Abstract
Solid-state nuclear magnetic resonance relaxation experiments were used to study the rigidity and spatial proximity of polymers in sugar beet (Beta vulgaris) cell walls. Proton T1ρ decay and cross-polarization patterns were consistent with the presence of rigid, crystalline cellulose microfibrils with a diameter of approximately 3 nm, mobile pectic galacturonans, and highly mobile arabinans. A direct-polarization, magic-angle-spinning spectrum recorded under conditions adapted to mobile polymers showed only the arabinans, which had a conformation similar to that of beet arabinans in solution. These cell walls contained very small amounts of hemicellulosic polymers such as xyloglucan, xylan, and mannan, and no arabinan or galacturonan fraction closely associated with cellulose microfibrils, as would be expected of hemicelluloses. Cellulose microfibrils in the beet cell walls were stable in the absence of any polysaccharide coating.
The term “hemicellulose” has a long history (Schulze, 1891), and its survival into current usage is perhaps surprising. The polysaccharides that it describes—xylans, xyloglucans, and glucomannans—have no common primary structure, but because they share the ability to adopt chain conformations similar to cellulose, they are all able to bind onto cellulose fibrils in a manner similar to the interchain bonding of cellulose itself. This has led to the supposition that they have common functions within the architecture of the plant cell wall, coating the microfibrils and cross-linking them, preventing their coalescence, or both (Hayashi, 1989; Carpita and Gibeaut, 1993).
To our knowledge, experimental evidence for these functions has thus far been restricted to xyloglucans. The cell walls of pea epicotyls contain at least enough xyloglucan to give a complete coating on the surface of the 3-nm microfibrils (Hayashi et al., 1993). High-resolution transmission electron microscopic images of onion and carrot cell walls revealed cross-links between microfibrils; these cross-links disappeared and the microfibrils aggregated when xyloglucans were removed with alkali (McCann et al., 1990). Extrusion of Acetobacter xylinum cellulose into xyloglucan solution gave a cross-linked network of fine microfibrils (Atalla et al., 1993). What would happen to the structure of a plant cell wall if there were not enough xyloglucan to coat the microfibril surface? Could other hemicelluloses or polysaccharides not normally classified as hemicelluloses fulfill the same functions and allow a normal cell wall to be formed? We have examined sugar beet (Beta vulgaris) cell walls, a good example of such a system, and discuss their functional architecture.
The swollen roots of sugar beets contain reserve parenchyma, but their cell walls differ in a number of key points from the primary cell walls of other dicots. Their pectins are substituted by ferulic acid on the arabinogalactan side chains, which seems to be a characteristic of the Chenopodiaceae, and by acetyl groups on the galacturonate backbone (Rombouts and Thibault, 1986a, 1986b). The cell walls appear to be almost devoid of xyloglucans, mannans, or xylans (Renard and Thibault, 1993). In spite of these differences, beet cell walls perform the same functions as cell walls whose cellulose fibrils are coated by xyloglucans. The question is, are there any other molecules coating the cellulose in beet cell walls?
Solid state 13C-NMR with CP and MAS is particularly useful for the investigation of mobility and domain size in polymer systems. The rate of relaxation (dissipation) of proton magnetization, which can be measured through the 13C spectrum by CP, is controlled by thermal motion and can therefore be used to probe the mobility of individual polymers within a complex structure (Schaefer et al., 1977; Kenwright and Say, 1993; Stejskal and Memory, 1994). In cell walls the proton-rotating, frame-spin-lattice relaxation time (1H-T1ρ) is the most suitable indicator of mobility for rigid components such as cellulose, and the spin-spin relaxation time (1H-T2) is appropriate for more mobile polymers (Tekely and Vignon 1987; Newman et al., 1994). The 1H-T1ρ decreases and the 1H-T2 increases with increasing molecular motion in materials of this type. Extremely mobile, hydrated polymers can also be identified, because the rate of CP from 1H to 13C is related to the 1H-T2 and becomes slow at very high levels of mobility (Ha et al., 1996). Proton T1ρ relaxation and CP occur simultaneously when the CP contact time is varied, but can be separated experimentally by inserting a variable delay with proton-spin locking before a constant CP contact time.
If two solid components are close together, proton magnetization within them is averaged by spin diffusion. Since the rate of proton spin diffusion is a constant (dependent inversely on the 1H-T2), the time required for this spatial averaging is a measure of the distance between the two components (Newman, 1992). This principle can be used to derive spatial information from NMR-relaxation experiments. Averaging of the expected 1H-T1ρ values of two polymer components known to differ in mobility, e.g. cellulose and hemicellulose, implies that they are at most 2 to 3 nm apart, or somewhat less if both are relatively mobile (Ha et al., 1998). Shorter spatial separations of approximately 1 nm or less are implied between components whose magnetization is averaged during the contact time of a 1H-T2 experiment, or those that show similar CP kinetics because the proton pools contributing to their CP have merged.
In hydrated onion (Ha et al., 1996, 1997) and citrus cell walls (Jarvis et al., 1996), the microfibril components were characteristically immobile, whereas the matrix polysaccharides were of greater mobility. The most mobile components were pectins, especially methylated segments of the backbone and, in onions, galactan side chains (Foster et al., 1996; Ha et al., 1996). The majority of the onion xyloglucans, identified as coating the cellulose in the microfibrils, had 1H-T1ρ and 1H-T2 relaxation rates similar to those of cellulose (Ha et al., 1997), indicating a close spatial association. A minor xyloglucan component showed considerably faster 1H-T1ρ and slower 1H-T2 relaxation rates than cellulose, and was identified with cross-linking segments of the xyloglucan chains (Ha et al., 1997).
Our aim was to identify any polymer in beet cell walls that was closely associated with cellulose in the manner expected of hemicelluloses. We have used both 1H-T1ρ relaxation and CP kinetics from protons to 13C nuclei and DP-MAS 13C experiments for the highly mobile component.
MATERIALS AND METHODS
Plants
Sugar beet (Beta vulgaris cv Saxon) plants were grown from seed in Hoagland solution for 5 months using a 14-h light cycle at 23°C. Swollen roots were collected and cleaned. The swollen part of the roots was peeled, cut into small pieces, and used immediately for cell wall preparation.
Cell Walls
Beet cell walls were isolated by the phenol-buffer method; a buffer simulating the ionic conditions in the apoplast (10 mm NaOAc, 3 mm KCl, 2 mm MgCl2, and 1 mm CaCl2) was used throughout the procedure (Jarvis, 1990). Fresh tissue (approximately 100 g) was suspended in chilled buffer (500 mL) plus Triton X-100 (2 g/L) and octanol (4 mL), and blended for six successive bursts of 15 s in a blender (Waring). The detergent was then washed out with chilled buffer through a 53-μm sieve. Washings were collected and assayed for total sugars by the phenol-sulfuric acid method (Dubois et al., 1956). The cell walls were then partially dried on G3-sintered glass and suspended in five times their weight of phenol for 1 h, and the saturated phenol solution was removed by washing with buffer on G3-sintered glass. The sample was finally solvent exchanged sequentially in 50%, 75%, and 100% acetone, and air dried. The first two 0.5-L aliquots of the washings were concentrated by rotary evaporation, filtered on nylon cloth (mesh size 0.1 mm), and precipitated by pouring into 3 volumes of cold 96% ethanol. The precipitate was collected by centrifugation at 2500 rpm for 10 min and redissolved in distilled water.
Cellulose was isolated from de-esterified beet pulp after controlled-acid hydrolysis (0.1 m HCl for 72 h at 80°C; Renard et al., 1998), followed by neutralization to and extensive washing at pH 7, and was dried by solvent exchange (ethanol followed by acetone). Beet arabinan was from British Sugar (Norwich, UK).
Analysis
Dry matter was determined after drying overnight at 80°C. Individual sugars were liberated by prehydrolysis in 13 mol/L H2SO4 (1 h at room temperature) followed by hydrolysis with 1 mol/L H2SO4 (3 h at 100°C). Cellulose was measured as the difference in Glc content with and without prehydrolysis. Monomeric sugars were reduced, acetylated, and analyzed by GLC according to the method of Englyst and Cummings (1984). Uronic acids were measured by the m-phenylphenol method (Blumenkrantz and Asboe-Hansen, 1973). After saponification (2 h in 0.5 mol/L KOH at room temperature), methanol was determined by an enzymatic oxidation method (Klavons and Bennet, 1986), acetyl groups were determined by HPLC on an HPX-87H column (Bio-Rad) eluted with 5 mmol/L H2SO4 at 0.6 mL/min (Voragen et al., 1986), and ferulic acid was determined by its UV absorption at 375 nm (Micard et al., 1994).
NMR
MAS spectra were collected on a spectrometer (Unity Plus 300, Varian Instruments, Sugarland, TX) operating at 75.34 MHz for 13C, with MAS rates of 2340 Hz (CP) or 2500 Hz (DP). Cell walls were hydrated to a 1.0:0.5 ratio of cell wall to water, and no loss of water occurred during spectral acquisition. A 1H spin-locking field of 60 kHz was applied throughout, but since the effective 1H field within the sample is reduced by water, the Hartman-Hahn condition was optimized by adjusting the 13C spin-locking field to maximize the total signal. Seven CP-MAS spectra were collected: five with CP contact times of 0.05, 0.2, 1.0, 3.0, and 15 ms, and two with a contact time of 1 ms after a 2- or 14-ms delay with proton-spin locking for 1H-T1ρ relaxation. A DP-MAS 13C spectrum was acquired using a limited recycle time of 100 ms, so that rigid components with 13C-T1 significantly greater than 100 ms were not observed (Foster et al., 1996). The 90° 13C pulse length was 12 μs. The proton-decoupling method differed from that of Foster et al. (1996). The Waltz-16 multiple-pulse decoupling sequence with a field of approximately 10 kHz was used and was gated off during the recycle period. For solution-state NMR, arabinan (approximately 25 mg) was 2H exchanged twice in 99.9% 2H2O before solubilization in 0.5 mL of 100% 2H2O. The 13C-NMR spectrum was recorded on a spectrometer (model ARX400, Bruker Analytik GmBH, Rheinstetten, Germany) at 298 K.
RESULTS
Chemical Characterization
The cell wall preparation represented 35 g/kg fresh beet tissue (190 g/kg dry matter). The cell walls were rich in Glc, GalUA, and Ara (Table I). Sugars characteristic of pectins (GalUA, Ara, rhamnose, and Gal) constituted approximately 430 mg/g of the cell walls. The degree of methylation was low (60). The high degree of acetylation (69) and the presence of ferulic acid are characteristic of beet cell walls. Very little pectin was extracted during the washing steps (approximately 3 mg/100 g fresh tissue), probably because of the presence of calcium in the washing buffer.
Table I.
Chemical characterization of the sugar beet cell wall and cellulose preparations
| Component | Cell Wall | Depectinated Cell Walls |
|---|---|---|
| mg g−1 dry matter | ||
| Rhamnose | 16 ± 1.1 | – |
| Fuc | 2 ± 0.2 | – |
| Ara | 165 ± 11.1 | 2 ± 0.2 |
| Xyl | 14 ± 1.6 | 31 ± 1 |
| Man | 14 ± 1.3 | 38 ± 3 |
| Gal | 55 ± 1.6 | trace |
| Glc (cellulose) | 266 ± 6.9 (254) | 837 ± 13 (816) |
| GalUA | 197 ± 11.0 | 59 ± 2 |
| Methanol (degree of methylation) | 10 ± 0.3 (60) | nda |
| AcOH (degree of acetylation) | 33 ± 1.9 (69) | nd |
| Ferulic acid | 20 ± 0.4 | nd |
| Calcium | 6 | nd |
nd, Not determined.
This cell wall preparation was deficient in sugars (Xyl, Man, and Fuc) that commonly indicate the presence of hemicelluloses. Only about 12 mg/g Glc could be liberated from the cell walls without prehydrolysis (noncellulosic Glc), a further indication of very low xyloglucan content. In total, the Xyl, Fuc, Man, and noncellulosic Glc accounted for approximately 42 mg/g, whereas there was approximately 250 mg/g cellulose.
After controlled-acid hydrolysis (Renard et al., 1998), repeated washings of the beet cell walls at pH 7 removed most of the homogalacturonans. However, at that point the solid residue was very fine and gave stable suspensions. It became very difficult to isolate, and no corresponding mass balance could be calculated. This depectinated residue contained more than 80% cellulose, and was enriched in Xyl and Man, with residual GalUA (Table I). Man, Xyl, and noncellulosic Glc accounted for only 90 mg/g.
NMR Spectroscopy
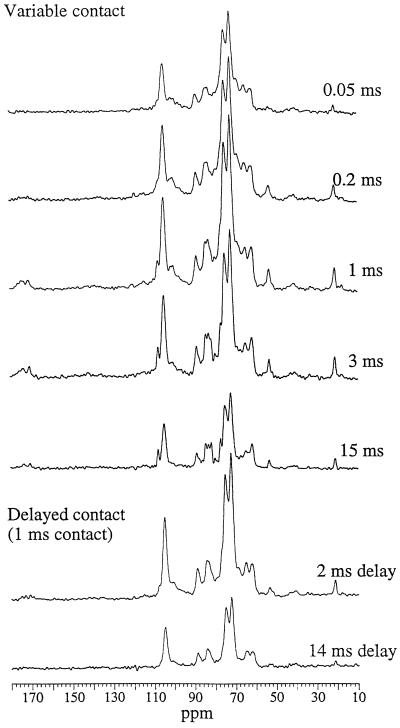
The CP-MAS spectra of sugar beet cell walls were recorded with varying contact times and with delays of 2 or 14 ms before a contact time of 1 ms (Fig. 1). Maximum intensity was observed with the 1-ms contact time. In all spectra, even at long contact times, the cellulose signals were clearly dominant: slow CP and relatively fast T1ρ decay lead to under-representation of the most mobile components, which can be a limitation in 13C CP-MAS studies of hydrated polysaccharide systems. The main peaks were the superimposed C-2, C-3, and C-5 signals at 72 to 75 ppm. Other prominent features were the cellulose C-1 (105 ppm), C-4 (89 and 85 ppm), and C-6 (65 and 62 ppm) and the pectin C-6 (at 172 and 175 ppm), C-1 (wide peak at approximately 100 ppm), and methoxy carbon at 54 ppm. The peak at 21 ppm (CH3 of acetyl groups) was unusually intense for primary cell walls, reflecting the high degree of acetylation found in beet cell walls. No signal characteristic of ferulic acid could be identified.
Figure 1.
13C CP-MAS NMR spectra of sugar beet cell walls at 1.0:0.5 ratio of cell wall to water. These spectra are not normalized and show the observed intensities in the varying experiments.
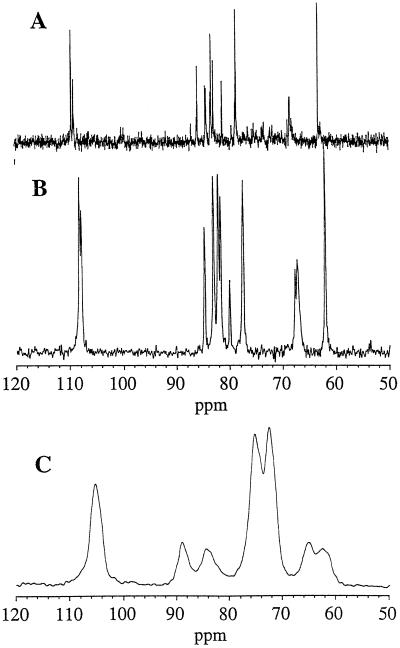
The beet spectrum showed one characteristic peak at 108 ppm that was not observed in onion or citrus cell walls, which was tentatively identified as the C-1 signal of sugar beet arabinans. To confirm this identification, a short-recycle, 13C DP-MAS spectrum was recorded (Fig. 2B). This spectrum showed substantial motional line narrowing and chemical shifts close to those observed for beet arabinan in aqueous solution (Fig. 2A). The CP-MAS spectrum of the depectinated residue (Fig. 2C) showed only the cellulose signals: C-1 (105 ppm), C-4 (89 and 85 ppm), and C-6 (65 and 62 ppm).
Figure 2.
Spectra of beet cell wall components. A, 13C-solution NMR spectrum of sugar beet arabinan. B, 13C DP-MAS NMR spectrum of sugar beet cell walls. C, 13C CP-MAS NMR of depectinated cell walls with 1-ms contact time.
T1ρ Decay
The delayed-contact experiments gave spectra derived essentially from cellulose, with a small contribution from xyloglucan at 82.5 ppm (i.e. strongly bound to cellulose; Ha et al., 1997). This was particularly evident after a 14-ms delay (i.e. a 15-ms T1ρ decay) to allow the signals from mobile components to decay. Signals associated with pectic polysaccharides in particular were absent from this spectrum.
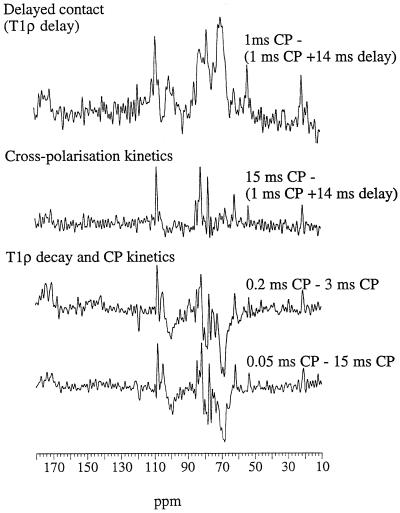
Subtraction of these delayed-contact spectra from the 1-ms spectrum (same CP time but no T1ρ delay) after normalization to give equal intensity in the cellulose C-4 region (84–91 ppm) gave subspectra (Fig. 3) corresponding to components of high and intermediate mobility. Approximate T1ρ was calculated on resolved peaks assuming a monocomponent exponential decay during the 14-ms T1ρ delay period (Table II); although it is an oversimplification, this gives the relative decay rate to a first approximation. The signals displayed in the mobile subspectra correspond to components with T1ρ < 10 ms, compared with 12 to 17 ms for the cellulose signals. These subspectra corresponded to pectic galacturonans (carboxy carbons at approximately 175–172 ppm, wide C-1 peak at 101–99 ppm, C-4 at approximately 80 ppm, and C-2, C-3, and C-5 at approximately 69 ppm) with both methoxy (54 ppm) and acetyl signals (21 ppm), plus some sharp signals at 108 (arabinan C-1), 77, and 62 ppm. The approximate T1ρ calculated for the depectinated cell wall residue was 16 to 19 ms for all signals; spectra acquired with different delays differed only in their overall intensity, and no subspectra could be extracted.
Figure 3.
Difference subspectra corresponding to the mobile elements and derived from rapid T1ρ decay (top spectrum), slow CP (middle spectrum), or both (bottom two spectra). Each point in the subspectrum corresponds to the difference in signal intensity (after normalization of the cellulose C-4 signals) between the corresponding points in the respective spectra.
Table II.
13C resonance assignments and approximate T1ρ (from delayed contact experiments) for hydrated sugar beet cell walls
| Assignment | Resonance | T1ρ |
|---|---|---|
| ppm | ms | |
| Acetyl carboxy | 175 | – |
| Galacturonan carboxy | 172 | – |
| Arabinan C-1 | 108 | 8 |
| Cellulose and galactan C-1 | 105 | 17 |
| Galacturonan and other C-1 | 101 to 99 | 7 |
| Crystalline cellulose C-4 | 89 | 17 |
| Crystal surface cellulose C-4 | 84 | 12 |
| Galacturonan C-4 | 80 | 9 |
| General carbohydrate C-2, C-3, C-5 | 75 to 72 | 16 to 14 |
| Galacturonan C-2, C-3 | 69 | 7 |
| Crystalline cellulose C-6 | 65 | 12 |
| Crystal surface cellulose, galactan C-6, and arabinan C-5 | 62 | 11 |
| Methoxy CH3 | 53.5 | 5 |
| Acetyl CH3 | 21 | 9 |
| Rhamnose CH3 | 18 | 5 |
CP Kinetics
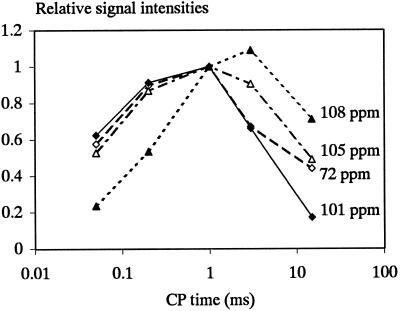
The total intensity of the 13C spectra varied with the contact time, as did the relative response of the different signals. Signals assigned to cellulose, galacturonans, and arabinans showed widely differing patterns of increase and subsequent decrease in intensity with increasing contact time (Figs. 1 and 4), and their CP kinetics and proton T1ρ values were clearly different. The spectrum obtained for 0.05-ms contact time, i.e. for the rapidly cross-polarizing, least-mobile components, showed mainly signals from cellulose. The pectic C-6 and methyl groups (172–175 and 54 ppm, respectively) were absent, as was the signal at 108 ppm. Compared with onion cell walls, beet cell walls appear to be depleted in relatively rigid pectic components, which may be an effect of acetylation. At the other end of the CP time scale, after a 15-ms contact time the signal at 108 ppm was enhanced and distinct peaks could be distinguished at 82 and 77 ppm, in good agreement with peaks recorded for beet arabinan. A subspectrum of the highly mobile components (Fig. 3) was obtained by subtracting a long-contact from a delayed-contact spectrum (normalizing of the cellulose C-4 signals throughout). This “mobile” subspectrum was dominated by signals from the arabinan components, giving a spectrum similar to the DP-MAS 13C spectrum (Table III). Signals for pectic methoxy and acetyl groups also appeared in this spectrum due to the slow CP kinetics of methyl carbons.
Figure 4.
Variation in the intensity of arabinan C-1 (108 ppm), cellulose C-1 (105 ppm), galacturonan C-1 (100 ppm), and general carbohydrate signal (72 ppm) as a function of the CP contact time relative to their intensity after 1 ms of CP.
Table III.
13C resonances and assignments for arabinan signals in sugar beet cell walls
| Assignment | Valueain solution | DP-MAS Spectrum | Highly Mobile Component |
|---|---|---|---|
| ppm | |||
| C-1 (terminal) | 109.9 | 108.2 | |
| C-1 (5-linked) | 108.8 | 107.9 | 108 |
| C-1 (3,5-linked) | 107.6 | ||
| C-4 (terminal) | 84.1 | 84.7 | 84.5 |
| C-4 (5- and 3,5-linked) | 82.8 | 83.1 | 83.5 (shoulder) |
| C-2 (terminal) | 81.4 | 81.8 | |
| C-2 (5- and 3,5-linked) | 81.8 | 82.2 | 82 |
| C-3 (terminal) | 77.7 | ||
| C-3 (5-linked) | 77.4 | 77.5 | 77 |
| C-3 (3,5-linked) | 79.8 | 80.1 | |
| C-5 (terminal) | 61.8 | 62.1 | 62 |
| C-5 (5- and 3,5-linked) | 67.5 | 68.0/67.3 | |
Vertical lines indicate that the resonances merged in a single peak.
Previous data are from Saulnier et al. (1988).
An alternative approach for obtaining spectral information on components of varying mobility is to compare spectra at different contact times. Using this approach the effects of CP kinetics and T1ρ delays are superimposed. Difference spectra for (long-short) contact times are shown in Figure 3. They all contain positive peaks assignable to arabinans and pectic methoxy and acetyl groups, which are linked to their slow CP behavior. They also show negative peaks assigned to GalUA because, on average, their signal intensities are more influenced by rapid T1ρ decay.
Crystallite Size within Cellulose Microfibrils
The proportion of cellulose chains inside the crystalline units can be estimated from the C-4 signals for the crystal-interior (89 ppm) and crystal-surface (84 ppm) chains, as described by Newman et al. (1994). The percentage of the internal cellulose C-4 signal (89 ppm) to the total cellulose C-4 signals (84–91 ppm) was calculated for spectra and rigid subspectra obtained from the proton spin-relaxation experiment. It was about 42% in the beet cell wall and 48% in the depectinated residue, indicating only a slight increase in mean crystallite size.
DISCUSSION
Although the plants used in the present study were young and had just begun to form thickened roots, the yield and composition of their cell walls were very similar to those found for mature beet (Renard and Thibault, 1993), with the characteristic high Ara content, the high degree of acetylation of the pectins, and the presence of ferulic acid (Thibault et al., 1994). The final beet cell walls contained enough calcium to neutralize about two-thirds of the free carboxyl groups of pectins. A procedure developed for pectin fractionation (Renard et al., 1998) made it possible to obtain a residue containing more than 80% of cellulose while avoiding concentrated alkali extractions that might lead to mercerization. A controlled-acid hydrolysis first solubilized the “hairy regions” of pectins as oligomers, leaving a solid consisting essentially of Glc and GalUA (Renard et al., 1998). The residual GalUA was present as homogalacturonans, which were insoluble under acidic conditions but became readily soluble upon careful neutralization. During this procedure there was only limited loss of Xyl (during the acid treatment) and almost no loss of Man relative to cellulose. The composition of the washed residue may be compared with that of the parenchymatous cellulose preparation from beets (Dinand et al., 1996), which contained 88% cellulose after treatment with 2% (0.5 m) NaOH and NaClO3 bleaching, and likewise formed stable suspensions.
The sugars denoting cross-linking polysaccharides (i.e. Fuc, Xyl, Man, and noncellulosic Glc) amounted to only one-sixth of the mass of the cellulose in the beet cell walls and one-ninth of that in the depectinated cell walls. There is convincing evidence for the absence of a large amount of typical hemicelluloses in beet cell walls. Oosterveld et al. (1996) extracted only 19% of the beet cell walls in 4 m NaOH, and this extract contained mainly pectic rhamnogalacturonans, galacturonans, and arabinans, with a quantitatively minor (but normal) fucogalactoxyloglucan (less than 10%, as estimated from the DEAE fractionation by Oosterveld [1997]) and traces of (gluco)mannans.
Although cellulose, pectic galacturonan, and pectic arabinan were present in similar amounts in the beet cell walls, the cellulose signals in the spectrum were far more intense. The total intensity of the arabinan signals was considerably less than that predicted from the arabinan content of the cell walls, as would be expected since their T1ρ decay was rapid and occurred simultaneously with particularly slow CP. Compared with hydrated citrus cell walls, the lower galacturonan content resulted in a decrease of the pectic galacturonan C-1 peak compared with the cellulose C-1 peak, and of the pectin C-4, C-2, and C-3 signals at approximately 80 and 69 ppm compared with the general C-2, C-3, and C-5 signals at 72 to 75 ppm.
Use of the Waltz-16 proton-decoupling sequence and a very short recycle time made it possible to record a DP-MAS 13C spectrum consisting exclusively of arabinan signals. Their chemical shifts were close to those of arabinans in solution, suggesting that the distribution of chain conformations was similar. The high degree of branching of beet arabinans and the abundance of nonreducing ends (Thibault and Rouau, 1990) explains the intensity of the C-5 and C-4 signals for terminal arabinofuranose residues, whereas the high degree of branching on C-3 led to a characteristic signal at 80.1 ppm. Cell walls investigated previously (Foster et al., 1996; Jarvis et al., 1996; Ha et al., 1997) did not have such high Ara contents, and no conclusions were drawn regarding the mobility of arabinan side chains. However, arabinans are known to be highly mobile in solution, because the glycosidic bonds in their backbone, Arafα(1→5), are through the primary alcohol group. The spectrum of the depectinated cell wall was that of a type-I cellulose (Attalla et al., 1980), with a high proportion of crystallite surface chains (Newman et al., 1996).
The proton T1ρ data made it possible to distinguish between cellulose, which is rigid and crystalline and thus has a longer T1ρ, and more mobile arabinans and galacturonans with shorter decay times. CP kinetics further separated the highly mobile arabinans from the galacturonans. The intensities of the arabinans were more influenced by their CP behavior, whereas those of galacturonans were more influenced by rapid T1ρ decay. The particularly slow CP of the arabinan component (C-1 at 108 ppm) indicated a high degree of mobility, as did the DP-MAS spectrum. The absence of signals associated with galacturonan or arabinans in delayed-contact experiments implied that these polymers were not associated closely enough with the cellulose microfibril to be made more rigid by the association, as occurs for xyloglucan (Ha et al., 1997), or to have their observed proton T1ρ significantly increased by averaging through proton spin diffusion. The minimum spatial separation between the mobile components themselves need not be large (minimum <1 nm) because mobility reduces the rate of spin diffusion.
In beet cell walls the total amount of hemicelluloses available to coat the cellulose surface was only one-sixth of the mass of the cellulose, and no other polysaccharide present in significant quantity fulfills a coating function. Beet cellulose may have a particularly low surface area, allowing it to be coated completely by a relatively small amount of hemicellulose. The surface area of cellulose fibrils depends on the fibril diameter. The percentage of interior cellulose chains was only 42% in the beet cell walls, compared with 48% for the depectinated beet residue. This corresponds to a crystallite size slightly greater than that in apple (38%; Newman et al., 1994) or Arabidopsis (40%; Newman et al., 1996). The data for beet indicate crystallites 2 to 3 nm wide in muro, the exact width depending on their shape and the packing density of the surface chains. After controlled-acid hydrolysis and washing there was no marked aggregation, although the cellulose content was >80%. Fibrils 2 to 4 nm in diameter have been isolated from beet cell walls by hydrolytic removal of the noncellulosic polysaccharides, thus avoiding highly alkali concentrations (Dinand et al., 1996). A slight tendency to aggregation where the fibrils contacted one another was observed by Dinand et al. (1996).
Assuming that a xyloglucan chain in the “flat” conformation described by Levy et al. (1991) is 1 nm wide and has a mean cross-sectional area of 0.7 nm2 (compared with 0.32 nm2 for cellulose), a 3-nm cellulose fibril will require an approximately equal mass of xyloglucan to provide a complete coating over its surface (Hayashi et al., 1993). We do not exclude the possibility that the crystallites of beet cellulose are aggregated into larger microfibrils and that these are disrupted upon removal of the noncellulosic polysaccharides. There is evidence (Ha et al., 1998) that this is the case in the onion cell wall, where the crystallite diameter is similar but the microfibril size (including the xyloglucan present) is 8 to 10 nm (McCann et al., 1990). However, 8- to 10-nm microfibrils would still require a xyloglucan:cellulose ratio of 1:2 by mass to provide a monolayer of xyloglucan at the surface.
Current cell wall models assume that hemicelluloses are effective only if they coat the cellulose microfibrils, but the data presented here show that beet microfibrils do not need such a coating to keep them separated. The aggregation observed after conventional extraction sequences that include strong alkali (McCann et al., 1990) may be due to the formation of cellulose II by mercerization rather than to the removal of hemicelluloses. The small amount of xyloglucan present in beet cell walls may be sufficient to cross-link the microfibrils, as the amounts that form the actual cross-links are minimal (McCann et al., 1990).
Although nature uses only a few building blocks for cell walls in terrestrial plants, we still do not understand the precise role of each of these blocks, nor how much redundancy or versatility there might be. Very few walls have actually been studied in detail, and we should pay more attention to variability in their function and design. Beet cell walls in particular are those of a storage tissue that is not undergoing fast growth or bearing weight.
CONCLUSIONS
The polymers of the beet cell walls can be divided in three classes of mobility: (a) cellulose, which is highly rigid; (b) pectic galacturonans of intermediate mobility; and (c) highly mobile arabinans. We have not located any quantitatively significant fraction of the beet galacturonan or arabinan that would display the close association with cellulose microfibrils characteristic of hemicellulose. Indeed, the high mobility of arabinans in muro confirms that the pectic side chains fill the pores of the cell wall network rather than play a cross-linking role. Their structural role could be as plasticizers and water-binding agents.
The beet cell wall contains so little xyloglucan, xylan, and mannan that in the absence of any other coating polysaccharide most of the surface area of the cellulose must be bare. The 2- to 3-nm crystallites appear to have considerable stability in the absence of noncellulosic polymers. It follows that a molecular mechanism that does not involve coating of microfibrils must be responsible for limiting the aggregation of cellulose within the beet cell wall.
ACKNOWLEDGMENTS
The authors thank Dr. Brett and Dr. Wende (Department of Botany, University of Glasgow) for the sugar beets. NMR spectra were recorded at the Engineering and Physical Sciences Research Council solid-state NMR service in Durham (UK).
Abbreviations:
- CP
cross-polarization
- DP
direct polarization
- MAS
magic-angle-spinning
Footnotes
This work was supported by a grant from the Institut National de la Recherche Agronomique (to C.M.G.C.R.) and by an Engineering and Physical Sciences Research Council award.
LITERATURE CITED
- Atalla RH, Gaast JC, Sindorf DW, Bartuska VJ, Maciel GE. 13C NMR spectra of cellulose polymorphs. J Am Chem Soc. 1980;102:3249–3251. [Google Scholar]
- Atalla RH, Hackney JM, Uhlin I, Thompson NS. Hemicelluloses as structure regulators in the aggregation of native cellulose. Int J Biol Macromol. 1993;15:109–112. doi: 10.1016/0141-8130(93)90007-9. [DOI] [PubMed] [Google Scholar]
- Blumenkrantz N, Asboe-Hansen G. New method for quantitative determination of uronic acids. Anal Biochem. 1973;54:484–489. doi: 10.1016/0003-2697(73)90377-1. [DOI] [PubMed] [Google Scholar]
- Carpita NC, Gibeaut DM. Structural models of primary cell walls in flowering plants: consistency of molecular structure with the physical properties of the walls during growth. Plant J. 1993;3:1–30. doi: 10.1111/j.1365-313x.1993.tb00007.x. [DOI] [PubMed] [Google Scholar]
- Dinand E, Chanzy H, Vignon MR. Parenchymal cell cellulose from sugar beet pulp: preparation and properties. Cellulose. 1996;3:183–188. [Google Scholar]
- Dubois M, Giles KA, Hamilton JK, Riebers PA, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;28:350–356. [Google Scholar]
- Englyst HN, Cummings JH. Simplified method for the measurement of total non-starch polysaccharides by gas-liquid chromatography of constituent sugars as alditol acetates. Analyst. 1984;109:937–942. doi: 10.1039/an9820700307. [DOI] [PubMed] [Google Scholar]
- Foster TJ, Ablett S, McCann MC, Gidley MJ. Mobility-resolved 13C-NMR spectroscopy of primary plant cell walls. Biopolymers. 1996;39:51–66. [Google Scholar]
- Ha M-A, Apperley DC, Evans BW, Huxham IM, Jardine WG, Viëtor RJ, Reis D, Vian C, Jarvis MC. Fine structure in cellulose microfibrils: NMR evidence from onion and quince. Plant J. 1998;16:183–190. doi: 10.1046/j.1365-313x.1998.00291.x. [DOI] [PubMed] [Google Scholar]
- Ha M-A, Apperley DC, Jarvis MC. Molecular rigidity in dry and hydrated onion cell walls. Plant Physiol. 1997;115:593–598. doi: 10.1104/pp.115.2.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha MA, Evans BW, Jarvis MC, Apperley DC, Kenwright AM. CP-MAS NMR of highly mobile hydrated biopolymers: polysaccharides of Allium cell walls. Carbohydr Res. 1996;288:15–23. [Google Scholar]
- Hayashi T. Xyloglucans in the primary cell wall. Annu Rev Plant Physiol. 1989;40:139–168. [Google Scholar]
- Hayashi T, Ogawa K, Mitsuishi Y. Characterisation of the adsorption of xyloglucan to cellulose. Plant Cell Physiol. 1993;35:1199–1205. [PubMed] [Google Scholar]
- Jarvis MC. Solid-state 13C-NMR spectra of Vigna primary cell walls and their polysaccharide components. Carbohydr Res. 1990;201:327–333. [Google Scholar]
- Jarvis MC, Fenwick K, Apperley D. Cross-polarisation kinetics and proton NMR relaxation in polymers of citrus cell walls. Carbohydr Res. 1996;288:1–14. [Google Scholar]
- Kenwright AM, Say BJ (1993) Solid-state NMR studies of polymers. In RN Ibbett, ed, NMR Spectroscopy of Polymers. Blackie, London, pp 232–274
- Klavons JA, Bennet RD. Determination of methanol using alcohol oxidase and its application to methyl ester content of pectins. J Agric Food Chem. 1986;34:597–599. [Google Scholar]
- Levy S, York WS, Stuike-Prill R, Meyer B, Staehelin LA. Simulations of the static and dynamic molecular conformations of xyloglucan: the role of the fucosylated side-chain in surface-specific sidechain folding. Plant J. 1991;1:195–215. [PubMed] [Google Scholar]
- McCann MC, Wells B, Roberts K. Direct visualization of cross-links in the primary plant cell wall. J Cell Sci. 1990;96:323–334. [Google Scholar]
- Micard V, Renard CMGC, Thibault J-F. Preliminary studies on enzymic release of ferulic acid from sugar-beet pulp. Lebensm-Wiss Technol. 1994;27:59–66. [Google Scholar]
- Newman RH. Nuclear magnetic resonance study of spatial relationships between chemical components in wood cell walls. Holzforschung. 1992;46:205–210. [Google Scholar]
- Newman RH, Davies LM, Harris PJ. Solid-state 13C nuclear magnetic resonance characterization of cellulose in the cell walls of Arabidopsis thaliana leaves. Plant Physiol. 1996;111:475–485. doi: 10.1104/pp.111.2.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman RH, Ha MA, Melton LD. Molecular ordering of cellulose in apple cell walls. J Agric Food Chem. 1994;42:1402–1406. [Google Scholar]
- Oosterveld A (1997) Pectic substances from sugar-beet pulp: structural features, enzymatic modification, and gel formation. PhD thesis. Wageningen Agricultural University, The Netherlands
- Oosterveld A, Beldman G, Schols HA, Voragen AGJ. Arabinose and ferulic acid rich pectic polysaccharides extracted from sugar beet pulp. Carbohydr Res. 1996;288:143–153. doi: 10.1016/s0008-6215(00)00095-1. [DOI] [PubMed] [Google Scholar]
- Renard CMGC, Lahaye M, Mutter M, Voragen FGJ, Thibault J-F. Isolation and characterization of rhamnogalacturonan oligomers generated by controlled acid hydrolysis of sugar-beet pulp. Carbohydr Res. 1998;305:271–280. doi: 10.1016/s0008-6215(97)10028-3. [DOI] [PubMed] [Google Scholar]
- Renard CMGC, Thibault J-F. Structure and properties of apple and sugar-beet pectins extracted by chelating agents. Carbohydr Res. 1993;244:99–114. [Google Scholar]
- Rombouts FM, Thibault J-F. Feruloylated pectic substances from sugar-beet pulp. Carbohydr Res. 1986a;154:177–187. [Google Scholar]
- Rombouts FM, Thibault J-F. Enzymic and chemical degradation and the fine structure of pectins from sugar-beet pulp. Carbohydr Res. 1986b;154:189–203. [Google Scholar]
- Saulnier L, Brillouet JM, Joseleau JP. Structural studies of pectic substances from the pulp of grape berries. Carbohydr Res. 1988;182:63–78. [Google Scholar]
- Schaefer J, Stejskal EO, Buchdahl R. Magic-angle 13C NMR analysis of motion in solid glassy polymers. Macromolecules. 1977;10:384–405. [Google Scholar]
- Schulze E. Zur Kenntniss der chemischen Zusammensetzung der Pflanzenzellmembranen. Berichte der Chemischen Gesellschaft. 1891;24:2277–2287. [Google Scholar]
- Stejskal EO, Memory JD (1994) High resolution NMR in the solid state. In Fundamentals of CP/MAS. Oxford University Press, New York
- Tekely P, Vignon MR. Proton T1 and T2 relaxation times of wood components using 13C CP/MAS NMR. J Polym Sci Part C Polym Lett. 1987;25:257–261. [Google Scholar]
- Thibault J-F, Renard CMGC, Guillon F. Physical and chemical analysis of dietary fibres in sugar beet and vegetables. In: Jackson JF, Linskens HF, editors. Modern Methods of Plant Analysis, Vol 16: Vegetables and Vegetable Products. Heidelberg: Springer-Verlag; 1994. pp. 23–55. [Google Scholar]
- Thibault J-F, Rouau X. Studies on enzymic hydrolysis of polysaccharides in sugar beet pulp. Carbohydr Polym. 1990;13:1–16. [Google Scholar]
- Voragen AGJ, Schols HA, Pilnik W. Determination of the degree of methylation and acetylation of pectins by H.P.L.C. Food Hydrocolloids. 1986;1:65–70. [Google Scholar]