Abstract
Chronic lower quadrant injuries constitute a significant percentage of the musculoskeletal cases seen by clinicians. While impairments may vary, pain is often the factor that compels the patient to seek medical attention. Traumatic injury from sport is one cause of progressive chronic joint pain, particularly in the lower quarter. Recent studies have demonstrated the presence of peripheral and central sensitization mechanisms in different lower quadrant pain syndromes, such as lumbar spine related leg pain, osteoarthritis of the knee, and following acute injuries such as lateral ankle sprain and anterior cruciate ligament rupture. Proper management of lower quarter conditions should include assessment of balance and gait as increasing pain and chronicity may lead to altered gait patterns and falls. In addition, quantitative sensory testing may provide insight into pain mechanisms which affect management and prognosis of musculoskeletal conditions. Studies have demonstrated analgesic effects and modulation of spinal excitability with use of manual therapy techniques, with clinical outcomes of improved gait and functional ability. This paper will discuss the evidence which supports the use of manual therapy for lower quarter musculoskeletal dysfunction.
Keywords: Lower quadrant, Pain, Sensitization, Low back pain, Hip, Knee, Ankle
Introduction
Low back and leg pain are complaints frequently addressed by healthcare practitioners, and constitute a significant source of medical expenditures. These medical conditions, including major health care burdens such as low back pain (LBP) and osteoarthritis (OA) of the hip, knee, and ankle, are often recurrent, progressive and lead to a significant decrease in quality of life.
A persistent musculoskeletal condition is more than simple connective tissue damage; rather, it entails a complex multisystem interaction of connective tissue changes, inflammation, and neuroplasticity of the nociceptive pathways. The end result is a chronic, painful, inflamed joint. In the lower extremity, unraveling the puzzle may be complicated by the weight-bearing role of these joints. Repetitive weight-bearing on an injured limb may contribute to chronicity through abnormal wear and tear, particularly when primary or secondary biomechanical abnormalities are present. In addition, noxious and/or non-noxious input from the affected joint may serve to maintain heightened central nociceptive processes, so that pain is more easily triggered.1
The purpose of this paper is to describe the clinical presentation of some common chronic lower quarter musculoskeletal conditions, and to discuss how an acute joint injury may progress to a chronically painful condition. A secondary purpose is to discuss the clinical assessment of altered nociceptive processing, and its potential relationship to functional deficits in lower quarter musculoskeletal conditions. Finally, an overview of the efficacy of manual therapy interventions will be provided, with particular emphasis on spine-related extremity pain (SREP), hip, knee, and ankle dysfunction.
Musculoskeletal Injury and Pain
Peripheral sensitization
Following musculoskeletal injury, increased pain sensitivity of primary afferent neurons at the site of injury occurs, mediated by peripheral sensitization. Peripheral sensitization is defined as increased responsiveness and reduced threshold of nociceptors to stimulation of their receptive fields.2 Clinically, mechanical stimulation of the tissue (e.g. with pressure or stretch) will more readily produce pain. This primary hyperalgesia is generally limited to the area of injury and serves to protect the site from further injury.
Central sensitization
Central sensitization is described as an increased responsiveness of nociceptive neurons in the central nervous system to normal or subthreshold afferent input leading to hyperalgesia.2 Central sensitization augments all sensory input from the periphery, such that noxious stimuli conveyed by nociceptive and non-nociceptive fibers in the joint is facilitated, increasing the pain response.3 Central sensitization has been reported in many chronic musculoskeletal conditions and has been demonstrated experimentally through heightened flexor withdrawal responses in individuals with knee OA4,5 and other chronic musculoskeletal conditions.6 Furthermore, the efferent secretion of neuropeptides such as substance P and calcitonin gene related peptide from sensory afferents (noxious and non-noxious) may accentuate pain and inflammatory processes, through a mechanism referred to as neurogenic inflammation.1
Hyperalgesia
A fundamental feature of central sensitization is the expansion of receptive fields, which may result in spread of symptoms and increased tenderness to palpation, both distally and proximally.7,8 In the lower quarter, LBP can refer distally, even beyond the knee,9 which may correlate to the clinical concept of ‘peripheralization’ of symptoms,10 although few if any studies have examined the mechanisms behind this clinical phenomenon. In individuals with knee OA, the pain pattern may be significantly expanded around the knee and into the lower leg.8 Clinically, the patient may describe a larger pattern of referred pain, sometimes in conjunction with other sensory symptoms. The diversity of symptoms may have neuropathic characteristics but typically do not correspond with a peripheral nerve or spinal nerve root distribution.7
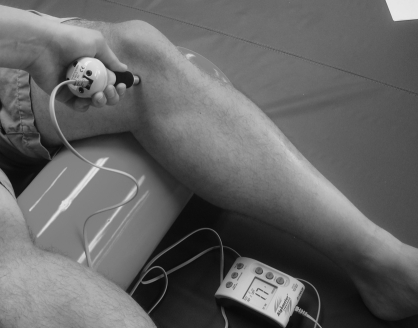
Regional spread may be found in the ipsilateral limb11 and potentially in the same distribution on the contralateral side. This regional manifestation of central sensitization is possibly distinct from widespread sensitization due to its presence in the affected quadrant(s) but not throughout the body, which may be commonly described in such conditions as fibromyalgia.11 However, widespread hyperalgesia has been reported as a component of some chronic musculoskeletal conditions and thus, widespread and regional hyperalgesia may be part of a continuum rather than mechanistically distinct.11,12 One method of clinically differentiating the two has been through assessment of deep tissue hyperalgesia using algometry (Fig. 1) locally (at site of injury), regionally (e.g. within the same limb) and at a remote upper quarter site such as the infraspinatus muscle,12 as an indication of widespread sensitization.
Figure 1.
Clinical assessment of hyperalgesia through use of algometry. Typical measures include pressure pain threshold and pressure pain tolerance.
Hypoesthesia
Hypoesthesia to mechanical and vibration stimuli (Fig. 2) has been demonstrated concurrently with hyperalgesia.13–15 Elevated vibrotactile thresholds have been found following experimental pain16 and in patients with painful articular disorders.14,15 Hypoesthesia and hyperalgesia are believed to be mediated by distinct neurophysiological mechanisms;17 however, both may be triggered by nociceptive input and may be centrally mediated, either at spinal and/or supraspinal levels. Clinically, these concurrent findings may make diagnosis more challenging and promote the use of vague diagnoses. Recent studies have focused on the differentiation between radicular and pseudoradicular lower quarter signs and symptoms. This is important clinically because the management of neuropathic pain, such as in the case of spinal nerve root pathology, may differ from that of non-neuropathic pain. Interestingly, Freynhagen et al.18 has suggested that the two may be part of a continuum rather than two separate entities.
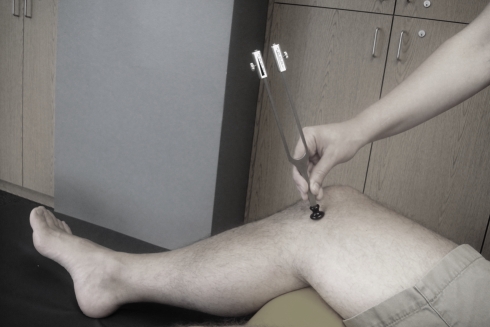
Figure 2.
Clinical assessment of vibratory perception through use of a Rydel–Seiffer graduated tuning fork. The device, typically applied at a bony prominence, allows an objective measurement of the intensity at which the vibration is no longer perceived.
Functional changes
Functional deficits found with chronic musculoskeletal pain such as decreased balance or gait status, may be related, in part, with concurrent somatosensory changes, although little evidence has connected these findings. Reduced proprioceptive acuity has been shown in LBP,19 knee OA,20 and chronic ankle instability (CAI)21 and postural control deficits have been reported as well.21,22 Impairments in dynamic or static stabilization and muscle strength are likely contributors to functional deficits following lower quarter injury; however, a relationship between pain-related quantitative sensory testing (QST) changes and functional instability may exist.23 Contralateral deficits have been found following unilateral lower extremity injury,20,24,25 supporting a spinal or supraspinal contribution.
Musculoskeletal disorders may benefit from classification in terms of pain mechanism,26 as treatment may be directed more appropriately for evidence-based modulation of aberrant nociceptive processing. Quantitative sensory testing has been recognized as one means of identifying altered pain mechanisms.27 In recent years, manual therapy has been more commonly proposed as a treatment for management of musculoskeletal pain, with beneficial outcomes reported in both acute28 and chronic29–31 conditions. Evidence supporting the use of manual therapy interventions in lower quarter musculoskeletal conditions will be discussed.
Clinical Findings in Lower Quarter Musculoskeletal Disorders
Lumbar Spine Related Extremity Pain
To provide the most appropriate treatment of LBP and SREP, clinicians may employ formal or informal classification schemes,32 with the intent of subgrouping based on suspected pathoanatomical insult,33 psychosocial status,34 clinical prediction rules,28 or combination of these. These classifications are usually not mutually exclusive, and reported outcomes of successful treatment have been variable, depending on the model. Accordingly, recent evidence over the last few years has called into question the validity of the pathoanatomical models of dysfunction in LBP and lumbar SREP.35
Recent studies have focused on the differentiation of neuropathic and non-neuropathic pain.18,36 With greater chronicity, the distribution of pain experienced following musculoskeletal injury of the lumbar spine will expand and often extend into the limbs. Some clinicians have referred to this as ‘peripheralization’ of symptoms.10 Central sensitization of nociceptive pathways has been identified as one mechanism explaining this spread of symptoms. Neuropathic-like signs and symptoms may be found during examination, such as spontaneous pain, paresthesias, and pain sensitivity that cross nerve territories.37 Functional changes may occur as well. Using a series of active lumbar movements, Luomajoki et al.38 demonstrated poorer movement control in subjects with non-specific LBP, with greater deficits found in those with longer duration of symptoms. These motor changes may persist even when the pain is in remission.39
Neuropathic pain defined by the International Association for the Study of Pain as ‘pain arising as a direct consequence of a lesion or disease affecting the somatosensory system’2 may represent up to 40% of the pain experienced by patients with chronic LBP and chronic pain, and is thought to be under diagnosed.40 Strictly speaking, the sequelae of central sensitization following joint or muscle injury cannot be defined as neuropathic pain, as no ‘lesion or disease’ has occurred. Thus, the differentiation of SREP, whether it is neuropathic in origin, or the sequelae of central sensitization due to a chronic spinal musculoskeletal injury, can be challenging. Not surprisingly, the clinical presentation of most patients is not exclusively neuropathic or non-neuropathic pain, but instead may be classified as a mixed type presentation.18
Traditionally, screening for myotomal related weakness, diminished reflexes, and altered sensation in the lower extremity has been used by clinicians to differentiate these conditions. Freynhagen and colleagues18 evaluated 27 patients with either low back or SREP using QST. Prior to testing, the SREP of each subject was classified as radicular pain, defined as symptoms radiating below the knee in a typical dermatomal pattern and demonstrating sensory or motor loss, or pseudoradicular pain, defined as symptoms presenting in a non-dermatomal manner with no radiation below the knee and without motor or sensory deficits. Interestingly, no significant differences were found between the two groups. The test that best differentiated the two groups was vibration detection threshold, which was affected in 73% of the radicular group and 47% in the pseudoradicular group. The authors concluded that ‘mixed’ neuropathic and non-neuropathic SREP may be more common than previously reported.18
Quantitative sensory testing
While Freynhagen et al. investigated the use of QST for the differentiation of SREP,18 other studies have focused on somatosensensory changes at the lumbar spine in individuals with LBP. Proprioception19 has been found to be increased (less sensitive), which parallels the findings in other chronic joint disorders of the lower quarter.20,41 Wand et al.42 demonstrated increased thresholds for two-point discrimination and graphesthesia at the lumbar spine in individuals with chronic LBP, suggesting supraspinal neuroplastic changes as a potential mechanism. Conversely, they found tactile thresholds to be unaltered as compared to age-matched controls, which is in agreement to previous work in the lumbar spine,43 but in contrast to other chronically painful joints.13,44
Regional and widespread hyperalgesia has been reported in individuals with chronic LBP. O’Neill and colleagues12 demonstrated that individuals diagnosed with lumbar disc herniation in the previous 6 to 24 months, confirmed by magnetic resonance imaging, had a significant reduction in PPT of the ipsilateral tibialis anterior but not of the infraspinatus muscle, suggesting regional but not necessarily widespread sensitization. In contrast, others have reported more global hyperalgesia in patients with LBP.45,46
PainDETECT questionnaire
A simple screening tool to assist identification of neuropathic origin is the painDETECT questionnaire.40 The painDETECT is a nine-item questionnaire that has been shown to classify patients as either ‘nociceptive’ (non-neuropathic) or neuropathic with a sensitivity and specificity of 85 and 80% respectively.40 Patients classified as neuropathic by painDETECT were more likely to have higher levels of pain and have co-morbidities such as depression, anxiety, and sleep disorders.39
Although the presence of leg pain is a common finding in individuals with SREP of neuropathic origin,46,47 it is not a sufficiently sensitive finding to rule out pain of non-neuropathic origin.36 In fact, perhaps one reason neuropathic SREP may be under-diagnosed is that its sensory symptom profile differs from other neuropathic pain syndromes.48 Using the painDETECT tool, it was found that neuropathic SREP, where the most focal pain was distal to the knee, differed from diabetic peripheral neuropathy and post-herpetic neuralgia. In particular, touch-evoked allodynia and thermal hyperalgesia were infrequent (occurred 10% or less) in neuropathic SREP (i.e., radicular pain), and the report of burning pain and prickling sensations were also less common.48
Neural provocation testing
One clinical test that may be valuable in recognizing neuropathic SREP is Lasegue’s test, also known as the passive straight leg raise (SLR) test. Scholz et al.47 found the presence of radicular pain on SLR a meaningful clinical finding for identifying a neuropathic pain component. Likewise, Beith et al.36 also found in their cohort of subjects with SREP a significant reduction in passive SLR, and the passive SLR with ankle dorsiflexion, that was concluded to be of neuropathic origin. However, it has been recommended that the test not be used in isolation, as neural provocation tests, such as the passive SLR, are not specific for neuropathic pain and may also test positive in the presence of centrally mediated pain.6
Hip
Approximately 20% of elder adults complain of hip pain, with degenerative joint disease as a major source of these symptoms.49 A potential factor which may accelerate degenerative changes and pain at the hip joint is a tear of joint labrum.50 While hip pain classically refers to the groin and medial thigh, pain from labral tears may also present in the region of the buttock and lateral trochanter.51 It has been proposed that labral tears may be a part of a continuum of joint degeneration leading to OA.50
In a study of patients with symptomatic hip pain, pain referral occurred most commonly into the buttock in 71% of patients. However, 22% of patients experienced pain distal to the knee, while foot pain was occasional, occurring in only 2% of patients.52 Accordingly, Khan et al.53 found 47% of patients awaiting hip arthroplasty had pain below the knee, particularly at the anterior shin. Expanded regions of pain, as found in these studies, have also been demonstrated in knee OA7,8 and like knee OA,54 no correlation was demonstrated between the pain distribution and radiographic findings of hip osteoarthritis. Thus, hip symptoms extending distal to the knee may be more common than previously believed, and as arthritic hip joint pain becomes chronic, the spread of symptoms may mimic SREP. Hip OA is associated with chronic pain and declining joint function, which affects weightbearing and balance during ambulation. Total hip replacement effectively restores hip function but restoration of balance during ambulation is multifactorial, depending on strength, motor control, and fear of falling.55
Spine and hip pain
Concurrent spine and hip pain are common and mostly attributed to biomechanical changes, particularly in relation to hip abductor weakness,56 yet nociceptive mechanisms such as neurogenic inflammation may contribute to degenerative joint changes and pain.57 Evidence of potential neurogenic inflammation has been demonstrated in the labra of human hips harvested during arthoplasty58 and in synovial tissues in individuals with prosthetic loosening after total hip replacement.59 The authors of these studies hypothesized that the pain of hip OA may be caused by invasion of nerve fibers into synovial tissues with efferent release of neuropeptides, which facilitates pain, inflammation, and joint degeneration.
The relationship between hip and lumbar spine pain has been labeled by some as the hip-spine syndrome,60 with evidence that treatment of the hip either surgically,61 or conservatively with physical therapy62 may alleviate LBP as well. Greater trochanteric pain syndrome, reported to affect between 10 and 25% of the general population,63 has also been associated with LBP, and ipsilateral and contralateral knee OA as well.55 Further research on the relationship of hip and lumbar spine pain is clearly warranted.
Quantitative sensory testing
Many clinical studies have utilized the hip OA population for the study of pain mechanisms and their clinical correlates. Individuals with hip OA have been found to have significantly lowered threshold perception to cutaneous stimulation in their areas of referred pain, and demonstrate increased hyperalgesia to the same stimuli when compared with healthy controls.64 Nikolajsen et al.65 also found punctate hyperalgesia in the same population, as well as changes in thermal detection threshold, but did not find hypoesthesia to tactile stimuli. Kosek and Ordeberg44 also demonstrated aberrant thermal sensation, which normalized six months following total hip arthroplasty. Vibratory perception threshold was found significantly reduced in subjects with symptomatic and radiographic hip OA when compared with age-matched controls without hip OA.15 While functional and somatosensory deficits have been reported separately in this population, no studies to date have investigated the relationship between these two impairments.
Knee
Knee pain may be idiopathic or occur following repetitive or traumatic injury. The relationship between joint injury and later onset of degenerative changes is not always evident. However, with acute injury such as anterior cruciate ligament (ACL) rupture, degenerative changes have been linked to the traumatic injury.66 Surgical reconstruction may be performed with the intention of restoring static stability to the joint, yet repair of the mechanical deficit does not completely impede the onset of degenerative changes.67 Furthermore, many of these individuals complain of pain and ‘giving way’ during functional activities, even with restored static stability and normal strength of the surrounding musculature.68 The factors that trigger arthritic mechanisms are likely complex, but in addition to abnormal wear and tear from altered arthrokinematics, facilitated nociceptive mechanisms may promote pain at the ipsilateral and potentially, the contralateral knee due to neurogenic inflammation.69 Heightened nociceptive reflexes, indicating central sensitization of nociceptive pathways, have been demonstrated in subjects following anterior cruciate rupture, in spite of the fact that all subjects were reportedly pain-free at the time of testing.70 Furthermore, the excitability of the reflex response was increased by application of a pain-free, passive anterior tibial translation, indicating that non-noxious stimuli (i.e., afferent input from stretch of joint tissues) may promote spinal nociceptive excitability. Given that central nociceptive excitability is considered a component of chronic knee OA as well,4 it follows that management of the patient with acute or chronic knee condition must address pain mechanisms in addition to impairments at the knee.
Quantitative sensory testing
Numerous studies have identified a proprioceptive deficit in both knee OA and ACL deficiency (see Refs. 20 and 71 for reviews). In both conditions, proprioceptive deficits are typically found in the affected and unaffected contralateral knee. Functional deficits, such as giving way and gait limitations, may be associated with diminished proprioceptive acuity20 but limited evidence supports this notion.
Cutaneous hypoesthesia has also been reported in knee joint disorders. Hendiani et al.13 noted that mechanical detection threshold was elevated in those with knee OA, particularly in the region superficial to the tibiofemoral joint line. In patients with longstanding unilateral patellofemoral syndrome, hypoesthesia was found in both the affected and unaffected knee.72 It was hypothesized that disturbed joint position sense and muscle recruitment patterns seen in some patients with patellofemoral pain syndrome may be due to dysfunction of mechanoreceptors in the skin close to the painful knee; however, proprioception was not measured in this study. Alternatively, other researchers have identified hypoesthesia to vibration in individuals with knee OA, and hypothesized a relationship between this finding and decreased proprioceptive acuity at the joint.14 Clearly, while pain-related hypoesthesia has been demonstrated in relation to various joint injuries and conditions, its relationship to function has yet to be unraveled.
Allodynia, described as the experience of pain with non-noxious stimuli, has been described in subjects with knee OA.73 This cutaneous finding is typically associated with neuropathic pain, and when identified in association with a joint injury, may also be an indicator central sensitization of nociceptive pathways.
Finally, hyperalgesia of deep somatic tissues has been demonstrated in patients with knee OA, using PPT.7,8,74 Nociceptors in deep somatic tissue, such as joint and muscle, show pronounced sensitization to mechanical stimuli in contrast to cutaneous nociceptors,7 thus, algometry may be a valuable tool for the clinician.
Ankle
Lateral ankle sprains are a common problem with recurrence rates as high as 70%,75 and with findings of at least one residual symptom 2 years following a single ankle injury.76 Valderrabano et al.77 reported a relationship between trauma ankle ligament injury and the development of OA. The exact mechanism of joint degeneration is unclear but likely due to both inflammatory factors and altered biomechanics.78
Mechanical ankle instability (MAI) is a result of a ligamentous tear that results in pathological joint laxity79 while CAI has been defined as a recurrent giving away of the ankle that occurs for greater than 1 year after an ankle sprain.80 The CAI patient may present with pain,81 recurrent ankle sprains,82 diminished neuromuscular control,83 weakness,84 impaired joint position sense,84 and decreased performance and self-report of functional and sport activity.85,86 The diversity of physical impairments reported in the literature is vast and the lack of correlation between MAI and CAI is perplexing. Due to the unpredictable clinical presentation of CAI, the clinician must rely on a thorough clinical exam and the use of evidence-based physical performance measures such as the Star Excursion Test87 and functional report scales such as the Foot and Ankle Ability Measure88 and Lower Extremity Functional Scale.88
Quantitative sensory testing
Quantitative sensory testing has the potential to help identify altered pain mechanisms following foot or ankle injury. For example, use of the contralateral limb as a reference standard has been found to be unreliable,89 potentially due to spinal and/or supraspinal mechanisms affecting the opposite corresponding joint.69 Contralateral balance impairments have been found in an acute unilateral ankle sprain population.25 Bilateral sensory impairments (elevated vibratory threshold) have also been documented in an ankle sprain population versus controls.90 Thus, the presence of local pain can trigger local peripheral, spinal and/or supraspinal mechanisms that alter neuromotor afferent and efferent processes locally and remotely.
The classic tissue strain model of ‘articular deafferentation’ as proposed by Freeman91 may not fully describe the diverse clinical presentation found in the CAI population. It was theorized that an ankle sprain produced disruption not only of collagenous connective tissue but also sensory mechanoreceptors within the ligament;91 however, poor correlation exists between true mechanical instability and complaints of functional instability.92 A recent systematic review by Menacho et al.93 showed consistent delayed peroneal muscle reaction time, while another by Munn et al.94 failed to demonstrate any consistent delay. However, impairments in joint position sense and postural control in FAI were routinely found.94 While some authors have reported delayed muscle response to rapid supination, Santos et al.95 showed shortened involved limb unloading time, evidence of heightened reflexive protective responses. They suggested that the functional sequelae of CAI may be due in part to hyperexcitability of central nociceptive pathways, as has been found with chronic knee conditions.4 This finding is in contrast to typical MAI findings and may be an indication of increased spinal excitability. Local sensory impairments (e.g. increased vibratory thresholds) have been reported in the severe ankle sprain population and hypothesized to originate from a tearing of type I/II joint mechanoreceptors; however, spinal/supraspinal sources may not have been considered. More recent research however has shown that the presence of pain alone (via capsaicin or electrical stimulation) is sufficient to impair cutaneous sensation, suggesting a presynaptic inhibition.17 Thus, two mechanisms have been proposed, the tissue strain (MAI/deafferentation) model which would result in delayed response (i.e. delay peroneal response time) and injury-induced CAI model, where increased excitability of nociceptive pathways can result in decreased reflex response time, more rapid unloading, local and wide spread sensorimotor changes and potentially, the feeling of instability or giving away.
Neural provocation testing
Few studies have reported the use of neural provocation tests for ankle and foot injuries. In a sample of subjects with lateral ankle sprain, Pahor and Toppenberg95 reported a decrease in knee extension, plantarflexion and inversion in the slump position with symptoms in a superficial peroneal nerve distribution. Release of cervical flexion from the slump position resulted in a significant reduction in symptoms, suggesting a positive test. It has been recommended that neural provocation testing not be used in isolation, as positive tests may be indicative of central mediated pain6 and therefore misinterpreted.
Manual Therapy in the Lower Quarter
The modulatory effects of manual therapy on nociception have been demonstrated experimentally in animal model studies97 and in chronic pain populations.5,98 Specifically, modulation of facilitated spinal reflexes (i.e., the nociceptive reflex) has been demonstrated with oscillatory joint mobilization at the affected joint, indicating that the analgesic effects of manual therapy are, at least in part, centrally mediated. However, the effects of manual therapy are likely multimodal.99 Clinical studies on treatment of lower quarter musculoskeletal pain have also shown it to be effective over the long term.28–31,100
Manual therapy for Spine Related Extremity Pain
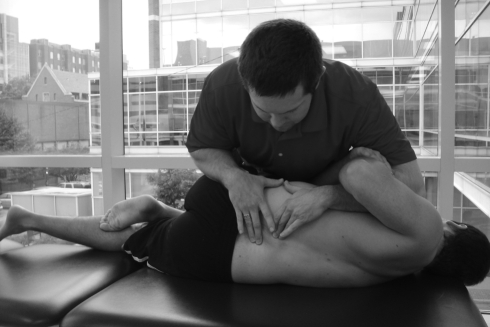
While support for manual therapy in musculoskeletal disorders has advanced, less evidence exists for its use with SREP. The clinical prediction rule for spinal manipulation28 has identified five factors that may predict success with this approach in the individual with LBP. These are symptom duration less than 16 days, no symptoms below the knee, at least one hypomobile lumbar segment, one hip with >35° internal rotation and Fear Avoidance Beliefs Questionnaire score <19 (work subscale). While this research provides evidence for use of lumbar spinal manipulation (Fig. 3), it may be less useful in the management of nerve root compression.28 Other types of manual therapy, however, may be beneficial in ‘centralizing’ symptoms of SREP.101
Figure 3.
Lumbar rotational thrust manipulation.
Specific to SREP of neuropathic origin, Schafer et al.102 found that those patients classified with ‘neuropathic sensitization’ had a poor result following ‘neural manual therapy’ which involved mobilization of the lumbar spine and exercises with the purpose of improving nerve excursion. In a recent systematic review of spinal manipulation or mobilization for SREP,103 the authors identified 11 trials that met their inclusion criteria. They concluded that the majority of these studies had a high risk of bias and that there is only moderate evidence at best to support manual therapy to treat SREP. However, there was no mention in the review of studies differentiating between extremity pain of neuropathic or non-neuropathic origin. The authors suggested that the strongest study cited in the review was undertaken by Santilli and colleagues104 which was a randomized double-blinded trial comparing chiropractic manipulation to a simulated sham treatment on patients with SREP due to a magnetic resonance imaging verified disc herniation. After 180 days, patients in the manipulation group had less leg pain than the simulated group. External validity of the study is limited by the fact that patient symptoms were acute (less than 10 days duration) and presence of motor or sensory signs was not indicated, thus making identification of the SREP as neuropathic or non-neuropathic difficult.
Manual therapy of the hip and knee
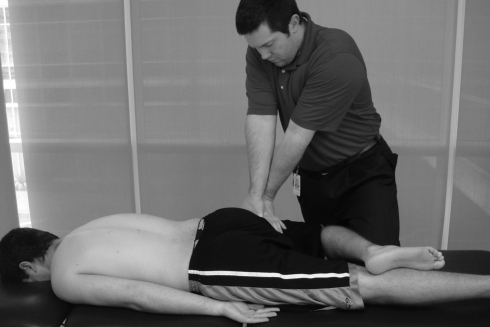
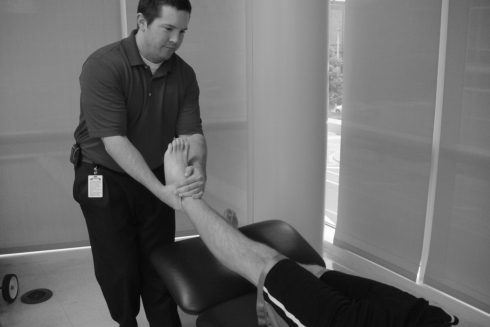
There is limited evidence on the effectiveness of manual therapy for painful hip disorders. In one randomized controled trial including patients with hip OA,31 manual therapy, consisting of mobilization (Fig. 4) and manipulation of the hip joint (Fig. 5), was compared with active exercises. Subjects receiving manual therapy demonstrated significantly better outcomes on pain, stiffness, hip range of motion, and function. Many of the outcome measures for this group showed lasting improvement at the 6-month follow-up. In a case series by MacDonald et al.,105 the combined effects of manual therapy and therapeutic exercise are reported. All seven of these patients responded with significant improvements in pain, range of motion and function (Harris Hip score in six of seven). Despite these promising results, insufficient numbers of high quality clinical trials exist. A recent systematic review by French et al.106 found only four studies that met their inclusion criteria for the comparison of manual therapy and therapeutic exercise in OA of the hip and knee. Quantitative sensory testing has not been reported as an outcome measure in manual therapy studies of the hip; however, Kosek and Ordeberg44 demonstrated that altered QST measures (pressure pain and thermal sensitivity) normalized in individuals with hip OA six months following total hip replacement surgery.
Figure 4.
Oscillatory mobilization technique at the hip.
Figure 5.
Longitudinal thrust manipulation at the hip.
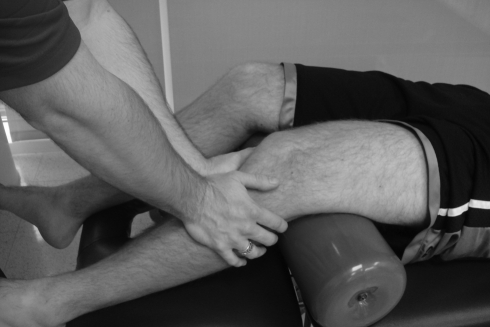
Manual therapy has also been demonstrated to be effective in the treatment of knee OA, although evidence is limited. In a randomized controled trial, mobilization techniques applied at the knee in addition to a clinical exercise program was found to produce significantly better outcomes, as measured by the Western Ontario and McMaster Universities Osteoarthritis Index and six-minute walk test, than a home exercise program, and these results were maintained at 1 year.30 Other studies have reported similar findings.29,107 In addition, there is evidence that manual therapy may diminish pressure pain sensitivity both at the medial joint line and at a distal non-painful site in subjects with knee OA. Most of these studies addressing knee OA have employed an oscillatory technique applied at the affected joint (Fig. 6) over several minutes;5,29,30,108 however, application of a thrust or impulse has also been reported as effective.107 There is little or no evidence supporting the use of manual therapy following acute knee injury, such as ligamentous or meniscal injury. Considering the evidence supporting its use in acute ankle sprain,109,110 this may be an important area of future clinical research.
Figure 6.
Oscillatory mobilization at the tibiofemoral joint.
Manual therapy of the foot and ankle
Evidence suggests that manual therapy following acute ankle sprain results in superior early dorsiflexion range of motion versus traditional exercise intervention alone.109,110 While most manual therapy study protocols use range of motion and functional outcome scales to determine successful outcome, a recent study examined changes in plantar load distribution (baropodometry) before and after manipulation.111 In this study, a caudal talocrural joint manipulation (Fig. 7) resulted in significant load distribution changes not found in a placebo group. This change may be attributed to mechanical joint alternation (i.e., change in stiffness) or alternatively, altered postural control mechanisms.
Figure 7.
Longitudinal thrust manipulation at the ankle.
Previous studies demonstrating significant improvements in ankle range of motion have failed to demonstrate significant changes in pressure or thermal pain threshold.110 However, a recent randomized controlled trial by Yeo and Wright produced significant improvement in both PPT and dorsiflexion range of motion from a single bout of talocrural joint mobilization.112
Conclusions
Recent studies have demonstrated evidence of peripheral and central sensitization in lower quarter musculoskeletal conditions in both acute and chronic conditions of the lumbar spine, hip, knee, and ankle, thereby changing the treatment focus from one directed solely at the musculoskeletal tissues, to a broader mode of management that considers altered neurophysiological mechanisms as well. Evidence exists for the analgesic effects and clinical effectiveness of manual therapy. Future research on the functional implications of central sensitization, as well as its impact on progression of degenerative joint disease is warranted. In addition, clinical biomarkers of altered nociceptive processing may aid in accurate and successful management of lower quarter musculoskeletal dysfunction.
References
- 1.Neumann S, Doubell TP, Leslie T, Woolf CJ. Inflammatory pain hypersensitivity mediated by phenotypic switch in myelinated primary sensory neurons. Nature 1996;384:360–4 [DOI] [PubMed] [Google Scholar]
- 2.IASP-Pain.org [Internet] Seattle, WA: International Association for the Study of Pain Proposed Pain Terminology. Available from: http://www.iasp-pain.org. Accessed in May 2011. [Google Scholar]
- 3.Schaible HG, Grubb BD. Afferent and spinal mechanisms of joint pain. Pain 1993;55:5–54 [DOI] [PubMed] [Google Scholar]
- 4.Courtney CA, Lewek MD, Witte PO, Chmell SJ, Hornby TG. Heightened flexor withdrawal responses in subjects with knee osteoarthritis. J Pain 2009;10:1242–9 [DOI] [PubMed] [Google Scholar]
- 5.Courtney CA, Witte PO, Chmell SJ, Hornby TG. Heightened flexor withdrawal response in individuals with knee osteoarthritis is modulated by joint compression and joint mobilization. J Pain 2010;11:179–85 [DOI] [PubMed] [Google Scholar]
- 6.Lim EC, Sterling M, Stone A, Vicenzino B. Central hyperexcitability as measured with nociceptive flexor reflex threshold in chronic musculoskeletal pain: a systematic review. Pain 2011;152:1811–20 [DOI] [PubMed] [Google Scholar]
- 7.Arendt-Nielsen L, Nie H, Laursen MB, Laursen BS, Madeleine P, Simonsen OH, et al. Sensitization in patients with painful knee osteoarthritis. Pain 2010;149:573–81 [DOI] [PubMed] [Google Scholar]
- 8.Bajaj P, Bajaj P, Graven-Nielsen T, Arendt-Nielsen L. Osteoarthritis and its association with muscle hyperalgesia: an experimental controlled study. Pain 2001;93:107–14 [DOI] [PubMed] [Google Scholar]
- 9.O’Neill S, Graven-Nielsen T, Manniche C, Arendt-Nielsen L. Ultrasound guided, painful electrical stimulation of lumbar facet joint structures: an experimental model of acute low back pain. Pain 2009;144:76–83 [DOI] [PubMed] [Google Scholar]
- 10.Aina A, May S, Clare H. The centralization phenomenon of spinal symptoms – a systematic review. Man Ther 2004;9:134–43 [DOI] [PubMed] [Google Scholar]
- 11.Graven-Nielsen T, Arendt-Nielsen L. Assessment of mechanisms in localized and widespread musculoskeletal pain. Nat Rev Rheumatol 2010;6:599–606 [DOI] [PubMed] [Google Scholar]
- 12.O’Neill S, Manniche C, Graven-Nielsen T, Arendt-Nielsen L. Generalized deep-tissue hyperalgesia in patients with chronic low-back pain. Eur J Pain 2007;11:415–20 [DOI] [PubMed] [Google Scholar]
- 13.Hendiani JA, Westlund KN, Lawand N, Goel N, Lisse J, McNearney T. Mechanical sensation and pain thresholds in patients with chronic arthropathies. J Pain 2003;4:203–11 [DOI] [PubMed] [Google Scholar]
- 14.Shakoor N, Agrawal A, Block JA. Reduced lower extremity vibratory perception in osteoarthritis of the knee. Arthritis Rheum 2008;59:117–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shakoor N, Lee KJ, Fogg LF, Block JA. Generalized vibratory deficits in osteoarthritis of the hip. Arthritis Rheum 2008;59:1237–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Apkarian AV, Stea RA, Bolanowski SJ. Heat-induced pain diminishes vibrotactile perception: a touch gate. Somatosens Mot Res 1994;11:259–67 [DOI] [PubMed] [Google Scholar]
- 17.Geber C, Magerl W, Fondel R, Fechir M, Rolke R, Vogt T, et al. Numbness in clinical and experimental pain – a cross-sectional study exploring the mechanisms of reduced tactile function. Pain 2008;139:73–81 [DOI] [PubMed] [Google Scholar]
- 18.Freynhagen R, Rolke R, Baron R, Tölle TR, Rutjes AK, Schu S, et al. Pseudoradicular and radicular low-back pain – a disease continuum rather than different entities? Answers from quantitative sensory testing. Pain 2008;135:65–74 [DOI] [PubMed] [Google Scholar]
- 19.Lee AS, Cholewicki J, Reeves NP, Zazulak BT, Mysliwiec LW. Comparison of trunk proprioception between patients with low back pain and healthy controls. Arch Phys Med Rehabil 2010;91:1327–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knoop J, Steultjens MP, van der Leeden M, van der Esch M, Thorstensson CA, Roorda LD, et al. Proprioception in knee osteoarthritis: a narrative review. Osteoarthritis Cartilage 2011;19:381–8 [DOI] [PubMed] [Google Scholar]
- 21.Hertel J. Sensorimotor deficits with ankle sprains and chronic ankle instability. Clin Sports Med 2008;27:353–70 [DOI] [PubMed] [Google Scholar]
- 22.Luomajoki H, Moseley GL. Tactile acuity and lumbopelvic motor control in patients with back pain and healthy controls. Br J Sports Med 2011;45:437–40 [DOI] [PubMed] [Google Scholar]
- 23.Emerson-Kavchak AJ, Fernández-de-las-Peñas C, Rubin LH, et al. Association between altered somatosensation, pain and knee stability in patients with severe knee osteoarthrosis. Clinical Journal of Pain 2011;(in review) [DOI] [PubMed] [Google Scholar]
- 24.Wikstrom EA, Naik S, Lodha N, Cauraugh JH. Bilateral balance impairments after lateral ankle trauma: a systematic review and meta-analysis. Gait Posture 2010;31:407–14 [DOI] [PubMed] [Google Scholar]
- 25.Evans T, Hertel J, Sebastianelli W. Bilateral deficits in postural control following lateral ankle sprain. Foot Ankle Int 2004;25:833–9 [DOI] [PubMed] [Google Scholar]
- 26.Woolf CJ, Bennett GJ, Doherty M, Dubner R, Kidd B, Koltzenburg M, et al. Towards a mechanism-based classification of pain? Pain 1998;77:227–9 [DOI] [PubMed] [Google Scholar]
- 27.Arendt-Nielsen L, Yarnitsky D. Experimental and clinical applications of quantitative sensory testing applied to skin, muscles and viscera. J Pain 2009;10:556–72 [DOI] [PubMed] [Google Scholar]
- 28.Childs JD, Fritz JM, Flynn TW, Irrgang JJ, Johnson KK, Majkowski GR, et al. A clinical prediction rule to identify patients with low back pain most likely to benefit from spinal manipulation: a validation study. Ann Intern Med 2004;141:920–8 [DOI] [PubMed] [Google Scholar]
- 29.Deyle GD, Henderson NE, Matekel RL, Ryder MG, Garber MB, Allison SC. Effectiveness of manual physical therapy and exercise in osteoarthritis of the knee. A randomized, controlled trial. Ann Intern Med 2000;132:173–81 [DOI] [PubMed] [Google Scholar]
- 30.Deyle GD, Allison SC, Matekel RL, Ryder MG, Stang JM, Gohdes DD, et al. Physical therapy treatment effectiveness for osteoarthritis of the knee: a randomized comparison of supervised clinical exercise and manual therapy procedures versus a home exercise program. Phys Ther 2005;85:1301–17 [PubMed] [Google Scholar]
- 31.Hoeksma HL, Dekker J, Ronday HK, Heering A, van der Lubbe N, Vel C, et al. Comparison of manual therapy and exercise therapy in osteoarthritis of the hip: a randomized clinical trial. Arthritis Rheum 2004;51:722–9 [DOI] [PubMed] [Google Scholar]
- 32.Brennan GP, Fritz JM, Hunter SJ, Thackeray A, Delitto A, Erhard RE. Identifying subgroups of patients with acute/subacute ‘nonspecific’ low back pain: results of a randomized clinical trial. Spine (Phila Pa 1976) 2006;31:623–31 [DOI] [PubMed] [Google Scholar]
- 33.Bernard TN, Jr, Kirkaldy-Willis WH. Recognizing specific characteristics of nonspecific low back pain. Clin Orthop Relat Res 1987;217:266–80 [PubMed] [Google Scholar]
- 34.Bergstrom C, Hagberg J, Bodin L, Jensen I, Bergstrom G. Using a psychosocial subgroup assignment to predict sickness absence in a working population with neck and back pain. BMC Musculoskelet Disord 2011;12:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Riddle DL. Classification and low back pain: a review of the literature and critical analysis of selected systems. Phys Ther 1998;78:708–37 [DOI] [PubMed] [Google Scholar]
- 36.Beith ID, Kemp A, Kenyon J, Prout M, Chestnut TJ. Identifying neuropathic back and leg pain: a cross-sectional study. Pain 2011;152(7):1511–6 [DOI] [PubMed] [Google Scholar]
- 37.Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain 2011;152:S2–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luomajoki H, Kool J, de Bruin ED, Airaksinen O. Movement control tests of the low back: evaluation of the difference between patients with low back pain and healthy controls. BMC Musculoskelet Disord 2008;9:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.MacDonald D, Moseley GL, Hodges PW. Why do some patients keep hurting their back? Evidence of ongoing back muscle dysfunction during remission from recurrent back pain. Pain 2009;142:183–8 [DOI] [PubMed] [Google Scholar]
- 40.Freynhagen R, Baron R, Tolle T, Stemmler E, Gockel U, Stevens M, et al. Screening of neuropathic pain components in patients with chronic back pain associated with nerve root compression: a prospective observational pilot study (MIPORT). Curr Med Res Opin 2006;22:529–37 [DOI] [PubMed] [Google Scholar]
- 41.Mendelsohn ME, Overend TJ, Petrella RJ. Effect of rehabilitation on hip and knee proprioception in older adults after hip fracture: a pilot study. Am J Phys Med Rehabil 2004;83:624–32 [DOI] [PubMed] [Google Scholar]
- 42.Wand BM, Di Pietro F, George P, O’Connell NE. Tactile thresholds are preserved yet complex sensory function is impaired over the lumbar spine of chronic non-specific low back pain patients: a preliminary investigation. Physiotherapy 2010;96:317–23 [DOI] [PubMed] [Google Scholar]
- 43.Peters ML, Schmidt AJ. A comparison of two-point discrimination threshold of tactual, non-painful stimuli between chronic low back pain patients and controls. Pain 1991;44:57–60 [DOI] [PubMed] [Google Scholar]
- 44.Kosek E, Ordeberg G. Abnormalities of somatosensory perception in patients with painful osteoarthritis normalize following successful treatment. Eur J Pain 2000;4:229–38 [DOI] [PubMed] [Google Scholar]
- 45.Giesecke T, Gracely RH, Grant MA, Nachemson A, Petzke F, Williams DA, et al. Evidence of augmented central pain processing in idiopathic chronic low back pain. Arthritis Rheum 2004;50:613–23 [DOI] [PubMed] [Google Scholar]
- 46.Giesbrecht RJ, Battie MC. A comparison of pressure pain detection thresholds in people with chronic low back pain and volunteers without pain. Phys Ther 2005;85:1085–92 [PubMed] [Google Scholar]
- 47.Scholz J, Mannion RJ, Hord DE, Griffin RS, Rawal B, Zheng H, et al. A novel tool for the assessment of pain: validation in low back pain. PLoS Med 2009;6(4):e1000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mahn F, Hullemann P, Gockel U, Brosz M, Freynhagen R, Tölle TR, et al. Sensory symptom profiles and co-morbidities in painful radiculopathy. PLoS One 2011;6(5):e18018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dawson J, Linsell L, Zondervan K, Rose P, Randall T, Carr A, et al. Epidemiology of hip and knee pain and its impact on overall health status in older adults. Rheumatology (Oxford) 2004;43:497–504 [DOI] [PubMed] [Google Scholar]
- 50.Neumann G, Mendicuti AD, Zou KH, Minas T, Coblyn J, Winalski CS, et al. Prevalence of labral tears and cartilage loss in patients with mechanical symptoms of the hip: evaluation using MR arthrography. Osteoarthritis Cartilage 2007;15:909–17 [DOI] [PubMed] [Google Scholar]
- 51.Arnold DR, Keene JS, Blankenbaker DG, Desmet AA. Hip pain referral patterns in patients with labral tears: analysis based on intra-articular anesthetic injections, hip arthroscopy, and a new pain ‘circle’ diagram. Phys Sportsmed 2011;39:29–35 [DOI] [PubMed] [Google Scholar]
- 52.Lesher JM, Dreyfuss P, Hager N, Kaplan M, Furman M. Hip joint pain referral patterns: a descriptive study. Pain Med 2008;9:22–5 [DOI] [PubMed] [Google Scholar]
- 53.Khan AM, McLoughlin E, Giannakas K, Hutchinson C, Andrew JG. Hip osteoarthritis: where is the pain? Ann R Coll Surg Engl 2004;86:119–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bedson J, Croft PR. The discordance between clinical and radiographic knee osteoarthritis: a systematic search and summary of the literature. BMC Musculoskelet Disord 2008;9:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Trudelle-Jackson E, Emerson R, Smith S. Outcomes of total hip arthroplasty: a study of patients one year postsurgery. J Orthop Sports Phys Ther 2002;32:260–7 [DOI] [PubMed] [Google Scholar]
- 56.Segal NA, Felson DT, Torner JC, Zhu Y, Curtis JR, Niu J, et al. Greater trochanteric pain syndrome: epidemiology and associated factors. Arch Phys Med Rehabil 2007;88:988–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schaible HG, Ebersberger A, von Banchet GS. Mechanisms of pain in arthritis. Ann NY Acad Sci 2002;966:343–54 [DOI] [PubMed] [Google Scholar]
- 58.Shirai C, Ohtori S, Kishida S, Harada Y, Moriya H. The pattern of distribution of PGP 9.5 and TNF-alpha immunoreactive sensory nerve fibers in the labrum and synovium of the human hip joint. Neurosci Lett 2009;450:18–22 [DOI] [PubMed] [Google Scholar]
- 59.Niissalo S, Li TF, Santavirta S, Takagi M, Hietanen J, Konttinen YT. Dense innervation in pseudocapsular tissue compared to aneural interface tissue in loose totally replaced hips. J Rheumatol 2002;29:796–803 [PubMed] [Google Scholar]
- 60.Offierski CM, MacNab I. Hip-spine syndrome. Spine (Phila Pa 1976) 1983;8:316–21 [DOI] [PubMed] [Google Scholar]
- 61.Ben-Galim P, Ben-Galim T, Rand N, Haim A, Hipp J, Dekel S, et al. Hip-spine syndrome: the effect of total hip replacement surgery on low back pain in severe osteoarthritis of the hip. Spine (Phila Pa 1976) 2007;32:2099–102 [DOI] [PubMed] [Google Scholar]
- 62.Burns SA, Mintken PE, Austin GP. Clinical decision making in a patient with secondary hip-spine syndrome. Physiother Theory Pract 2011;27:384–97 [DOI] [PubMed] [Google Scholar]
- 63.Strauss EJ, Nho SJ, Kelly BT. Greater trochanteric pain syndrome. Sports Med Arthrosc 2010;18:113–9 [DOI] [PubMed] [Google Scholar]
- 64.Gwilym SE, Keltner JR, Warnaby CE, Carr AJ, Chizh B, Chessell I, et al. Psychophysical and functional imaging evidence supporting the presence of central sensitization in a cohort of osteoarthritis patients. Arthritis Rheum 2009;61:1226–34 [DOI] [PubMed] [Google Scholar]
- 65.Nikolajsen L, Kristensen AD, Thillemann TM, Jurik AG, Rasmussen T, Kehlet H, et al. Pain and somatosensory findings in patients 3 years after total hip arthroplasty. Eur J Pain 2009;13:576–81 [DOI] [PubMed] [Google Scholar]
- 66.Louboutin H, Debarge R, Richou J, Selmi TA, Donell ST, Neyret P, et al. Osteoarthritis in patients with anterior cruciate ligament rupture: a review of risk factors. Knee 2009;16(4):239–44 [DOI] [PubMed] [Google Scholar]
- 67.Liden M, Sernert N, Rostgard-Christensen L, Kartus C, Ejerhed L. Osteoarthritic changes after anterior cruciate ligament reconstruction using bone-patellar tendon-bone or hamstring tendon autografts: a retrospective, 7-year radiographic and clinical follow-up study. Arthroscopy 2008;24:899–908 [DOI] [PubMed] [Google Scholar]
- 68.Eastlack ME, Axe MJ, Snyder-Mackler L. Laxity, instability, and functional outcome after ACL injury: copers versus noncopers. Med Sci Sports Exerc 1999;31:210–5 [DOI] [PubMed] [Google Scholar]
- 69.Kelly S, Dunham JP, Donaldson LF. Sensory nerves have altered function contralateral to a monoarthritis and may contribute to the symmetrical spread of inflammation. Eur J Neurosci 2007;26:935–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Courtney CA, Durr RK, Emerson-Kavchak AJ, Witte EO, Santos MJ. Heightened flexor withdrawal responses following ACL rupture are enhanced by passive tibial translation. Clin Neurophysiol 2010;122(5):1005–10 [DOI] [PubMed] [Google Scholar]
- 71.Ingersoll CD, Grindstaff TL, Pietrosimone BG, Hart JM. Neuromuscular consequences of anterior cruciate ligament injury. Clin Sports Med 2008;27:383–404 [DOI] [PubMed] [Google Scholar]
- 72.Jensen R, Hystad T, Kvale A, Baerheim A. Quantitative sensory testing of patients with long lasting patellofemoral pain syndrome. Eur J Pain 2007;11:665–76 [DOI] [PubMed] [Google Scholar]
- 73.Hochman JR, French MR, Bermingham SL, Hawker GA. The nerve of osteoarthritis pain. Arthritis Care Res (Hoboken) 2010;62:1019–23 [DOI] [PubMed] [Google Scholar]
- 74.Imamura M, Imamura ST, Kaziyama HH, Targino RA, Hsing WT, de Souza LP, et al. Impact of nervous system hyperalgesia on pain, disability, and quality of life in patients with knee osteoarthritis: a controlled analysis. Arthritis Rheum 2008;59:1424–31 [DOI] [PubMed] [Google Scholar]
- 75.Yeung MS, Chan KM, So CH, Yuan WY. An epidemiological survey on ankle sprain. Br J Sports Med 1994;28:112–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Anandacoomarasamy A, Barnsley L. Long term outcomes of inversion ankle injuries. Br J Sports Med 2005;39:e14; discussion e14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Valderrabano V, Hintermann B, Horisberger M, Fung TS. Ligamentous posttraumatic ankle osteoarthritis. Am J Sports Med 2006;34:612–20 [DOI] [PubMed] [Google Scholar]
- 78.Caputo AM, Lee JY, Spritzer CE, Easley ME, DeOrio JK, Nunley JA, et al. In vivo kinematics of the tibiotalar joint after lateral ankle instability. Am J Sports Med 2009;37:2241–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hubbard TJ, Hertel J. Mechanical contributions to chronic lateral ankle instability. Sports Med 2006;36:263–77 [DOI] [PubMed] [Google Scholar]
- 80.Freeman MA. Instability of the foot after injuries to the lateral ligament of the ankle. J Bone Joint Surg Br 1965;47:669–77 [PubMed] [Google Scholar]
- 81.Ogilvie-Harris DJ, Gilbart MK, Chorney K. Chronic pain following ankle sprains in athletes: the role of arthroscopic surgery. Arthroscopy 1997;13:564–74 [DOI] [PubMed] [Google Scholar]
- 82.Bahr R, Bahr IA. Incidence of acute volleyball injuries: a prospective cohort study of injury mechanisms and risk factors. Scand J Med Sci Sports 1997;7:166–71 [DOI] [PubMed] [Google Scholar]
- 83.Hertel J, Olmsted-Kramer LC. Deficits in time-to-boundary measures of postural control with chronic ankle instability. Gait Posture 2007;25:33–9 [DOI] [PubMed] [Google Scholar]
- 84.Willems T, Witvrouw E, Verstuyft J, Vaes P, de Clercq D. Proprioception and muscle strength in subjects with a history of ankle sprains and chronic instability. J Athl Train 2002;37:487–93 [PMC free article] [PubMed] [Google Scholar]
- 85.Denegar CR, Hertel J, Fonseca J. The effect of lateral ankle sprain on dorsiflexion range of motion, posterior talar glide, and joint laxity. J Orthop Sports Phys Ther 2002;32:166–73 [DOI] [PubMed] [Google Scholar]
- 86.Santos MJ, Liu W. Possible factors related to functional ankle instability. J Orthop Sports Phys Ther. 2008;38:150-157 [DOI] [PubMed] [Google Scholar]
- 87.Hertel J, Braham RA, Hale SA, Olmsted-Kramer LC. Simplifying the star excursion balance test: analyses of subjects with and without chronic ankle instability. J Orthop Sports Phys Ther 2006;36:131–7 [DOI] [PubMed] [Google Scholar]
- 88.Martin RL, Irrgang JJ. A survey of self-reported outcome instruments for the foot and ankle. J Orthop Sports Phys Ther 2007;37:72–84 [DOI] [PubMed] [Google Scholar]
- 89.Wikstrom EA, Naik S, Lodha N, Cauraugh JH. Bilateral balance impairments after lateral ankle trauma: a systematic review and meta-analysis. Gait Posture 2010;31:407–14 [DOI] [PubMed] [Google Scholar]
- 90.Bullock-Saxton JE. Local sensation changes and altered hip muscle function following severe ankle sprain. Phys Ther 1994;74:17–28; discussion 28–31 [DOI] [PubMed] [Google Scholar]
- 91.Freeman MA. Instability of the foot after injuries to the lateral ligament of the ankle. J Bone Joint Surg Br 1965;47:669–77 [PubMed] [Google Scholar]
- 92.Hertel J. Sensorimotor deficits with ankle sprains and chronic ankle instability. Clin Sports Med 2008;27:353–70 [DOI] [PubMed] [Google Scholar]
- 93.Menacho Mde O, Pereira HM, Oliveira BI, Chagas LM, Toyohara MT, Cardoso JR. The peroneus reaction time during sudden inversion test: systematic review. J Electromyogr Kinesiol 2010;20:559–65 [DOI] [PubMed] [Google Scholar]
- 94.Munn J, Sullivan SJ, Schneiders AG. Evidence of sensorimotor deficits in functional ankle instability: a systematic review with meta-analysis. J Sci Med Sport 2010;13:2–12 [DOI] [PubMed] [Google Scholar]
- 95.Santos MJ, Liu H, Liu W. Unloading reactions in functional ankle instability. Gait Posture 2008;27:589–94 [DOI] [PubMed] [Google Scholar]
- 96.Pahor S, Toppenberg R. An investigation of neural tissue involvement in ankle inversion sprains. Man Ther 1996;1:192–7 [DOI] [PubMed] [Google Scholar]
- 97.Sluka KA, Wright A. Knee joint mobilization reduces secondary mechanical hyperalgesia induced by capsaicin injection into the ankle joint. Eur J Pain 2001;5:81–7 [DOI] [PubMed] [Google Scholar]
- 98.Sterling M, Pedler A, Chan C, Puglisi M, Vuvan V, Vicenzino B. Cervical lateral glide increases nociceptive flexion reflex threshold but not pressure or thermal pain thresholds in chronic whiplash associated disorders: a pilot randomised controlled trial. Man Ther 2010;15:149–53 [DOI] [PubMed] [Google Scholar]
- 99.Wright A. Hypoalgesia post-manipulative therapy: a review of a potential neurophysiological mechanism. Man Ther 1995;1:11–6 [DOI] [PubMed] [Google Scholar]
- 100.Cleland JA, Abbott JH, Kidd MO, Stockwell S, Cheney S, Gerrard DF, et al. Manual physical therapy and exercise versus electrophysical agents and exercise in the management of plantar heel pain: a multicenter randomized clinical trial. J Orthop Sports Phys Ther 2009;39:573–85 [DOI] [PubMed] [Google Scholar]
- 101.Delitto A, Cibulka MT, Erhard RE, Bowling RW, Tenhula JA. Evidence for use of an extension-mobilization category in acute low back syndrome: a prescriptive validation pilot study. Phys Ther 1993;73:216–22; discussion 223–8 [DOI] [PubMed] [Google Scholar]
- 102.Schafer A, Hall T, Muller G, Briffa K. Outcomes differ between subgroups of patients with low back and leg pain following neural manual therapy: a prospective cohort study. Eur Spine J 2011;20:482–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Leininger B, Bronfort G, Evans R, Reiter T. Spinal manipulation or mobilization for radiculopathy: a systematic review. Phys Med Rehabil Clin N Am 2011;22:105–25 [DOI] [PubMed] [Google Scholar]
- 104.Santilli V, Beghi E, Finucci S. Chiropractic manipulation in the treatment of acute back pain and sciatica with disc protrusion: a randomized double-blind clinical trial of active and simulated spinal manipulations. Spine J 2006;6:131–7 [DOI] [PubMed] [Google Scholar]
- 105.MacDonald CW, Whitman JM, Cleland JA, Smith M, Hoeksma HL. Clinical outcomes following manual physical therapy and exercise for hip osteoarthritis: a case series. J Orthop Sports Phys Ther 2006;36:588–99 [DOI] [PubMed] [Google Scholar]
- 106.French HP, Brennan A, White B, Cusack T. Manual therapy for osteoarthritis of the hip or knee – a systematic review. Man Ther 2011;16:109–17 [DOI] [PubMed] [Google Scholar]
- 107.Pollard H, Ward G, Hoskins W, Hardy K. The effect of a manual therapy knee protocol on osteoarthritic knee pain: a randomised controlled trial. J Can Chiropr Assoc 2008;52:229–42 [PMC free article] [PubMed] [Google Scholar]
- 108.Moss P, Sluka K, Wright A. The initial effects of knee joint mobilization on osteoarthritic hyperalgesia. Man Ther 2007;12:109–18 [DOI] [PubMed] [Google Scholar]
- 109.van der Wees PJ, Lenssen AF, Hendriks EJ, Stomp DJ, Dekker J, de Bie RA. Effectiveness of exercise therapy and manual mobilisation in ankle sprain and functional instability: a systematic review. Aust J Physiother 2006;52:27–37 [DOI] [PubMed] [Google Scholar]
- 110.Collins N, Teys P, Vicenzino B. The initial effects of a mulligan’s mobilization with movement technique on dorsiflexion and pain in subacute ankle sprains. Man Ther 2004;9:77–82 [DOI] [PubMed] [Google Scholar]
- 111.Lopez-Rodriguez S, Fernandez de-Las-Penas C, Alburquerque-Sendin F, Rodriguez-Blanco C, Palomeque-del-Cerro L. Immediate effects of manipulation of the talocrural joint on stabilometry and baropodometry in patients with ankle sprain. J Manipulative Physiol Ther 2007;30:186–92 [DOI] [PubMed] [Google Scholar]
- 112.Yeo HK, Wright A. Hypoalgesic effect of a passive accessory mobilisation technique in patients with lateral ankle pain. Man Ther 2011:16(4):373–7 [DOI] [PubMed] [Google Scholar]