Abstract
Pregnancy has been associated with a low risk of HIV disease progression. Most pregnancies with HIV currently involve women who have not experienced AIDS-defining events, and are clinically classified as Centers for Disease Control and Prevention (CDC) groups A or B. We evaluated the main maternal outcomes among pregnant women with more advanced HIV disease, defined by CDC-C disease stage. Data from the Italian National Program on Surveillance on Antiretroviral Treatment in Pregnancy were used. A total of 566 HIV-infected mothers, 515 in stage A or B (CDC-AB group) and 51 in stage C (CDC-C group) were evaluated. The two groups had similar baseline characteristics. No differences were found in the main maternal and neonatal outcomes. Most of the women achieved viral suppression at end of pregnancy (>1000 copies per milliliter: CDC-C: 17.2%; CDC-AB: 13.7%). One year after delivery, HIV replication (HIV-RNA >1000 copies per milliliter) was present in 11.5% of CDC-AB women and 30.0% CDC-C women. Despite lower initial CD4 counts (300 versus 481 cells per microliter), CDC-C women maintained stable CD4 levels during pregnancy, and 1 year after delivery, a significant increase in CD4 count from preconception values was observed in both groups (CDC-C: +72 cells per microliter, p=0.031; CDC-AB: +43 cells per microliter, p<0.001). Only one AIDS event occurred in a woman with a previous diagnosis of AIDS. In CDC-C women, pregnancy is not associated with an increased rate of adverse maternal or neonatal outcomes, and a good immunovirologic response can be expected. During postpartum care, women with more advanced HIV infection should receive particular care to prevent loss of virologic suppression.
Introduction
The management and the outcome of pregnancy in women infected with HIV have dramatically improved in recent decades. In high-income countries, perinatal transmission rates as low as 1.5%1 have been achieved as a consequence of the use of highly active antiretroviral therapy (HAART) and appropriate management of labor, delivery, and neonatal feeding.2,3 In this new scenario, a growing number of HIV-infected women of childbearing age desire to become pregnant,4 and can expect in the vast majority of cases to give birth to healthy babies without a worsening of their clinical conditions.5
Although published studies suggest that pregnancy has little or no influence on the progression of HIV disease,5–8 most of these studies have not considered potential differences in HIV clinical status of pregnant women. Availability of HAART and progress in treatment and prevention of opportunistic infections have substantially increased the longevity and the quality of life of patients receiving a diagnosis of AIDS, who however may not reverse completely the immune damage already established, remaining more susceptible to opportunistic infections. Following delivery, antiretroviral therapy is often discontinued among women with HIV, with a potential increase in the risk of AIDS-defining events (ADE) or death.9,10 It is therefore important to compare pregnancy outcomes among women with different severity of HIV infection and assess on a longer term clinical outcomes, with particular reference to women with Centers for Disease Control and Prevention (CDC) stage C of HIV disease, who might be at higher risk of ADE.
Methods
Population
We used data from the National Program on Surveillance on Antiretroviral Treatment in Pregnancy, an ongoing multicenter observational study on HIV pregnant women established in Italy in 2001.11 All the results reported here are based on data extracted in July 2010 from the general database. We considered all the women with HIV diagnosed before pregnancy and undergoing antiretroviral therapy (ART) at conception, who had available data on pregnancy and delivery. Maternal preconception, pregnancy, delivery and postpartum data, and newborn information on birth weight, APGAR score, HIV infection, and congenital defects were collected. Information on sexually transmitted diseases (syphilis, gonorrhea, genital herpes, chlamydia) was also obtained from clinical records. Positivity to cytomegalovirus (CMV) was defined as presence of anti-CMV IgG; papillomavirus infection was defined by positive cervical sampling or clinical condylomatosis.
Cesarean section was defined as elective when performed before the onset of labor and with intact membranes. Gestational age at birth was determined on the basis of the last menstrual period, ultrasound biometry, or both. Gestational age-adjusted percentiles for birth weight were calculated according to the national reference standards by Bertino et al.,12 and birth defects were classified using the Antiretroviral Pregnancy Registry criteria.13 Maternal HIV clinical disease severity was classified according to the CDC definition,14 and the two groups of women with disease stage C of CDC classification (CDC-C) and with disease stages A or B (CDC-AB) were compared.
Statistical analysis
Differences between groups were evaluated using the χ2 test for trend and the Fisher's exact test for categorical variables and by the Mann-Whitney test for quantitative variables, and continuous variables were compared by using Wilcoxon test. Linear regression with Pearson's correlation coefficient were used to evaluate correlations between quantitative variables. Significance levels were set at the threshold of 0.05. All the analyses were performed using the SPSS software, version 17.0 (SPSS Inc., Chicago, IL).
Results
Population characteristics: demographics and coinfections
We identified 566 women who met the analysis criteria, 515 without previous AIDS events (CDC-AB group), and 51 with previous clinical ADE (CDC-C group). Their clinical and demographic characteristics are shown in Table 1. No differences in age, weight, route of infection, or ethnic origin were observed between the groups. Unintended pregnancy was significantly more common in the CDC-C group (p=0.040). The proportion of women with coinfections (hepatitis virus B or C [HBV, HCV], cytomegalovirus [CMV], human papillomavirus [HPV], and sexually transmitted diseases [STDs]) were similar in the two groups.
Table 1.
Population Characteristics
| CDC-C | CDC-AB | p Values | |
|---|---|---|---|
| Number | 51 | 515 | |
| Age (years) (median, range) | 34.0 (21.0–47.0) | 34.0 (16.0–44.0) | 0.485 |
| Caucasian (%) | 71.4 | 76.6 | 0.444 |
| BMI (median, range) | 20.5 (14.7–39.4) | 21.5 (15.2–40.7) | 0.291 |
| Months from HIV diagnosis (median, range) | 72 (5–266) | 94 (3–297) | 0.022 |
| CD4 nadir (cells/μl) (median, range) | 49 (0–260) | 230 (1–959) | <0.001 |
| Smokers (%)a | 22.2 | 17.3 | 0.435 |
| Recent substance use (%) | 7.8 | 6.2 | 0.652 |
| Route of HIV infection (%) | |||
| Sexual | 66.7 | 76.3 | 0.326 |
| IV | 29.2 | 20.3 | |
| Other | 4.2 | 3.4 | |
| Unplanned pregnancy (%) | 71.1 | 54.3 | 0.040 |
| HBV coinfection (%) | 14.6 | 12.4 | 0.650 |
| HCV coinfection (%) | 24.5 | 28.5 | 0.342 |
| HPV (%) | 35.3 | 25.5 | 0.225 |
| CMV (%) | 67.4 | 62.6 | 0.319 |
| Sexually transmitted diseases (%) | 14.0 | 16.3 | 0.427 |
More than 10 cigarettes/day.
CDC, Centers for Disease Control and Prevention; BMI, body mass index; IV, intravenous; HBV, hepatitis B virus; HCV, hepatitis C virus; HPV, human papillomavirus; CMV, cytomegalovirus.
HIV disease and antiretroviral therapy
The median interval from HIV diagnosis to pregnancy was 72 weeks (range, 5–266) in CDC-C women and 94 weeks (range, 3–297) in CDC-AB women (p=0.022). Information on CD4 nadir values was available for a small number of women (40/51 in CDC-C group and 250/515 in CDC-AB group), and values were significantly lower among CDC-C women (49 versus 230 cells per microliter, p<0.001). Based on available data, 97.5% (39/40) of CDC-C and 43.5% (111/250) of CDC-AB women had a CD4 nadir lower than 200 CD4 cells per microliter during their HIV infection history.
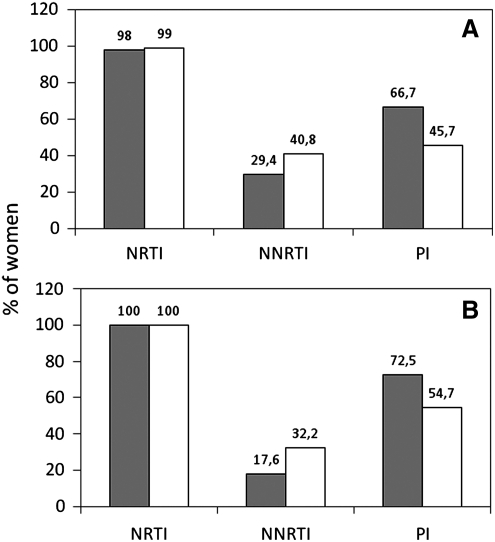
At conception, almost all the women of both groups had nucleotide reverse transcriptase inhibitors (NRTI) included in their regimens. Protease inhibitors (PI) were present more frequently in the CDC-C group (66.7% versus 45.7%, p=0.005), while non-nucleotide reverse transcriptase inhibitors (NNRTI) had an opposite trend (CDC-AB: 40.8%, CDC-C 29.4%, p=0.134; Fig. 1A). Within NNRTI-based regimens, use of efavirenz was more common in the CDC-C group compared to the CDC-AB group (66.7 versus 32.9%, p=0.010).
FIG. 1.
Proportion of women on nucleotide reverse transcriptase inhibitors (NRTI), non-nucleotide reverse transcriptase inhibitors (NNRTI), and protease inhibitors (PI) (A) at conception and (B) at delivery, by Centers for Disease Control and Prevention (CDC) HIV severity disease. Gray bars: CDC-C group; white bars: CDC-AB group.
Changes of regimens involved 52.0% of CDC-C women compared to 44.9% of CDC-AB women (p=0.208). Most of the changes represented drug switches for concerns related to fetal safety (CDC-C: 87%, CDC-AB: 86.1%, p=0.861) (Table 2). At delivery all regimens included at least one NRTI, with a maintained higher frequency of use of PI among CDC-C women (72.5%, compared to 54.7% in CDC-AB women, p=0.017), and an opposite trend for use of NNRTI (CDC-C: 17.6%; CDC-AB: 32.2%, p=0.038; Fig. 1B). Clinical data after delivery were available only for 24 CDC-C women and 197 CDC-AB women. Most of these women (198/221, 89%) continued ART: after 12 months only 1 of 24 (4.2%) CDC-C women and 22 of 197 (10.2%, p=0.338) CDC-AB women were not on ART.
Table 2.
Main Maternal and Neonatal Outcomes
| CDC-C | CDC-AB | p Values | |
|---|---|---|---|
| ART changes in pregnancy (%) | 52.0 | 44.9 | 0.208 |
| Type of change: Drug switch (%) | 87.0 | 86.1 | 0.861 |
| Intensification | 4.3 | 6.9 | |
| Simplification | 8.7 | 7.0 | |
| Delivery and neonates | |||
| Duration of pregnancy (weeks) (median, range) | 38.0 (30.0–40.0) | 37.0 (26.0–42.0) | 0.163 |
| Preterm deliverya (%) | 24.0 | 28.0 | 0.338 |
| Birthweight (g) (median, range) | 2700 (1624–3845) | 2820 (550–4130) | 0.304 |
| Birthweight by gestational age Z-score | −0.635 | −0.145 | 0.045 |
Before 37 completed weeks.
CDC, Centers for Disease Control and Prevention; ART, antiretroviral therapy.
HIV-RNA viral load and CD4 cell count
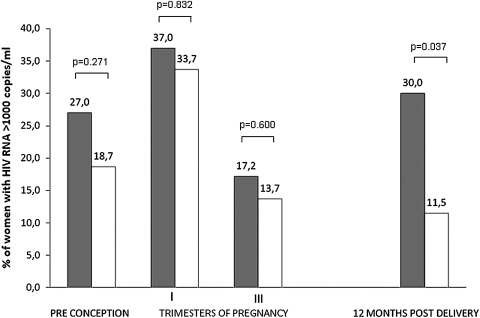
Preconception HIV viral load status was similar in the two groups: 27.0% of CDC-C and 18.7% of CDC-AB women had more than 1000 HIV-1 RNA copies per milliliter (p=0.271). During pregnancy, the percentage of women with more than 1000 HIV RNA copies per milliliter had a similar trend in both groups, with a limited and transient increase in the first trimester (37.0% versus 33.7%, p=0.832) followed by a decline at third trimester, when HIV-RNA viral load was above 1000 copies per milliliter in 17.2% of CDC-C women and in 13.7% of CDC-AB women (p=0.600). One year after delivery, the proportion of women with more than 1000 HIV-RNA copies returned to preconception levels in CDC-C women (30.0%), and remained low in CDC-AB women (11.5%), with a significant difference between the two groups (p=0.037, Fig. 2).
FIG. 2.
Proportion of women with HIV-1 RNA >1000 copies per milliliter at different times (preconception, pregnancy, postdelivery). Gray bars, CDC-C group; white bars, CDC-AB group. n: CDC-C: 37; CDC-AB: 316 (available preconception HIV-RNA values). CDC, Centers for Disease Control and Prevention.
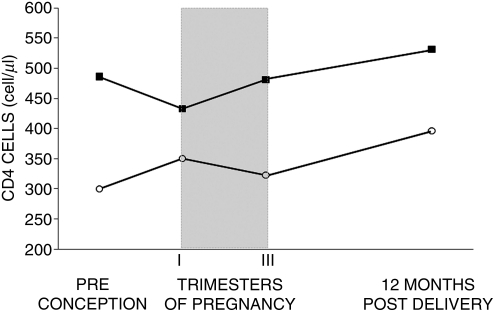
As expected, CDC-C women had lower preconception levels of CD4 cells compared to CDC-AB women (300 versus 481 per microliter, p<0.001). During pregnancy, the absolute number of CD4 cells significantly increased from first to third trimester in CDC-AB group (p<0.001), with no significant change in the CDC-C group (p=0.080). Similarly, the CD4 cell percentage between first and third trimester remained stable in the CDC-C group (20.0% and 20.8%, respectively, p=0.482), but significantly increased in the CDC-AB group (from 27.3% to 29.8%, p<0.001). Overall, CD4 cell count at the end of third trimester was similar to pre-pregnancy values in both groups (Fig. 3). One year after delivery, the CD4 count was significantly higher compared to preconception count in both groups (+72 cells per microliter in CDC-C, p=0.031; +43 cells per microliter in CDC-AB, p<0.001).
FIG. 3.
CD4 cell count at different times (preconception, pregnancy, postdelivery). ■, CDC-C group; ○, CDC-AB group. n: CDC-C: 44; CDC-AB: 366 (available preconception CD4 values). CDC, Centers for Disease Control and Prevention.
Pregnancy outcomes
Only one new ADE was observed during pregnancy, in a woman of the CDC-C group, who developed a non-Hodgkin's lymphoma at pregnancy week 24. A similar occurrence of other events (of any type) was observed in the two groups (39.3% and 32.7%, respectively, p=0.309). The most common among these events were represented by genitourinary infections, that were reported for 11.8% and 13.4% of CDC-C and CDC-AB women, respectively (p=1.000). Vaginal delivery accounted for less than 2% of deliveries. Nonelective cesarean section rate was 8.3% among CDC-C women and 18.1% among CDC-AB women (p=0.087). Delivery complications involved 4 (8.5%) CDC-C women and 25 (5.2%) CDC-AB women (p=0.343), and were represented by surgical wound complications in 3 of the 4 women of the CDC-C group and in 8 of the 25 women of the CDC-AB group. The most common postdelivery complications among CDC-AB women were anemia (n=11) and fever (n=8).
During the first year after delivery none of the CDC-AB women developed a new class C clinical event and no deaths occurred. In the CDC-C group, the woman with non-Hodgkin's lymphoma during pregnancy died 4 months after delivery. No other women developed ADEs.
Neonatal outcomes
Median gestational age (CDC-C: 38.0 weeks, CDC-AB: 37.0, p=0.163) and rate of preterm delivery (24.0% and 28.0%, respectively, p=0.338) (Table 2) were similar in the two groups. Four infants (0.7%) had neonatal death in the first days of life (CDC-C: 0/51, CDC-AB 4/515); three of them (gestational age from 25 to 35 weeks) suffered from neonatal respiratory distress syndrome with or without other complications related to prematurity. The fourth, a term neonate, died 1 week after birth of sepsis due to β-hemolytic streptococcal infection.
Three neonates (0.5%), all from CDC-AB mothers, were HIV infected. Two of the mothers had multiresistent HIV virus strains, and the other had poor adherence and high viral load at delivery. Birth defect rate was 3.7% (CDC-C: 0/51, CDC-AB 21/515, 4.2%). Newborns from CDC-C mothers had a slightly lower birth weight (2700 versus 2820 g, p=0.304), confirmed by a marginally significant difference in birth weight by gestational age, expressed as z-score (−0.640 versus −0.145, p=0.045). No differences were recorded in APGAR scores at 5 and 10 minutes postbirth (data not shown).
Discussion
Pregnancy can impact the course of various disease characterized by immunologic disorders, such as systemic lupus erythematosus, multiple sclerosis, and rheumatoid arthritis15,16 but does not seem to have a significant influence on HIV disease progression.5–8 Most of the published studies on pregnancy and HIV evaluated a population with non advanced disease; in our study we further confirm that pregnancy has limited or no influence on HIV progression, even in women with more advanced disease, represented by CDC-C stage of disease. Recent studies indicated that pregnancy incidence is lower among HIV-infected women with respect to an uninfected population of the same age, suggesting that more severe clinical disease can influence fertility rate.17,18 From our data we cannot draw conclusions on the incidence of pregnancy or on fertility rates among women with advanced disease. In the present analysis, CDC group C women represented 9% of all the pregnant women evaluated. However, we considered for the present analysis only those women who were already on HIV treatment at conception. The actual proportion within the entire population of pregnant women with HIV is likely to be lower, because also includes also women with no personal indication to treatment. In our global national sample of pregnant women with HIV, this proportion is below six per cent (National Program on Surveillance on Antiretroviral Treatment in Pregnancy, data not shown), consistent with other reports.19
In our study, women with and without a CDC-C diagnosis had similar demographic characteristics, body mass index, coinfections, and concomitant diseases, suggesting limited additional comorbidity in this group. The higher risk of unplanned pregnancy and the more frequent exposure to efavirenz among women with a CDC-C diagnosis, however, suggests that these women should receive more attention in terms of preconception counseling and treatment planning, not only to avoid exposure to contraindicated treatments, but also to prevent the frequent therapeutic changes in pregnancy that we observed in our study. Other studies have reported that reproductive counseling for HIV-infected women of childbearing age is still suboptimal, even in resource rich countries, underlining the need to improve the quality of communication between women with HIV and their care providers on issues regarding pregnancy, vertical transmission, and family planning.20,21
Although the teratogenicity potential of efavirenz in women of childbearing age is currently controversial,22–24 its potential fetal effects25 are likely to represent the underlying cause of some treatment switches from efavirenz to protease inhibitors observed during the first weeks of pregnancy. In our study, protease inhibitors were overall more commonly used than NNRTI, probably reflecting a more favorable safety profile in pregnancy1 and a very limited transplacental passage of these drugs.26 With respect to stage of disease, the more common use of PI among women with CDC-C disease stage is likely to be motivated by the expected higher potency and the higher genetic barrier to resistance of these regimens, which may be particularly relevant in patients with more advanced disease. As expected, in this particular group of women who had treatment indication for their own health before pregnancy, the vast majority of women were still on treatment 1 year after delivery.
We analyzed viral suppression considering of clinical relevance HIV plasma viremia above 1000 copies per milliliter, based on the correlation between values above this threshold and perinatal transmission of HIV-127 and on published reviews that used this cutoff.28 The observation that only a small percentage of women had an end-of-pregnancy viral load above 1000 copies per milliliter, independent from CDC disease stage, is reassuring in terms of risk of perinatal transmission. Conversely, 1 year after delivery, viral suppression was less frequently maintained among women with CDC-C disease, despite continuation of treatment. This finding suggests that women in CDC-C stage of HIV disease could develop a decline in therapeutic adherence during the first year of life of their offspring, and draws attention on the need to provide a more careful postdelivery care in these women.
Women with more advanced disease entered pregnancy at lower CD4 levels. The difference in CD4 counts at the beginning of pregnancy between the two groups could be explained by the more clinical advanced disease and/or a longer history of infection. Since CD4 cell count could be influenced by the physiologic hemodilution occurring in pregnancy,29–31 we analyzed both percentage and absolute number of CD4 cells during pregnancy, finding no major variations in CD4 count among more immunocompromised women, both as absolute values and as CD4 percentage. Importantly, 1 year after delivery CD4 count showed an upward trend and a significant increase in both groups compared to preconception values. This favorable immunologic trend was paralleled by a similar clinical course of pregnancy in the two groups and by a minimal occurrence of serious AIDS-related events: only one woman with a previous diagnosis of AIDS (CDC-C group) developed a new ADE, which was subsequently fatal. No ADEs were observed in the CDC-AB group, confirming that pregnancy is usually not associated with a progression of HIV disease in asymptomatic or mildly symptomatic HIV disease. Genitourinary infections were the most common clinical events observed; their prevalence was however in the range described for healthy pregnant women.32,33 Complications of cesarean delivery were also relatively infrequent, even in women with advanced disease. Neonatal outcomes were also similar in the two groups; the overall preterm birth rate of about 25% was similar to that reported in previous studies.34,35 The overall rates of neonatal death, birth defects, and vertical transmission were low and within expected ranges. The infrequent occurrence of such events did not allow to compare women with and without a CDC-C diagnosis for these outcomes. In general, there was no trend suggesting that stage C in HIV infection may significantly influence neonatal outcomes, and this is reassuring information.
Our study was has some limitations. Our follow-up analysis was limited by the low number of clinical data reported after delivery: after 1 year from delivery we had available data only on 221 of 566 women enrolled in this study, confirming the difficulties already observed in similar observational studies.9 We also have incomplete data on CD4 nadir, and we based our study on the HIV classification reported by clinicians. We therefore cannot exclude that clinical records may have not fully captured personal history of HIV-related events for those women with a long time from HIV diagnosis.
In summary, our findings are consistent with those of previous studies7,8,36,37 that demonstrated the scarce influence of pregnancy on HIV progression, but extend these considerations also to HIV women with a previous clinical diagnosis of AIDS, providing reassuring data on main pregnancy outcomes, changes in HIV-RNA levels, and CD4 response among women in stage C of HIV infection. The increased risk of unplanned pregnancy and treatment discontinuation one year after delivery, however, suggests that women with more advanced HIV disease may need particular attention from health providers, with needed improvements in preconception counseling and in postdelivery care.
Acknowledgments
We thank Cosimo Polizzi and Alessandra Mattei for providing technical secretarial for this study and all the women who participated in this study.
This work was supported by public grants 39C/A, 31D55, 31D56 from the Italian National Program on Research on AIDS and by public research grants from the Italian Medicines Agency (AIFA). No funding was received for this work from any of the following organizations: National Institutes of Health (NIH); Wellcome Trust; and the Howard Hughes Medical Institute (HHMI).
The Italian Group on Surveillance on Antiretroviral Treatment in Pregnancy includes: Project coordinators: M. Floridia, M. Ravizza, E. Tamburrini. Participants: M. Ravizza, E. Tamburrini, F. Mori, P. Ortolani, E.R. dalle Nogare, G. Sterrantino, M. Meli, S. Polemi, J. Nocentini, M. Baldini, G. Montorzi, M. Mazzetti, B. Borchi, F. Vichi, E. Pinter, E. Anzalone, R. Marocco, C. Mastroianni, V.S. Mercurio, A. Carocci, E. Grilli, A. Maccabruni, M. Zaramella, B. Mariani, G. Natalini Raponi, G. Guaraldi, K. Luzi, G. Nardini, C. Stentarelli, A. Degli Antoni, A. Molinari, P. Rogasi, M.P. Crisalli, A. Donisi, M. Piepoli, V. Cerri, A. Viganò, V. Giacomet, S. Coletto, F. Di Nello, G. Placido, M. D'Alessandro, A. Vivarelli, P. Castelli, F. Savalli, V. Portelli, F. Sabbatini, D. Francisci, S. Alberico, G. Maso, M. Tropea, A. Meloni, M. Dedoni, C. Cuboni, F. Ortu, P. Piano, A. Citernesi, I. Vicini, E. Periti, A. Spinillo, M. Roccio, A. Vimercati, E. Bassi, B. Guerra, F. Cervi, C. Puccetti, P. Murano, M. Contoli, E. Tridapalli, G. Brighi, M. Stella, G. Faldella, M. Sansone, P. Martinelli, A. Agangi, C. Tibaldi, L. Trentini, S. Marini, G. Masuelli, I. Cetin, A. Crepaldi, M.L. Muggiasca, E. Ferrazzi, C. Giaquinto, M. Fiscon, R. Rinaldi, E. Rubino, A. Bucceri, R. Matrone, G. Scaravelli, G. Anzidei, C. Fundarò, O. Genovese, C. Cafforio, C. Pinnetti, G. Liuzzi, V. Tozzi, P. Massetti, M. Anceschi, A.M. Casadei, F. Montella, A.F. Cavaliere, V. Finelli, C. Riva, L. Lazier, M. Cellini, S. Garetto, G. Castelli Gattinara, A.M. Marconi, S. Foina, S. Dalzero, M. Moneta, F. Di Lorenzo, C. Polizzi, A. Mattei, M.F. Pirillo, R. Amici, C.M. Galluzzo, S. Donnini, S. Baroncelli, M. Floridia. Pharmacokinetics: M. Regazzi, P. Villani, M. Cusato. Advisory Board: A. Cerioli, M. De Martino, P. Mastroiacovo, M. Moroni, F. Parazzini, E. Tamburrini, S. Vella. SIGO-HIV Group National Coordinators: P. Martinelli, M. Ravizza.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Panel on Treatment of HIV-Infected Pregnant Women and Prevention of Perinatal Transmission. Recommendations for Use of Antiretroviral Drugs in Pregnant HIV-1-Infected Women for Maternal Health and Interventions to Reduce Perinatal HIV Transmission in the United States. May 24, 2010. http://aidsinfo.nih.gov/ContentFiles/PerinatalGL.pdf. [Jan;2011 ]. pp. 1–117.http://aidsinfo.nih.gov/ContentFiles/PerinatalGL.pdf
- 2.UnAIDS/WHO. AIDS epidemic update: November 2009. WHO Library Cataloguing-in-Publication Data. 2009.
- 3.Paintsil E. Andiman WA. Update on successes and challenges regarding mother-to-child transmission of HIV. Curr Opin Pediatr. 2009;21:94–101. doi: 10.1097/MOP.0b013e32831ec353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen JL. Philips KA. Kanouse DE. Collins RL. Miu A. Fertility desires and intentions of HIV-positive men and women. Fam Plann Perspect. 2001;33:144–152, 165. [PubMed] [Google Scholar]
- 5.Saada M. Le Chenadec J. Berrebi A, et al. Pregnancy and progression to AIDS: results of the French prospective cohorts. SEROGEST and SEROCO Study Groups. AIDS. 2000;14:2355–2360. doi: 10.1097/00002030-200010200-00017. [DOI] [PubMed] [Google Scholar]
- 6.Weisser M. Rudin C. Battegay M. Pfluger D. Kully C. Egger M. Does pregnancy influence the course of HIV infection? Evidence from two large Swiss cohort studies. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;17:404–410. doi: 10.1097/00042560-199804150-00004. [DOI] [PubMed] [Google Scholar]
- 7.Watts DH. Lu M. Thompson B, et al. Treatment interruption after pregnancy: Effects on disease progression and laboratory findings. Infect Dis Obstet Gynecol. 2009;2009:456717. doi: 10.1155/2009/456717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tai JH. Udoji MA. Barkanic G, et al. Pregnancy and HIV disease progression during the era of highly active antiretroviral therapy. J Infect Dis. 2007;196:1044–1052. doi: 10.1086/520814. [DOI] [PubMed] [Google Scholar]
- 9.Onen NF. Nurutdinova D. Sungkanuparph S. Gase D. Mondy K. Overton ET. Effect of postpartum HIV treatment discontinuation on long-term maternal outcome. J Int Assoc Physicians AIDS Care. 2008;7:245–251. doi: 10.1177/1545109708325466. [DOI] [PubMed] [Google Scholar]
- 10.Melekhin VV. Shepherd BE. Jenkins CA, et al. Postpartum discontinuation of antiretroviral therapy and risk of maternal AIDS-defining events, non-AIDS–defining events, and mortality among a cohort of HIV-1–infected women in the United States. AIDS Patient Care STDS. 2010;24:279–286. doi: 10.1089/apc.2009.0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Floridia M. Ravizza M. Tamburrini E, et al. Diagnosis of HIV infection in pregnancy: data from a national cohort of pregnant women with HIV in Italy. Epidemiol Infect. 2006;134:1120–1127. doi: 10.1017/S0950268806006066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bertino E. Spada E. Occhi L, et al. Neonatal anthropometric charts: The Italian neonatal study compared with other European studies. J Pediatr Gastroenterol Nutr. 2010;51:353–361. doi: 10.1097/MPG.0b013e3181da213e. [DOI] [PubMed] [Google Scholar]
- 13.Scheuerle A. Covington D. Clinical review procedures for the Antiretroviral Pregnancy Registry. Pharmacoepidemiol Drug Saf. 2004;13:529–536. doi: 10.1002/pds.971. [DOI] [PubMed] [Google Scholar]
- 14.CDC. 1993 Revised Classification System for HIV Infection and Expanded Surveillance Case Definition for AIDS Among Adolescents and Adults. MMW. 1992;41:RR–17. [PubMed] [Google Scholar]
- 15.Confavreux C. Hutchinson M. Hours MM. Cortinovis-Tourniaire P. Moreau T. Rate of pregnancy-related relapse in multiple sclerosis. Pregnancy in Multiple Sclerosis Group. N Engl J Med. 1998;339:285–291. doi: 10.1056/NEJM199807303390501. [DOI] [PubMed] [Google Scholar]
- 16.Tincani A. Faden D. Tarantini M, et al. Systemic lupus erythematosus and pregnancy: a prospective study. Clin Exp Rheumatol. 1992;10:439–446. [PubMed] [Google Scholar]
- 17.Blair JM. Hanson DL. Jones JL. Dworkin MS. Trends in pregnancy rates among women with human immunodeficiency virus. Obstet Gynecol. 2004;103:663–668. doi: 10.1097/01.AOG.0000117083.33239.b5. [DOI] [PubMed] [Google Scholar]
- 18.Linas BS. Minkoff H. Cohen MH, et al. Relative time to pregnancy among HIV-infected and uninfected women in the Women's Interagency HIV Study, 2002–2009. AIDS. 2011;25:707–711. doi: 10.1097/QAD.0b013e3283445811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goetghebuer T. Haelterman E. Marvillet I, et al. Vertical transmission of HIV in Belgium: A 1986–2002 retrospective analysis. Eur J Pediatr. 2009;168:79–85. doi: 10.1007/s00431-008-0717-y. [DOI] [PubMed] [Google Scholar]
- 20.Arya M. Levison J. Giordano TP. Ongoing barriers to HIV testing during pregnancy: A need for media campaigns addressing low knowledge about perinatal HIV transmission among women in the United States. AIDS Patient Care STDs. 2010;24:71–72. doi: 10.1089/apc.2009.0212. [DOI] [PubMed] [Google Scholar]
- 21.Finocchario-Kessler S. Dariotis JK. Sweat MD, et al. Do HIV-infected women want to discuss reproductive plans with providers, and are those conversations occurring? AIDS Patient Care STDs. 2010;24:317–323. doi: 10.1089/apc.2009.0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ford N. Mofenson L. Kranzer K, et al. Safety of efavirenz in first-trimester of pregnancy: A systematic review and meta-analysis of outcomes from observational cohorts. AIDS. 2010;24:1461–1470. doi: 10.1097/QAD.0b013e32833a2a14. [DOI] [PubMed] [Google Scholar]
- 23.Hsu H. Rydzak C. Cotich K, et al. Quantifying the risks and benefits of efavirenz use in HIV-infected women of childbearing age in the USA. HIV Med. 2011;12:97–108. doi: 10.1111/j.1468-1293.2010.00856.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ekouevi DK. Coffie PA. Ouattara E, et al. Pregnancy outcomes in women exposed to efavirenz and nevirapine: an appraisal of the IeDEA West Africa and ANRS Databases, Abidjan, Côte d'Ivoire. J Acquir Immune Defic Syndr. 2011;56:183–187. doi: 10.1097/QAI.0b013e3181ff04e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brogly SB. Abzug MJ. Watts DH, et al. Birth defects among children born to human immunodeficiency virus-infected women: Pediatric AIDS clinical trials protocols 219 and 219C. Pediatr Infect Dis J. 2010;29:721–727. doi: 10.1097/INF.0b013e3181e74a2f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Watts DH. Treating HIV during pregnancy: an update on safety issues. Drug Saf. 2006;29:467–490. doi: 10.2165/00002018-200629060-00002. [DOI] [PubMed] [Google Scholar]
- 27.Cooper ER. Charurat M. Mofenson L, et al. Combination antiretroviral strategies for the treatment of pregnant HIV- 1–infected women and prevention of perinatal HIV-1 transmission. J Acquir Immune Defic Syndr. 2002;29:484–494. doi: 10.1097/00126334-200204150-00009. [DOI] [PubMed] [Google Scholar]
- 28.Ioannidis JP. Abrams EJ. Ammann A, et al. Perinatal transmission of human immunodeficiency virus type 1 by pregnant women with RNA virus loads <1000 copies/ml. J Infect Dis. 2001;183:539–545. doi: 10.1086/318530. [DOI] [PubMed] [Google Scholar]
- 29.Miotti PG. Liomba G. Dallabetta GA. Hoover DR. Chiphangwi JD. Saah AJ. T lymphocyte subsets during and after pregnancy: Analysis in human immunodeficiency virus type 1-infected and -uninfected Malawian mothers. J Infect Dis. 1992;165:1116–1119. doi: 10.1093/infdis/165.6.1116. [DOI] [PubMed] [Google Scholar]
- 30.Ekouevi DK. Inwoley A. Tonwe-Gold B, et al. Variation of CD4 count and percentage during pregnancy and after delivery: Implications for HAART initiation in resource-limited settings. AIDS Res Hum Retroviruses. 2007;23:1469–1474. doi: 10.1089/aid.2007.0059. [DOI] [PubMed] [Google Scholar]
- 31.Melo VH. Pinto JA. Freimanis-Hance L, et al. Postpartum changes in plasma viral load and CD4 percentage among HIV-infected women from Latin American and Caribbean countries: The NISDI Perinatal Study. Mem Inst Oswaldo Cruz. 2011;106:97–104. doi: 10.1590/s0074-02762011000100016. [DOI] [PubMed] [Google Scholar]
- 32.Valkenburg-van den Berg AW. Sprij AJ. Oostvogel PM, et al. Prevalence of colonisation with group B Streptococci in pregnant women of a multi-ethnic population in The Netherlands. Eur J Obstet Gynecol Reprod Biol. 2006;124:178–183. doi: 10.1016/j.ejogrb.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 33.Tolosa JE. Chaithongwongwatthana S. Daly S, et al. The International Infections in Pregnancy (IIP) study: Variations in the prevalence of bacterial vaginosis and distribution of morphotypes in vaginal smears among pregnant women. Am J Obstet Gynecol. 2006;195:1198–1204. doi: 10.1016/j.ajog.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 34.European Collaborative Study; Swiss Mother and Child HIV Cohort Study. Combination antiretroviral therapy and duration of pregnancy. AIDS. 2000;14:2913–2920. doi: 10.1097/00002030-200012220-00013. [DOI] [PubMed] [Google Scholar]
- 35.Cotter AM. Garcia AG. Duthely ML. Luke B. O'Sullivan MJ. Is antiretroviral therapy during pregnancy associated with an increased risk of preterm delivery, low birth weight, or stillbirth? J Infect Dis. 2006;193:1195–201. doi: 10.1086/503045. [DOI] [PubMed] [Google Scholar]
- 36.Martin F. Navaratne L. Khan W, et al. Pregnant women with HIV infection can expect healthy survival: Three-year follow-up. J Acquir Immune Defic Syndr. 2006;43:186–192. doi: 10.1097/01.qai.0000233311.28602.4d. [DOI] [PubMed] [Google Scholar]
- 37.Tungsiripat M. Drechsler H. Aberg JA. Discontinuation of antiretroviral therapy postpartum: No evidence for altered viral set point. J Acquir Immune Defic Syndr. 2007;44:116–117. doi: 10.1097/QAI.0b013e31802b9695. [DOI] [PubMed] [Google Scholar]