Abstract
Here, we show that a novel Rspo1-Wnt-Vegfc-Vegfr3 signaling pathway plays an essential role in developmental angiogenesis. A mutation in R-spondin1 (rspo1), a Wnt signaling regulator, was uncovered during a forward-genetic screen for angiogenesis-deficient mutants in the zebrafish. Embryos lacking rspo1 or the proposed rspo1 receptor kremen form primary vessels by vasculogenesis, but are defective in subsequent angiogenesis. Endothelial cell-autonomous inhibition of canonical Wnt signaling also blocks angiogenesis in vivo. The pro-angiogenic effects of Rspo1/Wnt signaling are mediated by Vegfc/Vegfr3(Flt4) signaling. Vegfc expression is dependent on Rspo1 and Wnt, and Vegfc and Vegfr3 are necessary to promote angiogenesis downstream from Rspo1-Wnt. As all of these molecules are expressed by the endothelium during sprouting stages, these results suggest that Rspo1-Wnt-VegfC-Vegfr3 signaling plays a crucial role as an endothelial-autonomous permissive cue for developmental angiogenesis.
Keywords: Angiogenesis, Vegfc, Zebrafish
INTRODUCTION
The Wnt and vascular endothelial growth factor (VEGF) signaling pathways are highly conserved mediators of cell fate specification and patterning (Rossant and Howard, 2002). VEGF signaling plays a well-documented central role in the vasculature, regulating endothelial specification, differentiation, migration and survival during angiogenesis and lymphangiogenesis (Olsson et al., 2006). Wnt signaling has also been implicated in endothelial function. Many Wnt signaling molecules are expressed in the developing vessels, and studies carried out in vitro using cultured endothelial cells have documented effects of Wnt signaling on endothelial proliferation, survival and migration (Franco et al., 2009; Liebner et al., 2008; Zerlin et al., 2008). Several in vivo studies on Wnt/β-catenin pathway signaling have also reported that this signaling pathway regulates various aspects of cardiovascular development, including artery/vein specification, blood-brain barrier formation and vascular smooth muscle integrity (Cohen et al., 2008; Corada et al., 2010; Hurlstone et al., 2003; Shu et al., 2002; Stenman et al., 2008). However, targeted disruption of Wnt signaling genes in mice has not resulted in widespread dramatic defects in developmental angiogenesis, perhaps owing to extensive functional overlap between these genes, and the in vivo role of Wnt signaling in the vasculature and the identity of crucial downstream pathways remain unclear (Franco et al., 2009; Ishikawa et al., 2001; Monkley et al., 1996; Xu et al., 2004; Ye et al., 2009).
A variety of genes have been shown to influence the Wnt signaling pathway or its output by modulating activities of Wnt pathway members (Chen et al., 2007; Cohen et al., 2008; Junge et al., 2009). Among these, R-spondins are a family of secreted ligands (Rspo1-4) that have been shown to enhance Wnt signaling (Kim et al., 2008). Frizzled, LRP6 and a specific receptor called kremen are believed to act as a receptor complex for Rspo signaling (Binnerts et al., 2007; Kazanskaya et al., 2004; Kim et al., 2008; Nam et al., 2007; Wei et al., 2007), although their mechanism of action is not well understood (Binnerts et al., 2007; Kinzler et al., 1991; Nam et al., 2006; Wei et al., 2007). Different R-spondin family members have been shown to modulate a wide variety of functions in different tissues (Hendrickx and Leyns, 2008; Kim et al., 2006), including in the vasculature. R-spondin 3 has been shown to promote expression of VEGFA in Xenopus and mouse embryos and to regulate endothelial specification and differentiation (Kazanskaya et al., 2008). Wnt signaling is also reported to promote expressions of VEGFA and VEGFC in cultured human endothelial and tumor cells (Skurk et al., 2005; Zhang et al., 2001). A recent study also reported that transgenic expression of an activated form of β-catenin affects vascular remodeling and arterial-venous specification by activating Dll4 expression and thus increasing Notch signaling (Corada et al., 2010). Dll4/Notch signaling regulates angiogenesis and tip cell specification in mouse and zebrafish embryos (Hellstrom et al., 2007; Lobov et al., 2007; Roca and Adams, 2007; Siekmann and Lawson, 2007). Despite these and other reports implicating Wnt signaling in various aspects of endothelial function, little is yet known about how its modulators affect blood vessel formation and vascular development in vivo.
Here, we show that a novel Rspo1-Wnt-Vegfc-Vegfr3 signaling pathway is essential for developmental angiogenesis. During embryogenesis, mesodermally derived endothelial progenitor cells (angioblasts) first come together to form the primary vascular bed by a process called vasculogenesis. The primary vascular bed subsequently undergoes remodelling, as well as sprouting and growth of new vessels from the pre-existing vessels by a process called angiogenesis. Using a newly identified mutant in rspo1 and variety of other methods for functionally manipulating different members of this pathway, we show that canonical Wnt signaling is required downstream of rspo1 for endothelial sprouting angiogenesis. We also further show that Rspo1/Wnt signaling promotes sprouting angiogenesis by upregulating endothelial vegfc expression. Our results reveal a new signaling pathway required for developmental angiogenesis.
MATERIALS AND METHODS
Zebrafish strains and genetic mapping
Tg(fli:EGFP)y1 fish were maintained on the EK background and used for ENU mutagenesis. Bulked segregant analysis and fine genetic mapping were carried out as described previously (Lawson et al., 2003). The Tg(fli:nEGFP)y7 is reported elsewhere (Roman et al., 2002). The Tg(flk:mCherry)y206 line was generated using a transgene construct in which cytoplasmic mCherry is driven by the endothelial kdrl/flk1 promoter (assembled using Gateway technology). The construct was injected into EK embryos and founders were screened for endothelial mCherry expression. The brightest mCherry-expressing transgenic zebrafish was propagated to establish the line. Zebrafish strains were maintained and bred, and embryos were staged as described elsewhere (Kimmel et al., 1995; Westerfield, 2000).
Measuring endothelial cell proliferation, endothelial cell count and ISV phenotype
Endothelial cell numbers were counted using Tg(fli:nEGFP)y7 transgenic zebrafish in which all endothelial cell nuclei are endogenously green fluorescent and can be easily counted. Mutant and wild-type embryos from the same clutch were mounted and trunk regions were imaged using 20× water immersion objective on an Olympus confocal microscope at described stages. Nuclei from the DA and CV of three middle trunk segments were counted from confocal z-stacks. At least ten embryos for each time point were imaged and counted for analysis. For dividing cells, mutant and sibling embryos derived from the Tg(fli:nEGFP)y7 line were fixed and processed for immunohistochemistry as described below. Labeled embryos imaged as described above, except the yolk was removed before imaging. Phospho-histone H3-positive nuclei were counted from confocal z-stacks of the axial vessels (dorsal aorta and cardinal vein). For ISV phenotype quantitation, 11 segments rostral to the end of the yolk extension were counted per embryo from a minimum of eight embryos per treatment. Phenotypes were divided in to three groups: no ISV sprouts, ISVs up to the horizontal myoseptum (half-way up the trunk) and ISVs up to the DLAV (fully extended dorsally). For calculating statistical significance, numbers or ISV reaching the DLAV were counted from at least two separate experiments each for control, rspo1, krm1, vegfc, vegfa or vegfr3 morpholino-injected animals, or from dtty135 or wild-type sibling animals. The results of replicate experiments were pooled and Student’s t-test was applied to compare control morpholino or wild-type animals with other morpholino or dtty135 mutant animals (supplementary material Table S1).
Whole-mount in situ hybridization, immunohistochemistry and TUNEL labeling
Whole-mount in situ hybridization was carried out as described previously (Gore et al., 2008). Immunohistochemistry was carried out using polyclonal anti-phosphohistone H3 (1:200) or monoclonal F59, S54 and Eng (all at 1:10 from hybridoma bank) antibodies as described previously (Srinivas et al., 2007). Anti-mouse Cy3 and anti-rabbit Cy3 secondary antibodies (Jackson Laboratories) were used at 1:400 dilution. For detection of apoptosis, the in situ cell death detection kit (TMR) from Roche was used as described previously (Gore et al., 2008).
Protein labeling and microinjections
Mouse R-spondin1/cristin 3 protein was purchased from R&D Systems. Alexa 555 microscale protein labeling kit was purchased from Invitrogen and labeling was carried out as per manufacturer’s instructions. Microangiography was carried out on 24 hpf embryos as described previously (Kamei et al., 2006; Weinstein et al., 1995). For trunk interstitial space injections, embryos were manually dechorionated and mounted in methylcellulose. A small bolus of labeled protein was injected between the somite boundaries at the 18- to 20-somite stages using a fine needle inserted into the trunk muscle.
Plasmid DNA cloning
Endothelial specific Tol2(Flk:mCherry-2A-vegfc) and Tol2(Flk:mCherry) constructs were assembled using Gateway technology (Kwan et al., 2007; Provost et al., 2007; Villefranc et al., 2007).
BIO drug treatment
A 100 mM stock solution of (2′Z,3′E)-6-bromoindirubin-3′-oxime (BIO; EMD biosciences, catalog number 361552) was prepared in DMSO and diluted in embryonic medium E3 to a final concentration of 0.5 mM. For early embryonic treatments, manually dechorionated embryos were placed in E3/BIO solution from high/dome stage (2/2.5 hpf) until shield stage (6 hpf), then fixed and processed for staining. For late-stage treatments, dechorionated embryos were placed in E3/BIO solution from 16 hpf to 40 hpf and analyzed at different stages by live imaging or staining. Embryos in E3 solution containing DMSO carrier alone were used as control for all experiments.
Microinjections
Morpholinos were injected into one- to two-cell stage zebrafish embryos at the indicated doses, as described previously (Gore et al., 2008). Morpholinos used in the study are as follows: rspo1 translation blocking MO (translation start site underlined), 5′-CCAGCGCCAGCAGTCCCAAATGCAT-3′; rspo1 exon 1 splice donor MO, 5′-AGAAACATCAGCACTCACTCCGTCT-3′; rspo1 exon 4 splice acceptor MO, 5′-CTTAGATCACATTGGACTGCAAAAT-3′; wnt2bb translation blocking MO (Ober et al., 2006), 5′-GTGTGCCATATAAAAGTATTCCCCG-3′; krm1 translation blocking MO (translation start site underlined), 5′-AAGCTGCGACTCTCCACGAATCCAT-3′; krm1 5′ UTR MO, 5′-TGTAGTCTGTGATCTGTCTGAGCTT-3′; krm1 exon 1 splice donor MO, 5′-GGTTCCTGTGTGAGAAAAACAGACT-3′; vegfc translation blocking MO (translation start site underlined) (Ober et al., 2004), 5′-GAAAATCCAAATAAGTGCATTTTAG-3′; flt4 splice donor MO (Covassin et al., 2006), 5′-TTAGGAAAATGCGTTCTCACCTGAG-3′. Synthetic mRNA and plasmid DNA injections were carried out as described previously (Gore et al., 2008).
Endothelial cell culture and treatments
Murine endothelial cell line MSS31 was kindly provided by Dr Yanai (Tohoku University, Japan) (Yanai et al., 1991) and cultured in α-minimal essential medium (αMEM, GIBCO) containing 10% FBS. For activation of Wnt signaling, cultured MSS31 endothelial cells were treated with 16 ng/ml Wnt3a (R&D) or with 6 μM (2′Z,3′E)-6-bromoindirubin-3′-oxime (BIO; EMD biosciences) for 2 days.
RT-PCR analysis
Total cellular RNA from morpholino injected embryos and cultured MSS31 endothelial cells was isolated using Trizol reagent and treated with DNAse I. Total cDNA was synthesized using the Thermoscript or SuperScript III first-strand synthesis supermix kit (Invitrogen) and PCR analysis was carried out using Qiagen Top Taq PCR kit or by Qiagen One-Step RT-PCR kit. Primers used in this study can be found in Table S2 (supplementary material).
RESULTS
dtty135 mutants have a specific defect in angiogenesis
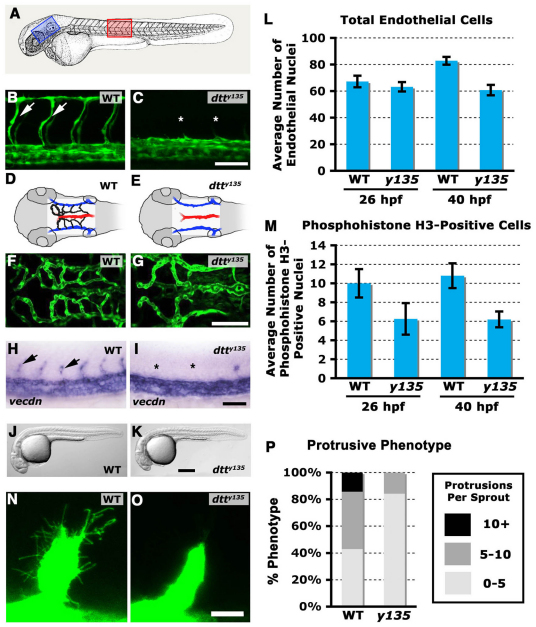
We identified down the tubes (dtty135), a zebrafish mutant with a specific defect in angiogenesis, in a Tg(fli-EGFP)y1 transgenic-based forward-genetic screen for vascular mutations. Initial vasculogenesis takes place normally in dtty135 mutants. Primary vessels in the head (e.g. primordial hindbrain channels) and trunk (e.g. dorsal aorta, DA, posterior cardinal vein, PCV) are properly formed and carry normal circulatory flow (data not shown). By contrast, the subsequent growth of angiogenic vessels is strongly inhibited. Trunk intersegmental vessels (ISV) are either absent or greatly reduced in number and length in 26 hpf dtty135 mutants compared with their wild-type siblings (Fig. 1A-C; see also Fig. 2L). There is some limited recovery of ISV later in development (after 2 dpf) but the vessels remain stunted and unable to form a complete trunk vascular network. The lymphatic network also fails to form in dtty135 mutants, although this probably reflects the lack of a properly formed primary vasculature (data not shown). Cranial central arteries (Isogai et al., 2001) are also absent in 26 hpf dtty135 mutants (Fig. 1D-G). Analysis of a variety of endothelial- or hematopoietic-specific markers reveals no defects in specification or differentiation of the endothelial or hematopoietic lineages (Fig. 1H,I; supplementary material Fig. S1), and no gross morphological defects, circulation defects or overall developmental delay are observed in dtty135 mutants (Fig. 1J,K and data not shown). In addition to a defect in angiogenic sprouting and endothelial cell migration, dtty135 mutants are also defective in angiogenic endothelial cell proliferation. dtty135 mutants initially have a normal number of endothelial cells in the DA and CV, but, unlike wild-type siblings, the number of endothelial cells does not increase at later (40 hpf) stages of development (Fig. 1L). The number of phosphohistone H3-positive endothelial cells is also reduced in mutant DA and CV compared with wild-type siblings (Fig. 1M). High-resolution imaging reveals that migration and protrusive activity of the angiogenic sprouts that do eventually form in dtty135 mutants is substantially reduced (supplementary material Movies 1 and 2), with fewer filopodial structures (Fig. 1N-P). In addition to endothelial effects, dtty135 mutants also show reduced skeletal musculature at later stages of trunk development. However, initial specification and patterning of muscle is normal (supplementary material Fig. S1).
Fig. 1.
dtty135 mutants have defects in angiogenesis, but not in endothelial specification or in vasculogenesis. (A) Diagram of a zebrafish embryo with the red box highlighting the region shown in B and C, and a blue box highlighting the region shown in D-G. (B,C) Confocal images of trunk vessels in 26 hpf Tg(fli-EGFP)y1 wild-type sibling (B) and dtty135 mutant (C) zebrafish, showing normal formation of the vasculogenic dorsal aorta and posterior cardinal vein but failure to form the angiogenic intersegmental vessels (arrows in B, asterisks show absence in C) in dtty135 mutants (lateral views, anterior towards the left). (D,E) The hindbrain vessels imaged in F,G. Primordial hindbrain channels (PHBC) are in blue, basilar artery (BA) is in red and the central arteries (CA) are in black. (F,G) Confocal images of hindbrain vessels in 48 hpf Tg(fli-EGFP)y1 wild-type sibling (F) and dtty135 mutant (G) zebrafish, showing normal formation of vasculogenic primary vessels, including the PHBC and BA, but failure to form the angiogenic CA that penetrate the hindbrain (dorsal views, anterior towards the left). (H,I) In situ hybridization of endothelium in the trunk of 26 hpf wild-type sibling (H) and dtty135 mutant (I) zebrafish with a probe for vecdn, showing normal expression levels in dtty135 mutants (lateral views, anterior towards the left). ISV are seen in wild-type siblings (arrows in H) but not in dtty135 mutants (asterisks in I). (J,K) Transmitted light images of 26 hpf Tg(fli-EGFP)y1 wild-type sibling (J) and dtty135 mutant (K) zebrafish, showing reduced width of yolk extension but otherwise normal morphology of dtty135 mutants (J) compared with their wild-type siblings (K). Images are lateral views, anterior towards the left. Endothelial proliferation is reduced in dtty135 mutants. (L) Total number of endothelial cells present in the three posteriormost trunk segments, measured in Tg(fli-nEGFP)y7 transgenic dtty135 mutant or wild-type sibling animals, at 26 hpf and 40 hpf. (M) Total number of phosphohistone H3-positive endothelial cells present in the three posteriormost trunk segments, measured in phosphohistone and GFP antibody-probed Tg(fli-nEGFP)y7 transgenic dtty135 mutant or wild-type sibling animals, at 26 hpf and 40 hpf. Data are mean±s.e.m. (N,O) Higher-magnification confocal images of ISV sprouts in wild-type sibling (N) and dtty135 mutant (O) embryos at 20 hpf, showing reduced filopodial protrusions in mutants. (P) Total number of filopodia formed by control and mutant sprouts. Scale bars: 50 μm in B,C,F,G; 100 μm in H-K; 20 μm in N,O.
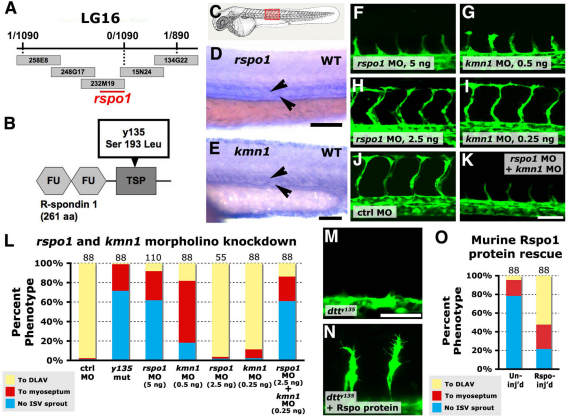
Fig. 2.
R-spondin 1 and its receptor kremen 1 are required together for angiogenesis. (A) dtty135 genetic interval on linkage group 16, with the number of recombinants per meiosis, position of BAC clones and position of the rspo1 locus indicated. (B) Rspo1 protein domain structure, with two furin domains (FU) and a thrombospondin domain (TSP). The dtty135 mutation changes serine 193 to leucine in the TSP domain. (C) A zebrafish embryo with the red box highlighting the region imaged in D-K. (D,E) In situ hybridization of the trunk of 24 hpf wild-type zebrafish embryos probed for rspo1 (D) or krm1 (E), with expression observed in the axial vasculature (arrowheads). (F-K) Confocal images of trunk vessels in 26 hpf Tg(fli-EGFP)y1 wild-type zebrafish injected with either 5 ng rspo1 MO (F), 0.5 ng krm1 MO (G), 2.5 ng rspo1 MO (H), 0.25 ng krm1 MO (I), 5 ng control MO (J) or 2.5 ng rspo1 MO + 0.25 ng krm1 MO (K). (L) Quantitation of the intersegmental vessel (ISV) phenotypes of 26 hpf Tg(fli-EGFP)y1 dtty135 mutant or morpholino-injected embryos. (M,N) Confocal images of ISV in 26 hpf Tg(fli-EGFP)y1 dtty135 mutant embryos that were either not injected (M) or were injected intramuscularly in the trunk with murine R-spondin (N). (O) Quantitation of the intersegmental vessel (ISV) phenotypes of uninjected or mouse R-spondin-injected dtty135 zebrafish at 28 hpf. In L and O the bars show the percentages of ISV that have failed to sprout (blue), ISV that have grown only up to the horizontal myoseptum half way up the trunk (red) and ISV that have grown all the way to the dorsal trunk to form the DLAV (yellow). The number of segments counted is shown above each bar on the graphs. Scale bars: 100 μm in D; 50 μm in E; 50 μm in F-K,M,N.
R-spondin 1 and kremen 1 are required together for angiogenesis
To examine the molecular nature of the dtty135 phenotype, we positionally cloned the defective locus. The mutation maps to a sequenced interval on linkage group 16 containing the R-spondin 1 (rspo1) gene (Fig. 2A). R-spondins are a family of secreted ligands (Rspo1-4) that have been shown to enhance Wnt signaling. Frizzled, LRP6 and a specific receptor called kremen are believed to act as a receptor complex for Rspo signaling (Binnerts et al., 2007; Kazanskaya et al., 2004; Kim et al., 2008; Nam et al., 2007; Wei et al., 2007), but their mechanism of action is not well understood (Binnerts et al., 2007; Kim et al., 2008; Wei et al., 2007). Sequence analysis of rspo1 cDNA from dtty135 mutants and their wild-type siblings reveals a C-to-T point mutation leading to a polar (serine) to non-polar (leucine) amino acid change at position 193 of the protein (Fig. 2B). Three additional lines of evidence confirm that the mutation in rspo1 is responsible for the dtty135 phenotype. First, rspo1 and its presumptive receptor kremen1 (krm1) are both expressed in the early zebrafish vasculature, consistent with a vascular function (Fig. 2C-E; supplementary material Fig. S2). The earliest vascular expression of rspo1 is detected during active stages of ISV sprouting. Second, injection of any one of three different antisense morpholino oligonucleotides (MO) targeting either rspo1 or krm1 transcripts at the translational start site or exon-intron splice sites (supplementary material Fig. S3) reproducibly phenocopies the angiogenesis phenotypes of dtty135 mutants (Fig. 2F,G,J,L). Co-injection of lower doses of rspo1 and krm1 morpholinos, that on their own fail to elicit a phenotype, results in a dramatic defective ISV phenotype (Fig. 2H,I,K,L; P<0.0038; supplementary material Table S1), providing evidence that rspo1 and krm1 function together during angiogenesis. Third, a single injection of murine Rspo1/cristin 3 protein into the trunk interstitial spaces partially rescues the ISV phenotypes of dtty135 mutants (Fig. 2M-O).
Canonical Wnt signaling is required endothelial cell-autonomously for angiogenesis
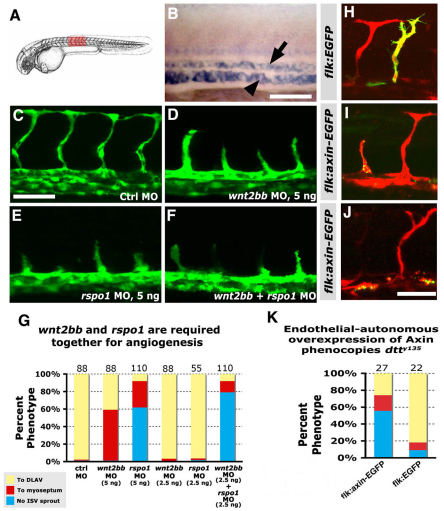
As noted above, previous data has suggested that Rspo/kremen signaling functions upstream from or together with the Wnt signaling pathway. During early embryogenesis, a large number of Wnt ligands are expressed in and around the developing vasculature. None of these Wnt ligands have been reported to show a strong vascular phenotype, however, suggesting that multiple Wnt ligands may contribute together to endothelial signaling in conjunction with Rspo1 in the developing trunk. We reasoned that if Rspo1 signaling is acting via the Wnt pathway then reduction of some of these Wnt ligands might show synergistic effects in combination with rspo1 knockdown. We chose the Wnt ligand wnt2bb to test this because a previous report (Ober et al., 2006) showed that wnt2bb is expressed in endothelial cells during early stages of zebrafish embryogenesis, although its function is required for liver specification at later stages (see also Fig. 3A,B). We found that in addition to these later liver defects, wnt2bb knockdown also causes defects in ISV sprouting and growth, although these defects are not as severe as those found in dtty135 mutants or rspo1 morphants (Fig. 3C-G). However, co-injection of lower doses of rspo1 and wnt2bb morpholinos that fail to elicit a phenotype on their own results in a severe ISV phenotype (Fig. 3F,G; P<0.0049; supplementary material Table S1), suggesting that rspo1 and wnt2bb are also required together for angiogenesis. To examine whether Wnt signaling is required cell-autonomously in the endothelium, we inhibited Wnt/β-catenin signaling in vivo by transgenic endothelial overexpression of Axin. Axin is a negative regulator of Wnt signaling that forms a complex with GSK3β and APC, marking β-catenin for destruction in the absence of active Wnt signaling (reviewed by Angers and Moon, 2009). We expressed Axin-EGFP fusion protein using the endothelial-specific flk promoter. Unlike endothelial cells expressing the control flk:EGFP transgene (Fig. 3H,K), flk:axin-EGFP transgene-expressing endothelial cells either failed to form ISV sprouts or formed stunted ISVs (Fig. 3I-K), as observed upon depletion of Wnt2bb, kremen or Rspo1 proteins. These data indicate that endothelial cell-autonomous Wnt/β-catenin signaling is required for in vivo angiogenesis.
Fig. 3.
Canonical Wnt signaling is required endothelial cell-autonomously for angiogenesis. (A) Diagram of a zebrafish embryo with the red box highlighting the region shown in B-F,H-J. (B) In situ hybridization of the trunk of a 26 hpf wild-type zebrafish embryo probed for wnt2bb, showing expression in the axial vessels (dorsal aorta, arrow; posterior cardinal vein, arrowhead). Lateral view, anterior towards the left. (C-F) Confocal images of trunk vessels in 26 hpf Tg(fli-EGFP)y1 wild-type zebrafish injected with either 5 ng control MO (C), 5 ng wnt2bb MO (D), 5 ng rspo1 MO (E) or 2.5 ng wnt2bb MO + 2.5 ng rspo1 MO (F). (G) Quantitation of the intersegmental vessel (ISV) phenotypes of morpholino-injected wild-type 26 hpf Tg(fli-EGFP)y1 zebrafish. (H-J) Confocal images of trunk vessels in 26 hpf wild-type Tg(flk-mCherry) zebrafish (red) injected with DNA for either control flk:EGFP (H) or flk:axin-EGFP (I,J) expression constructs (green), showing defects in sprouting (J) or growth (I) of ISV expressing the axin-EGFP fusion protein. (K) Quantitation of the intersegmental vessel (ISV) phenotypes of endothelium expressing flk:axin-EGFP or control flk:EGFP expression constructs. In G and K bars show the percentages of ISV that have failed to sprout (blue), ISV that have grown only up to the horizontal myoseptum half-way up the trunk (red) and ISV that have grown all the way to the dorsal trunk to form the DLAV (yellow). The number of segments counted is shown above each bar. Scale bars: 50 μm in B-F,H-J.
To confirm that activation of Wnt/β-catenin signaling is required downstream from Rspo1 function during angiogenesis, we examined zebrafish homozygous for apcmcr, a mutation previously shown to promote maintenance of active Wnt/β-catenin signaling by reducing degradation of β-catenin (Haramis et al., 2006; Hurlstone et al., 2003). We injected rspo1 morpholinos into embryos derived from apcmcr/+ incrosses (Fig. 4A-C). Rspo1 MO-injected wild type (+/+) or heterozygous (apcmcr/+) embryos showed reduced ISV formation (Fig. 4A,C), but rspo1 MO-injected homozygous (apcmcr/apcmcr) mutants developed relatively normal ISVs (Fig. 4B,C), showing that downstream activation of β-catenin signaling can rescue the angiogenic defects caused by loss of Rspo1. This was further confirmed using treatment with BIO [(2′Z,3′E)-6-bromoindirubin-3′-oxime], a small cell-permeable drug that upregulates β-catenin signaling by inhibiting GSK3β (Meijer et al., 2003). Zebrafish embryos treated with BIO during blastula stages (2.5-6.0 hpf) ectopically express the dorsal marker chordin, as expected for early upregulation of Wnt signaling, whereas treatment during early somitogenesis increases vascular expression of known canonical Wnt/β-catenin target gene cyclin D1 (ccnd1) (Shtutman et al., 1999) (Fig. 4D-G). BIO-treated rspo1 MO-injected embryos show improved sprouting and elongation of cranial central arteries compared with DMSO control-treated animals (Fig. 4H,I), whereas BIO-treated dtty135 mutant embryos show improved sprouting and elongation of trunk ISVs compared with DMSO control-treated dtty135 mutants (Fig. 4J-L). Activation of Wnt/β-catenin signaling in apcmcr mutants or BIO treatment of wild-type zebrafish embryos also promotes increased protrusive activity and branching of both trunk ISVs and cranial central arteries (supplementary material Fig. S4).
Fig. 4.
Upregulation of the Wnt/β-catenin pathway promotes angiogenesis. (A,B) Confocal images of trunk vessels in 48 hpf Rspo1 MO-injected wild-type (+/+) or heterozygous (apcmcr/+) embryo with ISV defects (A) or homozygous (apcmcr/apcmcr) mutant (B) with relatively normal ISV growth. (C) Quantitation of ISV phenotype of Rspo1 MO-injected wild type (+/+) or heterozygous (apcmcr/+) embryo and homozygous (apcmcr/apcmcr) mutant. (D,E) In situ hybridization of the trunks of 26 hpf wild-type zebrafish embryos treated from 16 hpf to 24 hpf with either carrier DMSO (D) or BIO (E), probed for cyclin D1. (F,G) In situ hybridization of the trunks of 6 hpf wild-type zebrafish embryos treated from 2.5 hpf to 6 hpf with either carrier DMSO (F) or BIO (G), probed for chordin. (H,I) Confocal images of hindbrain vessels in 48 hpf Rspo1 MO-injected embryos treated with carrier DMSO (H) or BIO (I). (J,K) Confocal images of trunk vessels in 40 hpf dtty135 mutant embryos treated with carrier DMSO (J) or BIO (K) from 16 hpf. (L) Quantitation of the intersegmental vessel (ISV) phenotypes of 40 hpf Tg(fli:EGFP)y1 dtty135 mutants treated from 16-40 hpf with either DMSO carrier or BIO. Bars show the percentages of ISV that have failed to sprout (blue), ISV that have grown only up to the horizontal myoseptum half-way up the trunk (red) and ISV that have grown all the way to the dorsal trunk to form the DLAV (yellow). The number of segments counted is shown above each bar. Scale bars: 50 μm in A,H,J.
Expression of Vegfc is specifically affected in dtty135 mutant embryos
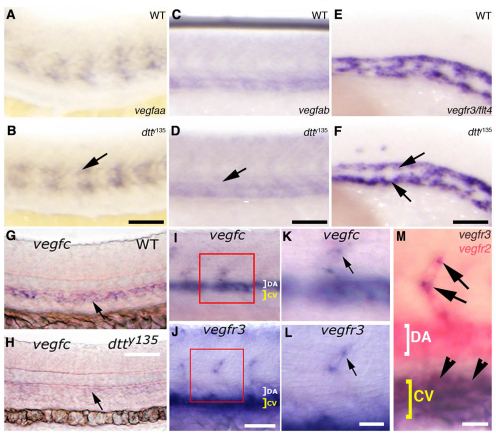
Wnt signaling has been reported to promote expression of VEGFA (Kazanskaya et al., 2008) or VEGFA and VEGFC (Skurk et al., 2005) in cultured human endothelial cells, so we examined the expression of zebrafish VEGFs, and VEGF receptors and other vascular marker genes, in dtty135 mutants and their wild-type siblings. We found no change in the expression of vegfaa, vegfab or any of the VEGF receptors, including vegfr3/flt4 (Fig. 5A-F; supplementary material Fig. S1), but noticed a strong decrease in expression of vegfc (Fig. 5G,H). vegfc has a dynamic expression pattern during early zebrafish development (Covassin et al., 2006; Ober et al., 2004). It is initially expressed in the hypochord, a trunk midline signaling structure located between the notochord and developing dorsal aorta (Covassin et al., 2006) (data not shown), but by 24 hpf vegfc becomes expressed exclusively in the adjacent dorsal aorta (Covassin et al., 2006) and, importantly, also in the growing ISV (Fig. 5G,I,K). Although vegfc is expressed in the hypochord at 18 hpf in dtty135 mutants, which is indistinguishable from wild-type animals (data not shown), by 24 hpf its expression in the dorsal aorta is absent or strongly reduced (Fig. 5H). vegfr3/flt4, the presumptive zebrafish receptor for vegfc, is also expressed in the vasculature, first in the dorsal aorta and posterior cardinal vein (Fig. 5E), which also express rspo1. Later during development, vegfr3/flt4 is expressed in posterior cardinal vein and growing ISV (Covassin et al., 2006; Hogan et al., 2009; Siekmann and Lawson, 2007) (Fig. 5K,L). We confirmed the tip cell expression of vegfr3/flt4 by performing double in situ hybridization (Fig. 5M). Similar vegfc expression by endothelial cells of the developing dorsal aorta and intersegmental vessels during mouse embryonic development was reported previously using a lacZ knock-in line (Tammela et al., 2008).
Fig. 5.
Expression of Vegfc is specifically affected in dtty135 mutant embryos. (A-F) In situ hybridization of the mid-trunk of wild-type sibling (A,C,E) and dtty135 mutant (B,D,F) zebrafish embryos probed for vegfaa (A,B), vegfab (C,D) or vegfr3 (E,F). The expression of vegfaa in the somites (arrow in B), vegfab in the axial vessels (arrow in D) and vegfr3 in the axial vessels (arrows in F) does not change in dtty135 mutant embryos compared with their wild-type siblings. (A-D) 24 hpf; (E,F) 20 hpf. (G,H) In situ hybridization of 26 hpf wild-type sibling (G) and dtty135 mutant (H) embryos probed for vegfc (arrows). (I,K) In situ hybridization of 26 hpf wild-type embryos probed for vegfc, showing expression in the DA and ISV (K shows a magnified image of the boxed region in I). (J,L) In situ hybridization of 26 hpf wild-type embryos probed for vegfr3, showing expression in the CV and ISV tip cells (L shows a magnified image of the boxed region shown in J). (M) Double in situ hybridization confirming expression of Vegfr3/flt4 (purple) in the tip cell (arrows) in addition to the cardinal vein (arrowheads), when compared with expression of Vegfr2/flk1 (pink) throughout the DA, CV and ISV. Scale bars: 50 μm in B,D,F,G; 45 μm in I,J; 25 μm in K-M.
Expression of vegfc is regulated by Wnt/β-catenin signaling
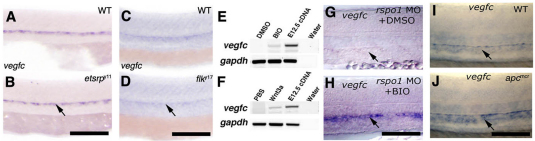
We examined whether vegfc expression is reduced in mutants other than dtty135 with similar defects in ISV formation. etsrpy11 and flky17 mutants, which are also defective in ISV formation, do not show reduced vegfc (Fig. 6A-D). To examine directly whether Wnt/β-catenin signaling regulates vegfc expression in endothelial cells in vitro, we treated mouse cultured endothelial cells with either Wnt/β-catenin signaling activator BIO or Wnt3a. Unlike control treatments, treatment with either BIO (Fig. 6E) or Wnt3a (Fig. 6F) activates vegfc expression in cultured mouse endothelial cells. Similarly, in zebrafish embryos, control DMSO-treated rspo1 morphants failed to express vegfc in the dorsal aorta (Fig. 6G), but BIO-treated morphants regained dorsal aorta expression of vegfc (Fig. 6H). Importantly, we also noted that higher level vegfc expression persists in the dorsal aorta at later stages of development in apcmcr/apcmcr mutant zebrafish when expression is reduced in their wild-type siblings (Fig. 6I,J).
Fig. 6.
Expression of vegfc is specifically regulated by Wnt/β-catenin signaling. (A-D) In situ hybridization of the mid-trunk of 24 hpf of wild-type sibling (A,C), etsrpy11 mutant (B) and flky17 mutant (D) embryos, probing for vegfc. Arrows show vegfc expression in the dorsal aorta, which is not affected in the two mutants. (E,F) RT-PCR analysis showing expression of vegfc by cultured mouse endothelial cells upon treatment with BIO (E) or Wnt3a (F). (G,H) In situ hybridization of 26 hpf rspo1 MO-injected embryos treated from 16-26 hpf with either 0.5% DMSO carrier (G) or 0.5 μM BIO in DMSO (H), probed for vegfc (arrows). (I,J) In situ hybridization of 36 hpf wild-type sibling (I) or homozygous (apcmcr/apcmcr) mutant (J) embryos probed for vegfc (arrows). Scale bars: 50 μm.
Vegfc signaling is required downstream of Rspo/Wnt signaling
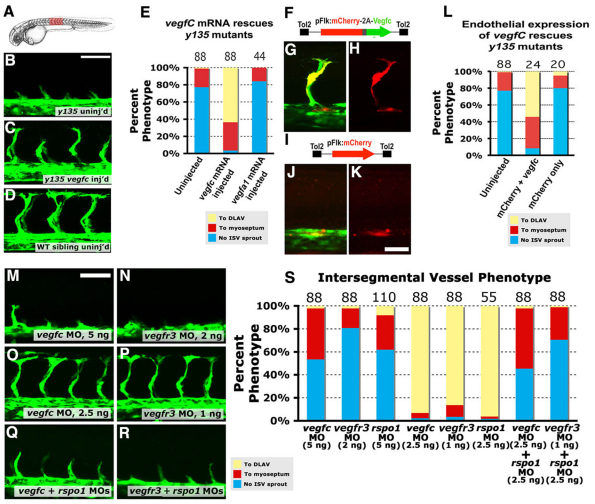
As our results indicated Rspo1/Wnt signaling promotes vegfc expression in the endothelium, we carried out additional experiments to test whether Vegfc/Vegfr3 signaling is required for angiogenesis downstream from Rspo1/Wnt. Injection of synthetic vegfc mRNA rescued ISV sprouting defects in dtty135 mutant embryos, whereas similar injection of vegfa1 mRNA failed to rescue (Fig. 7A-E), showing that exogenously supplied Vegfc, but not Vegfa, is sufficient to promote ISV sprouting in the absence of Rspo1. To determine whether endothelial-specific expression of vegfc can similarly rescue angiogenesis, we expressed either vegfc and mCherry together, or mCherry alone as a control, in dtty135 mutants using an endothelial-specific flk promoter (Fig. 7F-L). Endothelial cells expressing Vegfc and mCherry incorporated into normally sprouting and extending ISV (Fig. 7F-H,L), whereas endothelial cells expressing mCherry alone did not (Fig. 7I-L). These studies showed that vegfc is an essential component of Rspo1/Wnt downstream signaling required for sprouting angiogenesis.
Fig. 7.
Vegfc signaling is required downstream of Rspo/Wnt signaling. (A) Diagram of a zebrafish embryo with the red box highlighting the region shown in B-D. (B-D) Confocal images of the trunk vessels in 26 hpf dtty135 mutant (B), vegfc mRNA-injected dtty135 mutant (C) and uninjected wild-type sibling (D). (E) Quantitation of ISV growth phenotypes in 26 hpf uninjected and mRNA-injected zebrafish embryos. P values according to Student’s t-test: vegfc, P<0.0001; vegfa, P<0.0187. (F-H) Rescued ISV sprouts in 26 hpf Tg(fli-EGFP)y1 dtty135 mutant zebrafish injected with a Tol2(Flk:mCherry-2a-vegfc) construct (F) for Flk promoter-driven endothelial expression of mCherry and vegfC separated by a viral 2A peptide sequence. (I-K) No rescue of ISV sprouting in 26 hpf Tg(fli-EGFP)y1 dtty135 mutant zebrafish injected with a Tol2(Flk:mCherry) Tol2 construct (I) for Flk promoter-driven endothelial expression of mCherry reporter alone. (G,J) EGFP (green) and mCherry (red) combined fluorescence image. (H,K) Same fields as in G,J, viewing mCherry fluorescence alone. (L) Quantitation of ISV growth phenotypes in 26 hpf uninjected and Tol2(Flk:mCherry-2a-vegfc) or Tol2(Flk:mCherry) DNA-injected zebrafish embryos. The bars show the percentages of ISV that have failed to sprout (blue), ISV that have grown only up to the horizontal myoseptum half-way up the trunk (red) and ISV that have grown all the way to the dorsal trunk to form the DLAV (yellow). For DNA construct-injected animals in L, only mCherry-positive ISV phenotypes were assessed. The number of segments counted is shown above each bar on the graphs. (M-R) Confocal images of trunk vessels in 26 hpf Tg(fli-EGFP)y1 wild-type zebrafish injected with either 5 ng vegfc MO (M), 2 ng vegfr3 MO (N), 2.5 ng vegfc MO (O), 1 ng vegfr3 MO (P), 2.5 ng vegfc + 2.5 ng rspo1 MO (Q) or 1 ng vegfr3 MO + 2.5 ng rspo1 MO (R). (S) Quantitation of ISV growth phenotypes in 26 hpf different MO-injected zebrafish embryos. Color coding is the same as in L. Scale bars: 50 μm in B,M; 25 μm in K.
Vegfc signaling is believed to function primarily through Vegfr3 expressed by the tip cells during angiogenic sprouting. Blocking Vegfr3-mediated Vegfc signaling in mice leads to reduced angiogenic sprouting and vascular density (Tammela et al., 2008). To test whether Vegfc and Vegfr3 are required downstream of Rspo1/Wnt signaling, we injected MOs targeting the vegfc or Vegfr3/flt4 transcripts (Fig. 7M-S). Injections of full doses of these MOs resulted in absent or reduced ISV sprouting in wild-type embryos (Fig. 7M,N,S). Co-injection of low doses of either vegfc or vegfr3/flt4 MOs, which, on their own, failed to elicit significant phenotype (Fig. 7O,P,S), together with sub-phenotypic doses of rspo1 MO (Fig. 2H,L, Fig. 7S) yielded a strong effect on ISV sprouting and growth (P<0.0001; supplementary material Table S1), similar to that in dtty135 mutants (Fig. 7Q,S). Previous studies have reported a role for Notch signaling in angiogenesis and selection of endothelial tip cells (Hogan et al., 2009; Tammela et al., 2008). To examine whether Rspo1 signaling functions upstream of Notch, we injected rspo1 or control MO into a notch reporter transgenic line (Parsons et al., 2009). Vascular expression of the notch reporter was equivalent in rspo1 and control morphants (supplementary material Fig. S5). Furthermore, vascular expression of Notch pathway genes such as dll4, grl (supplementary material Fig. S1) and notch5 (data not shown) was unchanged in dtty135 mutants compared with their wild-type siblings. To determine whether Vegfc functions downstream from Notch, we examined vegfc expression following transgenic heat shock-inducible ubiquitous activation of Notch signaling, as we have reported previously (Lawson et al., 2001). The expression of vegfc was equivalent in Notch-activated and control embryos (supplementary material Fig. S5). These results suggest that Rspo-Wnt-Vegfc signaling functions independently from Notch signaling during embryonic angiogenesis.
DISCUSSION
Rspo1/Wnt signaling regulates angiogenesis
In this manuscript, we identify a novel signaling pathway essential for developmental angiogenesis. We provide multiple lines of evidence establishing the crucial role of Rspo1/Wnt signaling during developmental angiogenesis. Loss of Rspo1 function by means of genetic mutation or by morpholino mediated knockdown leads to suppression of angiogenic proliferation and to defects in both trunk and cranial vessel angiogenesis. Similarly, inhibiting functions of the presumptive receptor for R-spondin, kremen 1 (Binnerts et al., 2007) or the Wnt2bb ligand (both of which are expressed in the vasculature) also leads to defects in angiogenesis. Furthermore, inhibiting canonical Wnt/β-catenin pathway by transgenic overexpression of an Axin-GFP fusion protein under the control of the kdrl/flk1 promoter leads to sprouting defects similar to those resulting from loss of Rspo1 function, indicating that Wnt signaling is required for angiogenesis in an endothelial-autonomous fashion. We also demonstrate that genetic (via apcmcr mutation) or chemically induced (via BIO treatment) activation of Wnt signaling rescues Rspo1 angiogenesis defects and promotes increased vascular branching and sprouting. As noted above, multiple studies have reported roles for canonical Wnt signaling in endothelial cells during development and disease (Dejana, 2010; Franco et al., 2009; Zerlin et al., 2008). Canonical Wnt signaling appears to be active in the endothelium during development (Maretto et al., 2003), and a variety of studies have reported important roles for this signaling pathway during distinct steps of vascular development, including endothelial cell fate specification and endothelial cell proliferation, and vascular growth, integrity and regression (Goodwin et al., 2007; Liebner et al., 2008; Liu and Nathans, 2008; Stenman et al., 2008; Wang et al., 2007). In addition to canonical Wnt signaling, a recent study also reported an important role for non-canonical Wnt-Flt1 signaling in myeloid cells regulating retinal angiogenesis (Stefater et al., 2011). These studies underline the importance of this signaling pathway during vascular development. Our analysis of dtty135 mutants and downstream Wnt signaling shows that Rspo1/Wnt signaling is essential for all developmental angiogenesis, although it is not required for vasculogenesis.
Distinct roles of R-spondin family proteins during vascular development
R-spondin family proteins have been implicated in a diverse variety of developmental processes. Rspo2 regulates Xenopus muscle formation and tracheal and limb morphogenesis in mouse embryos (Bell et al., 2008; Kazanskaya et al., 2004). Rspo3 is required for mouse placental development and for endothelial specification and differentiation (Aoki et al., 2007; Kazanskaya et al., 2008). In this manuscript, we report that Rspo1 is dispensable for initial vasculogenesis and endothelial specification and differentiation, but is instead absolutely required for subsequent developmental angiogenesis. Different R-spondins also appear to act via different signaling pathways. We find that Rspo1 promotes angiogenesis during early embryogenesis through canonical Wnt signaling and Vegfc. By contrast, Rspo3 is reported to bind to syndecan 4 and initiate non-canonical Wnt/PCP signaling pathway during gastrulation and cartilage formation, influencing clathrin-mediated endocytosis (Ohkawara et al., 2011). Rspo3 also promotes endothelial specification and differentiation via Vegfa (Kazanskaya et al., 2008), whereas our results show that Rspo1 acts via Vegfc. Expression of vegfa1 and vegfa2 genes is not affected in rspo1 mutants, whereas expression of vegfc is strongly reduced, and this expression can be specifically restored by BIO-induced Wnt activation. The angiogenesis defects in rspo1 mutants can be rescued by forced expression of vegfc, but not by vegfa, and can be phoenocopied by knockdown of either vegfc or vegfr3. Thus, although it is likely that Rspo proteins use some common modes of action, in many cases these proteins may be using specialized mechanisms to regulate distinct developmental processes.
Vegfc/Vegfr3 signaling during angiogenesis
Vegfc is best-known as a pro-lymphangiogenic ligand for the Vegfr3 receptor, but recent evidence has shown that Vegfc-Vegfr3 signaling also has a role in angiogenesis during early development. Vegfr3 is highly expressed in angiogenic sprouts, and conditional genetic targeting of Vegfr3 or blocking of Vegfr3 signaling with monoclonal antibodies results in decreased sprouting, vascular density, vessel branching and endothelial cell proliferation in mice (Tammela et al., 2008). Complete embryonic inactivation of Vegfr3 causes defective early blood vessel development in mice (Dumont et al., 1998). In agreement with our findings, previous studies using morpholino knockdown of Vegfr3 or overexpression of soluble human Vegfr3 in the zebrafish also reported severe defects in blood vessel development (Covassin et al., 2006; Herbert et al., 2009; Ober et al., 2004). By contrast, a presumptive tyrosine phosphorylation-deficient zebrafish vegfr3 missense mutant shows primary defects in lymphatic development but no angiogenesis defects (Hogan et al., 2009), although the authors of this study suggest that this may reflect ‘very low-level, residual signaling in the presence of the I1042S mutation’. Overexpression of Vegfc during early murine development (prior to E16.5) is also highly angiogenic (Lohela et al., 2008). However, targeted inactivation of both known mouse Vegfr3 ligands (Vegfc and Vegfd) does not yield defects in blood vessel development analogous to those caused by loss of Vegfr3 (Haiko et al., 2008), suggesting that there are Vegfc/d-independent, or redundant, functions for Vegfr3 in murine blood vessel formation.
A permissive endothelial cue for developmental angiogenesis
Together, our results provide genetic evidence for a novel Rspo1-Wnt-Vegfc signaling pathway required for developmental angiogenesis (Fig. 8). The fact that expression of rspo1, krm1, wnt2bb, vegfc and vegfr3 is restricted to the vasculature during early development, and that endothelial-specific suppression of Wnt signaling phenocopies loss of Rspo1, suggests that this pathway serves as an endothelial-autonomous modulator of angiogenesis during embryogenesis. It remains to be determined whether the entire pathway can function in an autocrine fashion at the level of a single endothelial cell. Autocrine Vegfa signaling has been shown to promote endothelial maintenance and vascular homeostasis (Lee et al., 2007). Similarly, a Vegfc/Vegfr3 autocrine loop enhances tumor associated lymphangiogenesis and tumor progression in a murine tumor model (Matsuura et al., 2009). Vegfc is initially produced as a pro-Vegfc dimmer that is proteolytically cleaved at its N- and C-terminus to generate multiple processed secreted forms. Indeed, reduced activity is associated with cleavage mutants that fail to properly process the pro-Vegfc dimmer (Joukov et al., 1997). However, cleavage of pro-Vegfc does not appear to be essential for its secretion, as unprocessed pro-Vegfc and cleavage mutant forms of Vegfc are both secreted efficiently, although they have different abilities to bind and/or activate the Vegfr3 receptor (Joukov et al., 1997; Siegfried et al., 2003). Therefore, it is not possible using these data to distinguish between possible paracrine and autocrine modes of signaling.
Fig. 8.
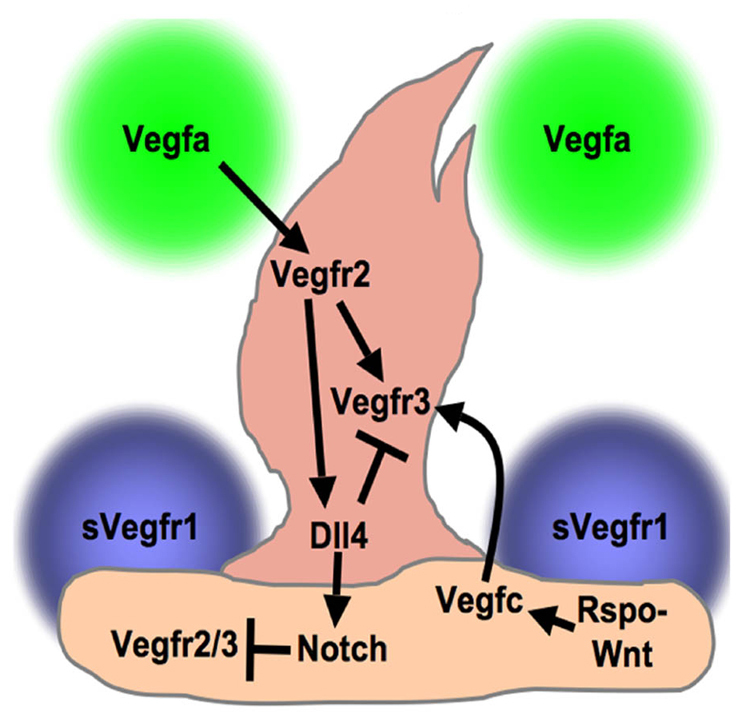
Schematic model for sprouting angiogenesis. A proposed model for regulation of angiogenic sprouting based on this and previous studies. Our data showing endothelial-specific expression strongly suggest that the Rspo1-Wnt-Vegfc-Vegfr3 pathway functions in an endothelial-specific fashion. The diagram as drawn shows autocrine Rspo1 and paracrine Vegfc endothelial signaling, but we are not able to determine from the data presented in this manuscript whether signaling is autocrine, juxtacrine or both. However, the pan-endothelial (not tip- or stalk-restricted) expression of the components of this pathway suggests that it provides a permissive cue for angiogenesis, rather than a selective cue for tip cells. Other published studies have shown that Vegfa produced by surrounding tissue signals through Vegfr2 to induce Vegfr3 and Dll4 in the tip cell (Lobov et al., 2007; Tammela et al., 2008). Supporting stalk cells express soluble Flt1 (sVegfr1) and Notch. sVegfr1 helps sharpen the concentration gradient of available Vegfa to direct sprouting (Chappell et al., 2009), whereas Dll4/Notch signaling limits the number of tip cells specified (Hellstrom et al., 2007; Lobov et al., 2007; Siekmann and Lawson, 2007). Notch limits Vegf receptor expression in stalk cells and Dll4 suppresses Vegfr3 in the tip cell to inhibit hyperbranching (Hogan et al., 2009; Roca and Adams, 2007; Tammela et al., 2008).
Using our in vivo model, we have shown a complete requirement of Vegfc signaling for developmental angiogenesis downstream of Rspo1/Wnt pathway. As abnormal Wnt and Vegfc signaling is frequently seen in different tumors and tumor cell lines (Laakkonen et al., 2007; Polakis, 2007; Stacker et al., 2002), the novel Rspo-Wnt-VegfC-Vegfr3 pathway we have identified may play an important role during tumor angiogenesis and, potentially, other vascular pathologies.
Supplementary Material
Acknowledgments
We thank Drs Igor Dawid, Karuna Sampath, Sudipto Roy and Ajay Chitnis, and the zebrafish community for sharing fish strains, reagents, protocols and discussions. We thank members of the Weinstein lab for technical help and critical suggestions. We also thank Drs Elisabetta Dejana and Monica Corada for sharing their unpublished results.
Footnotes
Funding
This research was supported by the intramural program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development [HD001011 to B.W.] and by the Leducq Foundation [to B.W.]. Deposited in PMC for release after 12 months.
Competing interests statement
The authors declare no competing financial interests.
Supplementary material
Supplementary material available online at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.068460/-/DC1
References
- Angers S., Moon R. T. (2009). Proximal events in Wnt signal transduction. Nat. Rev. Mol. Cell. Biol. 10, 468–477 [DOI] [PubMed] [Google Scholar]
- Aoki M., Mieda M., Ikeda T., Hamada Y., Nakamura H., Okamoto H. (2007). R-spondin3 is required for mouse placental development. Dev. Biol. 301, 218–226 [DOI] [PubMed] [Google Scholar]
- Bell S. M., Schreiner C. M., Wert S. E., Mucenski M. L., Scott W. J., Whitsett J. A. (2008). R-spondin 2 is required for normal laryngeal-tracheal, lung and limb morphogenesis. Development 135, 1049–1058 [DOI] [PubMed] [Google Scholar]
- Binnerts M. E., Kim K. A., Bright J. M., Patel S. M., Tran K., Zhou M., Leung J. M., Liu Y., Lomas W. E., 3rd, Dixon M., et al. (2007). R-Spondin1 regulates Wnt signaling by inhibiting internalization of LRP6. Proc. Natl. Acad. Sci. USA 104, 14700–14705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell J. C., Taylor S. M., Ferrara N., Bautch V. L. (2009). Local guidance of emerging vessel sprouts requires soluble Flt-1. Dev. Cell 17, 377–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Hu Y., Lu K., Flannery J. G., Ma J. X. (2007). Very low density lipoprotein receptor, a negative regulator of the wnt signaling pathway and choroidal neovascularization. J. Biol. Chem. 282, 34420–34428 [DOI] [PubMed] [Google Scholar]
- Cohen E. D., Tian Y., Morrisey E. E. (2008). Wnt signaling: an essential regulator of cardiovascular differentiation, morphogenesis and progenitor self-renewal. Development 135, 789–798 [DOI] [PubMed] [Google Scholar]
- Corada M., Nyqvist D., Orsenigo F., Caprini A., Giampietro C., Taketo M. M., Iruela-Arispe M. L., Adams R. H., Dejana E. (2010). The Wnt/beta-catenin pathway modulates vascular remodeling and specification by upregulating Dll4/Notch signaling. Dev. Cell 18, 938–949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covassin L. D., Villefranc J. A., Kacergis M. C., Weinstein B. M., Lawson N. D. (2006). Distinct genetic interactions between multiple Vegf receptors are required for development of different blood vessel types in zebrafish. Proc. Natl. Acad. Sci. USA 103, 6554–6559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejana E. (2010). The role of wnt signaling in physiological and pathological angiogenesis. Circ. Res. 107, 943–952 [DOI] [PubMed] [Google Scholar]
- Dumont D. J., Jussila L., Taipale J., Lymboussaki A., Mustonen T., Pajusola K., Breitman M., Alitalo K. (1998). Cardiovascular failure in mouse embryos deficient in VEGF receptor-3. Science 282, 946–949 [DOI] [PubMed] [Google Scholar]
- Franco C. A., Liebner S., Gerhardt H. (2009). Vascular morphogenesis: a Wnt for every vessel? Curr. Opin. Genet. Dev. 19, 476–483 [DOI] [PubMed] [Google Scholar]
- Goodwin A. M., Kitajewski J., D’Amore P. A. (2007). Wnt1 and Wnt5a affect endothelial proliferation and capillary length; Wnt2 does not. Growth Factors 25, 25–32 [DOI] [PubMed] [Google Scholar]
- Gore A. V., Lampugnani M. G., Dye L., Dejana E., Weinstein B. M. (2008). Combinatorial interaction between CCM pathway genes precipitates hemorrhagic stroke. Dis. Model. Mech. 1, 275–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haiko P., Makinen T., Keskitalo S., Taipale J., Karkkainen M. J., Baldwin M. E., Stacker S. A., Achen M. G., Alitalo K. (2008). Deletion of vascular endothelial growth factor C (VEGF-C) and VEGF-D is not equivalent to VEGF receptor 3 deletion in mouse embryos. Mol. Cell. Biol. 28, 4843–4850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haramis A. P., Hurlstone A., van der Velden Y., Begthel H., van den Born M., Offerhaus G. J., Clevers H. C. (2006). Adenomatous polyposis coli-deficient zebrafish are susceptible to digestive tract neoplasia. EMBO Rep. 7, 444–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellstrom M., Phng L. K., Hofmann J. J., Wallgard E., Coultas L., Lindblom P., Alva J., Nilsson A. K., Karlsson L., Gaiano N., et al. (2007). Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature 445, 776–780 [DOI] [PubMed] [Google Scholar]
- Hendrickx M., Leyns L. (2008). Non-conventional Frizzled ligands and Wnt receptors. Dev. Growth Differ. 50, 229–243 [DOI] [PubMed] [Google Scholar]
- Herbert S. P., Huisken J., Kim T. N., Feldman M. E., Houseman B. T., Wang R. A., Shokat K. M., Stainier D. Y. (2009). Arterial-venous segregation by selective cell sprouting: an alternative mode of blood vessel formation. Science 326, 294–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan B. M., Herpers R., Witte M., Helotera H., Alitalo K., Duckers H. J., Schulte-Merker S. (2009). Vegfc/Flt4 signalling is suppressed by Dll4 in developing zebrafish intersegmental arteries. Development 136, 4001–4009 [DOI] [PubMed] [Google Scholar]
- Hurlstone A. F., Haramis A. P., Wienholds E., Begthel H., Korving J., Van Eeden F., Cuppen E., Zivkovic D., Plasterk R. H., Clevers H. (2003). The Wnt/beta-catenin pathway regulates cardiac valve formation. Nature 425, 633–637 [DOI] [PubMed] [Google Scholar]
- Ishikawa T., Tamai Y., Zorn A. M., Yoshida H., Seldin M. F., Nishikawa S., Taketo M. M. (2001). Mouse Wnt receptor gene Fzd5 is essential for yolk sac and placental angiogenesis. Development 128, 25–33 [DOI] [PubMed] [Google Scholar]
- Isogai S., Horiguchi M., Weinstein B. M. (2001). The vascular anatomy of the developing zebrafish: an atlas of embryonic and early larval development. Dev. Biol. 230, 278–301 [DOI] [PubMed] [Google Scholar]
- Joukov V., Sorsa T., Kumar V., Jeltsch M., Claesson-Welsh L., Cao Y., Saksela O., Kalkkinen N., Alitalo K. (1997). Proteolytic processing regulates receptor specificity and activity of VEGF-C. EMBO J. 16, 3898–3911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junge H. J., Yang S., Burton J. B., Paes K., Shu X., French D. M., Costa M., Rice D. S., Ye W. (2009). TSPAN12 regulates retinal vascular development by promoting Norrin- but not Wnt-induced FZD4/beta-catenin signaling. Cell 139, 299–311 [DOI] [PubMed] [Google Scholar]
- Kamei M., Saunders W. B., Bayless K. J., Dye L., Davis G. E., Weinstein B. M. (2006). Endothelial tubes assemble from intracellular vacuoles in vivo. Nature 442, 453–456 [DOI] [PubMed] [Google Scholar]
- Kazanskaya O., Glinka A., del Barco Barrantes I., Stannek P., Niehrs C., Wu W. (2004). R-Spondin2 is a secreted activator of Wnt/beta-catenin signaling and is required for Xenopus myogenesis. Dev. Cell 7, 525–534 [DOI] [PubMed] [Google Scholar]
- Kazanskaya O., Ohkawara B., Heroult M., Wu W., Maltry N., Augustin H. G., Niehrs C. (2008). The Wnt signaling regulator R-spondin 3 promotes angioblast and vascular development. Development 135, 3655–3664 [DOI] [PubMed] [Google Scholar]
- Kim K. A., Zhao J., Andarmani S., Kakitani M., Oshima T., Binnerts M. E., Abo A., Tomizuka K., Funk W. D. (2006). R-Spondin proteins: a novel link to beta-catenin activation. Cell Cycle 5, 23–26 [DOI] [PubMed] [Google Scholar]
- Kim K. A., Wagle M., Tran K., Zhan X., Dixon M. A., Liu S., Gros D., Korver W., Yonkovich S., Tomasevic N., et al. (2008). R-Spondin family members regulate the Wnt pathway by a common mechanism. Mol. Biol. Cell 19, 2588–2596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel C. B., Ballard W. W., Kimmel S. R., Ullmann B., Schilling T. F. (1995). Stages of embryonic development of the zebrafish. Dev. Dyn. 203, 253–310 [DOI] [PubMed] [Google Scholar]
- Kinzler K. W., Nilbert M. C., Su L. K., Vogelstein B., Bryan T. M., Levy D. B., Smith K. J., Preisinger A. C., Hedge P., McKechnie D., et al. (1991). Identification of FAP locus genes from chromosome 5q21. Science 253, 661–665 [DOI] [PubMed] [Google Scholar]
- Kwan K. M., Fujimoto E., Grabher C., Mangum B. D., Hardy M. E., Campbell D. S., Parant J. M., Yost H. J., Kanki J. P., Chien C. B. (2007). The Tol2kit: a multisite gateway-based construction kit for Tol2 transposon transgenesis constructs. Dev. Dyn. 236, 3088–3099 [DOI] [PubMed] [Google Scholar]
- Laakkonen P., Waltari M., Holopainen T., Takahashi T., Pytowski B., Steiner P., Hicklin D., Persaud K., Tonra J. R., Witte L., et al. (2007). Vascular endothelial growth factor receptor 3 is involved in tumor angiogenesis and growth. Cancer Res. 67, 593–599 [DOI] [PubMed] [Google Scholar]
- Lawson N. D., Scheer N., Pham V. N., Kim C. H., Chitnis A. B., Campos-Ortega J. A., Weinstein B. M. (2001). Notch signaling is required for arterial-venous differentiation during embryonic vascular development. Development 128, 3675–3683 [DOI] [PubMed] [Google Scholar]
- Lawson N. D., Mugford J. W., Diamond B. A., Weinstein B. M. (2003). phospholipase C gamma-1 is required downstream of vascular endothelial growth factor during arterial development. Genes Dev. 17, 1346–1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., Chen T. T., Barber C. L., Jordan M. C., Murdock J., Desai S., Ferrara N., Nagy A., Roos K. P., Iruela-Arispe M. L. (2007). Autocrine VEGF signaling is required for vascular homeostasis. Cell 130, 691–703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebner S., Corada M., Bangsow T., Babbage J., Taddei A., Czupalla C. J., Reis M., Felici A., Wolburg H., Fruttiger M., et al. (2008). Wnt/beta-catenin signaling controls development of the blood-brain barrier. J. Cell Biol. 183, 409–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C., Nathans J. (2008). An essential role for frizzled 5 in mammalian ocular development. Development 135, 3567–3576 [DOI] [PubMed] [Google Scholar]
- Lobov I. B., Renard R. A., Papadopoulos N., Gale N. W., Thurston G., Yancopoulos G. D., Wiegand S. J. (2007). Delta-like ligand 4 (Dll4) is induced by VEGF as a negative regulator of angiogenic sprouting. Proc. Natl. Acad. Sci. USA 104, 3219–3224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohela M., Helotera H., Haiko P., Dumont D. J., Alitalo K. (2008). Transgenic induction of vascular endothelial growth factor-C is strongly angiogenic in mouse embryos but leads to persistent lymphatic hyperplasia in adult tissues. Am. J. Pathol. 173, 1891–1901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maretto S., Cordenonsi M., Dupont S., Braghetta P., Broccoli V., Hassan A. B., Volpin D., Bressan G. M., Piccolo S. (2003). Mapping Wnt/beta-catenin signaling during mouse development and in colorectal tumors. Proc. Natl. Acad. Sci. USA 100, 3299–3304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuura M., Onimaru M., Yonemitsu Y., Suzuki H., Nakano T., Ishibashi H., Shirasuna K., Sueishi K. (2009). Autocrine loop between vascular endothelial growth factor (VEGF)-C and VEGF receptor-3 positively regulates tumor-associated lymphangiogenesis in oral squamoid cancer cells. Am. J. Pathol. 175, 1709–1721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer L., Skaltsounis A. L., Magiatis P., Polychronopoulos P., Knockaert M., Leost M., Ryan X. P., Vonica C. A., Brivanlou A., Dajani R., et al. (2003). GSK-3-selective inhibitors derived from Tyrian purple indirubins. Chem. Biol. 10, 1255–1266 [DOI] [PubMed] [Google Scholar]
- Monkley S. J., Delaney S. J., Pennisi D. J., Christiansen J. H., Wainwright B. J. (1996). Targeted disruption of the Wnt2 gene results in placentation defects. Development 122, 3343–3353 [DOI] [PubMed] [Google Scholar]
- Nam J. S., Turcotte T. J., Smith P. F., Choi S., Yoon J. K. (2006). Mouse cristin/R-spondin family proteins are novel ligands for the Frizzled 8 and LRP6 receptors and activate beta-catenin-dependent gene expression. J. Biol. Chem. 281, 13247–13257 [DOI] [PubMed] [Google Scholar]
- Nam J. S., Turcotte T. J., Yoon J. K. (2007). Dynamic expression of R-spondin family genes in mouse development. Gene Expr. Patterns 7, 306–312 [DOI] [PubMed] [Google Scholar]
- Ober E. A., Olofsson B., Makinen T., Jin S. W., Shoji W., Koh G. Y., Alitalo K., Stainier D. Y. (2004). Vegfc is required for vascular development and endoderm morphogenesis in zebrafish. EMBO Rep. 5, 78–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ober E. A., Verkade H., Field H. A., Stainier D. Y. (2006). Mesodermal Wnt2b signalling positively regulates liver specification. Nature 442, 688–691 [DOI] [PubMed] [Google Scholar]
- Ohkawara B., Glinka A., Niehrs C. (2011). Rspo3 binds Syndecan 4 and induces Wnt/PCP signaling via clathrin-mediated endocytosis to promote morphogenesis. Dev. Cell 20, 303–314 [DOI] [PubMed] [Google Scholar]
- Olsson A. K., Dimberg A., Kreuger J., Claesson-Welsh L. (2006). VEGF receptor signalling-in control of vascular function. Nat. Rev. Mol. Cell. Biol. 7, 359–371 [DOI] [PubMed] [Google Scholar]
- Parsons M. J., Pisharath H., Yusuff S., Moore J. C., Siekmann A. F., Lawson N., Leach S. D. (2009). Notch-responsive cells initiate the secondary transition in larval zebrafish pancreas. Mech. Dev. 126, 898–912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polakis P. (2007). The many ways of Wnt in cancer. Curr. Opin. Genet. Dev. 17, 45–51 [DOI] [PubMed] [Google Scholar]
- Provost E., Rhee J., Leach S. D. (2007). Viral 2A peptides allow expression of multiple proteins from a single ORF in transgenic zebrafish embryos. Genesis 45, 625–629 [DOI] [PubMed] [Google Scholar]
- Roca C., Adams R. H. (2007). Regulation of vascular morphogenesis by Notch signaling. Genes Dev. 21, 2511–2524 [DOI] [PubMed] [Google Scholar]
- Roman B. L., Pham V. N., Lawson N. D., Kulik M., Childs S., Lekven A. C., Garrity D. M., Moon R. T., Fishman M. C., Lechleider R. J., et al. (2002). Disruption of acvrl1 increases endothelial cell number in zebrafish cranial vessels. Development 129, 3009–3019 [DOI] [PubMed] [Google Scholar]
- Rossant J., Howard L. (2002). Signaling pathways in vascular development. Annu. Rev. Cell Dev. Biol. 18, 541–573 [DOI] [PubMed] [Google Scholar]
- Shtutman M., Zhurinsky J., Simcha I., Albanese C., D’Amico M., Pestell R., Ben-Ze’ev A. (1999). The cyclin D1 gene is a target of the beta-catenin/LEF-1 pathway. Proc. Natl. Acad. Sci. USA 96, 5522–5527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu W., Jiang Y. Q., Lu M. M., Morrisey E. E. (2002). Wnt7b regulates mesenchymal proliferation and vascular development in the lung. Development 129, 4831–4842 [DOI] [PubMed] [Google Scholar]
- Siegfried G., Basak A., Cromlish J. A., Benjannet S., Marcinkiewicz J., Chretien M., Seidah N. G., Khatib A. M. (2003). The secretory proprotein convertases furin, PC5, and PC7 activate VEGF-C to induce tumorigenesis. J. Clin. Invest. 111, 1723–1732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siekmann A. F., Lawson N. D. (2007). Notch signalling limits angiogenic cell behaviour in developing zebrafish arteries. Nature 445, 781–784 [DOI] [PubMed] [Google Scholar]
- Skurk C., Maatz H., Rocnik E., Bialik A., Force T., Walsh K. (2005). Glycogen-Synthase Kinase3beta/beta-catenin axis promotes angiogenesis through activation of vascular endothelial growth factor signaling in endothelial cells. Circ. Res. 96, 308–318 [DOI] [PubMed] [Google Scholar]
- Srinivas B. P., Woo J., Leong W. Y., Roy S. (2007). A conserved molecular pathway mediates myoblast fusion in insects and vertebrates. Nat. Genet. 39, 781–786 [DOI] [PubMed] [Google Scholar]
- Stacker S. A., Achen M. G., Jussila L., Baldwin M. E., Alitalo K. (2002). Lymphangiogenesis and cancer metastasis. Nat. Rev. Cancer 2, 573–583 [DOI] [PubMed] [Google Scholar]
- Stefater J. A., 3rd, Lewkowich I., Rao S., Mariggi G., Carpenter A. C., Burr A. R., Fan J., Ajima R., Molkentin J. D., Williams B. O., et al. (2011). Regulation of angiogenesis by a non-canonical Wnt-Flt1 pathway in myeloid cells. Nature 474, 511–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenman J. M., Rajagopal J., Carroll T. J., Ishibashi M., McMahon J., McMahon A. P. (2008). Canonical Wnt signaling regulates organ-specific assembly and differentiation of CNS vasculature. Science 322, 1247–1250 [DOI] [PubMed] [Google Scholar]
- Tammela T., Zarkada G., Wallgard E., Murtomaki A., Suchting S., Wirzenius M., Waltari M., Hellstrom M., Schomber T., Peltonen R., et al. (2008). Blocking VEGFR-3 suppresses angiogenic sprouting and vascular network formation. Nature 454, 656–660 [DOI] [PubMed] [Google Scholar]
- Villefranc J. A., Amigo J., Lawson N. D. (2007). Gateway compatible vectors for analysis of gene function in the zebrafish. Dev. Dyn. 236, 3077–3087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Gilner J. B., Bautch V. L., Wang D. Z., Wainwright B. J., Kirby S. L., Patterson C. (2007). Wnt2 coordinates the commitment of mesoderm to hematopoietic, endothelial, and cardiac lineages in embryoid bodies. J. Biol. Chem. 282, 782–791 [DOI] [PubMed] [Google Scholar]
- Wei Q., Yokota C., Semenov M. V., Doble B., Woodgett J., He X. (2007). R-spondin1 is a high affinity ligand for LRP6 and induces LRP6 phosphorylation and beta-catenin signaling. J. Biol. Chem. 282, 15903–15911 [DOI] [PubMed] [Google Scholar]
- Weinstein B. M., Stemple D. L., Driever W., Fishman M. C. (1995). Gridlock, a localized heritable vascular patterning defect in the zebrafish. Nat. Med. 1, 1143–1147 [DOI] [PubMed] [Google Scholar]
- Westerfield M. (2000). The Zebrafish Book: A Guide for Laboratory Use of Zebrafish (Danio rerio). Eugene, OR: University of Oregon Press; [Google Scholar]
- Xu Q., Wang Y., Dabdoub A., Smallwood P. M., Williams J., Woods C., Kelley M. W., Jiang L., Tasman W., Zhang K., et al. (2004). Vascular development in the retina and inner ear: control by Norrin and Frizzled-4, a high-affinity ligand-receptor pair. Cell 116, 883–895 [DOI] [PubMed] [Google Scholar]
- Yanai N., Satoh T., Obinata M. (1991). Endothelial cells create a hematopoietic inductive microenvironment preferential to erythropoiesis in the mouse spleen. Cell Struct. Funct. 16, 87–93 [DOI] [PubMed] [Google Scholar]
- Ye X., Wang Y., Cahill H., Yu M., Badea T. C., Smallwood P. M., Peachey N. S., Nathans J. (2009). Norrin, frizzled-4, and Lrp5 signaling in endothelial cells controls a genetic program for retinal vascularization. Cell 139, 285–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerlin M., Julius M. A., Kitajewski J. (2008). Wnt/Frizzled signaling in angiogenesis. Angiogenesis 11, 63–69 [DOI] [PubMed] [Google Scholar]
- Zhang X., Gaspard J. P., Chung D. C. (2001). Regulation of vascular endothelial growth factor by the Wnt and K-ras pathways in colonic neoplasia. Cancer Res. 61, 6050–6054 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.