Abstract
The transition from acute to chronic musculoskeletal pain is not well understood. To understand this transition, it is important to know how peripheral and central sensitization are manifested and how they can be assessed. A variety of human pain biomarkers have been developed to quantify localized and widespread musculoskeletal pain. In addition, human surrogate models may be used to induce sensitization in otherwise healthy volunteers. Pain can arise from different musculoskeletal structures (e.g. muscles, joints, ligaments, or tendons), and differentiating the origin of pain from those different structures is a challenge. Tissue specific pain biomarkers can be used to tease these different aspects. Chronic musculoskeletal pain patients in general show signs of local/central sensitization and spread of pain to degrees which correlate to pain intensity and duration. From a management perspective, it is therefore highly important to reduce pain intensity and try to minimize the duration of pain.
Keywords: Sensitization, hyperalgesia, experimental pain, muscle, joint
Introduction
Musculoskeletal pain is a major clinical problem and further research into peripheral and central neurobiological mechanisms is required to improve understanding, diagnosis and management. Peripheral and central sensitization are important mechanisms for musculoskeletal pain conditions. This paper will focus on the clinical manifestations of localized and widespread musculoskeletal pain, and the methods to assess the underlying mechanisms involved. Several experimental techniques to assess deep tissue hyperalgesia, temporal summation of pain, descending pain control, and referred pain are available. These methods reflect different mechanisms relevant for symptoms seen in patients (e.g. spreading of pain, tenderness, widespread hyperalgesia) and offer additional information to be used for revisions of treatment strategies. A progressive sensitization of central mechanisms (central sensitization) is a potential mechanism involved in the transition from acute to chronic pain, and needs to be assessed by appropriate biomarkers.
This paper will follow the translational pain research strategy in the presentation where basic animal science is translated into human experimental pain models for investigating fundamental pain mechanisms in healthy volunteers. As healthy volunteers do not behave as for example a sensitized chronic patient, pain models are needed where the healthy volunteers can transiently be transformed into a surrogate model of a patient, i.e. the models act as a proxy for clinical symptoms where mechanisms such as peripheral or central sensitization can be mimicked. Finally, the discovered mechanisms can be further investigated and possibly modulated in individuals with musculoskeletal pain problems.
Basic Aspects of Musculoskeletal Pain
Basic aspects of muscle pain
Muscle pain presents as localized, regional or widespread pain. As the clinical pain condition transits from one to the other, more and more sensory abnormalities occur1 with widespread hyperalgesia in chronic conditions. There is evidence from the literature that the intensity of ongoing pain2 as well as the duration of pain3 determine the degree of generalized muscle hyperalgesia. This is important to note as it underpins the importance of the ongoing nociception for the chronification process.
The myofascial pain syndrome is an example of a regional muscle pain condition characterized by localized tenderness and pain caused by active myofascial trigger points. The affected muscles often display increased fatigability, stiffness, subjective weakness, pain in movement, and restricted range of motion that is unrelated to joint restrictions.4
The sensation of acute deep-tissue pain is the result of activation of group III (Adelta-fiber) and group IV (C-fiber) polymodal muscle nociceptors.5 The nociceptors can be sensitized by release of neuropeptides from nerve endings. This may eventually lead to hyperalgesia and central sensitization of dorsal horn neurons manifested as prolonged neuronal discharges, increased responses to defined noxious stimuli, response to non-noxious stimuli, and expansion of the receptive field.5–7 In humans, little information is available on the peripheral neuronal correlate of muscle nociceptor activation, and only few microneurographic studies have been published8,9 due to difficulties in recording and directly activating muscle nociceptors. Other quantitative techniques are therefore needed, and quantitative sensory testing may help to assess muscle pain, muscle hyperalgesia, and referred pain.
Basic aspects of tendon/ligament pain
Within the mixed group of musculoskeletal pain complaints are the tendinopathies. Rotator cuff, lateral elbow and Achilles tendinopathy are well-recognized examples. The wide range of treatments and lack of consensus among clinicians might reflect a lack of knowledge regarding, not only about etiology, but also basic sensitivity and nociceptive properties of tendon tissues. Alfredson and Lorentzon10 found high microdialysate glutamate levels in painful Achilles tendinopathy subjects. Cat tendon tissue was found to have a dense innervation of groups III and IV afferent fibers.11 Mense and Simons12 highlight the increased innervation density of neuropeptide containing fibers in rat calcaneal tendon peritendineum tissue compared to the associated muscle. Substance P and calcitonin gene-related peptide immunoreactivity has been demonstrated in human tendon tissue13,14 indicating a thin fiber sensory innervation (most likely serving a nociceptive function). The nerve endings are mostly found around small arterioles and blood vessels in the tissue.13,14
The nociceptive features of tendinous tissue have been described experimentally by tendon tissue injections of hypertonic saline (Fig. 1),15 glutamate, and capsaicin16 to study pain reactions and referred pain patterns. In general, tendons are more sensitive to experimental pain stimulus compared with similar injections in muscle tissues. Further, N-methyl-D-aspartate and transient receptor potential V1 receptors were shown to be functionally relevant in tendon tissue as peri-tendinous injections of glutamate and capsaicin, respectively, were effective in inducing tendon pain.16
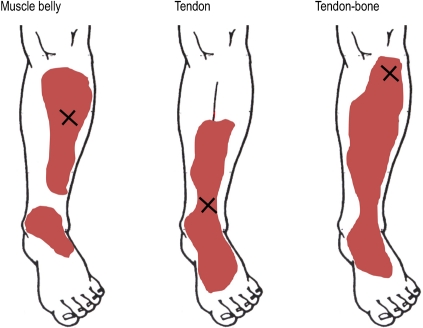
Figure 1.
Example of the pain distribution after injecting a small bolus of hypertonic saline into the muscle, tendon and tendon-bone junction of the tibialis anterior. Larger referred pain areas and higher pain intensities were often found after injecting the tendon-bone junction compared with tendon and muscle. Based on data from Gibson et al.15
Basic aspects of bone related pain
Bone-associated pain is very frequent in the clinic and is difficult to treat.17 A common bone disorder is osteoporosis, which leads to decreased density and bone fragility and thereby bone-associated pain.18 Cancer patients with bone metastases suffer from bone-associated pain, and animal models have been developed to delineate the underlying pain mechanisms and to help in the development of new and better treatment regimes.19
The underlying origin of bone-associated pain is still not fully understood in either animals or humans. Kellgren20 investigated the pain sensitivity of bone by drilling holes in human bone and found that this did not cause any pain when the periosteum was anesthetized. The periosteum is innervated by unmyelinated nociceptive afferents, and pressure stimulation seems capable of activating these fibers.21 Animal studies indicate that delta-opioid receptors are located on those peripheral endings and play an important role in controlling bone-associated nociception.22 This information could lead to design of better management regimes for bone-related pains as this is a significant clinical problem. Periosteum pain sensitivity has not been thoroughly investigated, although injection of hypertonic saline around the periost caused more pain than did intramuscular injections.23
Basic aspects of joint pain
Joint pain is a major clinical problem.24 Inflammatory joint diseases (e.g. rheumatoid arthritis) are the main reasons for joint pain at younger ages, whereas osteoarthritis (OA) is more prominent in the elderly. Osteoarthritis is one of the most common diseases worldwide, and the major source of OA-associated pain derives from nociceptive receptors in the damaged superficial bone and joint structures. OA pain is normally localized but it can be also referred (e.g. from hip OA to knee).
Most of the basic information on the neurobiology of joint pain comes from inflammatory models. Direct animal models exist for OA but they do not translate very well into humans. A model based on hypertonic saline injections into the patellar fat pad of the knee mimics some OA joint pain characteristics and is probably the best model so far.25 Continuous and intense nociceptive input from the OA-damaged knee joint may drive central sensitization in animals.26 Impairment of descending inhibition lowers the excitation threshold of spinal cord neurons to joint nociceptive input, increases the receptive fields of neurons, and increases ongoing discharges.27
Disease hallmarks in OA are articular cartilage degeneration and joint space narrowing but generally OA etiology is unclear. Peripheral nociceptors may be sensitized by, for example, inflamed synovium and damaged subchondral bone, and most often there is a discrepancy between physical damage of joint and pain symptoms.28 The joint afferent nerves contain Abeta, Adelta and C-fibers.29 Corpuscular endings of Abeta-fibers are identified in ligaments and in the fibrous capsule. C-fibers are identified in all structures of the joint except the normal cartilage. A particular group of C-fibers (silent nociceptors) do not respond to noxious mechanical stimuli under normal conditions but only with ongoing inflammation.30
It has been shown that muscle and joint tissue in rats show differing sensory responses to experimental pain, with prolonged allodynia in joint compared to muscle tissue.31 It would appear prudent to presume that human muscle and other deep tissue (joint, tendon, or tendon-bone junction) would similarly display differing sensory manifestations to experimental pain. From all human joint structures including ligaments, fibrous capsule, adipose tissue, meniscus, periosteum and synovial layer, but not cartilage, pain can be evoked in animals by mechanical, thermal and chemical stimuli.32,33
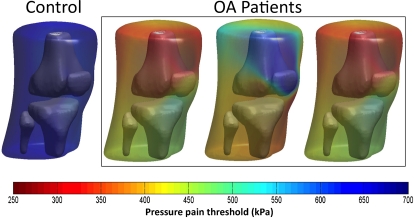
Recently a new joint pain mapping technology has been further developed where pressure pain thresholds are assessed from many locations over the joint (Fig. 2). By mapping the pain threshold levels onto the Magnetic Resonance Imaging extracted 3D joint surface it is now possible to get an impression of the regions of the joint (e.g. the knee) which are sensitized the most.34 This can later (1) be related to the radiological findings, or (2) be modulated by analgesic or anti-inflammatory compounds.
Figure 2.
Mapping of the pressure pain sensitivity. Pressure pain thresholds are assessed on several sites around the knee in a non-symptomatic healthy subject and in three different OA pain patients. A novel pain sensitivity mapping approach illustrates the individual pattern of sensitivity changes in patients. Based on data from Arendt-Nielsen et al.34
Central Mechanisms in Musculoskeletal Pain
Referred pain
Musculoskeletal conditions are often accompanied by local and/or referred pain20 where referred pain is based on a central mechanism.35 Pain located around the source of pain is termed local pain or primary pain, whereas pain felt in a different region away from the source of pain is termed referred pain. A clear distinction between spread of pain and referred pain is not possible at the moment, and these phenomena may also share common pathophysiological mechanisms. Central sensitization may be reflected by the size and location of referred pain.36 Animal studies show expansion and development of new receptive fields by noxious muscle stimuli.37 In the context of referred pain, the unmasking of new receptive fields due to central sensitization could mediate this phenomenon.35,38 The frequency of referred pain from prolonged mechanical stimulation on the anterior tibial muscle is significantly higher than for brief stimulation, indicating the time-dependency of referred pain.15 Moreover, saline-induced referred pain occurred less frequently in healthy subjects treated with ketamine compared with a placebo treatment,39 indicating the involvement of central sensitization.
Referred pain has been used extensively as a diagnostic tool in the clinic. In clinical practice, it is very common to see that pain in one region (e.g. the neck, shoulder or hip) spreads to another (e.g. the arm, hand or knee). Pain from muscles and joints is usually described as deep and diffuse and difficult to locate precisely.40 In patients with musculoskeletal pain, the symptoms may be the summation of referred pain from multiple tissues (e.g. ligament and muscles at the same time), making it more difficult to establish the proper diagnosis. Referred pain from muscle tissues may be similar to referred pain elicited by other tissues, for example, joints.41
Temporal summation of pain
The facilitated pain response to sequential stimuli of equal strength is defined as temporal summation and mimics the initial phase of the wind-up process measured in animal dorsal horn neurons.42 To elicit temporal summation, a stimulus is repeated at constant intervals, for example, five times with a frequency of 1 Hz, at constant intensity. The intensity of the five constant stimuli is increased gradually until the subject feels an increase in pain perception during the repeated stimulation. Repeated tapping on a muscle by a pressure probe has recently been used to assess the efficacy of temporal summation, and temporal summation was found to be more potent for deep tissue stimulation compared with skin stimulation.43
The facilitated degree of temporal summation indicates an enhanced central integrative mechanism (central sensitization). Therefore, facilitated temporal summation of pain in fibromyalgia and OA pain patients might suggest the involvement of central sensitization.34,44–46 The threshold for the withdrawal reflex during repeated stimulation is significantly lower in fibromyalgia and whiplash patients compared with healthy controls, indicating facilitated temporal summation (due to central sensitization) in these patients.47
Descending modulation
The manifestation of central sensitization may also be due to an imbalance between descending inhibition and facilitation, which can also be assessed experimentally. Painful heterotopic conditioning stimuli (thermal, mechanical, electrical, or chemical) have been utilized to evoke diffuse noxious inhibitory control or conditioning pain modulation, which is the decreased pain perception induced by phasic painful stimulation given elsewhere in the body than the heterotopic stimulus. It has been suggested that a dysfunction of diffuse noxious inhibitory control mechanisms may be an important contributor to the clinical manifestations of chronic pain48 as also found in e.g. OA patients with less efficient inhibition of pressure pain thresholds during experimental arm pain compared with healthy controls.34 In line, a recent study has demonstrated reduced activation of the rostral anterior cingulate cortex in fibromyalgia patients versus healthy control subjects; this brain area is probably essential in descending pain control.49
Transition from localized acute pain to chronic widespread pain
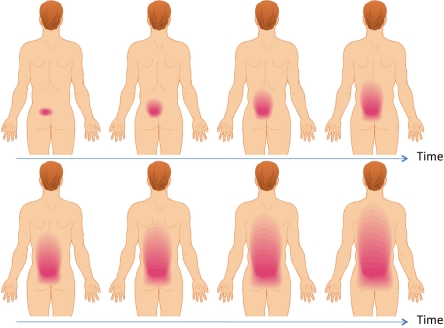
Today there are no definitive models explaining the transition from localized to widespread musculoskeletal pain conditions (Fig. 3). It is likely that that the initial excitation and sensitization of nociceptors due to tissue damage will cause sufficient nociceptive input to the central pain systems to cause central sensitization of dorsal horn neurons and/or at higher brain centers.36 The mechanisms of central sensitization may involve an imbalance between descending inhibition and facilitation. Reorganization of the higher brain centers may also take place in parallel or after the sensitization of second-order neurons.
Figure 3.
If a pain patient with an initial musculoskeletal pain problem is followed over years and if the problem is not resolved the pain starts to spread outside the origin of pain due to development of central sensitization. The pain will gradually spread as illustrated on this sketch and the spread can often not be explained by e.g. disease progression.
New and advanced quantitative pain assessment technologies have been developed to obtain more detailed information about the spreading of musculoskeletal hyperalgesia. By measuring pressure pain thresholds from many locations, the localized versus the generalized muscle hyperalgesia can be assessed and quantified50 and provides new diagnostic possibilities. Generalized hyperalgesia to pressure stimulation has been detected in conditions such as chronic osteoarthritis pain34 and whiplash pain patients.51
Biomarkers for Musculoskeletal Pain
In medicine, a biomarker has many definitions and can refer to many different tests (e.g. histology, serology, genetics, radiology, electrophysiology, imaging). A quantitative human pain biomarker developed for basic studies, diagnostics or drug screening is a technique to provoke pain and pain mechanisms in a standardized way combined with methods for quantitative assessment of the responses in healthy volunteers or patients.48
The challenge in developing novel pain management approaches is to translate pre-clinical data into a meaningful clinical context. It is estimated that maximally 10% of the pre-clinical findings on drug effects translate to pain patients, and hence it is very important to know which animal findings are predictive for a positive or negative effect in humans. In recent years, more and more translational pain biomarkers have been developed where techniques, mechanisms or procedures can be translated from animals via healthy volunteers to pain patients providing a mechanism-based approach for characterization of new and existing analgesic compounds and manual treatments.
In addition to traditional pain biomarkers, surrogate pain models are also very useful for understanding the complex pain mechanisms involved in musculoskeletal pain and for assessing the efficacy of novel pain management strategies. A surrogate pain model is used to mimic a specific clinical symptom (in particular sensitization such as allodynia or hyperalgesia) and hence acts as a proxy for alternations in the peripheral or central neural apparatus in pain patients.52 The surrogate models can be applied to healthy volunteers who transiently act as pain patients (e.g. short-lasting hyperalgesia), and the effect of a given treatment on a specific symptom can be investigated quantitatively under very standardized conditions. A similar approach has also been used for assessing the effects of a manual therapy technique in experimental lateral epicondylalgia.53 In addition, a surrogate model can be applied to patients to investigate how much a given pain mechanism will be facilitated54 and hence how a given drug can dampen this specific mechanism, e.g. capsaicin-induced facilitation of wind-up like pain in a chronic pain patient.
The biomarker approach in early drug development for managing musculoskeletal pain
Early phase trials applying pain biomarkers can be performed in healthy volunteers (phase I mechanism-based proof-of-concept experimental studies) or in small clinical studies (phase I mechanism-based proof-of-concept experimental clinical studies, phase II mechanism-based proof-of-concept clinical studies). In later phases, large clinical trials (phase III and VI pain trials) on, e.g. acute pain, inflammatory pain, neuropathic pain, musculoskeletal pain, visceral pain, headache and dysfunctional pain syndromes can besides standard clinical measures also include the most sensitive pain bio-markers to provide additional mechanistic information.55
Surrogate models of muscle pain and hyperalgesia
Classical studies based on exercise (eccentric) and the development of muscle soreness (delayed onset muscle soreness) is one good example of a surrogate model demonstrating deep tissue hyperalgesia.56 Based on fundamental studies, it is known that a variety of algogenic substances can provoke muscle pain,35 and hence many of those techniques have been transferred to humans for experimental, clinical and drug screening studies. The algogenic substances as surrogate models in humans are summarized below.
Bradykinin, serotonin, nerve growth factor (NGF) and substance P
Intramuscular injection of bradykinin, serotonin and substance P produces pain and hyperalgesia.57 NGF is of particularly interesting compound to use58,59 as many anti-NGF or TrkA-antagonists are under development. Moreover, NGF induces no pain at injection but widespread hyperalgesia develops within one day.
Hypertonic saline, glutamate and capsaicin
Intramuscular injection of hypertonic saline,39,60 glutamate61 and capsaicin62 induces local and referred pain areas. This technique has been used extensively to evaluate the effect of e.g. manual interventions, the N-methyl-D-aspartate receptor blocker ketamine, administered to block peripheral or central receptors, morphine, and alfentanil.
Acid (low pH)
Activation of acid-sensing ion channels results in mechanical hyperalgesia in animals.63 Recently, this observation has been confirmed in humans where intramulcular injection of an acidic buffer induced mechanical muscle hyperalgesia.64 Tissue acidosis is an important feature in many clinical conditions.65 This observation has prompted the pharmaceutical industry to start the development of activation of acid-sensing ion channel receptor antagonists, and the acid model can act as a biomarker in this area.
Assessing muscle hyperalgesia
Some of the chemical substances injected intramuscularly causes experimentally induced muscle pain hyperalgesia and hence can act as a proxy for clinical muscle hyperalgesia (surrogate model). Muscle hyperalgesia can be assessed by e.g. pressure stimulation (pressure algometry) or cuff algometry.
Pressure pain sensitivity can be assessed by means of a handheld pressure algometer where the probe can be applied to a hard body structure, such as the periost,66 joints, 34 soft tissue such as muscles,67or tendons.56 To determine the pressure pain threshold the intensity is increased, preferably by a fixed rate (kPa/second), until the person defines it as pain. The pressure can be increased further until the pain tolerance threshold is reached. Both Adelta- and C-fibers mediate pain induced by pressure stimulation.68 This technique is widely used for assessment of treatment (e.g. morphine, kappa-agonists, oxycodone, rofecoxib, tramadol, codeine, imipramine, ketamine) and recently reference values have been published.69 Automated pressure algometers are used in the most advanced laboratories as they are user-independent. The pressure is increased at a pre-defined rate for each assessment.34
The pain sensitivity of a musculoskeletal structure is dependent on the location of the stimulus application.70 Therefore, the pressure pain sensitivity map technology has been developed for volunteer and patient studies. Within a specific region (e.g. the knee joint) a set of locations71 are defined from where pressure pain thresholds are measured. These values are transferred to a color coded pain sensitivity map showing the regions of highest sensitivity (volunteers) or hyperalgesia before and after drug administration or pain management.
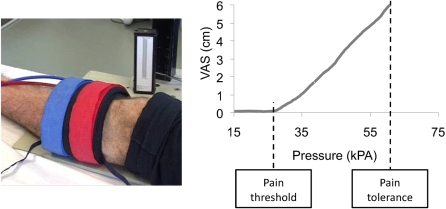
The different pressure devices are all designed to activate a relatively small area and a restricted structure. To get a more general response from musculoskeletal structures a cuff algometer (Fig. 4) has been developed and applied to healthy volunteers72 and pain patients.73 The standardized cuff is inflated at a pre-determined rate, and the volunteer rates the pain on an electronic pain scale and indicates when the pain detection and the pain tolerance thresholds are reached. This automated technique is user-independent and hence useful in drug trials.
Figure 4.
The pressure pain sensitivity can be assessed by the cuff-algometry method where a tourniquet is automatically is inflated and the subject simultaneously evaluates the pain intensity on an electronic Visual Analogue Scale (VAS). The pain threshold is defined as the first time the VAS score exceeds 0 and the Pain Tolerance is the pressure intensity where the subject stop the stimulation on a push button. Both the pain threshold and tolerance are relevant biomarkers of the pain sensitivity.
Conclusion
Musculoskeletal pain has a substantial socio-economical impact with only few available efficient pain management strategies. Musculoskeletal pain often develops over time resulting in more hyperalgesia and larger pain areas. Peripheral and spreading sensitizations are probably important mechanisms for the translation of acute local pain to chronic musculoskeletal pain conditions. Several mechanisms, such as sensitizations, descending control, central integration and expansion of receptive fields/referred pain have been identified in musculoskeletal nociception and pain. Quantitative methods for assessment of these mechanisms in chronic musculoskeletal pain conditions are available and offer additional information about involved mechanisms to be used for revisions of treatment strategies.
Acknowledgments
The Danish Advanced Technology Foundation is acknowledged by the support to authors LAN and TGN.
References
- 1.Carli G, Suman AL, Biasi G, Marcolongo R. Reactivity to superficial and deep stimuli in patients with chronic musculoskeletal pain. Pain 2002;100:259–69 [DOI] [PubMed] [Google Scholar]
- 2.Herren-Gerber R, Weiss S, Arendt-Nielsen L, Petersen-Felix S, Di SG, Radanov BP, et al. Modulation of central hypersensitivity by nociceptive input in chronic pain after whiplash injury. Pain Med 2004;5:366–76 [DOI] [PubMed] [Google Scholar]
- 3.Fernandez-de-Las-Penas C, Ge HY, Arendt-Nielsen L, Cuadrado ML, Pareja JA. The local and referred pain from myofascial trigger points in the temporalis muscle contributes to pain profile in chronic tension-type headache. Clin J Pain 2007;23:786–92 [DOI] [PubMed] [Google Scholar]
- 4.Simons DG, Travell JG, Simons L. Myofascial pain and dysfunction. The trigger point manual. Philadelphia, PA: Lippincott, Williams & Wilkins; 1999 [Google Scholar]
- 5.Mense S. Nociception from skeletal muscle in relation to clinical muscle pain. Pain 1993;54:241–89 [DOI] [PubMed] [Google Scholar]
- 6.Neugebauer V, Schaible HG. Evidence for a central component in the sensitization of spinal neurons with joint input during development of acute arthritis in cat’s knee. J Neurophysiol 1990;64:299–311 [DOI] [PubMed] [Google Scholar]
- 7.Sessle BJ, Hu JW, Yu X-M. Brainstem mechanisms of referred pain and hyperalgesia in the orofacial and temporomandibular region. : Vecchiet L, Albe-Fessard D, Lindblom U, editors. New trends in referred pain and hyperalgesia Amsterdam: Elsevier Science Publishers B.V.; 1993. 59–71 [Google Scholar]
- 8.Marchettini P, Simone DA, Caputi G, Ochoa JL. Pain from excitation of identified muscle nociceptors in humans. Brain Res 1996;740:109–16 [DOI] [PubMed] [Google Scholar]
- 9.Simone DA, Marchettini P, Caputi G, Ochoa JL. Identification of muscle afferents subserving sensation of deep pain in humans. J Neurophysiol 1994;72:883–9 [DOI] [PubMed] [Google Scholar]
- 10.Alfredson H, Lorentzon R. Chronic tendon pain: no signs of chemical inflammation but high concentrations of the neurotransmitter glutamate. Implications for treatment? Curr Drug Targets 2002;3:43–54 [DOI] [PubMed] [Google Scholar]
- 11.Mense S, Meyer H. Different types of slowly conducting afferent units in cat skeletal muscle and tendon. J Physiol 1985;363:403–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mense S, Simons DG. Muscle pain. Understanding its nature, diagnosis, and treatment. Philadelphia, PA: Lippincott Williams & Wilkins; 2001 [Google Scholar]
- 13.Bjur D, Alfredson H, Forsgren S. The innervation pattern of the human Achilles tendon: studies of the normal and tendinosis tendon with markers for general and sensory innervation. Cell Tissue Res 2005;320:201–6 [DOI] [PubMed] [Google Scholar]
- 14.Danielson P, Alfredson H, Forsgren S. Immunohistochemical and histochemical findings favoring the occurrence of autocrine/paracrine as well as nerve-related cholinergic effects in chronic painful patellar tendon tendinosis. Microsc Res Tech 2006;69:808–19 [DOI] [PubMed] [Google Scholar]
- 15.Gibson W, Arendt-Nielsen L, Graven-Nielsen T. Referred pain and hyperalgesia in human tendon and muscle belly tissue. Pain 2006;120:113–23 [DOI] [PubMed] [Google Scholar]
- 16.Gibson W, Arendt-Nielsen L, Sessle BJ, Graven-Nielsen T. Glutamate and capsaicin-induced pain, hyperalgesia and modulatory interactions in human tendon tissue. Exp Brain Res 2009;194:173–82 [DOI] [PubMed] [Google Scholar]
- 17.Delaney A, Fleetwood-Walker SM, Colvin LA, Fallon M. Translational medicine: cancer pain mechanisms and management. Br J Anaesth 2008;101:87–94 [DOI] [PubMed] [Google Scholar]
- 18.Downey PA, Siegel MI. Bone biology and the clinical implications for osteoporosis. Phys Ther 2006;86:77–91 [DOI] [PubMed] [Google Scholar]
- 19.Luger NM, Mach DB, Sevcik MA, Mantyh PW. Bone cancer pain: from model to mechanism to therapy. J Pain Symptom Manage 2005;29:S32–S46 [DOI] [PubMed] [Google Scholar]
- 20.Kellgren JH. On the distribution of pain arising from deep somatic structures with charts of segmental pain areas. Clin Sci 1939;4:35–46 [Google Scholar]
- 21.Gronblad M, Liesi P, Korkala O, Karaharju E, Polak J. Innervation of human bone periosteum by peptidergic nerves. Anat Rec 1984;209:297–9 [DOI] [PubMed] [Google Scholar]
- 22.Brainin-Mattos J, Smith ND, Malkmus S, Rew Y, Goodman M, Taulane J, et al. Cancer-related bone pain is attenuated by a systemically available delta-opioid receptor agonist. Pain 2006;122:174–81 [DOI] [PubMed] [Google Scholar]
- 23.Graven-Nielsen T, Arendt-Nielsen L, Svensson P, Jensen TS. Experimental muscle pain: a quantitative study of local and referred pain in humans following injection of hypertonic saline. J Musculoskel Pain 1997;5:49–69 [DOI] [PubMed] [Google Scholar]
- 24.Breivik H, Collett B, Ventafridda V, Cohen R, Gallacher D. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur J Pain 2006;10:287–333 [DOI] [PubMed] [Google Scholar]
- 25.Bennell K, Hodges P, Mellor R, Bexander C, Souvlis T. The nature of anterior knee pain following injection of hypertonic saline into the infrapatellar fat pad. J Orthop Res 2004;22:116–21 [DOI] [PubMed] [Google Scholar]
- 26.Martindale JC, Wilson AW, Reeve AJ, Chessell IP, Headley PM. Chronic secondary hypersensitivity of dorsal horn neurones following inflammation of the knee joint. Pain 2007;133:79–86 [DOI] [PubMed] [Google Scholar]
- 27.Schaible HG. Spinal mechanisms contributing to joint pain. Novartis Found Symp 2004;260:4–22 [PubMed] [Google Scholar]
- 28.Felson DT. The sources of pain in knee osteoarthritis. Curr Opin Rheumatol 2005;17:624–8 [DOI] [PubMed] [Google Scholar]
- 29.Schaible HG, Richter F, Ebersberger A, Boettger MK, Vanegas H, Natura G, et al. Joint pain. Exp Brain Res 2009;196:153–62 [DOI] [PubMed] [Google Scholar]
- 30.Schaible HG, Schmidt RF. Activation of groups III and IV sensory units in medial articular nerve by local mechanical stimulation of knee joint. J Neurophysiol 1983;49:35–44 [DOI] [PubMed] [Google Scholar]
- 31.Sluka KA. Stimulation of deep somatic tissue with capsaicin produces long-lasting mechanical allodynia and heat hypoalgesia that depends on early activation of the cAMP pathway. J Neurosci 2002;22:5687–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dye SF, Vaupel GL, Dye CC. Conscious neurosensory mapping of the internal structures of the human knee without intraarticular anesthesia. Am J Sports Med 1998;26:773–7 [DOI] [PubMed] [Google Scholar]
- 33.Kellgren JH, Samuel EP. The sensitivity and innervation of the articular capsule. J Bone Joint Surg 1950;4:193–205 [Google Scholar]
- 34.Arendt-Nielsen L, Nie H, Laursen MB, Laursen BS, Madeleine P, Simonsen OH, Graven-Nielsen T. Sensitization in patients with painful knee osteoarthritis. Pain 2010;149:573–81 [DOI] [PubMed] [Google Scholar]
- 35.Graven-Nielsen T. Fundamentals of muscle pain, referred pain, and deep tissue hyperalgesia. Scand J Rheumatol Suppl 2006;1–43 [DOI] [PubMed] [Google Scholar]
- 36.Graven-Nielsen T, Arendt-Nielsen L. Assessment of mechanisms in localized and widespread musculoskeletal pain. Nat Rev Rheumatol 2010;6:599–606 [DOI] [PubMed] [Google Scholar]
- 37.Hoheisel U, Mense S, Simons DG, Yu X-M. Appearance of new receptive fields in rat dorsal horn neurons following noxious stimulation of skeletal muscle: a model for referral of muscle pain? Neurosci Lett 1993;153:9–12 [DOI] [PubMed] [Google Scholar]
- 38.Mense S. Referral of muscle pain. New aspects. APS J 1994;3:1–9 [Google Scholar]
- 39.Schulte H, Graven-Nielsen T, Sollevi A, Jansson Y, Arendt-Nielsen L, Segerdahl M. Pharmacological modulation of experimental phasic and tonic muscle pain by morphine, alfentanil and ketamine in healthy volunteers. Acta Anaesthesiol Scand 2003;47:1020–30 [DOI] [PubMed] [Google Scholar]
- 40.Arendt-Nielsen L, Graven-Nielsen T. Muscle pain: sensory implications and interaction with motor control. Clin J Pain 2008;24:291–8 [DOI] [PubMed] [Google Scholar]
- 41.Bogduk N. The neck and headaches. Neurol Clin 2004;22:151–71 [DOI] [PubMed] [Google Scholar]
- 42.Arendt-Nielsen L, Graven-Nielsen T. translational aspects of musculoskeletal pain: from animals to patients. : Graven-Nielsen T, Arendt-Nielsen L, Mense S, editors. Fundamentals of Musculoskeletal Pain. Seattle, WA: IASP Press; 2008. 347–66 [Google Scholar]
- 43.Nie H, Arendt-Nielsen L, Andersen H, Graven-Nielsen T. Temporal summation of pain evoked by mechanical stimulation in deep and superficial tissue. J Pain 2005;6:348–55 [DOI] [PubMed] [Google Scholar]
- 44.Staud R, Cannon RC, Mauderli AP, Robinson ME, Price DD, Vierck CJ., Jr Temporal summation of pain from mechanical stimulation of muscle tissue in normal controls and subjects with fibromyalgia syndrome. Pain 2003;102:87–95 [DOI] [PubMed] [Google Scholar]
- 45.Staud R, Craggs JG, Perlstein WM, Robinson ME, Price DD. Brain activity associated with slow temporal summation of C-fiber evoked pain in fibromyalgia patients and healthy controls. Eur J Pain 2008;12:1078–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sorensen J, Graven-Nielsen T, Henriksson KG, Bengtsson M, Arendt-Nielsen L. Hyperexcitability in fibromyalgia. J Rheumatol 1998;25:152–5 [PubMed] [Google Scholar]
- 47.Banic B, Petersen-Felix S, Andersen OK, Radanov BP, Villiger PM, Arendt-Nielsen L, et al. Evidence for spinal cord hypersensitivity in chronic pain after whiplash injury and in fibromyalgia. Pain 2004;107:7–15 [DOI] [PubMed] [Google Scholar]
- 48.Arendt-Nielsen L, Yarnitsky D. Experimental and clinical applications of quantitative sensory testing applied to skin, muscles and viscera. J Pain 2009; 10:556–72 [DOI] [PubMed] [Google Scholar]
- 49.Jensen KB, Kosek E, Petzke F, Carville S, Fransson P, Marcus H, et al. Evidence of dysfunctional pain inhibition in Fibromyalgia reflected in rACC during provoked pain. Pain 2009;144:95–100 [DOI] [PubMed] [Google Scholar]
- 50.Binderup AT, Arendt-Nielsen L, Madeleine P. Cluster analysis of pressure pain threshold maps from the trapezius muscle. Comput Methods Biomech Biomed Engin 2010;13:677–83 [DOI] [PubMed] [Google Scholar]
- 51.Koelbaek JM, Graven-Nielsen T, Schou OA, Arendt-Nielsen L. Generalised muscular hyperalgesia in chronic whiplash syndrome. Pain 1999;83:229–34 [DOI] [PubMed] [Google Scholar]
- 52.Sandkuhler J. Models and mechanisms of hyperalgesia and allodynia. Physiol Rev 2009;89:707–58 [DOI] [PubMed] [Google Scholar]
- 53.Slater H, Arendt-Nielsen L, Wright A, Graven-Nielsen T. Effects of a manual therapy technique in experimental lateral epicondylalgia. Man Ther 2006;11:107–17 [DOI] [PubMed] [Google Scholar]
- 54.Holst H, Arendt-Nielsen L, Mosbech H, Serup J, Elberling J. Capsaicin-induced neurogenic inflammation in the skin in patients with symptoms induced by odorous chemicals. Skin Res Technol 2011;17:82–90 [DOI] [PubMed] [Google Scholar]
- 55.Lemming D, Sorensen J, Graven-Nielsen T, Arendt-Nielsen L, Gerdle B. The responses to pharmacological challenges and experimental pain in patients with chronic whiplash-associated pain. Clin J Pain 2005;21:412–21 [DOI] [PubMed] [Google Scholar]
- 56.Gibson W, Arendt-Nielsen L, Graven-Nielsen T. Delayed onset muscle soreness at tendon-bone junction and muscle tissue is associated with facilitated referred pain. Exp Brain Res 2006;174:351–60 [DOI] [PubMed] [Google Scholar]
- 57.Babenko VV, Graven-Nielsen T, Svensson P, Drewes AM, Jensen TS, Arendt-Nielsen L. Experimental human muscle pain induced by intramuscular injections of bradykinin, serotonin, and substance P. Eur J Pain 1999;3:93–102 [DOI] [PubMed] [Google Scholar]
- 58.Gerber RK, Nie H, Arendt-Nielsen L, Curatolo M, Graven-Nielsen T. Local pain and spreading hyperalgesia induced by intramuscular injection of nerve growth factor are not reduced by local anesthesia of the muscle. Clin J Pain 2011;27:240–7 [DOI] [PubMed] [Google Scholar]
- 59.Andersen H, Arendt-Nielsen L, Svensson P, Danneskiold-Samsoe B, Graven-Nielsen T. Spatial and temporal aspects of muscle hyperalgesia induced by nerve growth factor in humans. Exp Brain Res 2008;191:371–82 [DOI] [PubMed] [Google Scholar]
- 60.Graven-Nielsen T, Arendt-Nielsen L, Svensson P, Jensen TS. Quantification of local and referred muscle pain in humans after sequential i.m. injections of hypertonic saline. Pain 1997;69:111–7 [DOI] [PubMed] [Google Scholar]
- 61.Cairns BE, Svensson P, Wang K, Castrillon E, Hupfeld S, Sessle BJ, et al. Ketamine attenuates glutamate-induced mechanical sensitization of the masseter muscle in human males. Exp Brain Res 2006;169:467–72 [DOI] [PubMed] [Google Scholar]
- 62.Witting N, Svensson P, Gottrup H, Arendt-Nielsen L, Jensen TS. Intramuscular and intradermal injection of capsaicin: a comparison of local and referred pain. Pain 2000;84:407–12 [DOI] [PubMed] [Google Scholar]
- 63.Sluka KA, Price MP, Breese NM, Stucky CL, Wemmie JA, Welsh MJ. Chronic hyperalgesia induced by repeated acid injections in muscle is abolished by the loss of ASIC3, but not ASIC1. Pain 2003;106:229–39 [DOI] [PubMed] [Google Scholar]
- 64.Frey Law LA, Sluka KA, McMullen T, Lee J, Arendt-Nielsen L, Graven-Nielsen T. Acidic buffer induced muscle pain evokes referred pain and mechanical hyperalgesia in humans. Pain 2008;140:254–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Issberner U, Reeh PW, Steen KH. Pain due to tissue acidosis: a mechanism for inflammatory and ischemic myalgia? Neurosci Lett 1996;208:191–4 [DOI] [PubMed] [Google Scholar]
- 66.Curatolo M, Petersen-Felix S, Arendt-Nielsen L, Zbinden AM. Epidural epinephrine and clonidine: segmental analgesia and effects on different pain modalities. Anesthesiol 1997;87:785–94 [DOI] [PubMed] [Google Scholar]
- 67.Staahl C, Christrup LL, Andersen SD, Arendt-Nielsen L, Drewes AM. A comparative study of oxycodone and morphine in a multi-modal, tissue-differentiated experimental pain model. Pain 2006;123:28–36 [DOI] [PubMed] [Google Scholar]
- 68.Adriaensen H, Gybels J, Handwerker HO, Van Hees J. Nociceptor discharges and sensations due to prolonged noxious mechanical stimulation-a paradox. Hum Neurobiol 1984;3:53–8 [PubMed] [Google Scholar]
- 69.Neziri AY, Scaramozzino P, Andersen OK, Dickenson AH, Arendt-Nielsen L, Curatolo M. Reference values of mechanical and thermal pain tests in a pain-free population. Eur J Pain 2011;15:376–83 [DOI] [PubMed] [Google Scholar]
- 70.Andersen H, Arendt-Nielsen L, Danneskiold-Samsoe B, Graven-Nielsen T. Pressure pain sensitivity and hardness along human normal and sensitized muscle. Somatosens Mot Res 2006;23:97–109 [DOI] [PubMed] [Google Scholar]
- 71.Woolf CJ, Bennett GJ, Doherty M, Dubner R, Kidd B, Koltzenburg M, et al. Towards a mechanism-based classification of pain? Pain 1998;77:227–9 [DOI] [PubMed] [Google Scholar]
- 72.Polianskis R, Graven-Nielsen T, Arendt-Nielsen L. Computer-controlled pneumatic pressure algometry–a new technique for quantitative sensory testing. Eur J Pain 2001;5:267–77 [DOI] [PubMed] [Google Scholar]
- 73.Jespersen A, Dreyer L, Kendall S, Graven-Nielsen T, Arendt-Nielsen L, Bliddal H, et al. Computerized cuff pressure algometry: a new method to assess deep-tissue hypersensitivity in fibromyalgia. Pain 2007;131:57–62 [DOI] [PubMed] [Google Scholar]