Abstract
Ligand-dependent activation of the Hedgehog (Hh) signaling pathway has been implicated in both tumor initiation and metastasis of pancreatic ductal adenocarcinoma (PDAC). Prior studies in genetically engineered mouse models (GEMMs) have assessed the role of Hh signaling by cell autonomous expression of a constitutively active Gli2 within epithelial cells. On the contrary, aberrant pathway reactivation in the human exocrine pancreas occurs principally as a consequence of Sonic Hh ligand (Shh) overexpression from epithelial cells. To recapitulate the cognate pathophysiology of Hh signaling observed in the human pancreas, we examined GEMM where Hh ligand is conditionally overexpressed within the mature exocrine pancreas using a tamoxifen-inducible Elastase-Cre promoter (Ela-CreERT2;LSL-mShh). We also facilitated potential cell autonomous epithelial responsiveness to secreted Hh ligand by generating compound transgenic mice with concomitant expression of the Hh receptor Smoothened (Ela-CreERT2;LSL-mShh;LSL-mSmo). Of interest, none of these mice developed intraductal precursor lesions or PDAC during the follow-up period of up to 12 months after tamoxifen induction. Instead, all animals demonstrated marked expansion of stromal cells, consistent with the previously described epithelial-to-stromal paracrine Hh signaling. Hh responsiveness was mirrored by the expression of primary cilia within the expanded mesenchymal compartment and the absence within mature acinar cells. In the absence of cooperating mutations, Hh ligand overexpression in the mature exocrine pancreas is insufficient to induce neoplasia, even when epithelial cells coexpress the Smo receptor. This autochthonous model serves as a platform for studying epithelial stromal interactions in pancreatic carcinogenesis.
Introduction
Ductal adenocarcinoma of the pancreas (a.k.a. pancreatic cancer) is one of the most deadly of human malignancies to date and is associated with almost uniform lethality [1]. Accounting for an estimated 36,400 fatalities in the United States alone in 2010, pancreatic cancer represents the fourth most common cause of cancer-related mortality in the western world [2]. Moreover, approximately 80% of cases are diagnosed at locally advanced or metastatic tumor stages, usually precluding surgical resection and leaving patients without any curative therapeutic option. With an overall survival of less than 6 months and 5-year survival rates below 5%, the dismal overall prognosis of pancreatic cancer has not markedly improved during the past decades [1,3,4].
Multiple lines of evidence implicate the Hedgehog (Hh) signaling pathway playing a role in both pancreatic cancer initiation and progression [5,6], as well as representing a promising target for therapeutic intervention [7–13]. The basis for aberrant pathway activation in pancreatic cancer is usually not a consequence of oncogenic mutations within canonical Hh pathway components but rather secondary to endogenous overexpression of Hedgehog ligands by neoplastic epithelial cells [5,7,8,14]. Conversely, the nature of the Hh-receiving cells in the context of pancreatic cancer is less clearly defined. Various models have been proposed, including stimulation of “bulk” neoplastic epithelial cells in an autocrine or paracrine manner, maintenance of a putative subpopulation of neoplastic epithelial cells with enhanced tumorigenic potential (also referred to as “cancer stem cells” by some authors), and most recently, proliferative effects on stromal cells of mesenchymal origin within the tumor microenvironment, as well as combinations of these models [15,16].
Among the more powerful tools in understanding the mechanisms underlying pancreatic carcinogenesis has been the development of genetically engineered mouse models of pancreatic cancer [17–19]. In this present study, we describe a genetically engineered mouse model in which Sonic Hedgehog ligand (Shh) is overexpressed in the acinar cell compartment of adult murine pancreata. In contrast to prior reports that relied on the ectopic expression of a constitutively activated Gli2 in the murine pancreas [20], we recapitulated “physiological” conditions by inducing endogenous Hedgehog ligand expression from the adult exocrine cells. In this model, we fail to observe evidence of intraductal neoplasia in the absence of cooperating mutations, such as oncogenic Kras. Instead, the Shh ligand-overexpressing murine pancreata demonstrate a striking expansion of the periacinar mesenchymal compartment, consistent with paracrine epithelial-stromal signaling. This model should serve as a platform for elucidating epithelial-stromal interactions in the context of exocrine pancreatic neoplasia and, further, for developing relevant examples of oncogenic cooperation with Hh signaling.
Materials and Methods
Generation of Pdx1-Cre;LSL-mShh, Elastase-CreERT2;LSL-mShh, and Elastase-CreERT2;LSL-mShh;LSL-mSmo Mouse Cohorts
Generation of LSL-mShh mice has been described previously [21]. In brief, in this model, Cre-mediated recombination leads to excision of the lox-stop-lox (LSL) cassette and conditional expression of mShh ligand (Figure 1A). An identical strategy is used for driving expression of Smo receptor protein in the recombined cells of compound transgenic LSL-mShh, LSL-mSmo mice. Mice interbred and reproduced readily with an even distribution of female-to-male ratio of approximately 1:1. The presence of a constitutive fluorescent protein expression in nonrecombined tissues (e.g., tail snips) enables rapid detection of the LSL-mShh or LSL-mSmo allele, by green or red fluorescence, respectively. The “driver” Pdx1-Cre and Elastase (Ela)-CreERT2 mice have been previously described [22,23]. The presence of Cre or CreERT2 alleles, respectively, was determined by polymerase chain reaction analysis, as previously described [23,24].
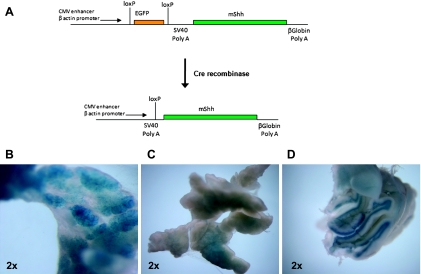
Figure 1.
Targeting endogenous Shh expression to the adult murine pancreas. (A) The presence of the transgenic construct in somatic cells can be verified by enhanced green fluorescent protein expression. Cre-mediated recombination of the LSL cassette leads to excision of the enhanced green fluorescent protein cassette and expression of Shh (described previously in Hingorani et al. [17]). (B) Positive X-Gal staining revealed robust Cre-mediated recombination in the exocrine pancreas of Ela-CreERT2;LSL-mShh;Rosa26R reporter mice after induction with tamoxifen. (C) Uninduced mouse. (D) Cerebellum of heterozygous Ptch-lacZ mice served as a positive control for X-Gal staining.
Two different reporter mouse crosses were generated in our studies: the first, to confirm the robustness of Cre-mediated recombination in the pancreatic acinar compartment, we generated Ela-CreERT2; Rosa26R mice, which harbor a beta-galactosidase gene downstream of a ubiquitous promoter and a LSL cassette. Second, to demonstrate Hh pathway activity within specific pancreatic compartments, the Ela-CreERT2;LSL-mShh mice were also crossed into a Ptch-lacZ background; in this latter strain, the lacZ allele is knocked into one of the Ptch loci, and the resulting beta-galactosidase activity (indicative of Hh pathway activity) can be visualized by X-Gal staining [24,25].
The following three cohorts of mice with pancreas-specific Shh ligand overexpression were generated for observation: Pdx1-cre; LSL-mShh, Ela-CreERT2;LSL-mShh, and Ela-CreERT2;LSL-mShh;mSmo. For the mice bearing tamoxifen-inducible ERT2 alleles, induction was carried out at the age of 6 weeks after birth by intraperitoneal injection of tamoxifen on five consecutive days as described previously [24]. Saline-injected mice of the same genotype were used as controls. Cohorts of at least five tamoxifen-induced, as well as at least two uninduced mice of each genotype were then killed at two monthly intervals, for the period ranging from 2 to 12 months after tamoxifen injection. At the end of the follow-up period, mice were killed by CO2 insufflation, and the pancreata were harvested and fixed in 10% neutral buffered formalin solution for paraffin embedding. In some cases, parts of the pancreata were immediately snap frozen in liquid nitrogen and then stored at -80°C until further use.
Immunohistochemistry
Immunolabeling of Shh and X-Gal staining were performed as previously described [24,26].
Immunofluorescence and Confocal Microscopy
Paraffin sections from Ela-CreERT2;LSL-mShh murine pancreata were collected and treated with antigen retrieval solution (Dako, Hamburg, Germany). The sections were probed overnight with the following antibodies: rabbit anti-insulin (Santa Cruz Biotechnology, Santa Cruz, CA), rabbit anti-Pan-cytokeratin (Abcam, Cambridge, United Kingdom), rabbit anti-amylase (Abcam), and mouse anti-acetylated-tubulin (Sigma, St Louis, MO). After washes in 0.1% PBS-Triton X-100, fluorescent detection was performed using Alexa Fluor 488 and 546 (Invitrogen, Carlsbad, CA)-conjugated secondary antibodies. Images were acquired using a Zeiss LSM 510 confocal microscope and digitized using Zeiss Image Browser (Zeiss, Jena, Germany).
Results
Developmental Overexpression of Shh Ligand in the Pancreatic Anlage Results in Malformation and Perinatal Lethality
In line with previous reports by others using comparable systems (Pdx1-Shh transgenic mice) [6], developmental overexpression of Shh led to pancreatic malformation and embryonic lethality in Pdx1-Cre; LSL-mShh mice (Figure W1). The pancreata were characterized by mucinous metaplastic glands and an expansion of intestinal-type mesenchyme, analogous to what has been described with transgenic ligand expression. These experiments confirmed the in vivo recombinatorial efficacy of the LSL cassette and the ability to generate an ectopic Shh ligand.
Aberrant Hh Ligand Expression in the Mature Pancreas Leads to Mesenchymal Expansion without Intraductal Lesions
In light of the developmental anomalies observed with Pdx1-Cre; LSL-mShh mice, we generated cohorts of Ela-CreERT2;LSL-mShh mice capable of producing Hh ligands in the adult exocrine pancreas. The Ela-CreERT2 allele is expressed within the mature acinar compartment on tamoxifen induction, as described [23]. To assess the efficiency of Cre-mediated recombination within the adult exocrine pancreas, we first generated tamoxifen-induced Ela-CreERT2;Rosa26R reporter mice, which confirmed the robustness of the Elastase promoter in conditional removal of the LSL cassette (Figure 1B); cerebellar beta-galactosidase expression in heterozygous Ptch-lacZ mice was used as positive control for the X-Gal staining (Figure 1C). In contrast to the Pdx1-Cre;LSL-mShh mice, embryonic malformations or subsequent growth defects were not observed in the Ela-CreERT2;LSL-mShh mice.
Cohorts of Ela-CreERT2;LSL-mShh mice were then observed for up to 1 year after tamoxifen induction, with at least five induced and two uninduced mice being killed at each interval of 2 months, beginning at the second month after tamoxifen injection. As expected, the uninduced mice did not demonstrate any microscopic abnormalities in the pancreas at any period during follow-up (Figure 2A). In contrast, tamoxifen-induced mice demonstrated a progressive mesenchymal expansion within the periacinar stroma, with the earliest histologic evidence for the spindle-shaped cells seen as early as 2 months after induction (Figure 2, B–D). Unlike the mutant Kras-driven mouse models of pancreatic neoplasia, aberrant expression of Shh ligand alone did not result in exocrine adenocarcinomas, intraductal precursor lesions, or even widespread acinar-ductal metaplasia, including that in mouse pancreata examined at 1 year after induction.
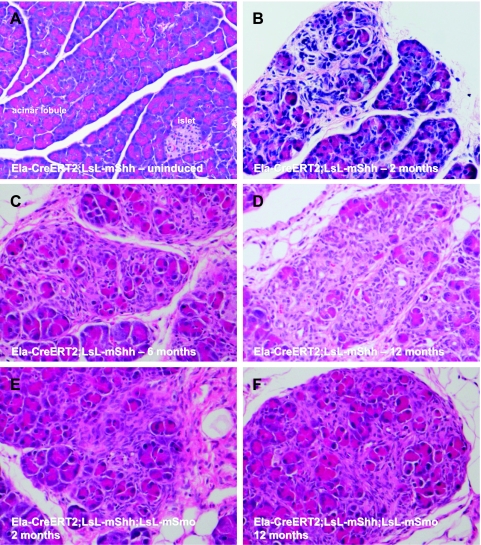
Figure 2.
Hh misexpression in the adult murine pancreas leads to mesenchymal expansion in the absence of intraductal lesions. (A) No morphologic abnormalities were observed in the pancreata of Ela-CreERT2;LSL-mShh mice in the absence of tamoxifen induction. (B–D) After tamoxifen induction, morphologic changes in the Ela-CreERT2;LSL-mShh pancreata were observed as early as 2 months and persisted at 1 year of follow-up, at which time the study was terminated. The most conspicuous alteration was a marked expansion of stromal cells between existing acinar lobules, displacing the acinar cells over time. Photomicrographs were obtained at 2 (B), 6 (C), and 12 months (D) after tamoxifen induction, respectively. In contrast, no epithelial alterations such as intraductal precursor lesions or exocrine cancers were found. (E and F) Comparable histologic alterations were observed in tamoxifen-induced Ela-CreERT2;LSL-mShh; LSL-mSmo mice, beginning as early as 2 months after induction (E) and persisting up to 1 year (F). No intraductal precursor lesions or exocrine cancers were seen in these cohorts either.
To address the possibility that the observed lack of Hh ligand responsiveness of the epithelial compartment was due to absence of the Smoothened (Smo) receptor, the entire study was repeated with acinar cell coexpression of mSmo along with mShh by generating Ela-CreERT2;LSL-mShh;LSL-mSmo compound transgenic mice. The animals were induced in the same manner as outlined above and observed for up to 12 months after tamoxifen induction. Notably, the pancreatic phenotype observed in the Ela-CreERT2;LSL-mShh;LSL-mSmo mice was identical to that in the Ela-CreERT2;LSL-mShh mice, that is, no intraductal lesions or neoplasms were identified, whereas a marked expansion of periacinar stromal cell compartment was found, as already described above (Figure 2, E–F). These results ruled out the possibility that the observed lack of Hh responsiveness of acinar cells was simply due to an overall lack of Smo expression as hypothesized originally.
Responsiveness to Ectopic Hh Ligand Is Restricted to the Stromal Compartment
To further characterize the dynamics of Hh ligand-dependent signaling in the exocrine pancreas, we assessed the localization of Shh, Smo, and the Hh gene target Ptch in tamoxifen-induced mice. Overexpression of Shh was observed in most acinar cells within the murine pancreata by immunohistochemistry (Figure 3A), whereas immunolabeling of Smo was essentially confined to adjacent stromal cells (Figure 3B). The localization of Ptch, assessed using Ela-CreERT2; LSL-mShh;Ptch-lacZ reporter mice, mirrored that of Smo protein, confirming that the stromal compartment was the principal recipient of the epithelial Hh ligand (Figure 3C). We were unable to demonstrate any convincing acinar-specific beta-galactosidase expression in the examined pancreata. In line with a mesenchymal nature of the Ptch-expressing periacinar stromal compartment, immunofluorescence demonstrated robust expression of nestin, a marker of mesenchymal cells in the adult pancreas [27], whereas the epithelial antigen E-cadherin was absent (Figure 3D).
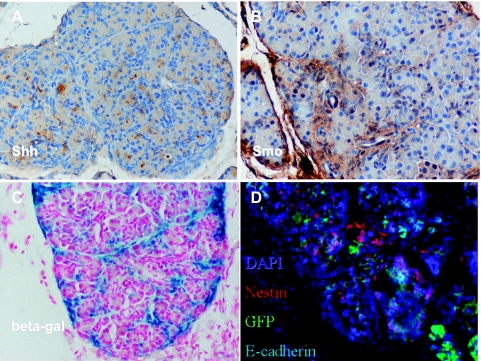
Figure 3.
Confirmation of stromal Hh pathway activity on Cre-mediated recombination in the exocrine pancreas. (A) Immunohistochemistry confirmed the expression of Shh ligand in most acinar cells in tamoxifen-induced Ela-CreERT2;LSL-mShh mice. (B) In contrast, Smo expression was essentially restricted to the expanded periacinar stromal compartment in tamoxifen-induced Ela-CreERT2; LSL-mShh mice. (C) The expanded stromal compartment had evidence of Hh activation, as confirmed by expression of beta-galactosidase (blue) in tamoxifen-induced Ela-CreERT2;LSL-mShh;Ptch-lacZ reporter mice. This pattern overlapped with that of Smo receptor expression in B. (D) The stromal population was negative for E-cadherin and expressed nestin, a feature of mesenchymal cells in the adult pancreas.
Primary Cilia Are Absent on Cells within the Acinar Compartment
Recent evidence suggests that the presence of primary cilia and recruitment of the receptor protein Smo to the cilia are prerequisites for the ability of a cell to respond to stimulation by Hh ligands, irrespective of the presence of other pathway components [28]. Therefore, the presence of primary cilia was analyzed in pancreatic cryosections using immunofluorescence for acetylated tubulin, a marker for primary cilia [29]. Acetylated tubulin expression was observed within the ductal epithelium, on pancreatic endocrine cells within islets of Langerhans, and the expanded periacinar stromal compartment, but not in acinar cells of Ela-CreERT2;LSL-mShh mice (Figure 4). The absence of primary cilia in acinar cells likely explains the lack of demonstrable cell autonomous effects from the secreted Hh ligand, including in compound transgenic Ela-CreERT2;LSL-mShh;LSL-mSmo mice.
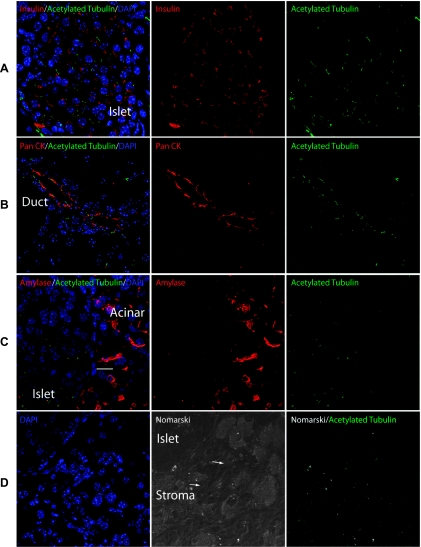
Figure 4.
The expanded stromal compartment expresses markers of primary cilia. Acetylated tubulin expression was observed in (A) islets marked by insulin and in (B) ducts marked by pan-cytokeratin (Pan CK) from Ela-CreERT2; LSL-mShh murine pancreata. In contrast, acetylated tubulin could not be detected by staining in amylase expressing acinar cells (C). (D) Stromal cells (white arrows) intermixed within exocrine structures were identified using Nomarski optics. Expression of acetylated tubulin in these cells was consistent with the presence of primary cilia within the stroma. Staining for insulin, pan CK, acetylated tubulin, and amylase as indicated. Scale bar, 10 µm.
Discussion
The identification of aberrant Hedgehog signaling in pancreatic cancer has led to significant research efforts aimed at exploiting this pathway for the development of novel therapeutic options during the past few years [5,6,30]. In fact, at present, there are several Hedgehog small-molecule inhibitors available that are undergoing initial clinical evaluation and have shown promising in vivo efficacy in various human cancers, including medulloblastoma and basal cell carcinoma [13,31–35].
However, despite this obviously encouraging progress in moving forward the translational component, there is an incomplete understanding of the mechanisms underlying the effects of aberrant Hh signaling in pancreatic cancer. Specifically, there have been conflicting reports as to the nature of cells within ductal adenocarcinomas that are competent to receive, and respond to, secreted Hh ligands [15,16]. The initial studies suggested that ligand expression and activation of the Hh pathway occurred within the “bulk” population of neoplastic epithelial cells in a cell autonomous manner [5,6]. Subsequent studies proposed a somewhat restricted subpopulation of Hh-dependent neoplastic cells within the epithelial compartment, specifically those with tumor-initiating and metastatic dissemination capacity [7,9,11,36,37].
The most recent series of studies in pancreatic adenocarcinoma suggest that the ability to respond to paracrine Hh ligand rests predominantly within stromal cells rather than the neoplastic cells themselves [38,39]. Bailey et al. [40] demonstrated that peritumoral desmoplasia, a feature most pronounced in pancreatic adenocarcinomas, is a consequence of the paracrine activity of epithelial Hh ligand on stromal cells. Furthermore, Olive et al. [10] showed that Hh inhibition using a small-molecule inhibitor led to marked stromal depletion and enhanced drug delivery in an autochthonous mouse model of pancreatic cancer. Similarly, Lauth et al. recently showed marked overexpression of the Hh target gene Gli1 in tumor-associated stromal cells, but only minimal expression in pancreatic adenocarcinoma, consistent with a high pathway activity in the former but not the latter compartment [41]. The dispensable nature of Hh signaling within the epithelial compartment is reiterated by recent studies, which confirmed that biallelic inactivation of Smo in pancreatic epithelial cells did not affect subsequent development of adenocarcinoma in an autochthonous model [42], whereas conversely, Smo inactivation in stromal cells led to delayed growth of pancreatic cancer xenografts [38].
Prior reports examining the role of aberrant Hh signaling in the epithelial compartment have largely relied on expression of a constitutively active mGli2 allele that lacks the N-terminal repressor domain (GLI2ΔN) [20]. Thus, Pdx1-Cre;CLEG2 mice conditionally overexpressing GLI2ΔN in the pancreas develop poorly differentiated cancers in the absence of concomitant mPanIN lesions. Notably, the use of a constitutively active mGli2 allele bypasses Hh ligand dependence, which is most relevant to the cognate pathophysiology in the human pancreas. Similarly, a study by Thayer et al. [43] reported on mice expressing a constitutively active form of Smoothened (R26-SmoM2) under the control of a ubiquitously expressed inducible Cre transgene (CAGGS-CreER). Interestingly, on tamoxifen induction, a high rate of novel cystic metaplastic lesions was observed in the pancreata of CAGGS-CreER;R26-SmoM2 mice, largely replacing the normal pancreatic architecture and showing histomorphologic features reminiscent of mucinous cystic neoplasms.
To the best of our knowledge, ours is the first study that examines ligand-dependent Hh signaling in the adult murine exocrine pancreas, with or without additional coexpression of an mSmo allele. We demonstrate that expression of Shh ligand in the adult exocrine pancreas is, by itself, not sufficient to cause the formation of either noninvasive intraductal mPanIN lesions or invasive adenocarcinomas. On the contrary, and not unexpectedly, our cohorts of mice demonstrate a marked expansion of a periacinar stromal cell compartment, reiterating that, in the absence of concomitant oncogene expression (e.g., Kras) or exocrine injury (i.e., pancreatitis), stromal cells remain the major Hh ligand recipients in the adult pancreas. Using Ptch-lacZ reporter mice, we observe X-Gal staining that is restricted to the expanded periacinar stromal cells, confirming their ability to respond to exogenous Hh signals.
Multiple lines of evidence have now demonstrated that the presence of primary cilia are a prerequisite for Hh signal transduction [44,45]. On binding of Hh ligand to the membrane receptor Ptch, the inhibition on Smo is released and Smo is recruited to the cilium [28,46]. Furthermore, SuFu represents a major negative regulator of Hh signal transduction in vertebrate cells [47–50], and ligand-driven activation of the Hh pathway might lead to subcellular translocation of a protein complex containing SuFu and Gli to the tip of primary cilia, where the inhibitory function of SuFu on Gli is released, so that the latter can shuttle to the nucleus and induce transcription of Hh target genes, thus exemplifying the pivotal role of primary cilia in this process [51]. Notably, we find expression of acetylated tubulin, a primary cilia marker, in islet cells, the ductal epithelium, and in the expanded periacinar stromal cells of the adult pancreas but none within acinar cells. The absence of primary cilia likely explains the inability of mature acinar cells to respond to secreted Hh ligand in a cell autonomous manner, even in the setting of enforced coexpression of the Smo receptor. A prior report by Seeley et al. [52] confirms that murine ductal epithelium and islets do harbor expression of ciliary markers, consistent with our own data. The lack of demonstrable pathology within islets or the ductal epithelium is somewhat surprising, given their apparent competence to respond to Hh signals and suggests that these compartments have other checkpoints in place, especially in the absence of additional oncogenic “driver” influences.
In conclusion, in this study, we describe a genetically engineered mouse model of Hh pathway activation through Shh ligand over-expression in acinar cells of the adult pancreas. Shh overexpression led to a striking expansion of a periacinar stromal cell compartment but did not induce formation of premalignant precursor lesions or overt pancreatic neoplasia, in line with stromal cells being the major Hh-responsive elements in the mature organ. This autochthonous model serves as a platform for studying epithelial stromal interactions in pancreatic carcinogenesis, as well as the role of cooperating genetic alterations with aberrant Hh signaling.
Supplementary Material
Acknowledgments
The authors thank Drs David Neil Watkins (Monash University, Australia) and Craig Peacock (Johns Hopkins University) for their assistance in providing LSL-mShh and LSL-mSmo breeder mice for this study.
Footnotes
G.F. was supported in part by a fellowship grant within the postdoctoral program of the German Academic Exchange Service, by the German Cancer Foundation (Deutsche Krebshilfe) grant number 109215, and by the European Community's Seventh Framework Program (FP7-2007-2013) under grant agreement HEALTH-F2-2011-256986. E.O. was supported by a Fight for Sight fellowship. N.K. was supported by the National Institutes of Health (NIH; RO1DK072301) and the Distinguished George W. Brumley Professorship. V.F. was supported by a Research Grant of the University Medical Center Giessen and Marburg. A.M. was supported by the Sol Goldman Pancreatic Cancer Research Center, the Michael Rolfe Foundation, and NIH (P01CA134292, R01CA134767, and R01CA113669). The authors have no conflicts of interest to declare.
This article refers to supplementary material, which is designated by Figure W1 and is available online at www.neoplasia.com.
References
- 1.Maitra A, Hruban RH. Pancreatic cancer. Annu Rev Pathol. 2008;3:157–188. doi: 10.1146/annurev.pathmechdis.3.121806.154305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 3.Carpelan-Holmstrom M, Nordling S, Pukkala E, Sankila R, Luttges J, Kloppel G, Haglund C. Does anyone survive pancreatic ductal adenocarcinoma? A nationwide study re-evaluating the data of the Finnish Cancer Registry. Gut. 2005;54:385–387. doi: 10.1136/gut.2004.047191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fujita H, Ohuchida K, Mizumoto K, Itaba S, Ito T, Nakata K, Yu J, Kayashima T, Souzaki R, Tajiri T, et al. Gene expression levels as predictive markers of outcome in pancreatic cancer after gemcitabine-based adjuvant chemotherapy. Neoplasia. 2010;12:807–817. doi: 10.1593/neo.10458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berman DM, Karhadkar SS, Maitra A, Montes De Oca R, Gerstenblith MR, Briggs K, Parker AR, Shimada Y, Eshleman JR, Watkins DN, et al. Widespread requirement for Hedgehog ligand stimulation in growth of digestive tract tumours. Nature. 2003;425:846–851. doi: 10.1038/nature01972. [DOI] [PubMed] [Google Scholar]
- 6.Thayer SP, di Magliano MP, Heiser PW, Nielsen CM, Roberts DJ, Castillo CF, Yajnik V, Antoniu B, McMahon M, Warshaw AL, et al. Hedgehog is an early and late mediator of pancreatic cancer tumorigenesis. Nature. 2003;425:851–856. doi: 10.1038/nature02009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feldmann G, Dhara S, Fendrich V, Bedja D, Beaty R, Mullendore M, Karikari C, Alvarez H, Iacobuzio-Donahue C, Jimeno A, et al. Blockade of Hedgehog signaling inhibits pancreatic cancer invasion and metastases: a new paradigm for combination therapy in solid cancers. Cancer Res. 2007;67:2187–2196. doi: 10.1158/0008-5472.CAN-06-3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feldmann G, Habbe N, Dhara S, Bisht S, Alvarez H, Fendrich V, Beaty R, Mullendore M, Karikari C, Bardeesy N, et al. Hedgehog inhibition prolongs survival in a genetically engineered mouse model of pancreatic cancer. Gut. 2008;57:1420–1430. doi: 10.1136/gut.2007.148189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feldmann G, Fendrich V, McGovern K, Bedja D, Bisht S, Alvarez H, Koorstra JB, Habbe N, Karikari C, Mullendore M, et al. An orally bioavailable small-molecule inhibitor of Hedgehog signaling inhibits tumor initiation and metastasis in pancreatic cancer. Mol Cancer Ther. 2008;7:2725–2735. doi: 10.1158/1535-7163.MCT-08-0573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, Madhu B, Goldgraben MA, Caldwell ME, Allard D, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457–1461. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jimeno A, Feldmann G, Suarez-Gauthier A, Rasheed Z, Solomon A, Zou GM, Rubio-Viqueira B, Garcia-Garcia E, Lopez-Rios F, Matsui W, et al. A direct pancreatic cancer xenograft model as a platform for cancer stem cell therapeutic development. Mol Cancer Ther. 2009;8:310–314. doi: 10.1158/1535-7163.MCT-08-0924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dai J, Ai K, Du Y, Chen G. Sonic Hedgehog expression correlates with distant metastasis in pancreatic adenocarcinoma. Pancreas. 2011;40:233–236. doi: 10.1097/MPA.0b013e3181f7e09f. [DOI] [PubMed] [Google Scholar]
- 13.Lorusso PM, Rudin CM, Reddy JC, Tibes R, Weiss GJ, Borad MJ, Hann CL, Brahmer JR, Chang I, Darbonne WC, et al. Phase I trial of Hedgehog pathway inhibitor GDC-0449 in patients with refractory, locally-advanced or metastatic solid tumors. Clin Cancer Res. 2011;17:2502–2511. doi: 10.1158/1078-0432.CCR-10-2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones S, Zhang X, Parsons DW, Lin JC, Leary RJ, Angenendt P, Mankoo P, Carter H, Kamiyama H, Jimeno A, et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008;321:1801–1806. doi: 10.1126/science.1164368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hidalgo M, Maitra A. The Hedgehog pathway and pancreatic cancer. N Engl J Med. 2009;361:2094–2096. doi: 10.1056/NEJMcibr0905857. [DOI] [PubMed] [Google Scholar]
- 16.Maitra A. Tracking down the Hedgehog's lair in the pancreas. Gastroenterology. 2010;138:823–825. doi: 10.1053/j.gastro.2010.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hingorani SR, Wang L, Multani AS, Combs C, Deramaudt TB, Hruban RH, Rustgi AK, Chang S, Tuveson DA. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell. 2005;7:469–483. doi: 10.1016/j.ccr.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 18.Hruban RH, Rustgi AK, Brentnall TA, Tempero MA, Wright CV, Tuveson DA. Pancreatic cancer in mice and man: the Penn Workshop 2004. Cancer Res. 2006;66:14–17. doi: 10.1158/0008-5472.CAN-05-3914. [DOI] [PubMed] [Google Scholar]
- 19.Fendrich V, Schneider R, Maitra A, Jacobsen ID, Opfermann T, Bartsch DK. Detection of precursor lesions of pancreatic adenocarcinoma in PET-CT in a genetically engineered mouse model of pancreatic cancer. Neoplasia. 2011;13:180–186. doi: 10.1593/neo.10956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pasca di Magliano M, Sekine S, Ermilov A, Ferris J, Dlugosz AA, Hebrok M. Hedgehog/Ras interactions regulate early stages of pancreatic cancer. Genes Dev. 2006;20:3161–3173. doi: 10.1101/gad.1470806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang DH, Clemons NJ, Miyashita T, Dupuy AJ, Zhang W, Szczepny A, Corcoran-Schwartz IM, Wilburn DL, Montgomery EA, Wang JS, et al. Aberrant epithelial-mesenchymal Hedgehog signaling characterizes Barrett's metaplasia. Gastroenterology. 2010;138:1810–1822. doi: 10.1053/j.gastro.2010.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hingorani SR, Petricoin EF, Maitra A, Rajapakse V, King C, Jacobetz MA, Ross S, Conrads TP, Veenstra TD, Hitt BA, et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell. 2003;4:437–450. doi: 10.1016/s1535-6108(03)00309-x. [DOI] [PubMed] [Google Scholar]
- 23.Desai BM, Oliver-Krasinski J, De Leon DD, Farzad C, Hong N, Leach SD, Stoffers DA. Preexisting pancreatic acinar cells contribute to acinar cell, but not islet beta cell, regeneration. J Clin Invest. 2007;117:971–977. doi: 10.1172/JCI29988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fendrich V, Esni F, Garay MV, Feldmann G, Habbe N, Jensen JN, Dor Y, Stoffers D, Jensen J, Leach SD, et al. Hedgehog signaling is required for effective regeneration of exocrine pancreas. Gastroenterology. 2008;135:621–631. doi: 10.1053/j.gastro.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goodrich LV, Milenkovic L, Higgins KM, Scott MP. Altered neural cell fates and medulloblastoma in mouse patched mutants. Science. 1997;277:1109–1113. doi: 10.1126/science.277.5329.1109. [DOI] [PubMed] [Google Scholar]
- 26.Fendrich V, Waldmann J, Esni F, Ramaswamy A, Mullendore M, Buchholz M, Maitra A, Feldmann G. Snail and Sonic Hedgehog activation in neuroendocrine tumors of the ileum. Endocr Relat Cancer. 2007;14:865–874. doi: 10.1677/ERC-07-0108. [DOI] [PubMed] [Google Scholar]
- 27.Esni F, Stoffers DA, Takeuchi T, Leach SD. Origin of exocrine pancreatic cells from nestin-positive precursors in developing mouse pancreas. Mech Dev. 2004;121:15–25. doi: 10.1016/j.mod.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 28.Rohatgi R, Milenkovic L, Scott MP. Patched1 regulates Hedgehog signaling at the primary cilium. Science. 2007;317:372–376. doi: 10.1126/science.1139740. [DOI] [PubMed] [Google Scholar]
- 29.Chen Y, Yue S, Xie L, Pu XH, Jin T, Cheng SY. Dual phosphorylation of suppressor of fused (Sufu) by PKA and GSK3beta regulates its stability and localization in the primary cilium. J Biol Chem. 2011;286:13502–13511. doi: 10.1074/jbc.M110.217604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Y, Laterra J, Pomper MG. Hedgehog pathway inhibitor HhAntag691 is a potent inhibitor of ABCG2/BCRP and ABCB1/Pgp. Neoplasia. 2009;11:96–101. doi: 10.1593/neo.81264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goppner D, Leverkus M. Basal cell carcinoma: from the molecular understanding of the pathogenesis to targeted therapy of progressive disease. J Skin Cancer. 2011;2011:650258. doi: 10.1155/2011/650258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dijkgraaf GJ, Alicke B, Weinmann L, Januario T, West K, Modrusan Z, Burdick D, Goldsmith R, Robarge K, Sutherlin D, et al. Small molecule inhibition of GDC-0449 refractory Smoothened mutants and downstream mechanisms of drug resistance. Cancer Res. 2011;71:435–444. doi: 10.1158/0008-5472.CAN-10-2876. [DOI] [PubMed] [Google Scholar]
- 33.Yauch RL, Dijkgraaf GJ, Alicke B, Januario T, Ahn CP, Holcomb T, Pujara K, Stinson J, Callahan CA, Tang T, et al. Smoothened mutation confers resistance to a Hedgehog pathway inhibitor in medulloblastoma. Science. 2009;326:572–574. doi: 10.1126/science.1179386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rudin CM, Hann CL, Laterra J, Yauch RL, Callahan CA, Fu L, Holcomb T, Stinson J, Gould SE, Coleman B, et al. Treatment of medulloblastoma with Hedgehog pathway inhibitor GDC-0449. N Engl J Med. 2009;361:1173–1178. doi: 10.1056/NEJMoa0902903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Von Hoff DD, LoRusso PM, Rudin CM, Reddy JC, Yauch RL, Tibes R, Weiss GJ, Borad MJ, Hann CL, Brahmer JR, et al. Inhibition of the Hedgehog pathway in advanced basal-cell carcinoma. N Engl J Med. 2009;361:1164–1172. doi: 10.1056/NEJMoa0905360. [DOI] [PubMed] [Google Scholar]
- 36.Rasheed ZA, Yang J, Wang Q, Kowalski J, Freed I, Murter C, Hong SM, Koorstra JB, Rajeshkumar NV, He X, et al. Prognostic significance of tumorigenic cells with mesenchymal features in pancreatic adenocarcinoma. J Natl Cancer Inst. 2010;102:340–351. doi: 10.1093/jnci/djp535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, Wicha M, Clarke MF, Simeone DM. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030–1037. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- 38.Yauch RL, Gould SE, Scales SJ, Tang T, Tian H, Ahn CP, Marshall D, Fu L, Januario T, Kallop D, et al. A paracrine requirement for Hedgehog signalling in cancer. Nature. 2008;455:406–410. doi: 10.1038/nature07275. [DOI] [PubMed] [Google Scholar]
- 39.Bailey JM, Mohr AM, Hollingsworth MA. Sonic Hedgehog paracrine signaling regulates metastasis and lymphangiogenesis in pancreatic cancer. Oncogene. 2009;28:3513–3525. doi: 10.1038/onc.2009.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bailey JM, Swanson BJ, Hamada T, Eggers JP, Singh PK, Caffery T, Ouellette MM, Hollingsworth MA. Sonic Hedgehog promotes desmoplasia in pancreatic cancer. Clin Cancer Res. 2008;14:5995–6004. doi: 10.1158/1078-0432.CCR-08-0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lauth M, Bergstrom A, Shimokawa T, Tostar U, Jin Q, Fendrich V, Guerra C, Barbacid M, Toftgard R. DYRK1B-dependent autocrine-to-paracrine shift of Hedgehog signaling by mutant RAS. Nat Struct Mol Biol. 2010;17:718–725. doi: 10.1038/nsmb.1833. [DOI] [PubMed] [Google Scholar]
- 42.Nolan-Stevaux O, Lau J, Truitt ML, Chu GC, Hebrok M, Fernandez-Zapico ME, Hanahan D. GLI1 is regulated through Smoothened-independent mechanisms in neoplastic pancreatic ducts and mediates PDAC cell survival and transformation. Genes Dev. 2009;23:24–36. doi: 10.1101/gad.1753809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mao J, Ligon KL, Rakhlin EY, Thayer SP, Bronson RT, Rowitch D, McMahon AP. A novel somatic mouse model to survey tumorigenic potential applied to the Hedgehog pathway. Cancer Res. 2006;66:10171–10178. doi: 10.1158/0008-5472.CAN-06-0657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huangfu D, Anderson KV. Cilia and Hedgehog responsiveness in the mouse. Proc Natl Acad Sci USA. 2005;102:11325–11330. doi: 10.1073/pnas.0505328102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Haycraft CJ, Banizs B, Aydin-Son Y, Zhang Q, Michaud EJ, Yoder BK. Gli2 and Gli3 localize to cilia and require the intraflagellar transport protein polaris for processing and function. PLoS Genet. 2005;1:e53. doi: 10.1371/journal.pgen.0010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Corbit KC, Aanstad P, Singla V, Norman AR, Stainier DY, Reiter JF. Vertebrate Smoothened functions at the primary cilium. Nature. 2005;437:1018–1021. doi: 10.1038/nature04117. [DOI] [PubMed] [Google Scholar]
- 47.Chen MH, Wilson CW, Li YJ, Law KK, Lu CS, Gacayan R, Zhang X, Hui CC, Chuang PT. Cilium-independent regulation of Gli protein function by Sufu in Hedgehog signaling is evolutionarily conserved. Genes Dev. 2009;23:1910–1928. doi: 10.1101/gad.1794109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Svard J, Heby-Henricson K, Persson-Lek M, Rozell B, Lauth M, Bergstrom A, Ericson J, Toftgard R, Teglund S. Genetic elimination of suppressor of fused reveals an essential repressor function in the mammalian Hedgehog signaling pathway. Dev Cell. 2006;10:187–197. doi: 10.1016/j.devcel.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 49.Cooper AF, Yu KP, Brueckner M, Brailey LL, Johnson L, McGrath JM, Bale AE. Cardiac and CNS defects in a mouse with targeted disruption of suppressor of fused. Development. 2005;132:4407–4417. doi: 10.1242/dev.02021. [DOI] [PubMed] [Google Scholar]
- 50.Humke EW, Dorn KV, Milenkovic L, Scott MP, Rohatgi R. The output of Hedgehog signaling is controlled by the dynamic association between suppressor of fused and the Gli proteins. Genes Dev. 2010;24:670–682. doi: 10.1101/gad.1902910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tukachinsky H, Lopez LV, Salic A. A mechanism for vertebrate Hedgehog signaling: recruitment to cilia and dissociation of SuFu-Gli protein complexes. J Cell Biol. 2010;191:415–428. doi: 10.1083/jcb.201004108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Seeley ES, Carriere C, Goetze T, Longnecker DS, Korc M. Pancreatic cancer and precursor pancreatic intraepithelial neoplasia lesions are devoid of primary cilia. Cancer Res. 2009;69:422–430. doi: 10.1158/0008-5472.CAN-08-1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.