus type 3 leon 12 a1b: comparison with poliovirus type 1. Nucleic Acids Res. 1983 Aug 25;11(16):5629–5643. doi: 10.1093/nar/11.16.5629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss E. G., Rice C. M., Strauss J. H. Complete nucleotide sequence of the genomic RNA of Sindbis virus. Virology. 1984 Feb;133(1):92–110. doi: 10.1016/0042-6822(84)90428-8. [DOI] [PubMed] [Google Scholar]
- Sweet R. M., Eisenberg D. Correlation of sequence hydrophobicities measures similarity in three-dimensional protein structure. J Mol Biol. 1983 Dec 25;171(4):479–488. doi: 10.1016/0022-2836(83)90041-4. [DOI] [PubMed] [Google Scholar]
- Toh H., Hayashida H., Miyata T. Sequence homology between retroviral reverse transcriptase and putative polymerases of hepatitis B virus and cauliflower mosaic virus. 1983 Oct 27-Nov 2Nature. 305(5937):827–829. doi: 10.1038/305827a0. [DOI] [PubMed] [Google Scholar]
- Wolfenden R. V., Cullis P. M., Southgate C. C. Water, protein folding, and the genetic code. Science. 1979 Nov 2;206(4418):575–577. doi: 10.1126/science.493962. [DOI] [PubMed] [Google Scholar]
- van Wezenbeek P., Verver J., Harmsen J., Vos P., van Kammen A. Primary structure and gene organization of the middle-component RNA of cowpea mosaic virus. EMBO J. 1983;2(6):941–946. doi: 10.1002/j.1460-2075.1983.tb01525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]



