ABSTRACT
Background:
Hepatocellular carcinoma (HCC) is frequently resistant to chemotherapy. However, epidermal growth factor receptor (EGFR) inhibition has demonstrated activity in HCC and overcomes chemotherapy resistance in other settings. We studied the efficacy of combining the anti-EGFR antibody cetuximab with capecitabine and oxaliplatin in advanced HCC.
Methods:
Patients who had chemotherapy-naive advanced/unresectable HCC and any Childs-Pugh–class chronic liver disease (provided bilirubin was <3 mg/dl) received capecitabine 850 mg/m2 bid days 1–14, oxaliplatin 130 mg/m2 day 1, and cetuximab 400 mg/m2 day 1 then 250 mg/m2 weekly for each 21 day cycle.
Results:
Twenty-nine patients received any protocol therapy, but 24 completed at least one cycle. Of the 24 patients evaluable for response, 3 had a partial response (12.5%, 95% confidence interval [CI], 3–32%) and 17 had stable disease (71%), for a disease control rate of 83%. Of patients with an elevated AFP, 57% had a >50% reduction in AFP. Median time to progression was 4.5 months (95% CI, 3.2–6.4), and overall survival was 4.4 months (95% CI, 2.4–7.3). Most common toxicities included diarrhea (13 patients, 45%), fatigue (12 patients, 41%), and hypomagnesemia (12 patients, 41%). Fatigue (6 patients) and diarrhea (5 patients) were the most common grade 3–4 toxicities. Three patients died within the first 30 days of treatment (one of toxicity, two of liver failure presumed to be related to disease progression).
Conclusions:
The capecitabine/oxaliplatin/cetuximab combination was tolerable, though diarrhea was pronounced, in this population. The combination was associated with a modest response rate, but a high rate of AFP response and radiographic stable disease. Time to progression and overall survival were shorter than would be expected for treatment with sorafenib.
Hepatocellular carcinoma (HCC) is a leading killer worldwide. With the incidence of HCC on the rise in the United States and elsewhere,1,2 lack of effective treatment for advanced HCC is a growing concern. Surgical resection or liver transplantation remains the only potentially curative therapies for HCC, but most patients are ineligible for such treatments. Locoregional strategies, such as chemoembolization, are the mainstay of treatment for patients with inoperable HCC, because few systemic options are available. Doxorubicin, until recently considered the standard chemotherapeutic for HCC, is associated with an objective response rate of approximately 10%.3,4 Sorafenib is the first agent to demonstrate a survival advantage over supportive care in HCC. Nevertheless, in a relatively fit group of sorafenib-treated patients (95% Childs-Pugh A), median survival was only 10.7 months.5
Epidermal growth factor receptor (EGFR) is overexpressed in HCC, compared with adjacent liver.6 Data for EGFR inhibition in HCC by the tyrosine kinase inhibitor erlotinib suggests a therapeutic effect of EGFR inhibition.7 Cetuximab, a chimeric murine-human monoclonal IgG1 to EGFR, prevents activation of EGFR by inhibiting ligand binding. Cetuximab may also inhibit heterodimerization of HER-family members, which could translate into greater efficacy in HCC compared with tyrosine kinase inhibition given the dual expression of HER-1/erbB-1 and HER-3/erbB-3 in HCC.8
A substantial body of literature now suggests that inhibition of the EGFR pathway can improve the response rate with chemotherapy and radiotherapy in tumors where EGFR is constitutively active. This effect likely results from lowering a tumor's apoptotic threshold by inhibiting important antiapoptotic signals that are constitutively activated by an overstimulated EGFR pathway in cancer.9 This sensitization phenomenon has already been convincingly demonstrated in colorectal cancer, where cetuximab added to irinotecan is able to overcome irinotecan resistance.10 Similar preclinical data exist for platinum compounds, with the suggestion of a synergistic interaction between EGFR inhibition and platinum agents.9
Oxaliplatin has demonstrated safety as well as modest single-agent activity in HCC.11 Oxaliplatin is also well tolerated in patients with hyperbilirubinemia,12 making it an excellent potential agent for HCC compared with doxorubicin, a drug contraindicated in those with abnormal bilirubin levels. The combination of oxaliplatin and capecitabine has also shown some promise in the phase II setting.13 This is an attractive combination for use in HCC because both drugs are tolerated in the setting of hepatic dysfunction.
We conducted a phase II trial of the combination of capecitabine/oxaliplatin (CapeOx) with cetuximab in patients with unresectable or metastatic HCC not previously treated with systemic therapy to evaluate the response rate, time to progression, and safety of this combination.
METHODS
Eligibility
This single-institution phase II trial enrolled patients from the gastrointestinal oncology clinic at the University of North Carolina. Eligible patients were 18 years of age or older and had a histologic diagnosis of hepatocellular carcinoma, or an alpha fetoprotein (AFP) level >400 ng/mL in the setting of a clinical picture (tumor characteristics on imaging studies in a patient with cirrhosis) consistent with HCC. EGFR positivity was not required because immunohistochemistry staining for EGFR is inaccurate for determining response in tumors such as colorectal cancer. All patients had either metastatic disease or disease not amenable to resection or immediate transplantation. Measurable disease or evaluable disease in combination with an AFP level two times the upper limit of normal (ULN) were also required. Prior locoregional therapy, including transarterial chemoembolization, was permitted, but prior systemic therapy was not.
Laboratory requirements included the following: total bilirubin ≤ 3 × ULN; aspartate aminotransferase (AST) and (alanine aminotransferase) ALT ≤ 5 × ULN; creatinine clearance (estimated by Cockcroft-Gault) > 50 ml/min; absolute neutrophil count ≥ 1.5 × 109 cells/L; hemoglobin ≥ 9 g/dl; international normalized ratio (INR) ≤ 1.5. An initial platelet cutoff of >100,000 × 109 cells/L was amended to 75,000 × 109 cells/L after the initial six patients.
Patients were ineligible if they had any of the following conditions: a comorbid illness with a life expectancy of <6 months or a second cancer other than nonmelanomatous skin cancer or cervical intraepithelial neoplasia; uncontrolled central nervous system metastases; variceal bleeding within 60 days; ongoing drug or alcohol abuse; or need for therapeutic anticoagulation with warfarin.
Treatment
Treatment consisted of oxaliplatin 130 mg/m2 intravenously (IV) over 120 min on day 1, cetuximab 400 mg/m2 IV over 90 min on day 1 of cycle 1 followed by 250 mg/m2 IV over 60 min weekly, and capecitabine 850 mg/m2 PO bid days 1–14 of each 21-day cycle.
Treatment with oxaliplatin and capecitabine was held for grade 3 or higher chemotherapy toxicity, with resumption at 80% of the initial dose after resolution to grade 0–1. For the following toxicities, however, treatment was held for grade 2 or higher toxicity: thrombocytopenia with dose reduction for grade 4 thrombocytopenia; diarrhea with dose reduction for grade 2 or higher; or hepatotoxicity with dose reductions for grade 2 or higher toxicity. CapeOx was held in the setting of grade 4 anemia. Cetuximab was held for grade 3 or higher skin toxicity and was held permanently in the case of grade 3 or 4 hypersensitivity reaction.
A protocol-specified halt in enrollment occurred after the first 10 patients completed cycle 1 of therapy in order to assess toxicity. According to the prespecified rules, treatment continued at the original doses for the subsequent patients.
Study Measures
Toxicity was measured using the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE; version 3.0). Toxicity was recorded by study personnel in the second week of cycle 1, then at the commencement of each subsequent cycle. Response was measured using the Response Evaluation Criteria in Solid Tumors (RECIST) criteria, with a radiographic assessment and repeat AFP for those with elevated AFP at baseline performed every other cycle.
Statistical Considerations
The primary objective was to ascertain the response rate (RR) of HCC to the combination of CapeOx and cetuximab. Secondary objectives included an assessment of safety of CapeOx/cetuximab in patients with HCC and compensated liver disease and estimation of median overall survival (OS), time to progression (TTP), and progression-free survival (PFS) in HCC patients treated with CapeOx/cetuximab.
A minimax two-stage design was used in which two responses were needed in the first 16 evaluable patients to go on to the second stage, after which 9 more evaluable patients were enrolled. Five or more responses among the 25 treated patients were required to consider CapeOx/cetuximab to merit further study. Enrollment was discontinued after 24 patients because the end point of five responses could not be reached.
TTP was defined as the time from study entry to disease progression. Deaths occurring in the absence of proven disease progression were censored. PFS was defined as time from study entry to disease progression or death from any cause. TTP, PFS, and OS were estimated using the Kaplan-Meier method, with cutoff for events of April 22, 2009. The trial was approved by the Biomedical Institutional Review Board of the University of North Carolina at Chapel Hill.
Role of the Funding Source
The authors were responsible for study design, data collection, analysis and interpretation of the data, and the decision to submit for publication. The principle investigator (B.H.O.) had full access to the data. The study sponsors had no role in design, data analysis, or decision to submit these results for publication.
RESULTS
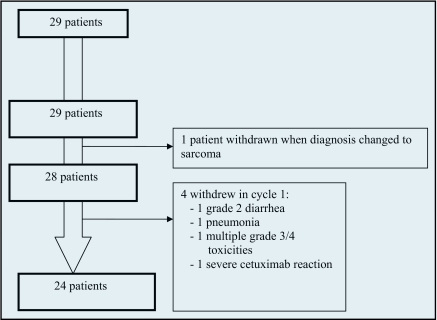
Twenty-nine patients received any protocol directed therapy (Figure 1), all of whom are included in toxicity assessment. One patient with a diagnosis of HCC based on fine-needle aspirate developed a hemothorax, necessitating surgical intervention during treatment. The surgical pathology specimen demonstrated sarcoma, and the patient was withdrawn from protocol. This patient is not included in any efficacy analysis. Four additional patients received some protocol treatment but withdrew before completing one cycle without assessment of response. Reasons for withdrawal included the following: one patient with an immediate cetuximab-associated anaphylaxis who received no further protocol directed therapy; one patient with grade 2 diarrhea in week 2; one patient with pneumonia in the setting of obstructive lung disease who required a 2-week hospitalization; one patient with hospitalization for multiple grade 3 and 4 toxicities who was discharged to hospice care. Two patients had hypersensitivity reactions to cetuximab and continued on protocol with CapeOx alone.
Figure 1.
Consort diagram.
The enrolled patients were predominantly male (82%) and white (61%), with a median age of 59 years (Table 1). Thirteen (45%) had received prior locoregional therapy including transarterial chemoembolization (8), resection (4), radiofrequency ablation (4), and external beam radiotherapy (1). Three of these patients received more than one prior treatment. At enrollment, 14 (52%) patients had an Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0, 12 (44%) were PS 1, and 1 patient had a PS of 2. All patients had a Childs-Pugh classification of A (18) or B. (6) Eighteen patients had an elevated AFP prior to treatment.
Table 1.
Patient characteristics
| N = 28* | |
|---|---|
| Median age (range) | 59 (46–79) |
| Sex (n, %) | |
| Male | 23 (82%) |
| Female | 5 (18%) |
| Race/ethnicity (n, %) | |
| White | 17 (61%) |
| Black | 9 (32%) |
| Latino | 1 (4%) |
| Other | 1 (4%) |
| ECOG PS (n, %) | |
| 0 | 14 (52%) |
| 1 | 12 (44%) |
| 2 | 1 (4%) |
| Childs-Pugh classification† (n, %) | |
| A | 18 |
| B | 6 |
| Prior therapy (n, %) | |
| None | 15 (54%) |
| Yes | 13 (46%) |
| Multiple | 3 |
| TACE | 8 |
| Resection | 4 |
| RFA | 4 |
| EBRT | 1 |
Rows may not sum to 100% because of rounding error.
28 points with HCC.
Childs-Pugh was available only for the 24 evaluable patients.
Abbreviations: ECOG = Eastern Cooperative Oncology Group; PS = performance status; RFA = radiofrequency ablation; TACE = transarterial chemoembolization.
Efficacy
Twenty-two patients underwent disease assessment following two cycles of protocol therapy. Two patients died of liver failure clinically considered to be attributable to rapid disease progression and are included as progressive disease. Of 24 evaluable patients, 3 (12.5%, 95% CI, 3%–32%, or 11% by intention to treat analysis [ITT]) had a partial response, and 17 patients (71%, 63% ITT) had stable disease for a disease control rate of 83%. One patient continues on protocol with stable disease for 19 months. Of the 18 patients with AFP >200 ng/ml at baseline, 8 of the 14 patients with serial testing (57%) had at least a 50% reduction in AFP during protocol therapy. The two patients treated with CapeOx alone following hypersensitivity to cetuximab did not respond.
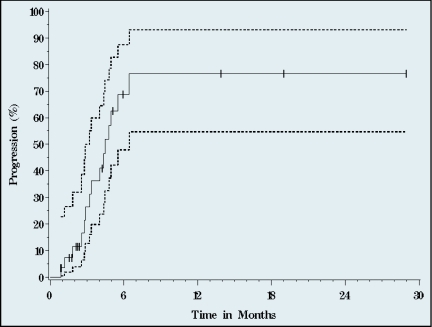
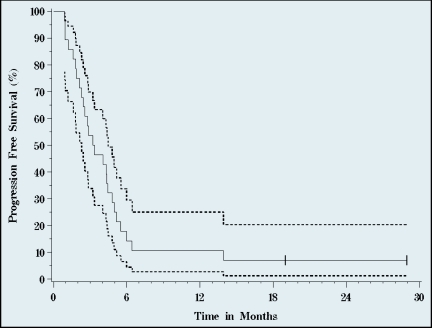
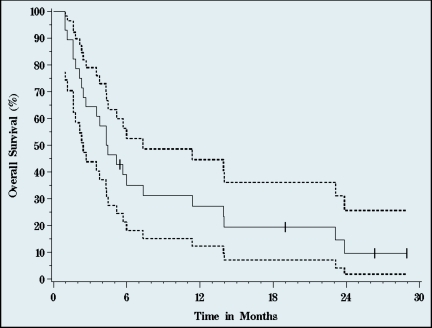
After a median 23 months follow-up of survivors, median TTP was 4.5 months (95% CI, 3.2–6.4) (Figures 2–4), median PFS 3.3 (95% CI, 2.3–4.5). Median OS was 4.4 months (95% CI, 2.4–7.3). Three of the 28 patients are alive at 19 (remain on study), 26, and 28 months. One-year survival was 27%, and 10% of patients were alive at 2 years.
Figure 2.
Time to progression. Median time to progression was 4.5 months (95% CI, 2.3–4.5 months). Solid line represents progression, dotted lines signify 95% CI (n=28, 15 events).
Figure 3.
Progression-free survival. Median progression-free survival was 3.3 months (95% CI, 2.3–4.5 months). Solid line represents progression, dotted lines signify 95% confidence intervals (n=28, 26 events).
Figure 4.
Overall survival. Median overall survival was 4.4 months (95% CI, 2.4–7.3 months). Solid line represents survival, dotted lines signify 95% confidence intervals (n=28, 24 events).
Toxicity
The most commonly experienced toxicities were diarrhea (13, 45%), hypomagnesemia (12, 41%), fatigue (12, 41%), mucositis (9, 31%), nausea (8, 28%), and hypocalcemia (8, 28%) (Table 2). The most common grade 3 or higher toxicities were fatigue (6, 21%), diarrhea (5, 17%), and mucositis (4, 14%). Grade 3 infection with grade 1–2 neutropenia occurred in four patients, three of whom had pneumonia and one who developed a simultaneous urinary tract infection and wound infection. Twenty-one percent of patients experienced cetuximab-associated acneiform rash, only one of which was grade 3.
Table 2.
Most common toxicities (n = 29)
| Toxicity | All grades n (%) | Grades 3–5 n |
|---|---|---|
| Fatigue | 13 (45%) | 6 |
| Diarrhea | 12 (41%) | 5 |
| Hypomagnesemia | 12 (41%) | 2 |
| Mucositis | 9 (31%) | 4 |
| Hypocalcemia | 8 (28%) | 3 |
| Nausea | 8 (28%) | 1 |
| Anemia | 7 (24%) | 2 |
| Hyperbilirubinemia | 7 (24%) | 3 |
| Thrombocytopenia | 7 (24%) | 3 |
| Vomiting | 7 (24%) | 1 |
| Sensory neuropathy | 7 (24%) | 1 |
| AST elevation | 6 (21%) | 3 |
| Acneiform rash | 6 (21%) | 1 |
| Infection with grade 1–2 neutropenia | 5 (17%) | 4 |
| Hyponatremia | 5 (17%) | 3 |
| Alkaline phosphatase elevation | 5 (17%) | 2 |
| ALT elevation | 5 (17%) | 1 |
| Hyperglycemia | 4 (14%) | 2 |
| Hypersensitivity reaction | 4 (14%) | 2 |
| Neutropenia | 4 (14%) | 1 |
| Hypokalemia | 3 (10%) | 3 |
| Dehydration | 3 (10%) | 3 |
| Hand-foot syndrome | 3 (10%) | 0 |
Abbreviations: ALT = alanine aminotransferase; AST = aspartate aminotransferase.
Severe toxicities reflective of worsening liver function included hyperbilirubinemia (3, 10%) and AST elevation (3, 10%). Grade 3 thrombocytopenia occurred in 10% of patients, though only one patient had any bleeding (grade 3 upper gastrointestinal bleed). Four patients (14%) had hypersensitivity reaction to cetuximab, two of which were severe. Three patients died within 30 days of initiating treatment: two of liver failure thought clinically to be due to rapid progression of disease, and one of treatment-associated toxicities.
DISCUSSION
In this single-institution phase II trial, treatment with CapeOx and cetuximab in patients with advanced or metastatic HCC resulted in a radiographic response rate of only 12.5%, thus failing to meet our prespecified criteria for further study of this combination. However, a disease control rate of 83% and an AFP response in 57% of patients suggest this regimen does have activity against HCC.
That the base chemotherapeutic regimen has activity in HCC is supported by a recently published French phase II trial of CapeOx, with capecitabine given at 1,000 mg/m2 bid compared with 850 mg/m2 bid in our study, that reported a response rate of 6% and a disease control rate of 72%.13 Median OS (9.3 months) and PFS (4.1 months) were better than what we observed (OS of 4.5 months). Eighty-six percent of patients in the French study had Childs-Pugh A liver disease, similar to the 75% in our study.
Another phase II trial of CapeOx with bevacizumab reported similar results, with a RR of 11%, disease control rate of 89%, and PFS of 5.4 months.14 Though OS seen in our patient population was substantially shorter than that of sorafenib-treated patients (median PFS 5.5 months and median OS 10.7 months),5 these other CapeOx trials support our findings that CapeOx does result in disease control in the majority of patients. The reason for the short TTP, PFS, and OS in our study is not clear. The mix of cases according to the Childs-Pugh classification and PS were similar across all of these studies. However, practice patterns and underlying disease may differ between the centers recruiting to these phase II trials and our institution.
To what extent the addition of cetuximab improved the disease control rate offered by this regimen is unclear. In fact, a study of single-agent cetuximab in HCC did not demonstrate significant activity,15 with stable disease seen in only 44% of patients. Of those with stable disease for longer than 8 weeks, however, time to progression was 22 weeks, which suggests that some small proportion of patients does glean benefit from single-agent cetuximab.16
It has recently been found in colorectal cancer that levels of EGFR ligands and lack of mutation of KRAS predict activity of cetuximab.17 As RAS mutation is infrequent in HCC,18 one might have predicted significant activity from cetuximab. Another factor that may influence response of colorectal cancer to anti-EGFR therapy is presence of excess levels of EGFR ligands, particularly amphiregulin and epiregulin.19 Interestingly, amphiregulin expression has been noted to be up-regulated in cirrhotic liver compared with normal liver in mouse models. To date, EGFR ligands have not been studied extensively in human HCC samples,20 and further study of this would seem warranted because such patients may make up a subset that would benefit from EGFR-based therapies.
Another recently reported phase II trial of the combination of gemcitabine with oxaliplatin and cetuximab reported a fairly good response rate (20%) and PFS time (4.5 months),21 though again, to what degree cetuximab contributed to this benefit is unclear.
The incidence of cetuximab-associated allergic reaction observed in HCC patients enrolled in this trial was considerably lower than we have previously reported for patients in the southeastern United States. Among 88 patients treated with cetuximab on clinical trials at cancer research centers in North Carolina and Tennessee (University of North Carolina, Vanderbilt University, and the Sarah Canon Cancer Institute), the rate of cetuximab hypersensitivity reactions was 28%.22 Twenty-two percent of patients had a severe grade 3 or 4 allergic reaction. In contrast, though still higher than expected, of the 29 HCC patients in this trial, only 14% had any allergic reaction, and two (7%) had a severe hypersensitivity reaction. It is hoped that ongoing correlative studies on cetuximab-associated reactions will explain why HCC patients might have a lower rate than the previously reported rate in colorectal, lung, and head and neck cancer patients.
CapeOx and cetuximab demonstrated modest activity in advanced hepatocellular carcinoma in this phase II trial. In light of the similar results of our trial with other reports of Cape/Ox alone in HCC, and that the most common severe toxicities we observed (fatigue, diarrhea, mucositis) were all likely exacerbated by cetuximab, pursuing further study of CapeOx with the addition of cetuximab in an unselected population of HCC patients does not seem warranted. Future work will need to focus on identifying molecular markers (eg, amphiregulin and epiregulin) predictive of clinical benefit from EGFR inhibitors in HCC.
Footnotes
Results of this trial were presented in part at the 2008 Gastrointestinal Cancers Symposium and the 2008 annual meeting of the American Society of Clinical Oncology.
This trial was supported by sanofi-aventis, Roche, Bristol-Myers Squibb, and the National Institutes of Health (Dr. Sanoff [KL2 RR025746] and Dr. O’Neil [NIH 5K23CA118431-02]).
Author Contributions
Study conception and design: H. K. Sanoff, B. H. O'Neil; Subject enrollment, data collection, and management: H. K. Sanoff, S. Bernard, R. M. Goldberg, R. Garcia, L. Woods, M. A. Morse, D. T. Moore, B. H. O'Neil; Data analysis: H. K. Sanoff, D. T. Moore, B. H. O'Neil; Manuscript preparation and approval: H. K. Sanoff, S. Bernard, R. M. Goldberg, R. Garcia, M. A. Morse, L. Woods, D. T. Moore, B. H. O'Neil.
Disclosures of Potential Conflicts of Interest
Dr. Goldberg serves as a consultant for sanofi-aventis, Bristol-Myers Squibb, and Roche., Dr. Morse has served as a consultant, received research funding, and/or participated in speakers' bureaus for sanofi-aventis, Bristol-Myers Squibb, and Roche. Dr. O'Neil has served as a consultant and/or participated in speakers' bureaus for sanofi-aventis, Bristol-Myers Squibb, and Roche.
This trial was supported by sanofi-aventis, Roche, Bristol-Myers Squibb, and the National Institutes of Health (Dr. Sanoff [KL2 RR025746] and Dr. O’Neil [NIH 5K23CA118431-02]).
REFERENCES
- 1. Ries L, Melbert D, Krapcho M, et al. : SEER Cancer Statistics Review, 1975–2004, National Cancer Institute. Bethesda, MD: Available at: http://seer.cancer.gov/csr/1975_2004/, based on November 2006 SEER data submission, posted to the SEER web site, 2007 [Google Scholar]
- 2. International Agency for Cancer Research: Available at: http://www-dep.iarc.fr/
- 3. Yeo W, Mok TS, Zee B, et al. : A randomized phase III study of doxorubicin versus cisplatin/interferon alpha-2b/doxorubicin/fluorouracil (PIAF) combination chemotherapy for unresectable hepatocellular carcinoma. J Natl Cancer Inst 97:1532–1538, 2005 [DOI] [PubMed] [Google Scholar]
- 4. Lai CL, Wu PC, Chan GC, et al. : Doxorubicin versus no antitumor therapy in inoperable hepatocellular carcinoma: a prospective randomized trial. Cancer 62:479–483, 1988 [DOI] [PubMed] [Google Scholar]
- 5. Llovet J, Ricci S, Mazzaferro V, et al. : Sorafenib in advanced hepatocellular carcinoma. N Eng J Med 359:378–390, 2008 [DOI] [PubMed] [Google Scholar]
- 6. Ito Y, Takeda T, Sakon M, et al. : Expression and clinical significance of erb-B receptor family in hepatocellular carcinoma. Br J Cancer 84:1377–1383, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Philip PA, Mahoney MR, Allmer C, et al. : Phase II study of Erlotinib (OSI-774) in patients with advanced hepatocellular cancer. J Clin Oncol 23:6657–6663, 2005 [DOI] [PubMed] [Google Scholar]
- 8. Mendelsohn J, Baird A, Fan Z, et al. : Growth factors and their receptors in epithelial malignancies, in Mendelsohn J, Howley PM, Israel M, Liotta L.(eds): The Molecular Basis of Cancer. Philadelphia, PA, W. B. Saunders Company, pp 137–144, 2001 [Google Scholar]
- 9. Huang SM, Harari PM: Epidermal growth factor receptor inhibition in cancer therapy: biology, rationale and preliminary clinical results. Invest New Drugs 17:259–269, 1999 [DOI] [PubMed] [Google Scholar]
- 10. Cunningham D, Humblet Y, Siena S, et al. : Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med 351:337–345, 2004 [DOI] [PubMed] [Google Scholar]
- 11. Yen Y, Doroshow J, Leong D, et al. : Phase II study of oxaliplatin in patients with unresectable, metastatic or recurrent hepatocellular cancer. J Clin Oncol 22(14S), 2004. (abstr 4169) [DOI] [PubMed] [Google Scholar]
- 12. Synold TW, Takimoto CH, Doroshow JH, et al. : Dose-escalating and pharmacologic study of oxaliplatin in adult cancer patients with impaired hepatic function: a National Cancer Institute Organ Dysfunction Working Group study. Clin Cancer Res 13:3660–3666, 2007 [DOI] [PubMed] [Google Scholar]
- 13. Boige V, Raoul JL, Pignon JP, et al. : Multicentre phase II trial of capecitabine plus oxaliplatin (XELOX) in patients with advanced hepatocellular carcinoma: FFCD 03–03 trial. Br J Cancer 97:862–867, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sun W, Haller D, Mykulowycz K, et al. : Combination of capecitabine, oxaliplatin with bevacizumab in treatment of advanced hepatocellular carcinoma (HCC): a phase II study. J Clin Oncol 25(18S), 2007. (abstr 4574) [Google Scholar]
- 15. Zhu AX, Stuart K, Blaszkowsky LS, et al. : Phase 2 study of cetuximab in patients with advanced hepatocellular carcinoma. Cancer 110:581–589, 2007 [DOI] [PubMed] [Google Scholar]
- 16. Gruenwald V, Wilkens L, Gebel M, et al. : A phase II open label study of cetuximab in unresectable hepatocellular carcinoma: final results. J Clin Oncol 25(18S), 2007. (abstr 4598) [Google Scholar]
- 17. Van Cutsem E, Kohne CH, Hitre E, et al. : Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Eng J Med 360:1408–1417, 2009 [DOI] [PubMed] [Google Scholar]
- 18. Wong CM, Ng IO: Molecular pathogenesis of hepatocellular carcinoma. Liver Int 28:160–174, 2008 [DOI] [PubMed] [Google Scholar]
- 19. Khambata-Ford S, Garrett CR, Meropol NJ, et al. : Expression of epiregulin and amphiregulin and K-ras mutation status predict disease control in metastatic colorectal cancer patients treated with cetuximab. J Clin Oncol 25:3230–3237, 2007 [DOI] [PubMed] [Google Scholar]
- 20. Berasain C, Garcia-Trevijano ER, Castillo J, et al. : Amphiregulin: an early trigger of liver regeneration in mice. Gastroenterology 128:424–432, 2005 [DOI] [PubMed] [Google Scholar]
- 21. Asnacios A, Fartoux L, Romano O, et al. : Gemcitabine plus oxaliplatin (GEMOX) combined with cetuximab in patients with progressive advanced stage hepatocellular carcinoma: results of a multicenter phase 2 study. Cancer 112:2733–2739, 2008 [DOI] [PubMed] [Google Scholar]
- 22. O'Neil BH, Allen R, Spigel DR, et al. : High incidence of cetuximab-related infusion reactions in Tennessee and North Carolina and the association with atopic history. J Clin Oncol 25:3644–3648, 2007 [DOI] [PubMed] [Google Scholar]