Abstract
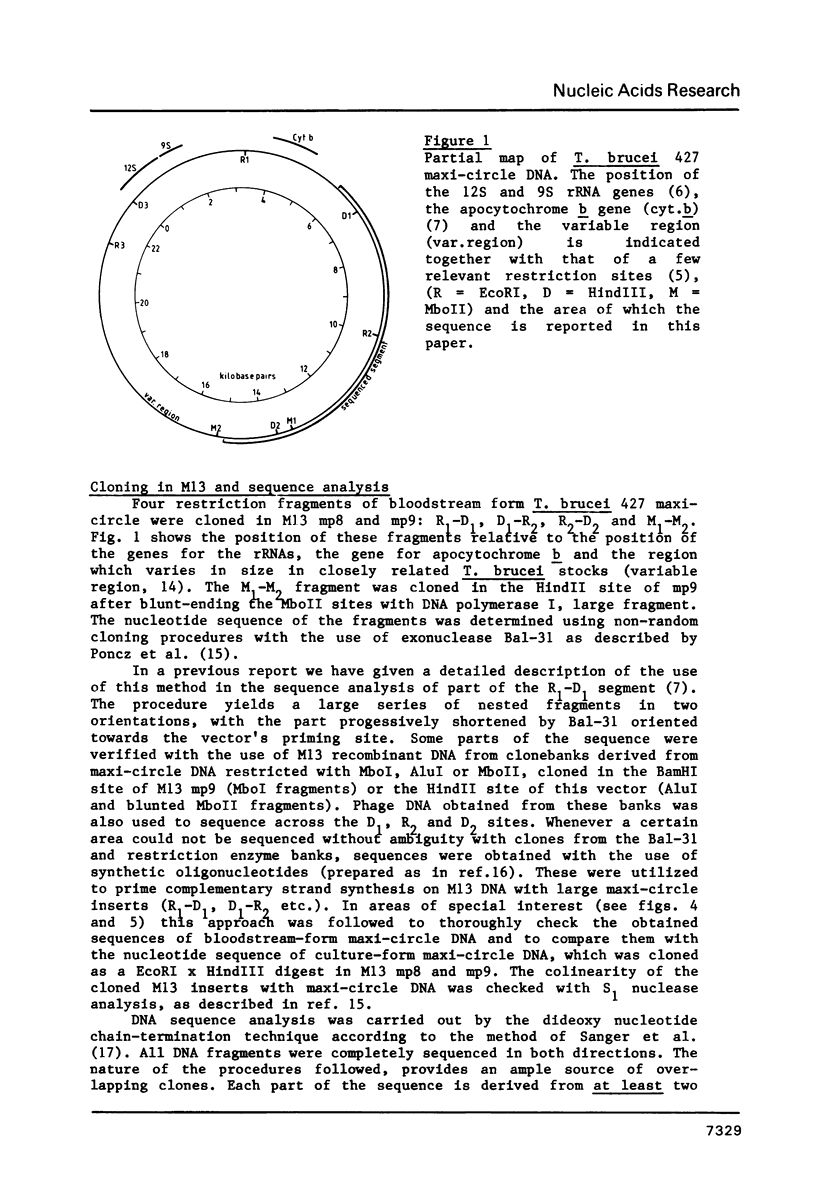
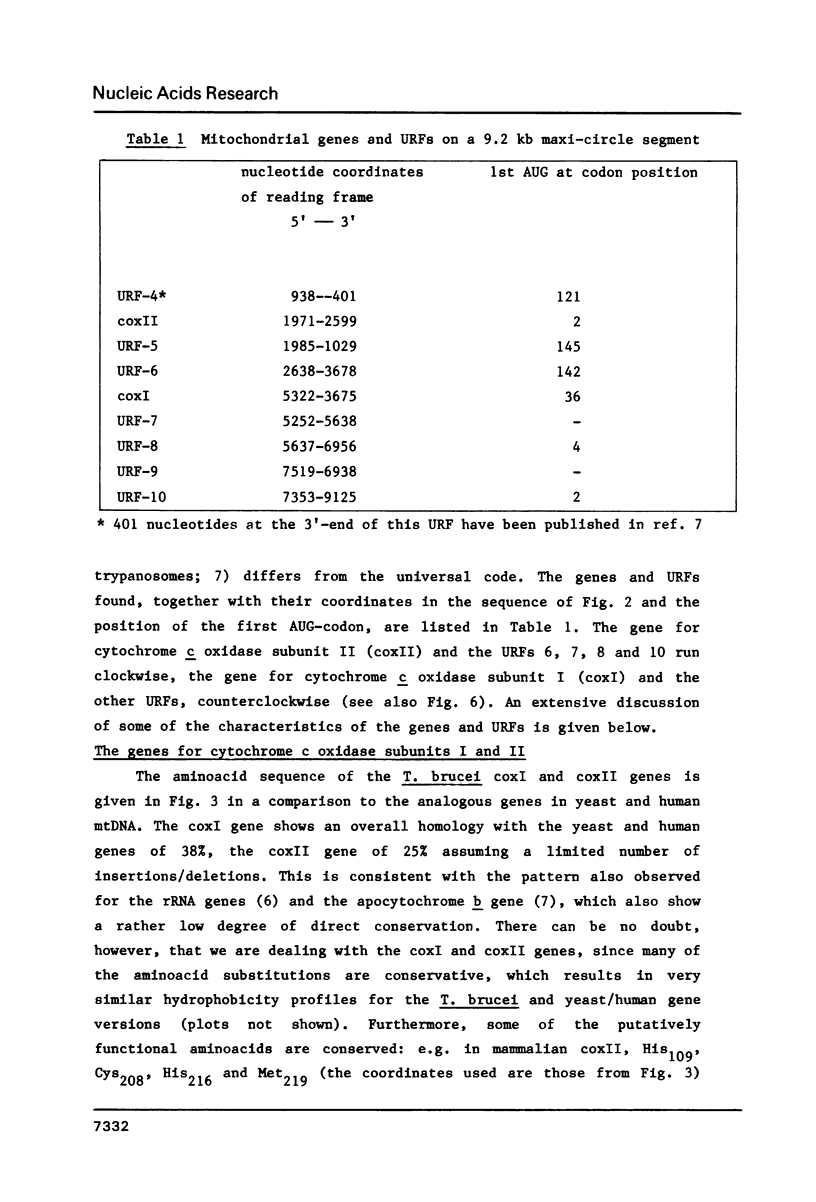
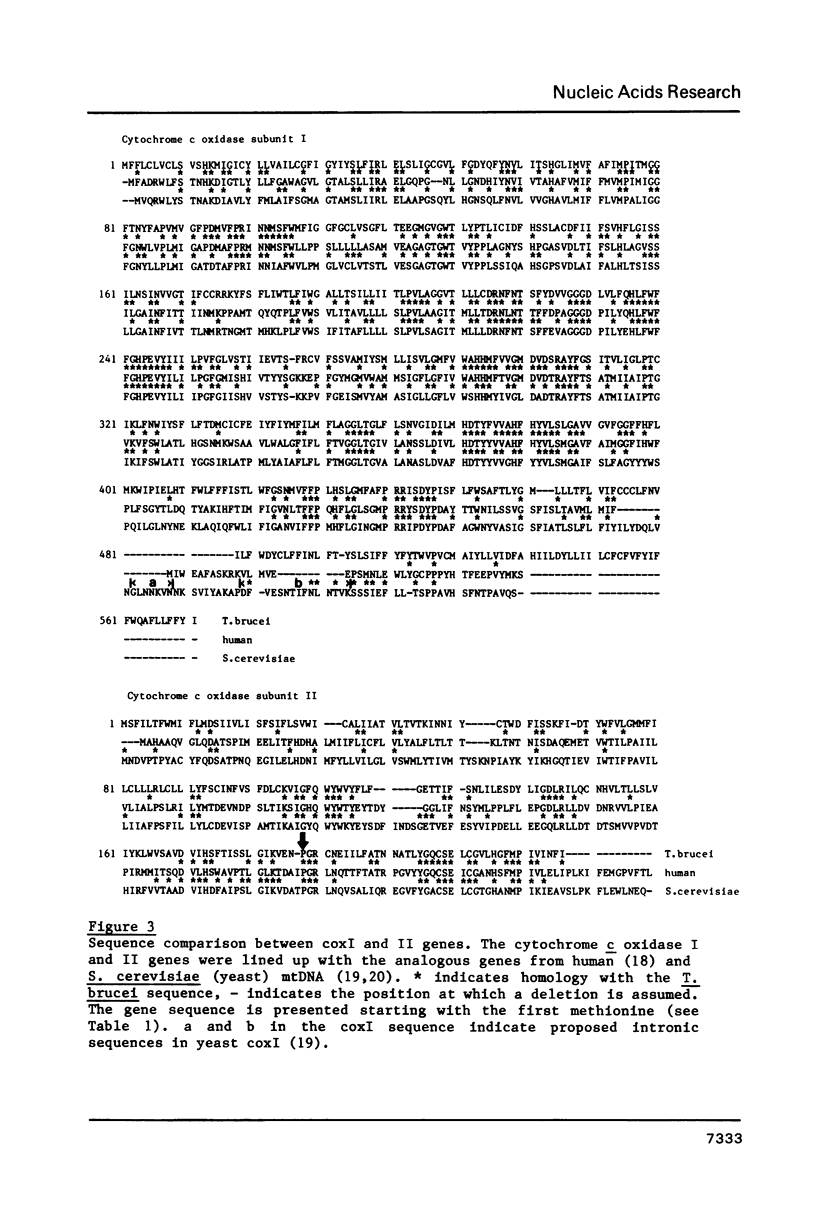
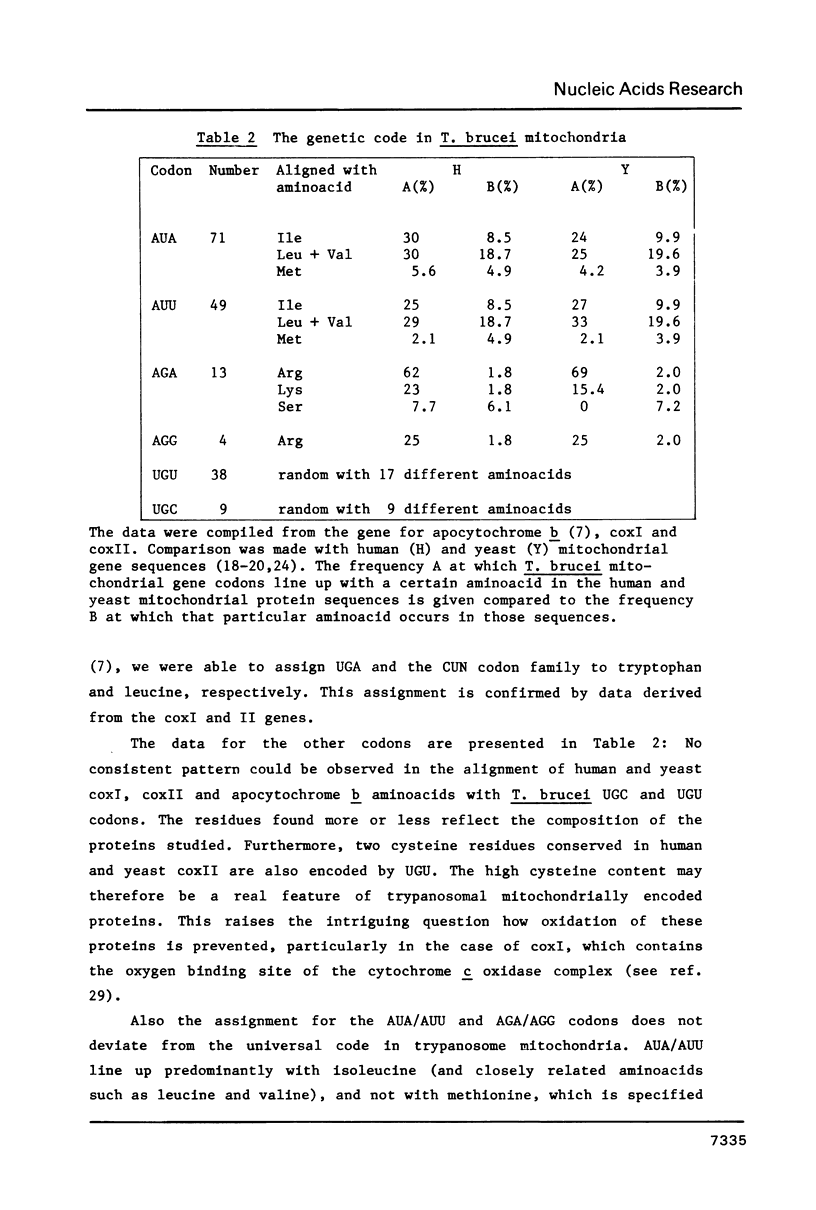
A 9.2 kb segment of the maxi-circle of Trypanosoma brucei mitochondrial DNA contains the genes for cytochrome c oxidase subunits I and II (coxI and coxII) and seven Unassigned Reading Frames ("URFs"). The genes for coxI and coxII display considerable homology at the aminoacid level (38 and 25%, respectively) to the corresponding genes in fungal and mammalian mtDNA, the only striking point of divergence being an unusually high cysteine content (about 4.5%). The reading frame coding for cytochrome c oxidase subunit II is discontinuous: the C-terminal portion of about 40 aminoacids, is present in the DNA-sequence in a -1 reading frame with respect to the N-terminal moiety. URF5, 8 and 10, show a low but distinct homology (about 20%) to mammalian mitochondrial URF-1, 4 and 5, respectively. In URF5, the first AUG is found at codon 145, whereas extensive homology to mammalian URF-1 sequences occurs upstream of this position. The possibility exists that UUG can serve as an initiator codon. URF7 and URF9 have a highly unusual aminoacid composition and do not possess AUG or UUG initiator codons. These URFs probably do not have a protein-coding function. The segment does not contain conventional tRNA genes.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S., Bankier A. T., Barrell B. G., de Bruijn M. H., Coulson A. R., Drouin J., Eperon I. C., Nierlich D. P., Roe B. A., Sanger F. Sequence and organization of the human mitochondrial genome. Nature. 1981 Apr 9;290(5806):457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- Benne R., De Vries B. F., Van den Burg J., Klaver B. The nucleotide sequence of a segment of Trypanosoma brucei mitochondrial maxi-circle DNA that contains the gene for apocytochrome b and some unusual unassigned reading frames. Nucleic Acids Res. 1983 Oct 25;11(20):6925–6941. doi: 10.1093/nar/11.20.6925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonitz S. G., Coruzzi G., Thalenfeld B. E., Tzagoloff A., Macino G. Assembly of the mitochondrial membrane system. Structure and nucleotide sequence of the gene coding for subunit 1 of yeast cytochrme oxidase. J Biol Chem. 1980 Dec 25;255(24):11927–11941. [PubMed] [Google Scholar]
- Borst P., Fase-Fowler F., Hoeijmakers J. H., Frasch A. C. Variations in maxi-circle and mini-circle sequences in kinetoplast DNAs from different Trypanosoma brucei strains. Biochim Biophys Acta. 1980 Dec 11;610(2):197–210. doi: 10.1016/0005-2787(80)90001-5. [DOI] [PubMed] [Google Scholar]
- Borst P., Hoeijmakers J. H. Kinetoplast DNA. Plasmid. 1979 Jan;2(1):20–40. doi: 10.1016/0147-619x(79)90003-9. [DOI] [PubMed] [Google Scholar]
- Capaldi R. A., Malatesta F., Darley-Usmar V. M. Structure of cytochrome c oxidase. Biochim Biophys Acta. 1983 Jul 15;726(2):135–148. doi: 10.1016/0304-4173(83)90003-4. [DOI] [PubMed] [Google Scholar]
- Clary D. O., Wahleithner J. A., Wolstenholme D. R. Sequence and arrangement of the genes for cytochrome b, URF1, URF4L, URF4, URF5, URF6 and five tRNAs in Drosophila mitochondrial DNA. Nucleic Acids Res. 1984 May 11;12(9):3747–3762. doi: 10.1093/nar/12.9.3747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coruzzi G., Bonitz S. G., Thalenfeld B. E., Tzagoloff A. Assembly of the mitochondrial membrane system. Analysis of the nucleotide sequence and transcripts in the oxi1 region of yeast mitochondrial DNA. J Biol Chem. 1981 Dec 25;256(24):12780–12787. [PubMed] [Google Scholar]
- Coruzzi G., Tzagoloff A. Assembly of the mitochondrial membrane system. DNA sequence of subunit 2 of yeast cytochrome oxidase. J Biol Chem. 1979 Sep 25;254(18):9324–9330. [PubMed] [Google Scholar]
- Eperon I. C., Janssen J. W., Hoeijmakers J. H., Borst P. The major transcripts of the kinetoplast DNA of Trypanosoma brucei are very small ribosomal RNAs. Nucleic Acids Res. 1983 Jan 11;11(1):105–125. doi: 10.1093/nar/11.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. A., Brown R. C. The effect of diphenylamine on terminal respiration in bloodstream and culture forms of Trypanosoma brucei. J Protozool. 1972 May;19(2):365–369. doi: 10.1111/j.1550-7408.1972.tb03478.x. [DOI] [PubMed] [Google Scholar]
- Fox T. D. Five TGA "stop" codons occur within the translated sequence of the yeast mitochondrial gene for cytochrome c oxidase subunit II. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6534–6538. doi: 10.1073/pnas.76.12.6534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox T. D., Weiss-Brummer B. Leaky +1 and -1 frameshift mutations at the same site in a yeast mitochondrial gene. Nature. 1980 Nov 6;288(5786):60–63. doi: 10.1038/288060a0. [DOI] [PubMed] [Google Scholar]
- Grivell L. A. Mitochondrial DNA. Sci Am. 1983 Mar;248(3):78–89. doi: 10.1038/scientificamerican0383-78. [DOI] [PubMed] [Google Scholar]
- Hoeijmakers J. H., Snijders A., Janssen J. W., Borst P. Transcription of kinetoplast DNA in Trypanosoma brucei bloodstream and culture forms. Plasmid. 1981 May;5(3):329–350. doi: 10.1016/0147-619x(81)90009-3. [DOI] [PubMed] [Google Scholar]
- Hoeijmakers J. H., Weijers P. J. The segregation of kinetoplast DNA networks in Trypanosoma brucei. Plasmid. 1980 Jul;4(1):97–116. doi: 10.1016/0147-619x(80)90086-4. [DOI] [PubMed] [Google Scholar]
- Johnson B. J., Hill G. C., Fox T. D., Stuart K. The maxicircle of Trypanosoma brucei kinetoplast DNA hybridizes with a mitochondrial gene encoding cytochrome oxidase subunit II. Mol Biochem Parasitol. 1982 Jun;5(6):381–390. doi: 10.1016/0166-6851(82)90011-1. [DOI] [PubMed] [Google Scholar]
- Kastelein R. A., Remaut E., Fiers W., van Duin J. Lysis gene expression of RNA phage MS2 depends on a frameshift during translation of the overlapping coat protein gene. Nature. 1982 Jan 7;295(5844):35–41. doi: 10.1038/295035a0. [DOI] [PubMed] [Google Scholar]
- Macino G., Tzagoloff A. Assembly of the mitochondrial membrane system: sequence analysis of a yeast mitochondrial ATPase gene containing the oli-2 and oli-4 loci. Cell. 1980 Jun;20(2):507–517. doi: 10.1016/0092-8674(80)90637-6. [DOI] [PubMed] [Google Scholar]
- Nobrega F. G., Tzagoloff A. Assembly of the mitochondrial membrane system. DNA sequence and organization of the cytochrome b gene in Saccharomyces cerevisiae D273-10B. J Biol Chem. 1980 Oct 25;255(20):9828–9837. [PubMed] [Google Scholar]
- Noller H. F., Kop J., Wheaton V., Brosius J., Gutell R. R., Kopylov A. M., Dohme F., Herr W., Stahl D. A., Gupta R. Secondary structure model for 23S ribosomal RNA. Nucleic Acids Res. 1981 Nov 25;9(22):6167–6189. doi: 10.1093/nar/9.22.6167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poncz M., Solowiejczyk D., Ballantine M., Schwartz E., Surrey S. "Nonrandom" DNA sequence analysis in bacteriophage M13 by the dideoxy chain-termination method. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4298–4302. doi: 10.1073/pnas.79.14.4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray S. K., Cross G. A. Branched electron transport chain in Trypanosoma mega. Nat New Biol. 1972 Jun 7;237(75):174–175. doi: 10.1038/newbio237174a0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson L., Spithill T. W., Simpson A. M. Identification of maxicircle DNA sequences in Leishmania Tarentolae that are homologous to sequences of specific yeast mitochondrial structural genes. Mol Biochem Parasitol. 1982 Oct;6(4):253–264. doi: 10.1016/0166-6851(82)90058-5. [DOI] [PubMed] [Google Scholar]
- Steffens G. J., Buse G. Studies on cytochrome c oxidase, IV[1--3]. Primary structure and function of subunit II. Hoppe Seylers Z Physiol Chem. 1979 Apr;360(4):613–619. [PubMed] [Google Scholar]
- Thalenfeld B. E., Tzagoloff A. Assembly of the mitochondrial membrane system. Sequence of the oxi 2 gene of yeast mitochondrial DNA. J Biol Chem. 1980 Jul 10;255(13):6173–6180. [PubMed] [Google Scholar]
- Welinder K. G., Mikkelsen L. The oxygen binding site of cytochrome oxidase. Structural predictions on subunit I from amino acid sequences. FEBS Lett. 1983 Jul 4;157(2):233–239. doi: 10.1016/0014-5793(83)80553-5. [DOI] [PubMed] [Google Scholar]
- Woese C. R., Gutell R., Gupta R., Noller H. F. Detailed analysis of the higher-order structure of 16S-like ribosomal ribonucleic acids. Microbiol Rev. 1983 Dec;47(4):621–669. doi: 10.1128/mr.47.4.621-669.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young I. G., Rogers B. L., Campbell H. D., Jaworowski A., Shaw D. C. Nucleotide sequence coding for the respiratory NADH dehydrogenase of Escherichia coli. UUG initiation codon. Eur J Biochem. 1981 May;116(1):165–170. doi: 10.1111/j.1432-1033.1981.tb05314.x. [DOI] [PubMed] [Google Scholar]




