ABSTRACT
Background:
Rectal cancer with anal involvement is typically treated with abdominoperineal resection (APR). However, patients treated with neoadjuvant chemoradiotherapy with good clinical response and tumor regression from the anus present a controversial management dilemma. This is a report of patients treated with low anterior resection (LAR) versus APR.
Methods:
Patients with T2–3N0–2M0 (IIA-IIIC) rectal cancer with anal canal involvement were eligible. Anal canal involvement was determined by sigmoidoscopy/colonoscopy or endoscopic ultrasound. Patients were treated in the prone position with the three-field technique to 45–50.4 Gy at 1.8 Gy/fraction given concurrently with 5-fluorouracil. Patients then underwent APR/LAR via total mesorectal excision 4–6 weeks after chemoradiotherapy. LAR was performed in patients with good sphincter function at presentation, in those with sufficient tumor regression away from anal canal to permit LAR, and in those compliant with close follow-up.
Results:
A total of 32 patients with rectal cancer with anal canal involvement were treated with neoadjuvant chemoradiotherapy. Local control was 85% and 89% for patients treated with APR and LAR, respectively. Overall survival was 76% and 86% in patients treated with APR and LAR, respectively. Pathologic complete response was seen in 24% of patients who underwent APR and 27% of patients who underwent LAR.
Conclusion:
Rectal cancers with anal involvement with good clinical response after neoadjuvant chemoradiotherapy are typically treated with APR. However, LAR may be a feasible alternative, particularly in those with excellent clinical response to neoadjuvant treatment with sufficient tumor regression away from the anal canal. In these patients close follow-up is necessary, and APR may be reserved as salvage when needed.
Approximately 41,000 patients were diagnosed with rectal cancer in the United States in 2007.1 Surgical options include abdominoperineal resection (APR) or low anterior resection (LAR). APR results in the formation of a permanent colostomy with associated decreased quality of life2,3 and long-term financial implications related to stoma care. APR also has an increased risk of perineal wound complications.4,5
In recent years the management of rectal cancer has evolved, and indications for APR have narrowed. This includes a clearer definition of the safe distal resection margin6,7 and improvement of surgical techniques, with the development and application of surgical stapling devices.8 In addition, there has been increased use of preoperative radiotherapy9,10 or neoadjuvant chemoradiotherapy10 and widespread adoption of total mesorectal excision (TME).11,12
Although the rates of APR have declined,13 its use is still considered essential in many cases.14 This is particularly true in low-lying rectal cancers and those with anal involvement. The funnel-shaped bony pelvis limits tumor access, visualization, and mobilization in resection of distal cancers. In addition, in the most distal segment of rectum, resection of the mesorectum is often incomplete, and lateral surgical margins are limited because of the proximity of other organs and bony structures.15
Neoadjuvant therapy has been employed in rectal cancers to facilitate tumor shrinkage and increase local control and sphincter preservation.9,10,16–19 Other advantages of preoperative radiotherapy include a lower total dose of radiation and easier displacement of the small bowel from the radiation field.20–24 Studies also suggest that the benefit from neoadjuvant chemoradiotherapy may be in improving results in the most distal cancers.18 These studies have shown that resection of low-lying rectal cancers followed by coloanal anastamosis can lead to high rates of anal function and preservation of quality of life.17–19
Although several reports about the utility of neoadjuvant chemoradiotherapy for low-lying rectal cancers have been published, the effectiveness of this approach in rectal cancers with anal canal involvement remains unclear. Interestingly, few published reports exist of patients with rectal cancer with anal canal extension treated with neoadjuvant chemoradiotherapy followed by LAR or APR. This is a retrospective report of our experience.
PATIENTS AND METHODS
Eligibility criteria included T2–3N0–2M0 (IIA-IIIC) rectal adenocarcinoma with anal involvement on pretreatment staging. Patients with distant metastases, squamous cell carcinoma, or transanal excision were excluded. The anal canal was considered to be involved if the tumor invaded beyond the dentate line by sigmoidoscopy/colonoscopy or endoscopic ultrasound (EUS). EUS and colonoscopy were performed by the same gastroenterologist at each institution, and findings were corroborated and confirmed by the same surgeon.
Patients with any rectal incontinence at presentation were excluded in this analysis. Distal margins of the rectal tumor ranged from the anal verge to 3 cm from the anal verge. Workup included history and physical, assessment of initial sphincter function, carcinoembryonic antigen assay, complete blood cell counts, chemistry panels, liver function tests, sigmoidoscopy/colonoscopy, EUS, biopsy, positron emission tomography scans, and computed tomography (CT) scans of the abdomen/pelvis.
All patients underwent neoadjuvant chemoradiotherapy at 1.8 Gy per fraction, 5 fractions per week for a total of 45–50.4 Gy. Patients were treated in the prone position on a belly board device to decrease radiation dose to the small bowel. Patients underwent CT simulation for CT planning. Patients were treated with a three-field technique of single posterior and two lateral portals. Superior borders were at the L5-S1 junction. Lateral borders extended 1–2 cm beyond the bony pelvis. The posterior border encompassed the entire sacrum with adequate margins on the lateral fields. Anteriorly, the field extended to the posterior symphysis pubis. Inferiorly, the anal canal was included with the field extending 2 cm inferior to the anal margin as seen by an anal marker placed at the time of simulation.
Irradiation of the bilateral inguinal nodes was left to the discretion of the treating physicians, as the addition of these fields was considered controversial and of uncertain benefit. Patients were treated concurrently with infusional 5 fluorouracil (5-FU) 1,000/m2/day every 4 weeks with leucovorin rescue or oral capecitabine.
From 4 to 6 weeks after neoadjuvant chemoradiotherapy, patients were reevaluated with CT scans, digital rectal examination, and sigmoidoscopy/colonoscopy for tumor downstaging and response. Patients were considered for LAR if they had good initial sphincter function at presentation and after neoadjuvant chemoradiotherapy, were compliant with follow-up, and had sufficient tumor regression from the anal canal. Sufficient tumor regression from the anal canal was documented as no visible tumor seen in the anal canal on repeat sigmoidoscopy/colonoscopy and physical exam with margin to allow for reanastamosis. Sufficient margin was considered to be 1 cm of noninvolved rectum. Repeat biopsies of the anal canal were not performed. All other patients underwent APR. No transanal resections or attempts at intersphincteric resections were performed. Surgery was performed via TME by the primary gastrointestinal surgical oncologist at each institution.
Patients were evaluated and underwent surgery after detailed discussion of whether to proceed with LAR or APR. Resections were performed by the combined transabdominal transanal approach. After resection, specimens were submitted for pathologic assessment. Downstaging was defined by comparison of the pretreatment TN stage determined by clinical, colonoscopy, EUS, and radiographic studies to the pathologic findings. Tumor regression away from the anal canal alone did not constitute downstaging. Negative margins were defined as an absence of tumor at the radial or proximal and distal inked margins.
Postoperative chemotherapy was continued at 500 m2/day for four 5-day cycles of 5-FU. Patients were then followed at 3-month intervals for 2 years and then at 6-month intervals for 3 years. Patients were assessed at follow-up for any recurrences and, in those who underwent LAR, for anal sphincter function. Sphincter function was assessed according to the Memorial Sloan-Kettering sphincter function scale both before and after treatment (Table 1).25 Sphincter function at the time of last follow-up was used. Soilage was defined according to Wagman et al17 as minimal leakage of mucus or liquid stool that occurred occasionally (1–2 episodes per week = mild soilage) or more frequently (> 2 episodes per week = moderate soilage).
Table 1.
Memorial Sloan-Kettering Sphincter Function Scale
| Excellent: | 1–2 bowel movements/day, no soilage |
| Good: | 3–4 bowel movements/day, and/or mild soilage |
| Fair: | Episodic >4 bowel movements/day, and/or moderate soilage |
| Poor: | Incontinence |
Statistical Analysis
End points were calculated from date of resection and included local control, locoregional control, distant-metastases–free survival, overall survival, and colostomy-free survival. Local-relapse–free survival was defined as no recurrence within the coloanal anastomosis, rectum, or stoma. Locoregional-relapse–free survival was defined as no recurrence in pelvic lymph nodal areas, bilateral inguinal nodal areas, coloanal anastomosis, rectum, or stoma. Distant-metastases–free survival was defined as survival with no evidence of distant dissemination of tumor. Overall survival was assessed in terms of all-cause mortality. Colostomy-free survival was defined as survival without the need for permanent colostomy for any reason. Local-relapse–free survival, locoregional-relapse–free survival, distant-metastases–free survival, overall survival. and colostomy-free survival was calculated using the Kaplan Meier method. Multivariate analysis was calculated using the proportional hazards Cox modeling with statistical inferences on the actuarial curves made using log rank tests.
RESULTS
From 1999 to 2005, 32 patients with rectal cancer with anal involvement were treated with neoadjuvant chemoradiotherapy. Abdominoperineal resection was performed in 21 patients and LAR in 11 patients. Median patient age was 60 years. Median follow-up was 45 months (6–131 months). There were 5 women and 27 men in this cohort. Patient characteristics are summarized in Table 2.
Table 2.
Patient characteristics
| APR (21 patients) | LAR (11 patients) | |
|---|---|---|
| Median age (years) | 61 | 60 |
| T stage | ||
| T2 | 1 | 2 |
| T3 | 20 | 9 |
| T4 | 0 | 0 |
| N stage | ||
| N0 | 11 | 8 |
| N1 | 9 | 3 |
| N2 | 1 | 0 |
| Overall stage | ||
| II | 11 | 8 |
| IIIA | 9 | 3 |
| IIIB | 1 | 0 |
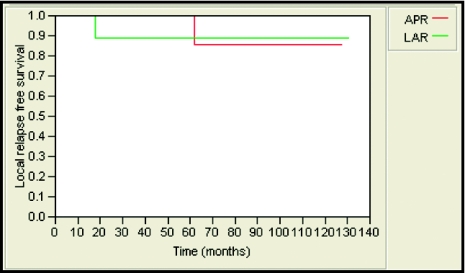
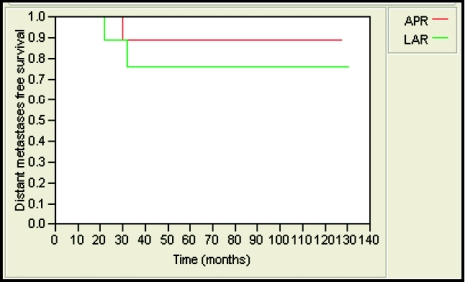
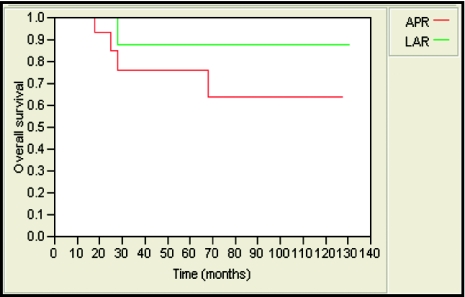
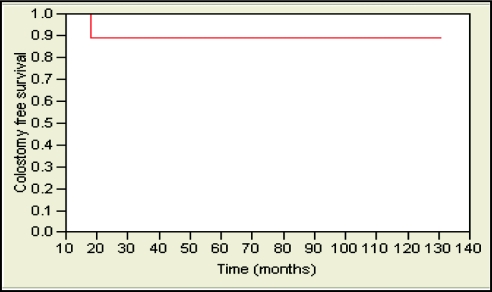
The 5-year local-relapse-free survival rate was 85% and 89% for patients treated with APR and LAR, respectively. This was not statistically significant (Figure 1). Likewise, no statistically significant differences were observed in 5-year locoregional-relapse–free survival rates (85% and 89, distant-metastases–free survival (89% and 76%; Figure 2), and overall survival (76% and 86%; Figure 3) in APR versus LAR groups, respectively. The 5-year colostomy-free survival rate was 89% for patients treated with LAR after neoadjuvant chemoradiotherapy (Figure 4).
Figure 1.
Five-year local-relapse–free survival was 85% and 89% for patients who underwent APR and LAR, respectively (P = .70).
Figure 2.
Five-year distant-metastases–free survival was 89% and 76% for patients who underwent APR and LAR, respectively (P = .44).
Figure 3.
Five-year overall survival was 76% and 86% for patients who underwent APR and LAR, respectively (P = .33).
Figure 4.
Five-year colostomy-free survival was 89% for patients who underwent LAR after neoadjuvant chemoradiotherapy.
Downstaging was possible in 13 (61%) of patients who underwent APR and in 7 (63%) patients who underwent LAR. Other patients had stable disease. However, it is important to note that all the patients who underwent LAR had sufficient tumor regression away from the anal canal to permit LAR. In the LAR group, pathologic complete response was seen in 3 (27%) patients and minimal residual tumor in 2 (18%). Pathologic complete response was seen in 5 (24%) patients who underwent APR, and an additional 2 (9%) patients were found to have no residual tumor in the anal canal. All patients had successful resections with negative margins in both the radial and longitudinal dimensions.
Function of the anal sphincter for patients who underwent LAR was assessed. According to Memorial Sloan-Kettering Sphincter Function Scale, 7 (64%) patients had good to excellent function of their sphincters. The other 4 (36%) patients had fair function. All patients with initial anal sphincter involvement achieved fair sphincter function. No patients experienced incontinence.
In univariate/multivariate analysis, distance from anal verge, APR, or LAR was not found to be prognostic for any outcomes. However, this analysis was limited by the small number of patients in this study.
DISCUSSION
Increased local control, downstaging of tumors, increased resectability, and sphincter preservation are the primary goals of neoadjuvant therapy for patients with rectal cancer. In the Swedish and Dutch Rectal Cancer Trials,9,10 increased local control was seen with preoperative radiotherapy alone. Sauer et al16 revealed increased local control and increased rates of sphincter preservation in patients who initially required APR treated with preoperative chemoradiotherapy in comparison with postoperative chemoradiotherapy.
Neoadjuvant chemoradiotherapy has been shown to reduce both the size and proliferative activity of rectal tumors when compared to pretreatment levels.26,27 With neoadjuvant chemoradiotherapy, Minskey et al26 revealed that unresectable tumors were rendered resectable with negative margins in 97% of cases, and pathologic complete response rates were seen in 11% of patients. This was similar to our cohort of patients, where pathologic complete response was 24% in patients who underwent APR and 27% in patients who underwent LAR. In addition, all of our patients had resections with negative margins.
Janjan et al19 revealed pathologic evidence of downstaging of more than 60%, and 27% of patients had a pathologic complete response with preoperative chemoradiotherapy. They found that in 72% of patients who had pathologic complete response, sphincter preservation was achieved. In patients whose tumors were downstaged, 63% underwent sphincter-sparing surgery. Wagman et al17 revealed that in patients prospectively identified as requiring an APR, neoadjuvant chemoradiotherapy converted 77% of these patients to LAR candidates. These studies have shown that when a tumor is located close to the dentate line, a decrease in tumor volume may allow the surgeon to perform a sphincter-sparing procedure that would not otherwise be possible. However, the use of sphincter-sparing procedures after neoadjuvant chemoradiotherapy for rectal cancers with anal canal involvement remains controversial.
Rectal cancers with anal involvement are difficult to resect because of the funnel shaped anatomy of the pelvis and proximity to other organs and structures. In addition, many surgeons are reluctant to perform sphincter-sparing surgery in these patients, regardless of response to neoadjuvant chemoradiotherapy. However, this treatment approach has been offered to patients at our facilities who are highly motivated, have no rectal incontinence at presentation, have a good clinical response to neoadjuvant therapy with tumor regression away from the anal canal, and are compliant with follow-up.
As mentioned before, sufficient tumor regression from the anal canal was defined as no visible tumor seen in the anal canal on repeat sigmoidoscopy or colonoscopy, with 1 cm margin to allow for reanastamosis. Kim et al28 evaluated the benefits of preoperative chemoradiotherapy in patients with rectal cancer located within 3 cm of the anal verge, similar to our cohort of patients. They reported that 35.5% of the patients who received preoperative chemoradiotherapy had sphincter preservation. However, they did not report rates of local control, distant-metastases–free survival, or overall survival. These are pertinent outcomes when assessing different treatments, and it is important not to violate oncologic principles when performing sphincter-sparing procedures.
Others have reported on their results.25,29 However, these studies also included patients that had tumors that were low lying but not directly involving the anal canal. To our knowledge, this is the first publication reporting on these important outcomes, specifically on rectal cancers with anal involvement treated with neoadjuvant chemoradiotherapy followed by LAR. Our results revealed good outcomes in patients who had good clinical response after neoadjuvant chemoradiotherapy followed by LAR.
Interestingly, Brierley et al30 reported on a cohort of rectal cancer patients treated with radiotherapy alone. They reported a local control rate of 30%. Although this did not approach the local control rate of radical surgery, it does reaffirm the radioresponsiveness/radiocurability of rectal tumors. We would expect an enhancement of this radioresponsiveness with the addition of chemotherapy.
These findings, as well as the pathologic complete response rates found in our patients, suggest that malignant cells initially present at the anal canal in a proportion of patients who undergo neoadjuvant chemoradiotherapy are no longer viable after treatment. In these patients an LAR would potentially not lead to increased rates of local recurrence. However, it is unclear which patients will have a clinical response to neoadjuvant chemoradiotherapy sufficient to convert from an APR indication to candidacy for an LAR. Magnetic resonance imaging (MRI) of the rectum and pelvis may help in this matter.
Kulkarni et al31 compared postneoadjuvant chemoradiotherapy MRI images with histologic examination of surgical specimens. They found that postchemoradiotherapy restaging MRI had an accuracy of 81%, with 87% specificity, but a sensitivity of 54%. They stated that the shortcomings of MRI are due to inability to identify small nodal and tumor deposits. Therefore, there is a risk that patients who undergo LAR will have microscopic residual tumor at the distal anus/rectum that was not sterilized by chemoradiotherapy. It is for this reason that the selection of patients for LAR include those likely to remain compliant with close follow-up.
Biopsies of the anal canal before deciding to proceed with APR may be useful in these situations and may have identified the 31% of patients who had either complete response or no residual tumor at the anal canal in our cohort of patients before they underwent APR. If these patients were found to have complete response rates after neoadjuvant therapy by biopsy, the option of LAR may have been considered. However, this remains controversial secondary to possible sampling error with biopsies.
As mentioned before, we offered LAR only to select patients who had good sphincter function and no rectal incontinence at presentation. Sphincter preservation without adequate function is meaningless. For patients who did not present with adequate sphincter function, APR was performed.
We assessed sphincter function after neoadjuvant therapy and LAR using the Memorial Sloan Kettering Cancer Center Sphincter Function Scale.32 According to this scale, 64% of our patients with LAR had good to excellent function. The other 36% of patients had fair function. This was comparable to the 85% of patients with good to excellent function seen by Wagman et al17 and to the 71% seen by Rouanet et al.33 Colostomy-free survival was 89%. This suggests that patients who have good initial sphincter function with tumor regression after neoadjuvant therapy followed by LAR will continue to have adequate sphincter function. It remains to be seen if different treatment approaches would increase these rates of sphincter preservation with good function. These include intersphincteric resection,34,35 use of colonic pouch,36,37 longer time interval between completion of neoadjuvant chemoradiotherapy and resection to allow for further tumor regression,38 and different chemotherapy, such as oxaliplatin-based regimens.39
Locoregional control was 87% for both treatment groups, despite no direct radiation fields to the bilateral inguinal canals. Distant-metastases–free survival and overall survival were found to be 83% and 81%, respectively, which was similar to several other randomized phase III studies investigating preoperative therapy.9,10,16 This suggested that in our cohort of patients, rectal cancer with anal involvement did not confer a worse prognosis than those without anal canal involvement. However, this may represent selection bias, and only patients with good response to neoadjuvant chemoradiotherapy were selected out in this study. In addition, our findings are not consistent with other studies, which revealed that distal tumors have a higher incidence of lymph node metastases40 with a 15%–20% worse overall prognosis compared with more proximal cancers of the rectum.41
Our study is limited by its retrospective nature and inherent selection bias. In addition, the population included a very select, small number of patients over an extended time period. Therefore, analysis of certain tumor and patient characteristics that would select out patients favorable for LAR was underpowered. This also holds true for comparison of outcomes between patients who underwent LAR and APR. However, our main purpose was to evaluate the safety of performing an LAR after neoadjuvant chemoradiotherapy for rectal cancer patients with anal involvement.
Another limitation in applying this study is that surgeons may have differing criteria for determining eligibility for LAR and the definition of safe resection margins.6,42 The surgical procedures used by our surgeons in this study were standardized. Nevertheless, our results should be interpreted cautiously and considered to be hypothesis generating. In addition, comparison with other studies will prove difficult, as many surgeons are reluctant to perform sphincter-sparing surgery on patients with initial anal canal involvement, despite response to preoperative treatment.
In conclusion, our findings suggest that neoadjuvant chemoradiotherapy followed by LAR can be performed in a highly select group of patients with rectal cancer with anal involvement. These patients include those with good sphincter function at presentation, those with good response to neoadjuvant chemoradiotherapy with tumor regression away from the anal canal, and those compliant with follow-up. This approach has led to acceptable outcomes in our cohort of patients. Postneoadjuvant chemoradiotherapy MRI may help increase accurate selection of patients suitable for LAR. In patients with local recurrence, APR as salvage can be attempted when technically feasible. Prospective studies should be performed to validate these provocative findings.
Footnotes
Disclosures of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
REFERENCES
- 1. American Cancer Society Cancer Facts and Figures 2007. Atlanta: American Cancer Society, 2007 [Google Scholar]
- 2. Sprangers MA, Taal BG, Aaronson NK: Quality of life in colorectal cancer. Stoma vs nonstoma patients. Dis Colon Rectum 38: 361–369, 1995 [DOI] [PubMed] [Google Scholar]
- 3. Williams NS, Johnston D: The quality of life after rectal excision for low rectal cancer. Br J Surg 70: 460–462, 1983 [DOI] [PubMed] [Google Scholar]
- 4. Bullard KM, Trudel KL, Baxter NN: Primary perineal wound closure after preoperative radiotherapy and abdominoperineal resection has a high incidence of wound failure. Dis Colon Rectum 48: 438–443, 2005 [DOI] [PubMed] [Google Scholar]
- 5. Christian CK, Kwaan MR, Betensky RA: Risk factors for perineal wound complications following abdominoperineal resection. Dis Colon Rectum 48: 43–48, 2005 [DOI] [PubMed] [Google Scholar]
- 6. Rullier E, Laurent C, Bretagnol F: Sphincter saving resection for all rectal carcinomas: The end of the 2 cm distal rule. Ann Surg 241: 465–469, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Marks GM, Mohuidden M, Marks J: New hope and promise for sphincter preservation in the management of cancer of the rectum. Semin Oncol 18: 388–398, 1991 [PubMed] [Google Scholar]
- 8. Tytherleigh MG, Mortenson NJ: Options for sphincter preservation in surgery for low rectal cancer. Br J Surg 90: 922–933, 2003 [DOI] [PubMed] [Google Scholar]
- 9. Swedish Rectal Cancer Trial Improved survival with preoperative radiotherapy in resectable rectal cancer. N Engl J Med 336: 980–987, 1997 [DOI] [PubMed] [Google Scholar]
- 10. Kapiteijn J, Marijnen CA, Nagtegaal ID: Preoperative radiotherapy combined with total mesorectal excision for respectable rectal cancer. N Engl J Med 345: 638–646, 2001 [DOI] [PubMed] [Google Scholar]
- 11. Heald RJ, Smedh RK, Kald A: Abdominoperineal excision of the rectum—an endangered operation. Norman Nigro Lectureship. Dis Colon Rectum 40: 747–751, 1997 [DOI] [PubMed] [Google Scholar]
- 12. Heald RJ: Total mesorectal excision is optimal surgery for rectal cancer: a Scandinavian consensus. Br J Surg 82: 1297–1299, 1995 [DOI] [PubMed] [Google Scholar]
- 13. Tilney HS, Heriot AG, Purkayastha S: A national perspective on the decline of abdomino-perineal resection for rectal cancer. Ann Surg 247: 77–84, 2008 [DOI] [PubMed] [Google Scholar]
- 14. Chuwa EW, Seow-Choen F: Outcomes for abdominoperineal resections are not worse than those of anterior resections. Dis Colon Rectum 49: 41–49, 2006 [DOI] [PubMed] [Google Scholar]
- 15. Mohiuddin M, Marks J, Marks G: Management of rectal cancer: short vs long course preoperative radiation. Int J Radiat Oncol Biol Phys 72: 636–643, 2008 [DOI] [PubMed] [Google Scholar]
- 16. Sauer R, Becker H, Hohenberger W: Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 351: 1731–1740, 2004 [DOI] [PubMed] [Google Scholar]
- 17. Wagman R, Minsky BD, Cohen AM: Sphincter preservation in rectal cancer with preoperative radiation therapy and coloanal anastamosis: long term follow up. Int J Radiat Oncol Biol Phys 42: 51–57, 1998 [DOI] [PubMed] [Google Scholar]
- 18. Mohiuddin M, Regine WF, Marks GJ: High dose preoperative radiation and the challenge of sphincter preservation surgery for cancer of the distal 2 cm of the rectum. Int J Radiat Oncol Biol Phys 40: 569–574, 1998 [DOI] [PubMed] [Google Scholar]
- 19. Janjan NA, Khoo VS, Abbruzzese J: Tumor downstaging and sphincter preservation with preoperative chemoradiation in locally advance rectal cancer—the M.D. Anderson Cancer Center experience. Int J Radiat Oncol Biol Phys 44: 1027–1038, 1999 [DOI] [PubMed] [Google Scholar]
- 20. Acker JC, Marks LB: The lack of impact of pelvic irradiation on small bowel mobility: implications for radiotherapy treatment planning. Int J Radiat Oncol Biol Phys 32: 1473–1475, 1995 [DOI] [PubMed] [Google Scholar]
- 21. Frykholm GJ, Glimelius B, Pahlman L: Preoperative or postoperative irradiation in adenocarcinoma of the rectum: final treatment results of a randomized trial and an evaluation of late secondary effects. Dis Colon Rectum 36: 564–572, 1993 [DOI] [PubMed] [Google Scholar]
- 22. Frykholm GJ, Isacsson U, Nygard K: Preoperative radiotherapy in rectal carcinoma-aspects of acute adverse effects and radiation technique. Int J Radiat Oncol Biol Phys 35: 1039–1048, 1996 [DOI] [PubMed] [Google Scholar]
- 23. Letschert JGJ, Lebesque JV, Aleman BMP: The volume effect in radiation-related late small bowel complications: Results of a clinical study of the EORTC Radiotherapy Cooperative Group in patients treated for rectal carcinoma. Radiother Oncol 32: 116–123, 1994 [DOI] [PubMed] [Google Scholar]
- 24. Minsky BD, Conti JA, Huang Y: Relationship of acute gastrointestinal toxicity and volume of irradiated small bowel in patients receiving combined modality therapy for rectal cancer. J Clin Oncol 13: 1409–1416, 1995 [DOI] [PubMed] [Google Scholar]
- 25. Crane C, Skibber J, Felg B: Response to preoperative chemoradiation increases the use of sphincter preserving surgery in patients with locally advanced low rectal carcinoma. Cancer 97: 517–524, 2003 [DOI] [PubMed] [Google Scholar]
- 26. Minsky BD, Cohen AM, Enker WE: Preoperative 5 FU, low dose leucovorin, and radiation therapy for locally advanced and unresectable rectal cancer. Int J Radiat Oncol Biol Phys 37: 289–295, 1997 [DOI] [PubMed] [Google Scholar]
- 27. Willett CG, Warland G, Hagan MP: Tumor proliferation in rectal cancer following preoperative irradiation. J Clin Oncol 13: 1417–1424, 1995 [DOI] [PubMed] [Google Scholar]
- 28. Kim DW, Lim SB, Kim DY: Pre-operative chemo-radiotherapy improves the sphincter preservation rate in patients with rectal cancer located within 3 cm of the anal verge. EJSO 32: 162–167, 2006 [DOI] [PubMed] [Google Scholar]
- 29. Weiser M, Quah HM, Shia J: Sphincter preservation in low rectal cancer is facilitated by preoperative chemoradiation and intersphincteric dissection. Ann Surg 249: 236–242, 2009 [DOI] [PubMed] [Google Scholar]
- 30. Brierley JD, Cummings BJ, Wong CS: Adenocarcinoma of the rectum treated by radical external beam therapy. Int J Radiat Oncol Biol Phys 31: 255–259, 1995 [DOI] [PubMed] [Google Scholar]
- 31. Kulkarni T, Gollins S, Maw A: Magnetic resonance imaging in rectal cancer downstaged using neoadjuvant chemoradiation: accuracy of prediction of tumour stage and circumferential resection margin status. Colorectal Dis 10: 479–489, 2008. Jun [DOI] [PubMed] [Google Scholar]
- 32. Minsky BD, Cohen AM, Enker WE: Sphincter preservation with preoperative radiation therapy and coloanal anastamosis. Int J Radiat Oncol Biol Phys 31: 553–559, 1995 [DOI] [PubMed] [Google Scholar]
- 33. Rouanet P, Fabre JM, Dubois JB: Conservative surgery for low rectal carcinoma after high dose radiation. Functional and oncologic results. Ann Surg 221: 67–72, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schiessel R, Karner-Hanusch J, Herbst F: Intersphincteric resection for low rectal tumors. Br J Surg 81: 1376–1378, 1994 [DOI] [PubMed] [Google Scholar]
- 35. Chih-Chien Chin, Chien-Yuh Yeh, Wen-Shih Huang: Clinical outcome of intersphincteric resection for ultra low rectal cancer. World J Gastroenterol 12: 640–643, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Joo JS, Latulippe JF, Alabaz O: Long term functional evaluation of straight coloanal anastamosis and colonic J-pouch: is the functional superiority of colonic J-pouch sustained? Dis Colon Rectum 41: 740–746, 1998 [DOI] [PubMed] [Google Scholar]
- 37. Sailer M, Fuchs KH, Fein M: Randomized clinical trial comparing quality of life after straight and pouch coloanal reconstruction. Br J Surg 89: 1108–1117, 2002 [DOI] [PubMed] [Google Scholar]
- 38. Moore HG, Gittleman AE, Minsky BD: Rate of pathologic complete response with increased interval between preoperative combined modality therapy and rectal cancer resection. Dis Colon Rectum 47: 279–286, 2004 [DOI] [PubMed] [Google Scholar]
- 39. Freyer G, Bossard N, Romestaing P: Addition of oxaliplatin to continuous fluorouracil, 1-folinic acid and concomitant radiotherapy in rectal cancer: the Lyon R 97-03 phase 1 trial. J Clin Oncol 19: 2433–2438, 2001 [DOI] [PubMed] [Google Scholar]
- 40. Brodsky J, Richard GK, Cohen AM: Variables correlated with the risk of lymph node metastases in early rectal cancer. Cancer 69: 322–326, 1992 [DOI] [PubMed] [Google Scholar]
- 41. Mohiuddin M, Marks C: Adjuvant radiation therapy for colon and rectal cancer. Semin Oncol 18: 411–420, 1991 [PubMed] [Google Scholar]
- 42. Tjandra JJ, Kilkenny JW, Buie WD: Practice parameters for the management of rectal cancer. Dis Colon Rectum 48: 411–423, 2005 [DOI] [PubMed] [Google Scholar]