Abstract
In recent years, increased knowledge of the pathogenesis of upper quadrant pain syndromes has translated to better management strategies. Recent studies have demonstrated evidence of peripheral and central sensitization mechanisms in different local pain syndromes of the upper quadrant such as idiopathic neck pain, lateral epicondylalgia, whiplash-associated disorders, shoulder impingement, and carpal tunnel syndrome. Therefore, a treatment-based classification approach where subjects receive matched interventions has been developed and, it has been found that these patients experience better outcomes than those receiving non-matched interventions. There is evidence suggesting that the cervical and thoracic spine is involved in upper quadrant pain. Spinal manipulation has been found to be effective for patients with elbow pain, neck pain, or cervicobrachial pain. Additionally, it is known that spinal manipulative therapy exerts neurophysiological effects that can activate pain modulation mechanisms. This paper exposes some manual therapies for upper quadrant pain syndromes, based on a nociceptive pain rationale for modulating central nervous system including trigger point therapy, dry needling, mobilization or manipulation, and cognitive pain approaches.
Keywords: Upper quadrant, Pain, Sensitization, Neck, Thoracic, Manual therapy
Introduction
In the twenty-first century, upper quadrant syndromes are common and cause substantial pain and disability. It has been estimated that 70% of the population experience neck or arm pain at some time during their life.1,2 In fact, musculoskeletal disorders represent the majority of occupational ill-health and upper quadrant pain is second only to back pain as a cause of work-related illness.3,4 In addition, upper quadrant pain represents high costs for health care systems as up to 58% of patients will make use of healthcare within the next 12 months.5
Walker-Bone et al. found that pain experienced in the upper quadrant region is frequently perceived in the dominant arm and the neck.6 Upper quadrant pain can arise from several widely different conditions. In fact, different terms, i.e. cumulative trauma disorders, cervicobrachial disorders, repetitive strain injury, and work-related upper limb disorders, have been used to describe pain at different sites in the neck and upper limb with no confirmed pathoanatomical abnormalities.7 Pain symptoms in the neck, shoulder, or arm, which are not based on acute trauma or underlying systemic diseases, have been defined as ‘complaints of the arm, neck and/or shoulder region’. This term suggests that symptoms in the upper quadrant may have different causes.
In this paper, we will discuss: (1) the relevance of the cervical and thoracic spine in upper quadrant pain syndromes and their management with manual therapy; (2) the presence of common sensitization mechanisms in different local pain syndromes of the upper quadrant; and (3) manual therapies proposed for upper quadrant pain syndromes based on nociceptive pain rationale.
The Cervical and Thoracic Spine in Upper Quadrant Pain
Any innervated structure in the cervical and thoracic spine can be a source of nociception and provide an input mechanism for the experience of upper quadrant pain. However, just as with low back pain, identifying the exact anatomical sources of neck and arm pain is often not possible. Current research is encouraging a paradigm shift in clinical decision making away from the traditional tissue-based (biomedical) models of pain towards a more comprehensive biopsychosocial model.8,9 The biopsychosocial model encompasses more than just the biological factors (anatomy, physiology, and pathoanatomy) in upper quadrant function, by addressing psychological (thoughts, emotions, and behaviors), and social (work and playing status, culture, and religion) factors known to play a significant role in upper quadrant function in the context of injury or illness. A true biopsychosocial model includes a greater understanding of how the nervous system processes injury, disease, pain, threat, and emotions.10
The limitations of a tissue-based approach for managing upper quadrant pain have led to current best evidence advocating a treatment-based classification (TBC) approach.11,12 With such an approach, less emphasis is placed on locating the probable tissue sources of upper quadrant pain. Instead, greater emphasis is placed on matching the patient to optimal interventions based on the identification of signs and symptoms collected during the interview and physical examination.12–14 Preliminary studies of a TBC approach demonstrated that subjects receiving matched interventions were found to experience better outcomes (neck disability scores and pain ratings) than individuals receiving non-matched interventions.12 The current TBC system for patients with neck and arm pain is composed of five classification subgroups.12 The classification subgroups are: mobility, centralization, exercise and conditioning, pain control, and headache. An algorithm has been proposed to aid the clinician in determining which appropriate classification subgroup their patient should be assigned to (Fig. 1). The interventions proposed as a match for the respective subgroups include combinations of joint mobilization and/or manipulation; therapeutic exercises including neuromuscular reeducation, stretching and strengthening, cervical retraction, and manual/mechanical traction.15 We propose that neuroscience education (i.e. explaining the patient’s pain) is an intervention that should be provided across all subgroups if physical therapists wish to follow a true biopsychosocial approach to the management of upper quadrant pain.
Figure 1.
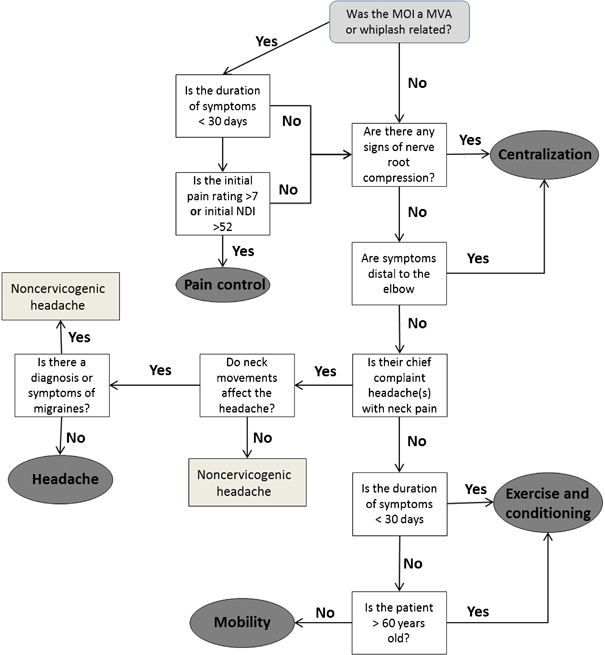
The treatment-based algorithm as outlined by Fritz and Brennan.12 MOI: mechanism of injury; MVA: motor vehicle accident; NDI: neck disability index.
Manual therapy interventions for neck pain
The use of manipulation of the cervical spine remains controversial because of the reported adverse reactions and subsequent concerns about safety. These reported adverse reactions range from minor and transient conditions, e.g. headaches, stiffness, increased pain, and limitations in motion,16–19 to the more serious injuries including permanent neurological deficits, dissection of carotid or vertebral arteries, and death.20–25 Despite the potential for adverse reactions, there is evidence to suggest that manipulation is effective in immediately improving cervical range of motion and decreasing neck pain when it is applied to the cervical26 and thoracic spine.27–29 Because of concerns about the safety of applying manipulation to the cervical spine and the concept of regional interdependence,30,31 researchers have investigated the value of manipulating the thoracic spine in patients with mechanical neck pain. Studies have shown that thoracic spine manipulation results in immediate clinically significant reduction in neck pain when compared to placebo;27 and significantly greater reduction in pain and disability, as measured by the neck disability index, when compared to non-thrust mobilization or TENS, exercise, and massage.32,33
Cleland et al.15 conducted a prospective cohort study involving 78 patients with neck pain and developed a clinical prediction rule (CPR) for patients who would achieve clinically meaningful improvement on the global rating of change scale following thoracic spine manipulation. Predictor variables included: symptoms less than 30 days; no symptoms below the shoulder; looking up does not aggravate symptoms; fear avoidance beliefs questionnaire physical activity subscale score of less than 12; diminished upper thoracic kyphosis; and cervical extension range of motion less than 30°. A CPR was developed which demonstrated that patients meeting three out of the six above predictors had an 86% post-test probability of experiencing success with thoracic manipulation. A follow-up validation study34 found that the CPR was not effective in identifying patients with neck pain who would experience benefit from thoracic manipulation. They found that patients with neck pain and no contraindications to thoracic manipulation experienced greater improvements in pain and disability if they received thoracic manipulation when compared to patients who received exercise only.
We completed a recent randomized clinical trial35 involving 24 consecutive patients presenting to physical therapy with a primary complaint of neck pain who met four out of six of the CPR criteria for thoracic spine thrust manipulation. The patients were randomly assigned to one of two treatment groups: a thoracic group who received thoracic thrust manipulation (Figs. 2–4) for the first two sessions followed by a standardized exercise program for an additional three sessions, and a cervical group who received a cervical thrust manipulation (Fig. 5) for the first two sessions and the identical exercise program as the thoracic group for the next three sessions. Results demonstrated that patients who received cervical manipulation demonstrated greater improvements in neck disability index (P⩽0.001) and NPRS (P⩽0.003) at all follow-up time periods.35 There was also a statistically significant improvement in the fear avoidance beliefs questionnaire (PA) at all follow-ups for the cervical group (P⩽0.004).35 Importantly, we found no serious adverse events during the treatment period and 6-month follow-up in either group. There were some minor side effects which were defined as short-term, mild in nature, non-serious, transient, and reversible consequences of the treatment such as increase in neck pain, headache, and fatigue. Interestingly, these were more common in the thoracic group than in the cervical group, and may suggest that, when used appropriately and for the right patient with mechanical neck pain, cervical manipulation may be less harmful than thoracic manipulation.
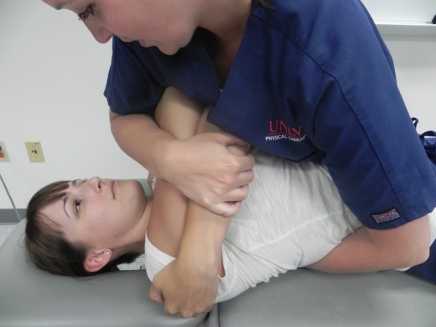
Figure 2.
Supine middle to lower thoracic spine thrust manipulation. The therapist uses the manipulative hand to stabilize the inferior vertebra of the motion segment targeted and uses the body to push down through the patient’s arms to perform a high-velocity, low-amplitude thrust.
Figure 4.
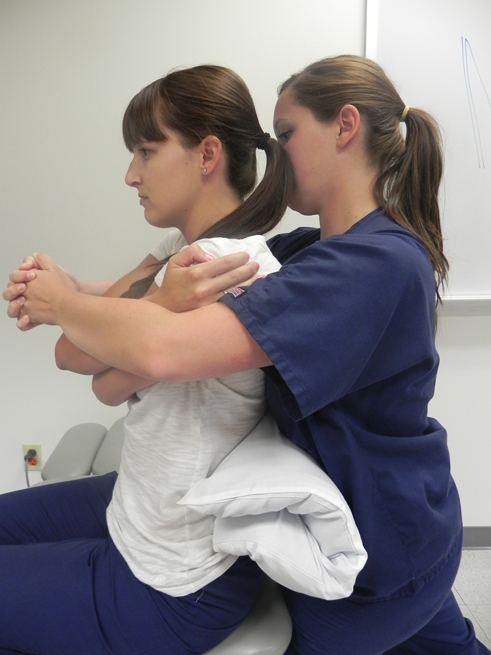
Seated thoracic spine distraction thrust manipulation. The therapist places the upper chest at the level of the patient’s middle thoracic spine and grasps the patient’s elbows. A high-velocity distraction thrust is performed in an upward direction.
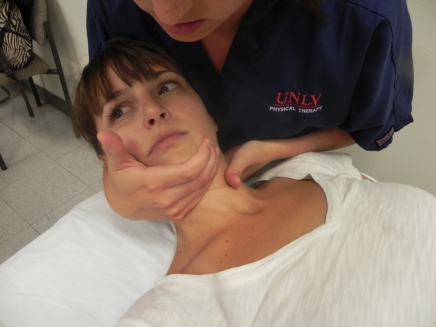
Figure 5.
Cervical spine thrust joint manipulation. The therapist uses the manipulative (left) hand to localize the motion segment targeted and uses both hands to perform a high-velocity, low-amplitude thrust into rotation, which was directed up towards the patient’s contra-lateral eye.
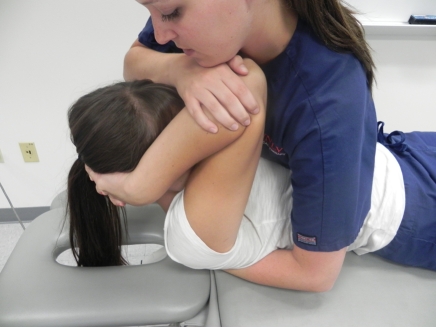
Figure 3.
Supine upper thoracic on mid-thoracic spine thrust manipulation in cervico-thoracic flexion. The therapist uses the manipulative hand to stabilize the inferior vertebra of the motion segment targeted and uses the body to push down through the patient’s arms, to perform a high-velocity, low-amplitude thrust.
Manual therapy interventions for upper extremity pain
Patients with a primary complaint of pain in their upper extremity (shoulder, upper arm, elbow, forearm, and hand) may be helped with manual therapy interventions to the cervical and thoracic spine. A recent case study found that pain, hyperesthesia, paresthesia, and hyperpigmentation of the middle back (subscapular area) associated with the condition known as notalgia paresthetica, were improved with thoracic spine manipulation.36 A randomized, blinded, placebo-controlled, cross-over trial involving 21 subjects with shoulder pain found that cervical lateral glide mobilizations to the cervical spine resulted in significant decreases in shoulder abduction painful arc and shoulder pain.37 Boyles et al.38 demonstrated that thoracic manipulation resulted in a statistically significant decrease in self-reported pain and disability in patients with shoulder impingement syndrome at 48 hours follow-up. Finally, Mintken et al.39 were able to develop a CPR for patients with a primary complaint of shoulder pain who demonstrated rapid improvement in pain and disability following cervical and thoracic spine manipulation. The prospective single-arm trial found if three out of five prognostic variables were present (pain-free shoulder flexion <127°; shoulder internal rotation <53° at 90° of abduction; negative Neer test; no medication use for their shoulder pain; and symptoms <90 days), the chance of experiencing rapid improvement following manipulation improved from 61 to 89% (positive likelihood ratio = 5.3).39
As identified in a prognostic analysis, patients with upper extremity symptoms and concomitant neck pain have a poorer outcome.40 However, there are studies that show benefits of applying treatment to the cervical spine in patients with elbow pain. In a retrospective case audit of 112 cases, Cleland et al.41 showed that significantly fewer treatments were required for those who received additional manual therapy to the cervical spine in the form of non-thrust oscillatory manipulations, mobilization with movement, and/or muscle energy techniques. More recently in a pilot trial of 10 cases, Cleland et al. reported a better result on pain-free grip force and the disability of the arm, shoulder and hand questionnaire.42
Furthermore, there are a number of studies that show both high- and low-velocity manipulations of the cervical spine produce an initial improvement in pain at the elbow.43–45 This evidence provides a basis for the cervical spine to be treated if found to be implicated on physical examination, especially since there have been reported significant differences in pain provocation on manual examination of the cervical spine and significant reductions in sagittal plane motion in patients with lateral epicondylalgia when compared to age-matched controls.46,47
Sensitization Mechanisms in Upper Quadrant Syndromes
In the last years, there has been an increasing interest in nociceptive mechanisms in chronic pain syndromes. In fact, a mechanism-based classification or understanding of pain syndromes is based on the hypothesis that different clinical signs and symptoms reflect different underlying pathophysiological mechanisms of pain generation.48 For that purpose, different quantitative sensory tests have been used for investigating A-beta, A-delta, or C fibers.49
Musculoskeletal pain syndromes are characterized by mechanical hyperalgesia and allodynia. Therefore, mechanical and thermal pain thresholds are the most useful quantitative tests used for investigating pressure pain hypersensitivity in musculoskeletal pain.50,51 In fact, in the last decade, different studies have consistently reported increased pressure pain sensitivity in both painful and distant pain-free areas, suggesting both extra-segmental spreading of sensitization in different local pain syndromes of the upper quadrant. In the current manuscript, we will use lateral epicondylalgia, shoulder pain, and carpal tunnel syndrome as examples of upper quadrant pain syndromes.
For instance, it is claimed that hyperalgesia plays an important role in etiology of lateral epicondylalgia, as different studies demonstrated higher levels of glutamate,52 calcitonin gene-related peptide,53 or substance P54 at the extensor carpi radialis brevis muscle in patients with lateral epicondylalgia, underlining the role of the nociceptive system in this musculoskeletal pain condition. Additionally, several studies have found that lateral epicondylalgia is characterized by pressure hypersensitivity.55–57 In addition, we have shown that lateral epicondylalgia may also exhibit cold pain hyperalgesia.58 We recently demonstrated that this pressure pain hyperalgesia is heterogeneously distributed around the elbow region, while thermal pain hyperalgesia was homogenous around the elbow area in patients with unilateral lateral epicondylalgia.59 These studies support an important role specific to the peripheral sensitization mechanism in this pain syndrome. In addition, our group also demonstrated that patients with unilateral lateral epicondylalgia also exhibit widespread pressure pain hyperalgesia.60 In this study, we found bilateral decreases in pressure pain thresholds (pressure pain hyperalgesia) over peripheral nerve trunks of the upper extremity, C5–C6 zygapophyseal joint, the elbow, and tibialis anterior muscle, suggesting the presence of a central sensitization process originating from local pain at the elbow.
Similar results have been recently reported for individuals with carpal tunnel syndrome. We have found bilateral widespread pressure hypersensitivity61 and bilateral thermal hyperalgesia62 in women with unilateral and moderate carpal tunnel syndrome, suggesting widespread sensitization in a local neuropathy. Furthermore, Zanette et al. showed that women with carpal tunnel syndrome exhibiting extra-median symptoms demonstrated thermal and mechanical pain hyperalgesia, and enhanced wind-up pain in the territories related to the median, ulnar, and radial nerves, confirming the presence of impairments in nociceptive gain in this local neuropathy.63 Additionally, an important clinical finding of central sensitization is the fact that patients with local pain, with time, usually also exhibit pain in distant areas. In fact, while symptoms in patients with carpal tunnel syndrome are mainly located over the median nerve distribution, extra-median sensory symptoms64 resulting in whole-hand involvement65 and proximal arm/shoulder pain66 were experienced by almost 50% of subjects with carpal tunnel syndrome, suggesting involvement of the central nociceptive mechanisms and plasticity.67
In a third example, Hidalgo-Lozano et al. found pressure pain hyperalgesia over the levator scapulae, supraspinatus, infraspinatus, biceps brachii, pectoralis major, and tibialis anterior muscles in patients with unilateral shoulder impingement syndrome.68 These findings reveal the presence of segmental sensitization, as the examined muscles receive innervation from the same segment of the cervical spine (C4–C6 segments), and also central sensitization, as distant pain-free areas not innervated by the cervical spine (tibialis anterior muscles) also exhibited pressure hyperalgesia.
Finally, it should be noted that there is evidence for these sensitization processes in other pain syndromes of the upper quadrant, such as repetitive strain injury,69 tension type headache,70 temporomandibular pain,71 and also in pain syndromes of the lower quadrant, e.g. low back pain,72 or knee osteoarthritis.73 It seems that upper quadrant pain syndromes may exhibit similar sensitization processes, but with different underlying pathophysiological mechanisms of pain generation.
It is interesting to note that individuals with these local pain syndromes exhibit similar nociceptive changes as patients with more widespread pain syndromes such as whiplash74 or fibromyalgia.75 This is an important topic as greater hyper-excitability of the central nervous system has been found to be a poor prognostic factor for physical therapy improvement in subjects with chronic whiplash-associated disorders.76 This study found that widespread pressure pain hypersensitivity and cold hyperalgesia were associated with a poor response to physical therapy in individuals with chronic whiplash-associated disorders.76 Nevertheless, the relationship between central sensitization and physical therapy response is more complex than expected, as this central sensitization is not always associated with a poor outcome. Fernández-de-las-Peñas et al. recently demonstrated that the presence of peripheral sensitization, instead of widespread central sensitization, may be related to a positive physical therapy response in individuals with carpal tunnel syndrome.77 This study found that pressure hyperalgesia over the cervical spine and heat hyperalgesia over the carpal tunnel, but not widespread pressure or cold pain hyperalgesia, were associated with a successful outcome after the application of physical therapy.77
Manual Therapy in Upper Quadrant Pain Syndromes
It has been suggested that more knowledge is needed regarding the impact of abnormal sensory features on the effectiveness of physical therapy before any clinical trial is conducted. The challenge facing clinicians is how to select the proper treatment approach for each individual patient, who is likely to be somewhat different in their individual clinical presentations. In choosing the proper multimodal management, consideration must be given to the interpretation of the clinical manifestations of peripheral and central sensitization processes (discussed previously) involved in musculoskeletal pain disorders of the upper quadrant.78 In addition, the potential neurophysiologic and tissue mechanisms underlying the effects (positive and negative) of any intervention should also be considered. In fact, multiple interacting tissue and pain mechanisms are likely to contribute to pain modulatory effects of physical manual therapy.79 Therefore, clinical management of patients with upper quadrant pain syndromes needs to be extended beyond local tissue-based pathology, to incorporate strategies directed at normalizing central nervous system sensitivity. The existence of a wide range of conservative treatments (i.e. medication, electro-physical agents, exercise, cognitive interventions, and manual therapies) advocated for chronic pain, is an indication that no one treatment has proven superior, and also, in part, a product of an inconclusive understanding of the underlying pathology of chronic pain.
Clinicians should consider that the presence of sustained peripheral noxious inputs is likely to play a role in the initiation and maintenance of central sensitization mechanisms.80 This hypothesis is supported by Herren-Gerber et al. who found that injection of lidocaine in tender points in the neck of patients with whiplash decreased pressure pain hypersensitivity depending on the effect of injection on ongoing pain.81 A recent study found that a single intramuscular anesthetic injection into the midpoint of the upper trapezius muscle significantly increases pain thresholds and decreases remote secondary heat hyperalgesia in women with fibromyalgia.82 These studies support the notion that central hyper-sensitivity is a dynamic condition influenced by the presence and activity of a nociceptive source. Nevertheless, once central sensitization has been established, only minimal nociception is required to maintain this process and non-nociceptive input might also contribute to the subsequent pain and mechanical allodynia.83
Therefore, from a clinical viewpoint, when a patient with a local pain syndrome in the upper quadrant appears mediated primarily by peripheral mechanisms (dominantly peripheral sensitization), early and appropriate local treatments and functional activity should be encouraged. For instance, in a patient with lateral epicondylalgia where the peripheral input is mainly dominant, a multimodal approach including inactivation of trigger points (TrPs) of the forearm muscles84 with manual strategies (Fig. 6), elbow joint mobilization/manipulation85 (Fig. 7), and specific exercises may be appropriate. Similarly, in a patient with shoulder pain where a peripheral input is dominant, shoulder joint mobilization (Fig. 8), TrPs manual therapy86 (Fig. 9), and specific exercises for the upper limb may be also sufficient. Finally, a patient with carpal tunnel syndrome in an initial stage, and hence, with a peripheral input dominance, may be successfully treated with manual techniques targeted at the carpal tunnel (Fig. 10) and passive neurodynamic technique targeted to the median nerve87,88 (Fig. 11). Clinicians should remember that the aim of these techniques is the restoration of normal function by limiting the chance of sustained central nervous system facilitation.
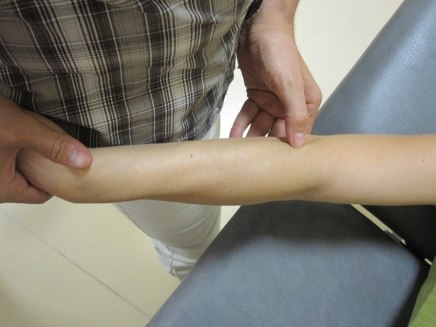
Figure 6.
Manual therapy techniques addressing trigger points (TrPs) in the forearm muscles. One hand of the therapist stabilizes the skin of the patient and the other hand performs a longitudinal stoker over the TrP taut band.
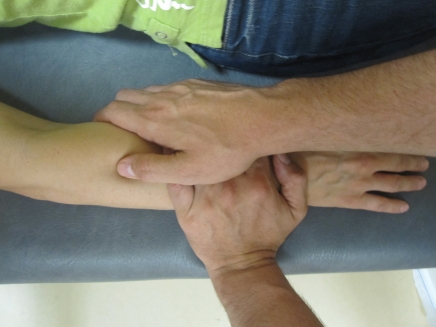
Figure 7.
Elbow joint mobilization/manipulation. The patients’ forearm is supinated at the point of hypo-mobility. The pad of the thumb is placed posteriorly against the radial head. The technique consists of applying a posterior–anterior glide of the radial head.
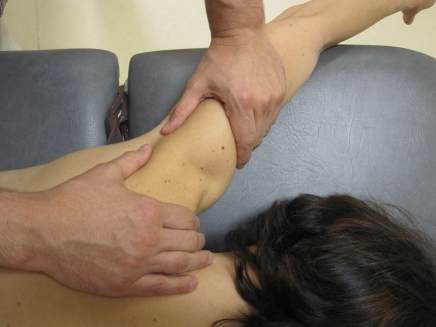
Figure 8.
Shoulder joint mobilization. Both hands of the therapist cup the humeral head of the patient. The therapist applies a lateral–medial or posterior–anterior glide of the humerus along joint plane of glenoid fossa.
Figure 9.
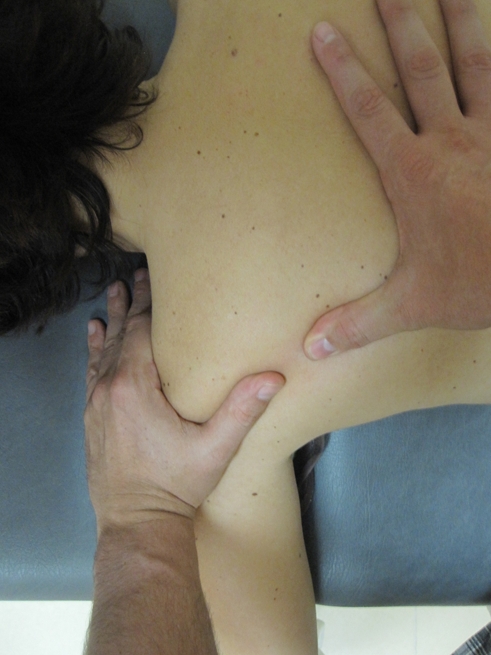
Trigger point (TrPs) manual therapy to the shoulder. The fingers grasp the taut band from both sides of the TrP and strokes centrifugally away from the TrP.
Figure 10.
Manual therapy technique targeting the carpal tunnel. The therapist places his thumbs on the region of the carpal tunnel and flexes the index fingers over the back of the wrist forming a clamp. Holding the patient’s wrist, the therapist applies a three-dimensional traction while slightly extending the wrist.
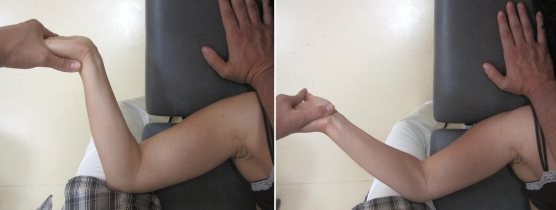
Figure 11.
Neurodynamic technique targeted to the median nerve: (left) shoulder girdle depression, gleno-humeral abduction and lateral rotation, supination of the forearm, elbow flexion and wrist, thumb, and finger extension; (right) shoulder girdle depression, gleno-humeral abduction and lateral rotation, supination of the forearm, elbow extension and wrist, thumb, and finger flexion.
On the other side, in a patient with chronic pain in the upper quadrant who appears to be mainly mediated by central processes (dominantly central sensitization), a multimodal physical and cognitive approach should be encouraged. For example, depending on the chronicity of the disorder and the associated level of impairment and disability, patients would be educated on optimizing normal functional movements and undertaking active and specific or more global exercises. In such cases, manual therapists should consider the neurophysiological mechanisms involved in manual therapies. For instance, several manual therapies have demonstrated ability to induce hypoalgesic and motor effects (for spinal manipulation see previous section). Interestingly, mobilization with movement applied over the elbow region (Fig. 12) has also exhibited an hypoalgesic effect and a concurrent sympatho-excitation consisting in increased grip force and pressure pain thresholds as well as changes in heart rate, blood pressure, and cutaneous sudomotor and vasomotor function in individuals with lateral epicondylalgia.89 Others have shown that manual treatment of active TrPs within the shoulder muscles reduces spontaneous pain and pressure hypersensitivity in patients with shoulder impingement.90 In fact, it seems that TrP treatment induces segmental anti-nociceptive effects.91,92 This finding may be related to the fact that levels of chemical mediators and algogenic substances, e.g. bradykinin, substance P, or serotonin, are higher in active TrPs.93 Additionally, Hsieh et al. showed that dry needling of active TrPs in the infraspinatus muscle (Fig. 13) decreased the pain intensity and mechanical pain sensitivity on the treated arm in patients with shoulder pain.94 Additionally, the fact that TrP therapy also decreased pressure pain hypersensitivity in distant pain-free areas, e.g. tibialis anterior muscle, indicates a generalized anti-nociceptive effect.95 Therefore, in a patient with chronic pain where central sensitization processes are dominant, a multimodal approach targeted to desensitize the central nervous system should be applied.
Figure 12.
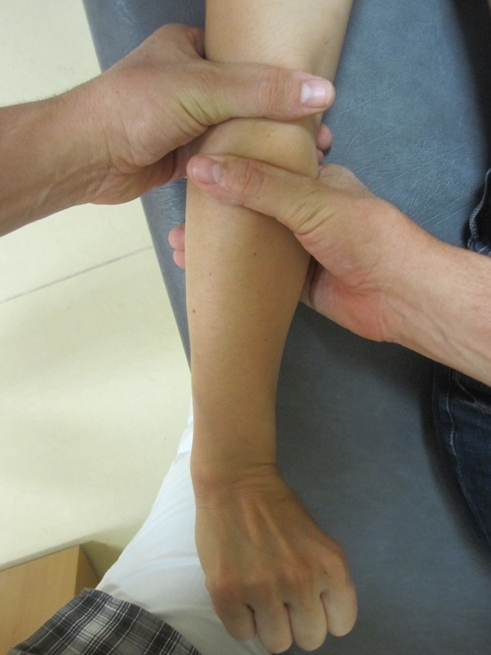
Mobilization with movement applied over the elbow region. One hand of the therapist is used to glide the proximal forearm laterally, while the other hand fixed the distal end of the humerus while the patient performed a pain-free gripping action. Ten repetitions are performed with an approximate 15-second rest interval between repetitions.
Figure 13.
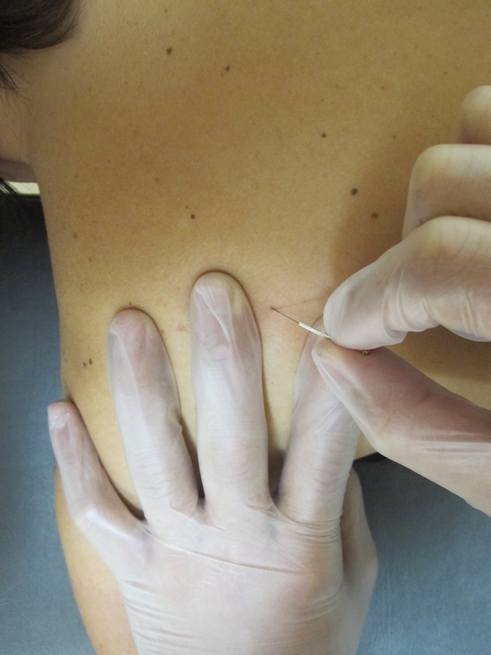
Dry needling of active trigger points (TrPs) in the infraspinatus muscle.
Conclusions
There is recent evidence demonstrating the presence of peripheral and central sensitization mechanisms in local pain syndromes of the upper quadrant: mechanical neck pain, lateral epicondylalgia, whiplash neck pain, shoulder impingement, or carpal tunnel syndrome. Therefore, a TBC approach where patients receive matched interventions has been advocated. There is evidence supporting that the cervical and thoracic spine is involved in upper quadrant pain as spinal manipulation has been found to be effective for the management of cervicobrachial pain. Clinicians should be aware of using therapeutic strategies, based on nociceptive pain rationale, that exert a modulating effect on the central nervous system for the management of upper quadrant pain syndromes.
References
- 1.Huisstede BM, Bierma-Zeinstra SM, Koes BW, Verhaar JA. Incidence and prevalence of upper-extremity musculoskeletal disorders. A systematic appraisal of the literature. BMC Musculoskelet Disord 2006;7:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walker-Bone KE, Palmer KT, Reading I, Cooper C. Soft-tissue rheumatic disorders of the neck and upper limb: prevalence and risk factors. Semin Arthritis Rheum 2003;33:185–203 [DOI] [PubMed] [Google Scholar]
- 3.Palmer KT, Cooper C. Work-related disorders of the upper limb. Arthritis Res Campaign: Top Rev 2006;10:1–7 [Google Scholar]
- 4.Manchikanti L, Singh V, Datta S, Cohen SP, Hirsch JA. Comprehensive review of epidemiology, scope, and impact of spinal pain. Pain Physician 2009;12:E35–70 [PubMed] [Google Scholar]
- 5.Huisstede BM, Wijnhoven HA, Bierma-Zeinstra SM, Koes BW, Verhaar JA, Picavet S. Prevalence and characteristics of complaints of the arm, neck, and/or shoulder (CANS) in the open population. Clin J Pain 2008;24:253–9 [DOI] [PubMed] [Google Scholar]
- 6.Walker-Bone K, Reading I, Coggon D, Cooper C, Palmer KT. The anatomical pattern and determinants of pain in the neck and upper limbs: an epidemiologic study. Pain 2004;109:45–51 [DOI] [PubMed] [Google Scholar]
- 7.Robinson H, Walker-Bone K. Occupation and soft tissue neck and upper limb disorders: cause and or effect? Rheumatol Pract 2009;7:7–10 [Google Scholar]
- 8.Foster NE, Delitto A. Embedding psychosocial perspectives within clinical management of low back pain: integration of psychosocially informed management principles into physical therapist practice — challenges and opportunities. Phys Ther 2011;91:790–803 [DOI] [PubMed] [Google Scholar]
- 9.Linton SJ, Shaw WS. Impact of psychological factors in the experience of pain. Phys Ther 2011;91:700–11 [DOI] [PubMed] [Google Scholar]
- 10.Puentedura EJ, Louw A. A neuroscience approach to managing athletes with low back pain. Phys Ther Sport 2011;in review [DOI] [PubMed] [Google Scholar]
- 11.Fritz JM, Cleland JA, Childs JD. Subgrouping patients with low back pain: evolution of a classification approach to physical therapy. J Orthop Sports Phys Ther 2007;37:290–302 [DOI] [PubMed] [Google Scholar]
- 12.Fritz JM, Brennan GP. Preliminary examination of a proposed treatment-based classification system for patients receiving physical therapy interventions for neck pain. Phys Ther 2007;87:513–24 [DOI] [PubMed] [Google Scholar]
- 13.Childs JD, Fritz JM, Piva SR, Whitman JM. Proposal of a classification system for patients with neck pain. J Orthop Sports Phys Ther 2004;34:686–96 [DOI] [PubMed] [Google Scholar]
- 14.Childs JD, Cleland JA, Elliott JM, Teyhen DS, Wainner RS, Whitman JM, et al. Neck pain: clinical practice guidelines linked to the International Classification of Functioning, Disability, and Health from the Orthopedic Section of the American Physical Therapy Association. J Orthop Sports Phys Ther 2008;38:A1–34 [DOI] [PubMed] [Google Scholar]
- 15.Cleland JA, Childs JD, Fritz JM, Whitman JM, Eberhart SL. Development of a clinical prediction rule for guiding treatment of a subgroup of patients with neck pain: use of thoracic spine manipulation, exercise, and patient education. Phys Ther 2007;87:9–23 [DOI] [PubMed] [Google Scholar]
- 16.Senstad O, Leboeuf-Yde C, Borchgrevink C. Frequency and characteristics of side effects of spinal manipulative therapy. Spine (Phila Pa 1976) 1997;22:435–440 [DOI] [PubMed] [Google Scholar]
- 17.Leboeuf-Yde C, Rasmussen LR, Klougart N. The risk of over-reporting spinal manipulative therapy-induced injuries: a description of some cases that failed to burden the statistics. J Manipulative Physiol Ther 1996;19:536–8 [PubMed] [Google Scholar]
- 18.Cagnie B, Vinck E, Beernaert A, Cambier D. How common are side effects of spinal manipulation and can these side effects be predicted? Man Ther 2004;9:151–6 [DOI] [PubMed] [Google Scholar]
- 19.Hurwitz EL, Morgenstern H, Vassilaki M, Chiang LM. Frequency and clinical predictors of adverse reactions to chiropractic care in the UCLA neck pain study. Spine (Phila Pa 1976) 2005;30:1477–84 [DOI] [PubMed] [Google Scholar]
- 20.Di Fabio RP. Manipulation of the cervical spine: risks and benefits. Phys Ther 1999;79:50–65 [PubMed] [Google Scholar]
- 21.Ernst E. Manipulation of the cervical spine: a systematic review of case reports of serious adverse events, 1995–2001. Med J Aust 2002;176:376–80 [DOI] [PubMed] [Google Scholar]
- 22.Haldeman S, Kohlbeck FJ, McGregor M. Stroke, cerebral artery dissection, and cervical spine manipulation therapy. J Neurol 2002;249:1098–104 [DOI] [PubMed] [Google Scholar]
- 23.Haldeman S, Kohlbeck FJ, McGregor M. Unpredictability of cerebrovascular ischemia associated with cervical spine manipulation therapy: a review of sixty-four cases after cervical spine manipulation. Spine (Phila Pa 1976) 2002;27:49–55 [DOI] [PubMed] [Google Scholar]
- 24.Licht PB, Christensen HW, Hoilund-Carlsen PF. Is cervical spinal manipulation dangerous? J Manipulative Physiol Ther 2003;26:48–52 [DOI] [PubMed] [Google Scholar]
- 25.Oppenheim JS, Spitzer DE, Segal DH. Nonvascular complications following spinal manipulation. Spine J 2005;5:660–6 [DOI] [PubMed] [Google Scholar]
- 26.Martinez-Segura R, Fernández-de-las-Peñas C, Ruiz-Saez M, Lopez-Jimenez C, Rodriguez-Blanco C. Immediate effects on neck pain and active range of motion after a single cervical high-velocity low-amplitude manipulation in subjects presenting with mechanical neck pain: a randomized controlled trial. J Manipulative Physiol Ther 2006;29:511–7 [DOI] [PubMed] [Google Scholar]
- 27.Cleland JA, Childs JD, McRae M, Palmer JA, Stowell T. Immediate effects of thoracic manipulation in patients with neck pain: a randomized clinical trial. Man Ther 2005;10:127–35 [DOI] [PubMed] [Google Scholar]
- 28.Fernández-de-las-Peñas C, Palomeque-del-Cerro L, Rodriguez-Blanco C, Gomez-Conesa A, Miangolarra-Page JC. Changes in neck pain and active range of motion after a single thoracic spine manipulation in subjects presenting with mechanical neck pain: a case series. J Manipulative Physiol Ther 2007;30:312–20 [DOI] [PubMed] [Google Scholar]
- 29.Krauss J, Creighton D, Ely JD, Podlewska-Ely J. The immediate effects of upper thoracic translatoric spinal manipulation on cervical pain and range of motion: a randomized clinical trial. J Man Manip Ther 2008;16:93–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wainner RS, Whitman JM, Cleland JA, Flynn TW. Regional interdependence: a musculoskeletal examination model whose time has come. J Orthop Sports Phys Ther 2007;37:658–60 [DOI] [PubMed] [Google Scholar]
- 31.Bialosky JE, Bishop MD, George SZ. Regional interdependence: a musculoskeletal examination model whose time has come. J Orthop Sports Phys Ther 2008;38:159–60 [DOI] [PubMed] [Google Scholar]
- 32.Cleland JA, Glynn P, Whitman JM, Eberhart SL, MacDonald C, Childs JD. Short-term effects of thrust versus nonthrust mobilization/manipulation directed at the thoracic spine in patients with neck pain: a randomized clinical trial. Phys Ther 2007;87:431–40 [DOI] [PubMed] [Google Scholar]
- 33.Gonzalez-Iglesias J, Fernández-de-las-Peñas C, Cleland JA, Gutierrez-Vega Mdel R. Thoracic spine manipulation for the management of patients with neck pain: a randomized clinical trial. J Orthop Sports Phys Ther 2009;39:20–7 [DOI] [PubMed] [Google Scholar]
- 34.Cleland JA, Mintken PE, Carpenter K, Fritz JM, Glynn P, Whitman J, et al. Examination of a clinical prediction rule to identify patients with neck pain likely to benefit from thoracic spine thrust manipulation and a general cervical range of motion exercise: multi-center randomized clinical trial. Phys Ther 2010;90:1239–50 [DOI] [PubMed] [Google Scholar]
- 35.Puentedura EJ, Landers MR, Cleland JA, Mintken PE, Huijbregts P, Fernández-de-las-Peñas C. Thoracic spine thrust manipulation versus cervical spine thrust manipulation in patients with acute neck pain: a randomized clinical trial. J Orthop Sports Phys Ther 2011;41:208–20 [DOI] [PubMed] [Google Scholar]
- 36.Richardson BS, Way BV, Speece AJ., 3rd Osteopathic manipulative treatment in the management of notalgia paresthetica. J Am Osteopath Assoc 2009;109:605–8 [PubMed] [Google Scholar]
- 37.McClatchie L, Laprade J, Martin S, Jaglal SB, Richardson D, Agur A. Mobilizations of the asymptomatic cervical spine can reduce signs of shoulder dysfunction in adults. Man Ther 2009;14:369–74 [DOI] [PubMed] [Google Scholar]
- 38.Boyles RE, Ritland BM, Miracle BM, Barclay DM, Faul MS, Moore JH, et al. The short-term effects of thoracic spine thrust manipulation on patients with shoulder impingement syndrome. Man Ther 2009;14:375–80 [DOI] [PubMed] [Google Scholar]
- 39.Mintken PE, Cleland JA, Carpenter KJ, Bieniek ML, Keirns M, Whitman JM. Some factors predict successful short-term outcomes in individuals with shoulder pain receiving cervicothoracic manipulation: a single-arm trial. Phys Ther 2010;90:26–42 [DOI] [PubMed] [Google Scholar]
- 40.Smidt N, van der Windt DA. Tennis elbow in primary care. BMJ 2006;333:927–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cleland JA, Whitman JM, Fritz JM. Effectiveness of manual physical therapy to the cervical spine in the management of lateral epicondylalgia: a retrospective analysis. J Orthop Sports Phys Ther 2004;34:713–22 [DOI] [PubMed] [Google Scholar]
- 42.Cleland JA, Flynn TO, Palmer J. Incorporation of manual therapy directed at the cervicothoracic spine in patients with lateral epicondylalgia: a pilot clinical trial. J Man Manip Ther 2005;13:143–51 [Google Scholar]
- 43.Fernandez-Carnero J, Fernández-de-las-Peñas C, Cleland JA. Immediate hypoalgesic and motor effects after a single cervical spine manipulation in subjects with lateral epicondylalgia. J Manipulative Physiol Ther 2008;31:675–81 [DOI] [PubMed] [Google Scholar]
- 44.Vicenzino B, Collins D, Wright A. The initial effects of a cervical spine manipulative physiotherapy treatment on the pain and dysfunction of lateral epicondylalgia. Pain 1996;68:69–74 [DOI] [PubMed] [Google Scholar]
- 45.Vicenzino B, Collins D, Benson H, Wright A. An investigation of the interrelationship between manipulative therapy-induced hypoalgesia and sympathoexcitation. J Manipulative Physiol Ther 1998;21:448–53 [PubMed] [Google Scholar]
- 46.Waugh EJ, Jaglal SB, Davis AM, Tomlinson G, Verrier MC. Factors associated with prognosis of lateral epicondylitis after 8 weeks of physical therapy. Arch Phys Med Rehabil 2004;85:308–18 [DOI] [PubMed] [Google Scholar]
- 47.Berglund KM, Persson BH, Denison E. Prevalence of pain and dysfunction in the cervical and thoracic spine in persons with and without lateral elbow pain. Man Ther 2008;13:295–9 [DOI] [PubMed] [Google Scholar]
- 48.Jensen TS, Baron R. Translation of symptoms and signs into mechanisms in neuropathic pain. Pain 2003;102:1–8 [DOI] [PubMed] [Google Scholar]
- 49.Rolke R, Baron R, Maier C, Tölle TR, Treede RD, Beyer A, et al. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): standardized protocol and reference values. Pain 2006;123:231–43 [DOI] [PubMed] [Google Scholar]
- 50.Chesterton LS, Barlas P, Foster NE, Baxter GD, Wright CC. Gender differences in pressure pain threshold in healthy humans. Pain 2003;101:259–66 [DOI] [PubMed] [Google Scholar]
- 51.Rolke R, Andrews Campbell K, Magerl W, Treede RD. Deep pain thresholds in the distal limbs of healthy human subjects. Eur J Pain 2005;9:39–48 [DOI] [PubMed] [Google Scholar]
- 52.Alfredson H, Ljung BO, Thorsen K, Lorentzon R. In vivo investigation of ECRB tendons with microdialysis technique — no signs of inflammation but high amounts of glutamate in tennis elbow. Acta Orthop Scand 2000;71:475–9 [DOI] [PubMed] [Google Scholar]
- 53.Ljung BO, Forsgren S, Friden J. Substance P and calcitonin gene-related peptide expression at the extensor carpi radialis brevis muscle origin: implications for the etiology of tennis elbow. J Orthop Res 1999;17:554–9 [DOI] [PubMed] [Google Scholar]
- 54.Ljung BO, Alfredson H, Forsgren S. Neurokinin 1-receptors and sensory neuropeptides in tendon insertions at the medial and lateral epicondyles of the humerus. Studies on tennis elbow and medial epicondylalgia. J Orthop Res 2004;22:321–7 [DOI] [PubMed] [Google Scholar]
- 55.Sran M, Souvlis T, Vicenzino B, Wright A. Characterisation of chronic lateral epicondylalgia using the McGill pain questionnaire, visual analog scales, and quantitative sensory tests. Pain Clin 2002;13:251–9 [Google Scholar]
- 56.Wright A, Thurnwald P, O’Callaghan J, Smith J, Vicenzino B. Hyperalgesia in tennis elbow patients. J Musculoskeletal Pain 1994;2:83–97 [Google Scholar]
- 57.Smith J, Wright A. The effect of selective blockade of myelinated afferent neurons on mechanical hyperalgesia in lateral epicondylalgia. Pain Clin 1993;6:9–16 [Google Scholar]
- 58.Fernandez-Carnero J, Fernández-de-las-Peñas C, Sterling M, Souvlis T, Arendt-Nielsen L, Vicenzino B. Exploration of the extent of somato-sensory impairment in patients with unilateral lateral epicondylalgia. J Pain 2009;10:1179–85 [DOI] [PubMed] [Google Scholar]
- 59.Ruiz-Ruiz B, Fernández-de-las-Peñas C, Ortega-Santiago R, Arendt-Nielsen L. Topographical pressure and thermal pain sensitivity mapping in patients with unilateral lateral epicondylalgia. J Pain 2011;in press. [DOI] [PubMed] [Google Scholar]
- 60.Fernandez-Carnero J, Fernández-de-las-Peñas C, de-la-Llave-Rincón AI, Ge HY, Arendt-Nielsen L. Widespread mechanical pain hypersensitivity as sign of central sensitization in unilateral epicondylalgia: a blinded, controlled study. Clin J Pain 2009;25:555–61 [DOI] [PubMed] [Google Scholar]
- 61.Fernández-de-las-Peñas C, de-la-Llave-Rincón AI, Fernandez-Carnero J, Cuadrado ML, Arendt-Nielsen L, Pareja JA. Bilateral widespread mechanical pain sensitivity in carpal tunnel syndrome: evidence of central processing in unilateral neuropathy. Brain 2009;132:1472–9 [DOI] [PubMed] [Google Scholar]
- 62.de-la-Llave-Rincón AI, Fernández-de-las-Peñas C, Fernandez-Carnero J, Padua L, Arendt-Nielsen L, Pareja JA. Bilateral hand/wrist heat and cold hyperalgesia, but not hypoesthesia, in unilateral carpal tunnel syndrome. Exp Brain Res 2009;198:455–63 [DOI] [PubMed] [Google Scholar]
- 63.Zanette G, Cacciatori C, Tamburin S. Central sensitization in carpal tunnel syndrome with extraterritorial spread of sensory symptoms. Pain 2010;148:227–36 [DOI] [PubMed] [Google Scholar]
- 64.Zanette G, Marani S, Tamburin S. Extra-median spread of sensory symptoms in carpal tunnel syndrome suggests the presence of pain-related mechanisms. Pain 2006;122:264–70 [DOI] [PubMed] [Google Scholar]
- 65.Nora DB, Becker J, Ehlers JA, Gomes I. What symptoms are truly caused by median nerve compression in carpal tunnel syndrome? Clin Neurophysiol 2005;116:275–83 [DOI] [PubMed] [Google Scholar]
- 66.Zanette G, Marani S, Tamburin S. Proximal pain in patients with carpal tunnel syndrome: a clinical-neurophysiological study. J Peripher Nerv Syst 2007;12:91–7 [DOI] [PubMed] [Google Scholar]
- 67.Campbell JN, Meyer RA. Mechanisms of neuropathic pain. Neuron 2006;52:77–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hidalgo-Lozano A, Fernández-de-las-Peñas C, Alonso-Blanco C, Ge HY, Arendt-Nielsen L, Arroyo-Morales M. Muscle trigger points and pressure pain hyperalgesia in the shoulder muscles in patients with unilateral shoulder impingement: a blinded, controlled study. Exp Brain Res 2010;202:915–25 [DOI] [PubMed] [Google Scholar]
- 69.Greening J, Lynn B. Vibration sense in the upper limb in patients with repetitive strain injury and a group of at-risk office workers. Int Arch Occup Environ Health 1998;71:29–34 [DOI] [PubMed] [Google Scholar]
- 70.Fernández-de-las-Peñas C, Cuadrado ML, Arendt-Nielsen L, Ge HY, Pareja JA. Increased pericranial tenderness, decreased pressure pain threshold, and headache clinical parameters in chronic tension-type headache patients. Clin J Pain 2007;23:346–52 [DOI] [PubMed] [Google Scholar]
- 71.Fernández-de-las-Peñas C, Galan-del-Rio F, Fernandez-Carnero J, Pesquera J, Arendt-Nielsen L, Svensson P. Bilateral widespread mechanical pain sensitivity in women with myofascial temporomandibular disorder: evidence of impairment in central nociceptive processing. J Pain 2009;10:1170–8 [DOI] [PubMed] [Google Scholar]
- 72.O’Neill S, Manniche C, Graven-Nielsen T, Arendt-Nielsen L. Generalized deep-tissue hyperalgesia in patients with chronic low-back pain. Eur J Pain 2007;11:415–20 [DOI] [PubMed] [Google Scholar]
- 73.Arendt-Nielsen L, Nie H, Laursen MB, Laursen BS, Madeleine P, Simonsen OH, et al. Sensitization in patients with painful knee osteoarthritis. Pain 2010;149:573–81 [DOI] [PubMed] [Google Scholar]
- 74.Sterling M, Jull G, Vicenzino B, Kenardy J. Sensory hypersensitivity occurs soon after whiplash injury and is associated with poor recovery. Pain 2003;104:509–17 [DOI] [PubMed] [Google Scholar]
- 75.Desmeules JA, Cedraschi C, Rapiti E, Baumgartner E, Finckh A, Cohen P, et al. Neurophysiologic evidence for a central sensitization in patients with fibromyalgia. Arthritis Rheum 2003;48:1420–9 [DOI] [PubMed] [Google Scholar]
- 76.Jull G, Sterling M, Kenardy J, Beller E. Does the presence of sensory hypersensitivity influence outcomes of physical rehabilitation for chronic whiplash? — A preliminary RCT. Pain 2007;129:28–34 [DOI] [PubMed] [Google Scholar]
- 77.Fernández-de-las-Peñas C, Cleland JA, Ortega-Santiago R, de-la-Llave-Rincón AI, Martinez-Perez A, Pareja JA. Central sensitization does not identify patients with carpal tunnel syndrome who are likely to achieve short-term success with physical therapy. Exp Brain Res 2010;207:85–94 [DOI] [PubMed] [Google Scholar]
- 78.Nijs J, Van Houdenhove B, Oostendorp RA. Recognition of central sensitization in patients with musculoskeletal pain: Application of pain neurophysiology in manual therapy practice. Man Ther 2010;15:135–41 [DOI] [PubMed] [Google Scholar]
- 79.Wright A. Pain-relieving effects of cervical manual therapy. : Grant R, editor. Physical therapy of the cervical and thoracic spine New York: Churchill-Livingstone; 2002. 217–38 [Google Scholar]
- 80.Mendell LM, Wall PD. Responses of single dorsal cord cells to peripheral cutaneous unmyelinated fibres. Nature 1965;206:97–9 [DOI] [PubMed] [Google Scholar]
- 81.Herren-Gerber R, Weiss S, Arendt-Nielsen L, Petersen-Felix S, Di Stefano G, Radanov BP, et al. Modulation of central hypersensitivity by nociceptive input in chronic pain after whiplash injury. Pain Med 2004;5:366–76 [DOI] [PubMed] [Google Scholar]
- 82.Staud R, Nagel S, Robinson ME, Price DD. Enhanced central pain processing of fibromyalgia patients is maintained by muscle afferent input: a randomized, double-blind, placebo-controlled study. Pain 2009;145:96–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.DeSantana JM, Sluka KA. Central mechanisms in the maintenance of chronic widespread noninflammatory muscle pain. Curr Pain Headache Rep 2008;12:338–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fernandez-Carnero J, Fernández-de-las-Peñas C, de-la-Llave-Rincón AI, Ge HY, Arendt-Nielsen L. Prevalence of and referred pain from myofascial trigger points in the forearm muscles in patients with lateral epicondylalgia. Clin J Pain 2007;23:353–60 [DOI] [PubMed] [Google Scholar]
- 85.Slater H, Fernández-de-las-Peñas C. Joint mobilization and manipulation of the elbow. : Fernández-de-las-Peñas C, Cleland J A, Huijbregts P, editors. Neck and arm pain syndromes: evidence-informed screening, diagnosis and conservative management London: Churchill-Livingstone; 2011. 328–34 [Google Scholar]
- 86.Ge HY, Fernández-de-las-Peñas C, Madeleine P, Arendt-Nielsen L. Topographical mapping and mechanical pain sensitivity of myofascial trigger points in the infraspinatus muscle. Eur J Pain 2008;12:859–65 [DOI] [PubMed] [Google Scholar]
- 87.Butler DS, Coppieters MW. Neurodynamics in a broader perspective. Man Ther 2007;12:e7–8 [DOI] [PubMed] [Google Scholar]
- 88.Coppieters MW, Butler DS. Do ‘sliders’ slide and ‘tensioners’ tension? An analysis of neurodynamic techniques and considerations regarding their application. Man Ther 2008;13:213–21 [DOI] [PubMed] [Google Scholar]
- 89.Paungmali A, O’Leary S, Souvlis T, Vicenzino B. Hypoalgesic and sympathoexcitatory effects of mobilization with movement for lateral epicondylalgia. Phys Ther 2003;83:374–83 [PubMed] [Google Scholar]
- 90.Hidalgo-Lozano A, Fernández-de-las-Peñas C, Diaz-Rodriguez L, Gonzalez-Iglesias J, Palacios-Cena D, Arroyo-Morales M. Changes in pain and pressure pain sensitivity after manual treatment of active trigger points in patients with unilateral shoulder impingement: a case series. J Bodyw Mov Ther 2011;in press. [DOI] [PubMed] [Google Scholar]
- 91.Srbely JZ, Dickey JP, Lee D, Lowerison M. Dry needle stimulation of myofascial trigger points evokes segmental anti-nociceptive effects. J Rehabil Med 2010;42:463–8 [DOI] [PubMed] [Google Scholar]
- 92.Srbely JZ, Dickey JP, Lowerison M, Edwards AM, Nolet PS, Wong LL. Stimulation of myofascial trigger points with ultrasound induces segmental antinociceptive effects: a randomized controlled study. Pain 2008;139:260–6 [DOI] [PubMed] [Google Scholar]
- 93.Shah JP, Phillips TM, Danoff JV, Gerber LH. An in vivo microanalytical technique for measuring the local biochemical milieu of human skeletal muscle. J Appl Physiol 2005;99:1977–84 [DOI] [PubMed] [Google Scholar]
- 94.Hsieh YL, Kao MJ, Kuan TS, Chen SM, Chen JT, Hong CZ. Dry needling to a key myofascial trigger point may reduce the irritability of satellite MTrPs. Am J Phys Med Rehabil 2007;86:397–403 [DOI] [PubMed] [Google Scholar]
- 95.Niddam DM, Chan RC, Lee SH, Yeh TC, Hsieh JC. Central modulation of pain evoked from myofascial trigger point. Clin J Pain 2007;23:440–8 [DOI] [PubMed] [Google Scholar]