Abstract
Aims
β-Phenethyl isothiocyanate (PEITC) is a natural product with potent anticancer activity against human leukemia cells including drug-resistant primary leukemia cells from patients. This study aimed at investigating the key mechanisms that contribute to the potent anti-leukemia activity of PEITC and at evaluating its therapeutic potential.
Results
Our study showed that PEITC caused a rapid depletion of mitochondrial glutathione (GSH) and a significant elevation of reactive oxygen species (ROS) and nitric oxide, and induced a disruption of the mitochondrial electron transport complex I manifested by an early degradation of NADH dehydrogenase Fe-S protein-3 and a significant suppression of mitochondrial respiration. Using biochemical and pharmacological approaches, we further showed that inhibition of mitochondrial respiration alone by rotenone caused only a moderate cytotoxicity in leukemia cells, whereas a combination of respiratory inhibition and an ROS-generating agent exhibited a synergistic effect against leukemia and lymphoma cells.
Innovation and Conclusion
Although PEITC is a reactive compound and might have multiple mechanisms of action, we showed that a rapid depletion of GSH and inhibition of mitochondrial respiration are two important early events that induced synergistic cytotoxicity in leukemia cells. These findings not only suggest that PEITC is a promising compound for potential use in leukemia treatment, but also provide a basis for developing new therapeutic strategies to effectively kill leukemia cells by using a novel combination to modulate ROS and inhibit mitochondrial respiration. Antioxid. Redox Signal. 15, 2911–2921.
Introduction
Recent studies suggest that the natural products β-phenethyl isothiocyanate (PEITC) is able to induce apoptosis in various types of tumor cells and exhibit potential anticancer activity with relatively low cytotoxicity toward normal cells. As a single agent, PEITC is effective against solid tumor cells as well as leukemia cells, including those isolated from patients with chronic lymphocytic leukemia (CLL) resistant to fludarabine (37), and those from patients with chronic myelogenous leukemia resistant to Gleevec (47). Further, human myeloid leukemia cells with multi drug-resistant phenotype such as HL-60/ADR cells (MRP-1-positive) and HL60/VCR cells (Pgp-1-positive) are also sensitive to PEITC (24, 46). Although it has been shown that PEITC is able to cause redox imbalance and oxidative stress in cancer cells by causing depletion of cellular glutathione (GSH), the exact mechanisms responsible for its potent anticancer activity remain to be elucidated.
Innovation.
This study demonstrated that inhibition of the mitochondrial respiratory chain complex I through disruption of NDUFS3 and a rapid depletion of mitochondrial GSH were two major mechanisms of action that contributed to the potent anti-leukemia activity of PEITC. This led to the development of a novel strategy to effectively kill leukemia cells by a combination of inhibition of mitochondrial respiration and depletion of GSH. Such a synergistic drug combination may have potential therapeutic implications, as it is possible to use clinically relevant drugs such as BSO to deplete GSH and ATO to inhibit mitochondrial respiration (18, 26, 27) as a new strategy to increase therapeutic activity. Importantly, PEITC as a single agent is able to induce a rapid depletion of GSH and to inhibit mitochondrial respiration, and, thus, may have promising potential as a novel therapeutic agent for cancer treatment.
Due to its reactive chemical properties, PEITC is likely to interact with various cellular molecules and have multiple mechanisms of action. It has been reported that this compound directly binds to alpha- and beta-tubulins and promotes their degradation, thus leading to cell cycle arrest in various cancer cells (28, 29). PEITC may also interact with 26S and 20S proteasomes and inhibits the enzyme activity (30). A recent study suggests that this compound seems to bind to mutant p53 and cause its conformational change, thus leading to its depletion (41). Interestingly, PEITC induces phosphorylation of p66Shc, which may translocate to mitochondria and enhance reactive oxygen species (ROS) production and DNA fragmentation (45). It has also been observed that PEITC causes aberrant regulation of cellular signaling pathways involving p38 mitogen-activated protein kinases, hypoxia-inducible factor, nuclear factor kappa-light-chain-enhancer of activated B cells, c-Jun N-terminal kinases, and signal transducer and activator of transcription 3 (5, 17, 22, 42). Thus, the cytotoxic effect of PEITC is likely a consequence of various mechanisms of action. The degree of alterations in cellular redox status and signaling pathways and the specific molecules involved may depend on the PEITC concentrations and treatment duration. However, the critical early events induced by PEITC and that are pivotal for its potent anticancer activity still remain to be defined.
Several studies have shown that a rapid decrease of mitochondrial GSH can lead to instability of mitochondrial anti-apoptosis proteins, such as Bcl-2 and Bcl-xl (1, 2, 40, 48), and trigger apoptosis through the opening of mitochondrial permeability transition pore and the release of apoptosis factors such as cytochrome c and apoptosis inducing factor (12, 15). The oxidative form of GSH, glutathione disulfide (GSSG), cannot be exported from the mitochondria, as there is no GSSG transporter on the mitochondrial membrane. This can reduce the mitochondrial GSH/GSSG ratio, causing further glutathionylation of a number of mitochondrial proteins including aconitase (9, 19, 34), pyruvate dehydrogenase (32), certain subunits of the respiratory chain complexes I and IV, and α-ketoglutarate dehydrogenase (13, 14, 31), and thus disrupt the normal mitochondrial function.
In the current study, we examined the effect of PEITC on mitochondrial respiration and its correlation with alterations in mitochondrial GSH and ROS/nitric oxide (NO) levels. Our study revealed that mitochondrial respiratory chain complex I was a key target of PEITC, which caused a rapid degradation of the complex I component NADH dehydrogenase Fe-S subunit 3. PEITC also caused an early depletion of mitochondrial GSH, which was associated with elevated mitochondrial ROS and NO, the reactive chemical species capable of inhibiting mitochondrial respiration. We also found a novel drug combination strategy that was highly effective in killing leukemia based on a simultaneous inhibition of mitochondrial respiration and depletion of GSH.
Results
Inhibition of mitochondrial respiration by PEITC and its association with elevated NO
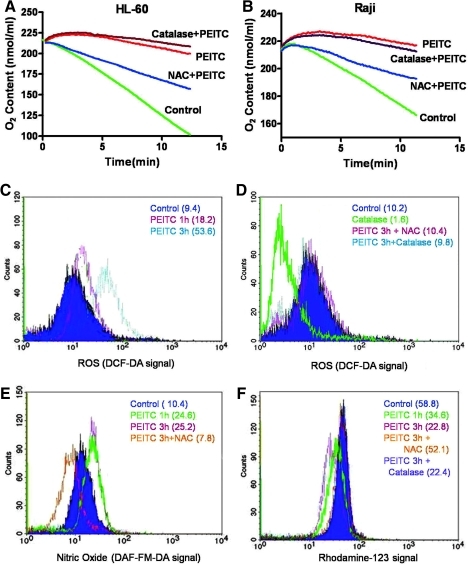
Using human leukemia HL-60 cells, we observed that incubation with 10 μM PEITC for 3 h led to a significant suppression of mitochondrial respiration, as evidenced by a substantial decrease in oxygen consumption from 8.6 to 1.6 nmole oxygen/min (Fig. 1A). Similarly, treatment of human lymphoma cells (Raji) with the same concentration of PEITC caused a reduction of their respiration rate from 4.6 to 0.8 nmole oxygen/min (Fig. 1B). Pretreatment of cells with antioxidant N-acetyl cysteine (NAC, 2 mM, 2 h) substantially reversed the PEITC-mediated inhibition of respiration in both cell lines, thus suggesting that inhibition of mitochondrial respiration by PEITC might be associated with alteration in ROS, which are known to suppress the respiratory chain activity. However, pretreatment of cells with catalase (2000 unit/ml), a hydrogen peroxide (H2O2) scavenger, did not significantly reverse the respiratory inhibition by PEITC in either cell line.
FIG. 1.
Inhibition of mitochondrial respiration by PEITC and its association with increases of ROS and nitric oxide (NO). (A) HL-60 cells were treated with 10 μM PEITC for 3 h with or without a 2-h pretreatment with NAC (2 mM) or catalase (2000 unit/ml). Oxygen contents were monitored by using the Oxythem system (6 million cells/ml). Representative results of three experiments are shown. (B) Raji cells were treated with 10 μM PEITC for 3 h with or without a 2-h preincubation with NAC (2 mM) or catalase (2000 unit/ml). Oxygen contents were then monitored. (C) HL-60 cells were treated with 10 μM PEITC for 1–3 h, cellular ROS levels were determined by flow cytometry by using DCF-DA dye. (D) HL-60 cells were treated with 10 μM PEITC for 3 h with or without NAC or catalase pretreatment. ROS levels were determined by flow cytometry by using DCF-DA dye. (E) HL-60 cells were treated with 10 μM PEITC for 1–3 h with/without NAC pretreatment. Cellular NO levels were determined by flow cytometry with DAF-FM-DA dye. (F) HL-60 cells were treated with 10 μM PEITC for 1–3 h with/without NAC or catalase as indicated. Mitochondrial membrane potential was determined by flow cytometry by using rhodamine-123 as a fluorescent dye. The numbers in parentheses indicate the mean values of the relative fluorescent intensity. PEITC, β-phenethyl isothiocyanate; ROS, reactive oxygen species; NAC, N-acetyl cysteine; DAF-FM-DA, 4-amino-5-methylamino-2′,7′-difluorescein diacetate; DCF-DA, dichlorodihydrofluorescein diacetate.
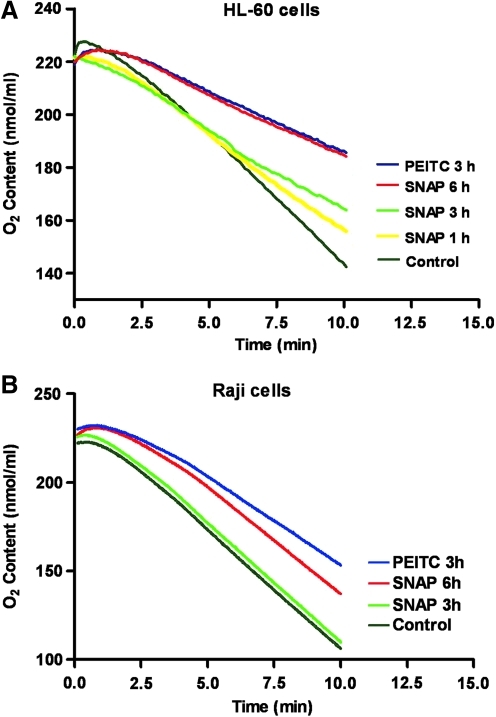
We then used flow cytometry to analyze cellular H2O2 and NO, using the redox-sensitive dyes 5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate (CM-H2DCF-DA) and 4-amino-5-methylamino-2′,7′-difluorescein diacetate (DAF-FM-DA), respectively. We found that cellular H2O2 levels were markedly increased 1–3 h after PEITC treatment (Fig. 1C). Either NAC or catalase could effectively reverse H2O2 increase induced by PEITC and decrease the cellular ROS to its baseline level (Fig. 1D). Interestingly, PEITC also caused a rapid increase of cellular NO, which could be reserved by NAC (Fig. 1E), but not by catalase (data not shown). The mitochondrial transmembrane potential was disrupted by PEITC in a time-dependant manner. NAC, but not catalase, reversed this effect (Fig. 1F). Since NAC could effectively suppress both H2O2 and NO (via enhancing GSH synthesis to maintain GSH level under oxidative stress), whereas catalase could only scavenge H2O2, it seemed likely that the increase in NO might contribute to the inhibition of mitochondrial respiration and the decrease of transmembrane potential. To test this possibility, we used the NO donor S-nitroso-N-acetylpenicillamine (SNAP) to test whether the release of NO from this compound could suppress mitochondrial respiration. As shown in Figure 2, incubation of HL-60 cells with 4 mM SNAP led to a time-dependent inhibition of respiration (Fig. 2A). Similar results were also observed in Raji cells (Fig. 2B). These findings are consistent with the previous observation that NO is an inhibitor of mitochondrial respiratory chain (35), and suggest that the induction of NO generation by PEITC might, in part, contribute to the ability of this compound to inhibit mitochondrial respiration.
FIG. 2.
Effect of PEITC or NO donor SNAP on mitochondrial respiration. (A) HL-60 cells were treated with 5 μM PEITC for 3 h or 4 mM SNAP for 1–6 h as indicated. Oxygen content was recorded by using the Oxytherm system at a cell density of 6 million/ml. (B) Raji cells were treated with PEITC or SNAP under the same conditions as in (A), and oxygen consumption was monitored by using the Oxytherm system. SNAP, S-nitroso-N-acetylpenicillamine.
PEITC caused disruption of mitochondrial respiratory complex I
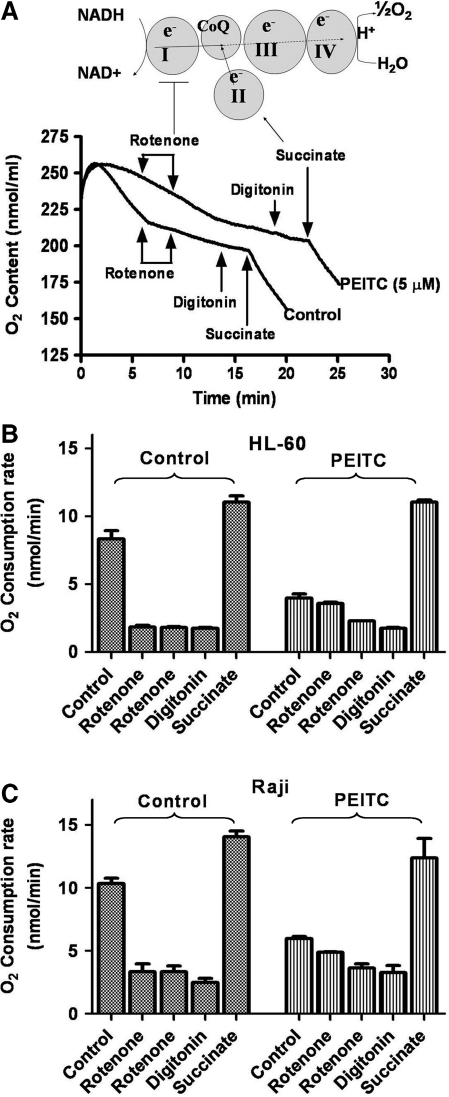
To further examine which respiratory chain complex might be inhibited by PEITC, we use a combination of specific respiratory complex inhibitors and substrates to assess the individual mitochondrial complex activity. As shown in Figure 3, HL-60 cells treated with or without PEITC were suspended in oxygenated culture medium (5 million cells/ml) and placed in a sealed chamber for measurement of oxygen consumption rate. At 5 and 8 min time points, two 10-μl aliquots of the complex I inhibitor rotenone (10 μM) were added to the chamber to ensure that the complex I activity was inhibited. After another 5 min, the mitochondrial membrane was made permeable by addition of digitonin (30 μg/ml), and the complex II substrate succinate (5 mM) was then added to measure the activity of complexes II-III-IV segment of the respiratory chain. We found that rotenone significantly inhibited the respiration rate in HL-60 cells by suppressing complex I activity in the control cells (Fig. 3A). In the cells treated with PEITC, oxygen consumption was substantially reduced, and rotenone only caused a slight further inhibition, thus suggesting that complex I was likely the main site of inhibition by PEITC. Consistently, when the complex II substrate succinate was added to the cells, the respiration rates of the control cells and the PEITC-treated cells were recovered to a similar degree (Fig. 3A, B), thus confirming that the activity of complexes II-III-IV was not significantly inhibited by PEITC. Similar results were observed in Raji cells (Fig. 3C).
FIG. 3.
Inhibition of mitochondrial respiratory chain complex I by PEITC. (A) Analysis of individual mitochondrial complex activity. The top panel shows the assay principle, and the lower panel shows assay results. HL-60 cells (6 million cells/ml) were incubated with or without 5 μM PEITC for 2 h, and oxygen consumption was monitored by using the Oxytherm system. Rotenone (100 nM), digitonin (30 μg/ml) and the complex II substrate succinate (5 mM) were added as indicated. Representative results of three experiments are shown. (B) Quantitative analysis of oxygen consumption rates of HL-60 cells shown in (A) assay conditions. Each column indicates Mean±SE. (C) Quantitative analysis of oxygen consumption rates of Raji cells treated with or without PEITC under the indicated conditions. Each column shows Mean±SE.
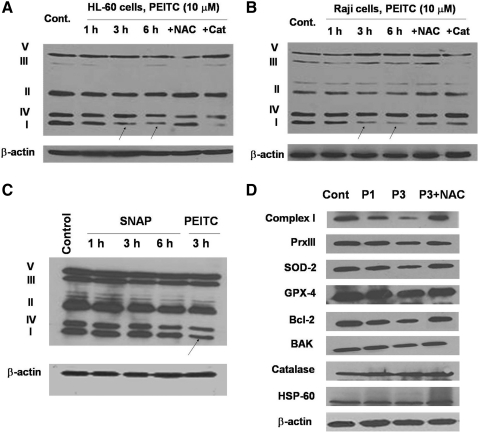
As shown in Figure 4A, PEITC treatment caused a decrease in the protein component of complex I and NAC, thus again preventing this degradation (Fig. 4B). These data are consistent with the observations shown in Figure 1, where PEITC inhibited respiration and NAC (but not catalase) reversed the inhibitory effect, and suggest that NO might contribute to triggering the degradation of complex I. However, when the ability of SNAP to cause degradation of complex I was compared with that of PEITC, it appeared that SNPA could only cause a moderate decrease of complex I, whereas PEITC was more effective (Fig. 4C).
FIG. 4.
Effect of PEITC and SNAP on the expression of representative protein components of the mitochondrial respiratory complexes. (A) HL-60 cells were treated with 10 μM PEITC with or without pretreatment with NAC or catalase for 1, 3, and 6 h, analyzed on a 12% SDS-PAGE, and then probed for representative components of the mitochondrial complexes using the respective specific antibody cocktail. The arrows indicate a decrease in complex I component. (B) Raji cells were treated with 10 μM PEITC with or without pretreatment with NAC or catalase for 1, 3, and 6 h, and the representative components of mitochondrial respiration complexes were analyzed by western blotting. (C) HL-60 cells were treated with 4 mM SNAP for 1, 3, and 6 h or 5 μM PEITC for 3 h. Cell lysates were separated by 12% SDS-PAGE and blotted with a cocktail of antibodies against representative mitochondrial complex components. The arrow indicates a decrease in complex I component. (D) HL-60 cells were treated with 10 μM PEITC with/without NAC pretreatment, and cell lysates were separated by 12% SDS-PAGE and blotted with various redox-related proteins by using specific antibodies as indicated. SDS-PAGE, sodium dodecyl sulfate–polyacrylamide gel electrophoresis.
When the protein extracts of mitochondria isolated from HL-60 cells were analyzed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) to determine the levels of mitochondrial anti-oxidant molecules and Bcl-2 family proteins, it was found that in addition to the decrease of complex I component, there was a decrease in peroxiredoxin III (Prx III), superoxide dismutase-2 (SOD-2), and Bcl-2. Glutathione peroxidase 4 (GPX-4) was slightly decreased. Bcl-2 homologous antagonist/killer, catalase, and HSP-60 showed no change (Fig. 4D). Pretreating the cells with NAC prevented the PEITC-induced decrease in complex I, Prx III, SOD-2, and Bcl-2 proteins.
Decrease of the mitochondrial Fe-S–containing protein NADH dehydrogenase Fe-S protein-3 in PEITC treated cells
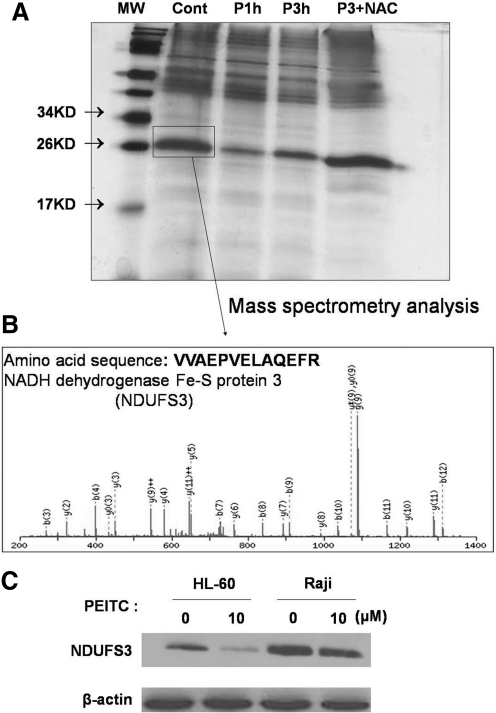
We used beads conjugated with antibodies against mitochondrial complex I to pull down mitochondrial proteins from the control and the PEITC-treated cells. The precipitated proteins were separated by a 12% SDS-PAGE and visualized by silver staining. As shown in Figure 5A, a protein band migrating slightly above the 26 kD marker showed a substantial decrease in the PEITC-treated cells, and pretreatment with NAC reversed this degradation. The protein from this band was recovered and analyzed by mass spectrometry (MS) analysis, which revealed the identity of this protein as NADH dehydrogenase Fe-S protein-3 (NDUFS3), a critical component of complex I (Fig. 5B). To further confirm the results of MS analysis, protein lysates from HL-60 and Raji cells treated with or without PEITC (10 μM, 3 h) were analyzed by western blotting, using a specific monoclonal antibody against NDUFS3. As shown in Figure 5C, PEITC indeed caused a decrease of NDUFS3 in both cell lines.
FIG. 5.
Decrease of mitochondrial respiratory complex I subunit NDUFS3 induced by PEITC. (A) HL-60 cells were treated with 10 μM PEITC for 1 and 3 h in the presence or absence of pretreatment with NAC. Mitochondria were isolated, and complex I proteins were immunoprecipitated. The precipitated products were separated by 12% SDS-PAGE and stained with a sliver staining kit. The protein band near 26 kD was cut out for mass spectrometry analysis. (B) Mass spectrometry analysis showing the amino acid sequence of the protein fragment (VVAEPVELAQEFR), which matched to NDUFS3. (C) HL-60 and Raji cells were treated with 10 μM PEITC for 3 h, and the cell lysates were separated by 12% SDS-PAGE and blotted with a NDUFS3 monoclonal antibody. NDUFS3, NADH dehydrogenase Fe-S protein-3.
PEITC induced a rapid depletion of mitochondrial GSH before the depletion of cytosolic GSH
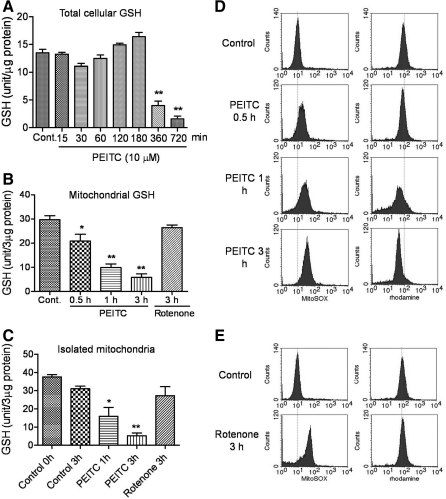
We treated HL-60 cells with 10 μM PEITC for various times and measured mitochondrial GSH in comparison with total cellular GSH. As shown in Figure 6A, total cellular GSH retained steady during the first 3 h of PEITC treatment, deceased at 6 h, and further diminished at 12 h. Surprisingly, analysis of mitochondrial GSH in the PEITC treated cells showed that the GSH in mitochondria started to decrease as early as 30 min after PEITC treatment, and 80% GSH was depleted by 3 h (Fig. 6B). In contrast, incubation of cells with rotenone for 3 h did not cause any depletion of mitochondrial GSH, thus suggesting that inhibition of respiration was not the cause of GSH depletion, and that GSH depletion by PEITC likely led to respiration inhibition, as supplements of the GSH precursor NAC reverse such inhibition (Fig. 1A, B). Interestingly, when the isolated mitochondria from untreated HL-60 cells were incubated with PEITC in vitro, the mitochondrial GSH was also rapidly depleted, with only 15% GSH remaining at 3 h (Fig. 6C), thus suggesting that depletion of mitochondrial GSH was a primary event induced by PEITC. Treatment of isolated mitochondria with rotenone did not reduce the mitochondrial GSH (Fig. 6C).
FIG. 6.
Effect of PEITC on cellular and mitochondrial GSH levels and ROS and membrane potential. (A) HL-60 cells were treated with 10 μM PEITC for various time points up to 12 h, and cellular GSH contents were determined. Each bar indicates the mean±SD from three experiments. (B) HL-60 cells were treated with 10 μM PEITC for 0.5, 1, and 3 h or 100 nM rotenone for 3 h. Mitochondria were then isolated, and the mitochondrial GSH contents were determined. Each bar indicates the mean±SD from three experiments. (C) Mitochondria were isolated from HL-60 cells, re-suspended in buffer, and then incubated with 10 μM PEITC for 0.5, 1, and 3 h or with 100 nM rotenone for 3 h. Mitochondrial GSH contents were determined. Each bar indicates the mean±SD from three experiments. *p<0.01; **p<0.001 by comparing to control. (D) HL-60 cells were treated with 10 μM PEITC for 0.5, 1, and 3 h; mitochondrial transmembrane potential and ROS levels were measured by flow cytometry by using rhodamine-123 and MitoSOX dye, respectively. (E) HL-60 cells were treated with 100 nM rotenone for 3 h, and mitochondrial membrane potential and ROS level were determined by flow cytometry by using rhodamine-123 and MitoSOX dye, respectively. GSH, glutathione.
The rapid depletion of mitochondrial GSH by PEITC was associated with a time-dependent increase of mitochondrial ROS detected by MitoSOX and a concurrent decrease of mitochondrial transmembrane potential (Fig. 6D). In contrast, although rotenone was able to inhibit the respiratory chain leading to elevated mitochondrial ROS, there was no loss of mitochondrial transmembrane potential (Fig. 6E), again suggesting that the depletion of mitochondrial GSH by PEITC, but not the inhibition of respiration, was a critical event that caused loss of mitochondrial integrity.
Depletion of GSH and inhibition of mitochondrial respiration synergistically enhanced cytotoxicity in leukemia cells
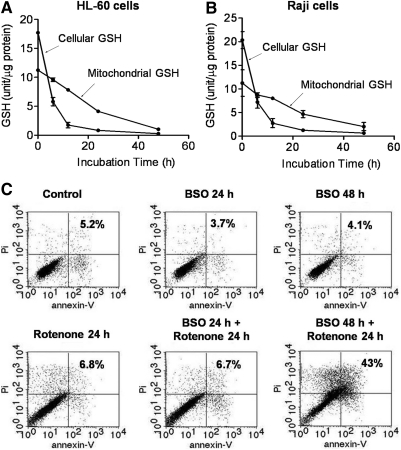
We postulated that a depletion of GSH and an inhibition of respiration might have synergistic effect on cell viability. To test this possibility, we first examined if L-buthionine sulfoximine (BSO), an inhibitor of GSH synthesis, could enhance the cytotoxic effect of rotenone. As shown in Figure 7A, incubation of HL-60 cells with BSO causes a time-dependent decrease of cellular and mitochondrial GSH, although the depletion of mitochondrial GSH occurred at a much slower rate compared with that induced by PEITC. Flow cytometry analysis of cell viability showed that BSO (500 μM) or rotenone (100 nM) as a single agent caused minimum cytotoxicity (5% cell death) in a 24 h incubation (Fig. 7B). However, combination of the two compounds resulted in a greater-than-additive cytotoxic effect (43% cell death).
FIG. 7.
Effect of BSO on cellular and mitochondrial GSH levels and rotenone-induced cytotoxicity. (A) HL-60 cells were treated with 500 μM BSO for various time points for up to 48 h, and cellular and mitochondrial GSH contents were determined. Each data point indicates the mean±SD from three experiments. (B) Raji cells were treated with 500 μM BSO for various time points up to 48 h, and cellular and mitochondrial GSH contents were determined. Each data point indicates the mean±SD from three experiments. (C) HL-60 cells were pretreated with BSO (500 μM) for 0 and 24 h, then each sample was incubated with rotenone (100 nM) in the presence of BSO for an additional 24 h. Cell viability was determined by annexin-V/PI double staining by using flow cytometry analysis. The number in each panel indicated % of death in cells. BSO, L-buthionine sulfoximine; PI, propidium iodide.
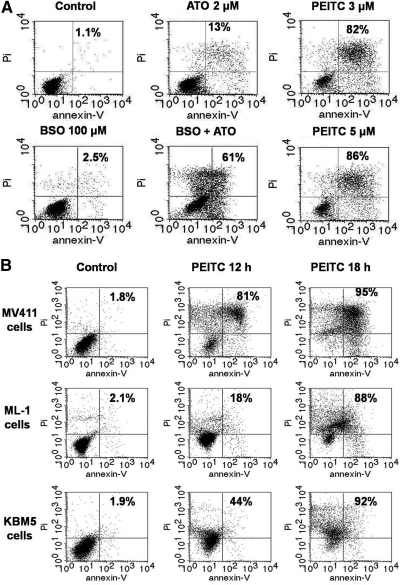
We also tested the combination of BSO with arsenic trioxide (ATO), a clinical drug with an ability to inhibit mitochondrial respiration. The results showed that this drug combination also had a greater-than-additive effect (Fig. 8A), and since PEITC could induce a rapid depletion of GSH and inhibition of respiration, this compound alone exhibited very potent anticancer activity in multiple leukemia cell lines (Fig. 8A, B), thus suggesting that PEITC has a promising potential as a new anticancer agent owing to its unique mechanisms of action.
FIG. 8.
Effect of BSO, ATO, and PEITC on cell viability in various leukemia cell lines. (A) HL-60 cells were treated with ATO (2 μM), BSO (500 μM), ATO+BSO, or PEITC for 18 h. Cell viability was determined by annexin-V/PI double staining followed by flow cytometry analysis. (B) MV411, ML-1, and KBM5 cells were treated with 5 μM PEITC for 18 h, cell viability was measured by flow cytometry using annexin-V/PI double staining. The number in each panel indicated % of death in cells. ATO, arsenic trioxide.
Discussion
PEITC effectively kills various cancer cells with low toxicity to normal cells (16, 36, 44, 46), suggesting that this compound may have a promising therapeutic potential due to its potent anticancer activity and selectivity. One likely mechanism that contributes to the anticancer selective of PEITC is the ability of this compound to disable the cellular GSH antioxidant system through depletion of GSH and inhibition of GPX enzyme activity (36, 46). Since cancer cells are under intrinsic ROS stress and, thus, highly dependent on GSH to keep redox balance, abrogation of the GSH system by PEITC would have a detrimental impact on cancer cells, whereas such redox modulation can be better tolerated by normal cells due to their low basal ROS output and intact redox regulatory mechanisms (38). Further, PEITC has been shown to have a significant hormetic protective effect due to its ability to induce the expression of various detoxification enzymes and antioxidant molecules, which may contribute to its chemopreventive activity (6). The potent anticancer activity of PEITC is likely attributed to its multiple mechanisms of action, including abrogation of the GSH system, direct binding to certain proteins that are important for cell survival, inducing phosphorylation of p66(Shc), and inhibition of enzymes such as GPX and proteasomes (30, 36, 41, 45). In our study, we found that 10 μM PEITC dramatically inhibited respiration by >80% in HL-60 and Raji cells with only a 3-h treatment. We also found a significant increase in H2O2 and NO associated with a decrease of mitochondrial transmembrane potential. When HL-60 and Raji cells were pretreated with the antioxidant NAC to decrease H2O2 and NO, the PEITC-induced respiratory inhibition was largely prevented in both cell lines. Interestingly, the H2O2-scavenger catalase could not suppress the PEITC-induced increase of NO and was unable to prevent respiratory suppression by PEITC. Recent studies suggest that the effect of PEITC on cellular NO may be cell-type dependent, as a positive effect was observed in osteogenic sarcoma cells (43), whereas suppression of NO was reported in macrophages (39). Thus, it would be interesting to test the effect of PEITC on respiration in macrophages to further evaluate the role of NO in mediating PEITC-induced respiratory inhibition.
Our study suggested that mitochondrial respiratory complex I was likely the main target site where PEITC exerted its inhibitory effect on the respiratory chain. Figure 9 illustrates the proposed mechanism of action of PEITC based on the results of this study. Treatment of cells with PEITC caused a rapid disruption of complex I manifested by an early degradation of the complex I component NDUFS3 and a loss of complex I function. The elevated NO likely contributed to the degradation of complex I proteins. Depletion of mitochondrial GSH by PEITC might also contribute to the instability of complex I protein, as GSH is known to affect protein stability through S-glutathionylation (8). It is also possible that PEITC itself might directly bind to certain respiratory complex components and cause their degradation. Functional analysis of individual complex activity showed that PEITC preferentially inhibited complex I, whereas complex II-IV segment of the respiratory chain was less impacted (Fig. 3). Interestingly, a recent study showed that induction of ROS increase by PEITC resulted in an inhibition of mitochondrial complex III activity, leading to apoptotic and autophagic cell death in human prostate cancer cells (44). Our study suggests that inhibition of complex I is likely a major effect of PEITC on leukemia cells.
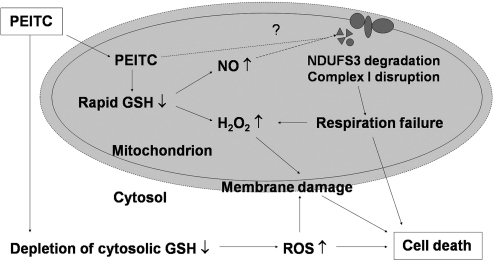
FIG. 9.
Proposed model for mechanisms of action of PEITC. PEITC causes a rapid depletion of mitochondrial GSH and an increase of mitochondrial ROS and NO. This is associated with a degradation of the mitochondrial complex I component NDUFS3 and a loss of respiratory function. It is unclear whether PEITC could directly target NDUFS3 and cause its instability (indicated by the question mark). The severe depletion of mitochondrial GSH, elevated ROS, and respiratory failure induced by PEITC leads to cell death. See text for further explanation.
In our study, cells treated with PEITC exhibited a rapid GSH depletion at early time points. The mitochondrial GSH level decreased as early as 1 h after PEITC incubation, whereas no noticeable change in cellular GSH level was observed until 6 h after PEITC treatment (Fig. 6A–C). Intriguingly, when the cells were treated with the complex I inhibitor rotenone, there was no significant change in mitochondrial GSH. Although rotenone caused an increase in mitochondrial ROS level, it did neither disrupt the mitochondrial membrane potential nor induce apoptosis (Fig. 6B). These findings indicate that mitochondrial GSH plays a key role in maintaining the function of mitochondria and in determining cellular function and viability in leukemia cells, consistent with the observations in other experimental systems (10, 11, 20, 25).
Human mitochondrial complex I (NADH-quinone oxidoreductase) is a membrane-bound enzyme complex consisting of 45 protein subunits (4, 7). Complex I is essential for oxidative phosphorylation and is a major source of ROS generation. Damage to complex I contributes to a range of pathological processes. There has been considerable interest in understanding how the function of complex I is affected by oxidative stress (3, 21). It has recently been reported that the mitochondrial thiol oxidant diamide can induce the glutathionylation of Cys-531 and Cys-704 of the 75 kD subunit of complex I (23). In our study, PEITC caused the rapid degradation of the NDUFS3 subunit, a core component involved in electron transfer from NADH to ubiquinone, and led to a substantial inhibition of respiration. One possible mechanism could be a protein conformational change when the subunit component is conjugated with PEITC in a low GSH/GSSG environment, thus leading to a loss of the complex stability. However, the exact mechanism responsible for the decrease of NDUFS3 is still unclear and requires further investigation. It would also be interesting to test whether PEITC would also cause degradation of other Fe-S proteins.
Materials and Methods
Reagents
PEITC, N-acetyl-L-cysteine (NAC), bovine catalase, rotenone, digitonin, succinate, BSO, and ATO were purchased from Sigma-Aldrich. Rhodamine-123, CM-H2DCF-DA, DAF-FM-DA, SNAP, and MitoSOX were obtained from Invitrogen/Molecular Probes.
Cell lines and cell culture
Human leukemia cell lines HL-60, ML-1, MV411, and KBM5 and human lymphoma cell line Raji were cultured in RPMI 1460 medium (Invitrogen) containing 10% fetal bovine serum. All cell lines were maintained in a cell culture incubator at 37°C in humidified air with 5% CO2.
Assays for cytotoxicity
Cell death was determined by flow cytometry after the cells had been double stained with annexin-V and propidium iodide, using an assay kit from BD PharMingen as previously described (33).
Analyses of cellular/mitochondrial ROS and mitochondrial transmembrane potential
Cellular H2O2, NO, and mitochondrial transmembrane potential were separately measured by first incubating the control or drug-treated cells (HL-60 or Raji cells in suspension culture) with 3 μM CM-H2DCF-DA, 3 μM DAF-FM-DA, or 100 nM rhodamine-123, respectively, for 30 min. The cells were then washed with phosphate-buffered saline (PBS), re-suspended in PBS, and analyzed by flow cytometry by using a FACSCalibur equipped with CellQuestPro software as previously described (33). To measure the mitochondrial ROS contents, the control or drug-treated cells were first collected by centrifugation (1000 rpm for 3 min), re-suspended in PBS containing 10 μM MitoSOX, and then incubated for 10 min. After washing with PBS and re-suspension in PBS, the cells were analyzed by flow cytometry by using a FACSCalibur equipped with CellQuestPro software.
Measurement of mitochondrial respiratory activity
The oxygen consumption rate in intact cells was measured as an indication of the mitochondrial respiratory activity. The control or drug-treated cells were suspended in 1 ml of culture medium pre-equilibrated with 21% oxygen and were then placed in a sealed respiration chamber to monitor oxygen consumption by using the Oxytherm system (Hansatech Instrument) as previously described (33). Rotenone (100 nM) was used to inhibit the complex I respiratory activity, and digitonin (30 μg/ml) was used to permeabilize the cells. An excess amount of succinate (5 mM) was then added as the complex II substrate.
Determination of cellular and mitochondrial GSH contents
A glutathione assay kit (Cayman Chemical Co.) was used to measure the total cellular and mitochondrial GSH levels. Cell extracts were prepared by sonication and deproteination, using the conditions recommended by the manufacturer. Mitochondria were isolated using the following procedures. Cells were harvested, re-suspended in 1 ml ice-cold RSB buffer (10 mM NaCl, 1.5 mM MgCl2, and 10 mM Tris-HCl, pH 7.5), and incubated for 10 min to allow them to swell under hypotonic conditions. The samples were then transferred to a 2-ml Dounce homogenizer, and the cells were broken with 15 strokes, followed by addition of 0.66 ml of 2.5× MS buffer (525 mM mannitol, 175 mM sucrose, 12.5 mM Tris-HCl, pH 7.5, 2.5 mM EDTA, and pH 7.5) and mixing with 5 more strokes. The homogenate was centrifuged for 5 min at 1500×g at 4°C to remove nuclei and unbroken cells. The supernatant was transferred to another centrifuge tube for further centrifugation (17,000×g, 4°C, 15 min). The mitochondrial pellets were washed twice with 1× MS buffer and then harvested by centrifugation at 17,000×g. The samples were transferred to 96-well plates, and total GSH was determined by using a glutathione assay kit. The reaction product glutathionylated 5,5′-dithiobis-(2-nitrobenzoic acid) was quantified by measuring the optical density at 405 nm by using a plate reader. The GSH levels were calculated by using a standard curve generated in parallel experiments. Cellular protein concentrations were determined by the bicinchoninic acid assay (Pierce) and were used for normalization of the GSH concentrations.
Immunoprecipitation of mitochondrial complex I proteins and MS
Mitochondria were isolated as just described, and complex I proteins were isolated by using an immunocapture kit (Mitosciences, Inc.) according to the assay procedures recommended by the manufacturer. The mitochondrial complex I proteins were then separated on a 12% SDS-PAGE and visualized using a color silver staining kit (Thermo Scientific, Inc.) according to the manufacturer's instructions. The protein bands of interest were excised from the gel, subjected to limited trypsin digestion, desalted through a small reversed-phase column (POROS 20R2), and directly eluted into a metallized nanospray needle (Proxeon Biosystems) in 5% formic acid and 60% acetonitrile. The needle was then positioned a few millimeters from the orifice of a QqTOF mass spectrometer (Qstar Pulsar-i), and a 1 kV spray potential was applied to acquire MS and MS-MS spectra (506.7 m/z).
Statistical analysis
The statistical significance of the differences in cytotoxicity and cellular/mitochondria GSH levels between two sample sets was evaluated by using Student's t-test. A p-value of <0.05 was considered statistically significant.
Abbreviations Used
- ATO
arsenic trioxide
- BSO
L-buthionine sulfoximine
- CLL
chronic lymphocytic leukemia
- CM-H2DCF-DA
5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate
- GPX4
glutathione peroxidase 4
- GSH
glutathione
- H2O2
hydrogen peroxide
- MS
mass spectrometry
- NAC
N-acetyl cysteine
- NDUFS3
NADH dehydrogenase Fe-S protein-3
- NO
nitric oxide
- PBS
phosphate-buffered saline
- PEITC
β-phenethyl isothiocyanate
- PI
propidium iodide
- Prx III
peroxiredoxin III
- ROS
reactive oxygen species
- SDS-PAGE
sodium dodecyl sulfate–polyacrylamide gel electrophoresis
- SNAP
S-nitroso-N-acetylpenicillamine
- SOD-2
superoxide dismutase-2
Acknowledgments
The authors thank L. Feng for technical assistance. This work was supported in part by grants CA085563, CA100428, CA109041, and CA16672 from the National Institutes of Health, and a grant for the CLL Global Research Foundation.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Armstrong JS. Jones DP. Glutathione depletion enforces the mitochondrial permeability transition and causes cell death in Bcl-2 overexpressing HL60 cells. FASEB J. 2002;16:1263–1265. doi: 10.1096/fj.02-0097fje. [DOI] [PubMed] [Google Scholar]
- 2.Bojes HK, et al. Bcl-xL overexpression attenuates glutathione depletion in FL5.12 cells following interleukin-3 withdrawal. Biochem J. 1997;325(Pt 2):315–319. doi: 10.1042/bj3250315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brandt U. Energy converting NADH:quinone oxidoreductase (complex I) Annu Rev Biochem. 2006;75:69–92. doi: 10.1146/annurev.biochem.75.103004.142539. [DOI] [PubMed] [Google Scholar]
- 4.Chen X, et al. Kinetics and regulation of mammalian NADH-ubiquinone oxidoreductase (Complex I) Biophys J. 2010;99:1426–1436. doi: 10.1016/j.bpj.2010.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheung KL, et al. PEITC induces G1 cell cycle arrest on HT-29 cells through the activation of p38 MAPK signaling pathway. AAPS J. 2008;10:277–281. doi: 10.1208/s12248-008-9032-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheung KL. Kong AN. Molecular targets of dietary phenethyl isothiocyanate and sulforaphane for cancer chemoprevention. AAPS J. 2010;12:87–97. doi: 10.1208/s12248-009-9162-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chomova M. Racay P. Mitochondrial complex I in the network of known and unknown facts. Gen Physiol Biophys. 2010;29:3–11. doi: 10.4149/gpb_2010_01_3. [DOI] [PubMed] [Google Scholar]
- 8.Dalle-Donne I, et al. S-glutathionylation in human platelets by a thiol-disulfide exchange-independent mechanism. Free Radic Biol Med. 2005;38:1501–1510. doi: 10.1016/j.freeradbiomed.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 9.Eaton P, et al. Detection, quantitation, purification, and identification of cardiac proteins S-thiolated during ischemia and reperfusion. J Biol Chem. 2002;277:9806–9811. doi: 10.1074/jbc.M111454200. [DOI] [PubMed] [Google Scholar]
- 10.Fernandez-Checa JC, et al. Mitochondrial glutathione: importance and transport. Semin Liver Dis. 1998;18:389–401. doi: 10.1055/s-2007-1007172. [DOI] [PubMed] [Google Scholar]
- 11.Fernandez-Checa JC, et al. Oxidative stress: role of mitochondria and protection by glutathione. Biofactors. 1998;8:7–11. doi: 10.1002/biof.5520080102. [DOI] [PubMed] [Google Scholar]
- 12.Franco R. Cidlowski JA. Apoptosis and glutathione: beyond an antioxidant. Cell Death Differ. 2009;16:1303–1314. doi: 10.1038/cdd.2009.107. [DOI] [PubMed] [Google Scholar]
- 13.Fratelli M, et al. Identification by redox proteomics of glutathionylated proteins in oxidatively stressed human T lymphocytes. Proc Natl Acad Sci USA. 2002;99:3505–3510. doi: 10.1073/pnas.052592699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fratelli M, et al. Identification of proteins undergoing glutathionylation in oxidatively stressed hepatocytes and hepatoma cells. Proteomics. 2003;3:1154–1161. doi: 10.1002/pmic.200300436. [DOI] [PubMed] [Google Scholar]
- 15.Friesen C. Kiess Y. Debatin KM. A critical role of glutathione in determining apoptosis sensitivity and resistance in leukemia cells. Cell Death Differ. 2004;11(Suppl 1):S73–S85. doi: 10.1038/sj.cdd.4401431. [DOI] [PubMed] [Google Scholar]
- 16.Gao N, et al. Phenethyl isothiocyanate exhibits antileukemic activity in vitro and in vivo by inactivation of Akt and activation of JNK pathways. Cell Death Dis. 2011;2:e140. doi: 10.1038/cddis.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gong A, et al. Phenethyl isothiocyanate inhibits STAT3 activation in prostate cancer cells. Mol Nutr Food Res. 2009;53:878–886. doi: 10.1002/mnfr.200800253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haga N. Fujita N. Tsuruo T. Involvement of mitochondrial aggregation in arsenic trioxide (As2O3)-induced apoptosis in human glioblastoma cells. Cancer Sci. 2005;96:825–833. doi: 10.1111/j.1349-7006.2005.00114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Han D, et al. Sites and mechanisms of aconitase inactivation by peroxynitrite: modulation by citrate and glutathione. Biochemistry. 2005;44:11986–11996. doi: 10.1021/bi0509393. [DOI] [PubMed] [Google Scholar]
- 20.Han D, et al. Mechanisms of liver injury. III. Role of glutathione redox status in liver injury. Am J Physiol Gastrointest Liver Physiol. 2006;291:G1–G7. doi: 10.1152/ajpgi.00001.2006. [DOI] [PubMed] [Google Scholar]
- 21.Hirst J, et al. The nuclear encoded subunits of complex I from bovine heart mitochondria. Biochim Biophys Acta. 2003;1604:135–150. doi: 10.1016/s0005-2728(03)00059-8. [DOI] [PubMed] [Google Scholar]
- 22.Hu R, et al. The roles of JNK and apoptotic signaling pathways in PEITC-mediated responses in human HT-29 colon adenocarcinoma cells. Carcinogenesis. 2003;24:1361–1367. doi: 10.1093/carcin/bgg092. [DOI] [PubMed] [Google Scholar]
- 23.Hurd TR, et al. Complex I within oxidatively stressed bovine heart mitochondria is glutathionylated on Cys-531 and Cys-704 of the 75-kDa subunit: potential role of CYS residues in decreasing oxidative damage. J Biol Chem. 2008;283:24801–24815. doi: 10.1074/jbc.M803432200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jakubikova J. Bao Y. Sedlak J. Isothiocyanates induce cell cycle arrest, apoptosis and mitochondrial potential depolarization in HL-60 and multidrug-resistant cell lines. Anticancer Res. 2005;25:3375–3386. [PubMed] [Google Scholar]
- 25.Kaplowitz N. Aw TY. Ookhtens M. The regulation of hepatic glutathione. Annu Rev Pharmacol Toxicol. 1985;25:715–744. doi: 10.1146/annurev.pa.25.040185.003435. [DOI] [PubMed] [Google Scholar]
- 26.Kim HR, et al. Combination treatment with arsenic trioxide and sulindac augments their apoptotic potential in lung cancer cells through activation of caspase cascade and mitochondrial dysfunction. Int J Oncol. 2006;28:1401–1408. [PubMed] [Google Scholar]
- 27.Li JJ, et al. Role of oxidative stress in the apoptosis of hepatocellular carcinoma induced by combination of arsenic trioxide and ascorbic acid. Acta Pharmacol Sin. 2006;27:1078–1084. doi: 10.1111/j.1745-7254.2006.00345.x. [DOI] [PubMed] [Google Scholar]
- 28.Mi L, et al. Covalent binding to tubulin by isothiocyanates. A mechanism of cell growth arrest and apoptosis. J Biol Chem. 2008;283:22136–22146. doi: 10.1074/jbc.M802330200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mi L, et al. Cancer preventive isothiocyanates induce selective degradation of cellular alpha- and beta-tubulins by proteasomes. J Biol Chem. 2009;284:17039–17051. doi: 10.1074/jbc.M901789200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mi L. Gan N. Chung FL. Isothiocyanates inhibit proteasome activity and proliferation of multiple myeloma cells. Carcinogenesis. 2011;32:216–223. doi: 10.1093/carcin/bgq242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nulton-Persson AC, et al. Reversible inactivation of alpha-ketoglutarate dehydrogenase in response to alterations in the mitochondrial glutathione status. Biochemistry. 2003;42:4235–4242. doi: 10.1021/bi027370f. [DOI] [PubMed] [Google Scholar]
- 32.Odin JA, et al. Bcl-2-dependent oxidation of pyruvate dehydrogenase-E2, a primary biliary cirrhosis autoantigen, during apoptosis. J Clin Invest. 2001;108:223–232. doi: 10.1172/JCI10716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pelicano H, et al. Mitochondrial respiration defects in cancer cells cause activation of Akt survival pathway through a redox-mediated mechanism. J Cell Biol. 2006;175:913–923. doi: 10.1083/jcb.200512100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Queiroga CS, et al. Glutathionylation of adenine nucleotide translocase induced by carbon monoxide prevents mitochondrial membrane permeabilization and apoptosis. J Biol Chem. 2010;285:17077–17088. doi: 10.1074/jbc.M109.065052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sarti P, et al. Nitric oxide and mitochondrial complex IV. IUBMB Life. 2003;55:605–611. doi: 10.1080/15216540310001628726. [DOI] [PubMed] [Google Scholar]
- 36.Trachootham D, et al. Selective killing of oncogenically transformed cells through a ROS-mediated mechanism by beta-phenylethyl isothiocyanate. Cancer Cell. 2006;10:241–252. doi: 10.1016/j.ccr.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 37.Trachootham D, et al. Effective elimination of fludarabine-resistant CLL cells by PEITC through a redox-mediated mechanism. Blood. 2008;112:1912–1922. doi: 10.1182/blood-2008-04-149815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Trachootham D. Alexandre J. Huang P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat Rev Drug Discov. 2009;8:579–591. doi: 10.1038/nrd2803. [DOI] [PubMed] [Google Scholar]
- 39.Tsai JT. Liu HC. Chen YH. Suppression of inflammatory mediators by cruciferous vegetable-derived indole-3-carbinol and phenylethyl isothiocyanate in lipopolysaccharide-activated macrophages. Mediators Inflamm. 2010;2010:293642. doi: 10.1155/2010/293642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Voehringer DW. BCL-2 and glutathione: alterations in cellular redox state that regulate apoptosis sensitivity. Free Radic Biol Med. 1999;27:945–950. doi: 10.1016/s0891-5849(99)00174-4. [DOI] [PubMed] [Google Scholar]
- 41.Wang X, et al. Selective Depletion of mutant p53 by cancer chemopreventive isothiocyanates and their structure-activity relationships. J Med Chem. 2011;54:809–816. doi: 10.1021/jm101199t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang XH, et al. Inhibition of hypoxia inducible factor by phenethyl isothiocyanate. Biochem Pharmacol. 2009;78:261–272. doi: 10.1016/j.bcp.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 43.Wu CL, et al. Benzyl isothiocyanate (BITC) and phenethyl isothiocyanate (PEITC)-mediated generation of reactive oxygen species causes cell cycle arrest and induces apoptosis via activation of caspase-3, mitochondria dysfunction and nitric oxide (NO) in human osteogenic sarcoma U-2 OS cells. J Orthop Res. 2011;29:1199–1209. doi: 10.1002/jor.21350. [DOI] [PubMed] [Google Scholar]
- 44.Xiao D, et al. Phenethyl isothiocyanate inhibits oxidative phosphorylation to trigger reactive oxygen species-mediated death of human prostate cancer cells. J Biol Chem. 2010;285:26558–26569. doi: 10.1074/jbc.M109.063255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xiao D. Singh SV. p66Shc is indispensable for phenethyl isothiocyanate-induced apoptosis in human prostate cancer cells. Cancer Res. 2010;70:3150–3158. doi: 10.1158/0008-5472.CAN-09-4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xu K. Thornalley PJ. Involvement of glutathione metabolism in the cytotoxicity of the phenethyl isothiocyanate and its cysteine conjugate to human leukaemia cells in vitro. Biochem Pharmacol. 2001;61:165–177. doi: 10.1016/s0006-2952(00)00526-8. [DOI] [PubMed] [Google Scholar]
- 47.Zhang H, et al. Effective killing of Gleevec-resistant CML cells with T315I mutation by a natural compound PEITC through redox-mediated mechanism. Leukemia. 2008;22:1191–1199. doi: 10.1038/leu.2008.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zimmermann AK, et al. Glutathione binding to the Bcl-2 homology-3 domain groove: a molecular basis for Bcl-2 antioxidant function at mitochondria. J Biol Chem. 2007;282:29296–29304. doi: 10.1074/jbc.M702853200. [DOI] [PMC free article] [PubMed] [Google Scholar]