Abstract
Sensory axons must develop appropriate connections with both central and peripheral targets. Whereas the peripheral cues have provided a classic model for neuron survival and guidance, less is known about the central cues or the coordination of central and peripheral connectivity. Here we find that type III Nrg1, in addition to its known effect on neuron survival, regulates axon pathfinding. In type III Nrg1–/– mice, death of TrkA+ nociceptive/thermoreceptive neurons was increased, and could be rescued by Bax elimination. In the Bax and type III Nrg1 double mutants, axon pathfinding abnormalities were seen for TrkA+ neurons both in cutaneous peripheral targets and in spinal cord central targets. Axon guidance phenotypes in the spinal cord included penetration of axons into ventral regions from which they would normally be repelled by Sema3A. Accordingly, sensory neurons from type III Nrg1–/– mice were unresponsive to the repellent effects of Sema3A in vitro, which might account, at least in part, for the central projection phenotype, and demonstrates an effect of type III Nrg1 on guidance cue responsiveness in neurons. Moreover, stimulation of type III Nrg1 back-signaling in cultured sensory neurons was found to regulate axonal levels of the Sema3A receptor neuropilin 1. These results reveal a molecular mechanism whereby type III Nrg1 signaling can regulate the responsiveness of neurons to a guidance cue, and show that type III Nrg1 is required for normal sensory neuron survival and axon pathfinding in both central and peripheral targets.
Keywords: Sensory neuron, Neuregulin 1, Axon guidance, Mouse
INTRODUCTION
Sensory neurons in the dorsal root ganglion (DRG) are a heterogeneous population of cells that convey information related to pain, temperature, pressure and position to the central nervous system. The correct perception of external stimuli by the central nervous system requires that, during development, each class of DRG sensory neurons must project axons to establish appropriate connectivity with peripheral targets, such as muscle or skin, and also with central targets in the spinal cord. In the periphery, extensive work has identified attractant and repellent cues that guide sensory axons along specific routes to their targets and has also shown that after reaching their target fields, sensory neurons depend for their survival on limiting amounts of target-derived neurotrophins (Davies and Lumsden, 1990; Tessier-Lavigne and Goodman, 1996; White et al., 1996). For the central projections, less is known about the mechanisms and molecular identities of the complement of factors that may coordinate axon guidance and neuron survival.
The functionally distinct classes of sensory neurons depend for their survival on different peripheral target-derived neurotrophins. The majority of DRG neurons are nociceptive or thermoreceptive, innervate the epidermis, express TrkA (Ntrk1) and require nerve growth factor (Ngf) for survival (Snider and Silos-Santiago, 1996; White et al., 1996). By contrast, proprioceptive neurons innervate muscle spindles or tendons, express TrkC (Ntrk3) and are dependent on the neurotrophin NT3 (Ntf3) (Snider and Silos-Santiago, 1996). These distinct classes of sensory neurons also project to different locations within the spinal cord. Proprioceptive axons penetrate the dorsal horn medially and project to deeper areas of the spinal cord where they can connect with interneurons in the intermediate zone or directly with motoneurons in the ventral horn to form the reflex arc (Ozaki and Snider, 1997). Unlike proprioceptive axons, sensory axons involved in nociception and thermoreception enter the dorsal horn more laterally and terminate in superficial laminae I and II, where they connect to interneurons (Ozaki and Snider, 1997). In contrast to the periphery, central target-derived neurotrophins do not seem essential for correct innervation of the spinal cord (Oakley et al., 1995; Patel et al., 2000; Patel et al., 2003).
Although guidance cues for sensory axon navigation within the spinal cord are not thoroughly understood, one molecule known to have a role is semaphorin 3A (Sema3A). Sema3A is expressed in ventral spinal cord and acts in vitro as a chemorepellent that is selective for TrkA+ axons (Behar et al., 1996; Messersmith et al., 1995; Wright et al., 1995). Correspondingly, studies in mice show that loss-of-function of Sema3A or of its co-receptor neuropilin 1 (Nrp1) results in abnormal penetration of TrkA+ sensory axons into the ventral spinal cord (Behar et al., 1996; Gu et al., 2003). The selective Sema3A responsiveness of different classes of sensory axons correlates with their expression of Nrp1: TrkA+ axons have high Nrp1 expression and do not penetrate the ventral horn where Sema3A is expressed, whereas proprioceptive axons have lower Nrp1 expression and are able to enter the ventral horn (Pond et al., 2002; Puschel et al., 1996).
Neuregulin 1 (Nrg1) is a key regulator of the development of the peripheral nervous system (Gassmann et al., 1995; Kramer et al., 1996; Meyer and Birchmeier, 1995; Meyer et al., 1997; Morris et al., 1999; Riethmacher et al., 1997; Woldeyesus et al., 1999; Wolpowitz et al., 2000). Type III Nrg 1 is the predominant isoform expressed by sensory neurons within the DRG and is known to be essential for the survival of sensory neurons and for maintaining peripheral target innervation (Bermingham-McDonogh et al., 1997; Hippenmeyer et al., 2002; Ho et al., 1995; Meyer et al., 1997; Wolpowitz et al., 2000). Expressed along sensory axons, type III Nrg1 is essential for the proper development and migration of Schwann cells, as well as for the initiation and extent of axonal myelination (Birchmeier and Nave, 2008; Chen et al., 2006; Lemke, 2006; Michailov et al., 2004; Taveggia et al., 2005). Among the family of Nrg1 isoforms, type III Nrg1 uniquely signals in a juxtacrine, bi-directional manner, acting as a receptor for ErbB proteins and sending a back-signal into the cell carrying type III Nrg1 (Bao et al., 2004; Bao et al., 2003; Falls, 2003; Hancock et al., 2008; Wang et al., 2001; Zhong et al., 2008). Prior studies by us and others have shown that back-signaling by ErbB-type III Nrg1 interactions can activate at least two intracellular signaling pathways, one resulting in gamma secretase-dependent proteolytic release and nuclear translocation of the intracellular domain of type III Nrg1 (Bao et al., 2004; Bao et al., 2003; Chen et al., 2010), and the other involving activation of phosphoinositide 3-kinase signaling (Hancock et al., 2008). Activation of these pathways contributes to hippocampal neuronal survival (Bao et al., 2003), the elaboration of dendrites by cortical pyramidal neurons (Chen et al., 2010), and regulates the levels of neurotransmitter receptors at the synapse (Hancock et al., 2008; Zhong et al., 2008).
Here we find that in type III Nrg1–/– mice, TrkA+ sensory neurons showed reduced survival, as well as abnormal pathfinding in the spinal cord. When the survival phenotype was rescued by elimination of Bax, a strategy used previously to distinguish survival and pathfinding phenotypes (Patel et al., 2000; Patel et al., 2003), central axon defects were still present, supporting an independent effect on axonal pathfinding. This axonal pathfinding phenotype included abnormal penetration into ventral regions of the spinal cord, comparable to the abnormalities previously seen after gene knockout of Sema3a or its receptor Nrp1 (Behar et al., 1996; Gu et al., 2003). Furthermore, in vitro assays revealed that sensory neurons from type III Nrg1–/– mice showed no detectable response to Sema3A. Correspondingly, loss of type III Nrg1 resulted in reduced levels of the Sema3A receptor Nrp1, whereas activation of type III Nrg1 signaling by soluble ErbB4 ectodomain increased cell surface levels of Nrp1. Together, our findings identify a novel role for type III Nrg1 as a factor intrinsic to sensory neurons that regulates axon pathfinding.
MATERIALS AND METHODS
Animals
Type III Nrg1 (Nrg1tm1/Lwr) heterozygous mice were bred as described previously (Wolpowitz et al., 2000). Baxtm1Sjk (Bax+/–) heterozygous mice were obtained from Jackson Laboratory (Deckwerth et al., 1996). Bax+/– mice were crossed with type III Nrg1+/– mice to generate the Bax–/–; type III Nrg1+/+ and Bax–/–; type III Nrg1–/– embryos. The age of the embryos was determined by the plug date, which was considered to be E0.5.
Immunolabeling
Embryos were fixed in 4% paraformaldehyde (PFA) in PBS for 4 hours at 4°C, incubated in 15% then 30% sucrose in PBS at 4°C, OCT (Tissue-Tek) embedded and transverse sectioned by cryostat every 20 μm. For type III Nrg1 immunolabeling, fixed tissue was paraffin embedded and sectioned every 10 μm. Sections were permeabilized, blocked and incubated overnight at 4°C with the following primary antibodies: anti-cleaved caspase 3 (rabbit, 1:200, Cell Signaling Technology); anti-CGRP (rabbit, 1:200, Peninsula); anti-Islet1/2 (rabbit, 1:5000, T. M. Jessell, Columbia University, USA); anti-MAP2 (mouse, 1:500, Chemicon); anti-NF200 (mouse, 1:500, Chemicon); anti-parvalbumin (rabbit, 1:1000, Swant); anti-peripherin (rabbit, 1:500, Chemicon); anti-PGP9.5 (guinea pig, 1:200, Abcam); anti-Sema3A (rabbit, 1:1000, Abcam); anti-substance P (rabbit, 1:1000, Swant); anti-TrkA (rabbit, 1:5000, L. Reichardt, UCSF, USA); anti-TrkB (rabbit, 1:1000, Chemicon); or anti-type III Nrg1 (sheep, crude serum, 1:1000) (Yang et al., 1998). Sections were incubated in Alexa 488- or 568-conjugated secondary antibodies (Molecular Probes) for 45 minutes at room temperature. Confocal images were captured with an LSM510 confocal microscope (Zeiss). To generate summary plots of axon collaterals in the spinal cord, confocal z-stack images of TrkA labeling were selected for each genotype. A grid with eight regions was superimposed on representative spinal cord images, and individual TrkA+ fiber endings along the dorsoventral axis of the spinal cord were marked on the grid.
Cell counts
To count DRG neurons, thoracic-lumbar spinal cords matched for position on the rostral-caudal axis from each genotype were processed as described above. Neurons that contained a clear nucleus were counted and summed as described (Wolpowitz et al., 2000). Statistical significance was determined by ANOVA or Student’s t-test (when only two groups were analyzed).
Cell culture and immunofluorescence
E14.5 DRG were dissected and cultured as described (Hancock et al., 2008). Where indicated, neurons were treated with 5 μg/ml recombinant mouse ErbB4-Fc (B4-ECD; R&D Systems) for 2 hours. For surface labeling of receptors, cultures were fixed with 4% PFA and blocked with 10% normal donkey serum for 1 hour. Cells were incubated overnight at 4°C in anti-Nrp1 (rabbit, 1:1000, A. Kolodkin, Johns Hopkins University, USA), and incubated in Alexa 488-conjugated secondary antibody (Molecular Probes) for 45 minutes at room temperature. Images were captured with an Eclipse 80i microscope (Nikon). Outlines of extending growth cones were traced digitally and the average fluorescence intensity of Nrp1 was calculated using MetaMorph software (Version 7.6; Molecular Devices). Statistical significance was determined by Student’s t-test.
Live imaging
DRG explants were cultured overnight, then placed on a motorized stage enclosed by a CO2 incubation chamber at 37°C. Growth cones were randomly selected, and time-lapse images were acquired using differential interference contrast optics on a TE2000 microscope (Nikon). Sema3A-Fc (600 ng/ml, R&D Systems) was bath-applied to cultures during time-lapse imaging. Images were captured every 30 seconds for 50 minutes using MetaMorph software. Axon extension or retraction lengths were measured from images captured before and after addition of Sema3A-Fc using MetaMorph software. Statistical significance was determined by Student’s t-test.
Growth cone collapse
DRG explants were cultured overnight in medium containing serum and Ngf (50 ng/ml). Medium supplemented with vehicle alone (control) or Sema3A-Fc was bath-applied to cultures for 1 hour. Explants were fixed with 4% PFA, permeabilized, blocked, and labeled with Alexa 647-conjugated phalloidin (Molecular Probes). For quantification of growth cone collapse, the area of each growth cone was determined by digital tracing and the number of filopodia per growth cone was counted using MetaMorph software. A growth cone was considered collapsed if it had an area that was less than 50% of the mean for control-treated growth cones (of the same genotype) and had between zero and two filopodia. Statistical significance was determined by Student’s t-test.
Isolation of axons, biotinylation of surface receptors and immunoblotting
Tissues or cells were homogenized in lysis buffer [150 mM NaCl, 20 mM Tris-HCl pH 7.5, 10 mM dithiothreitol, 1 mM EDTA, 1% NP40, protease and phosphate inhibitors (Roche)] on ice, centrifuged at 10,000 g for 20 minutes at 4°C, and protein extracts (15-25 μg) were resolved by electrophoresis on 10% NuPAGE gels (Invitrogen) and transferred to nitrocellulose filters. The following primary antibodies were used: anti-Erk1/2 (rabbit, 1:1000, Santa Cruz); anti-Nrp1 (rabbit, 1:200, Calbiochem); anti-peripherin (rabbit, 1:2000, Chemicon); anti-plexin A4 (rabbit, 1:250, Abcam); anti-TrkA (rabbit, 1:5000, L. Reichardt, UCSF, USA); or anti-β-tubulin (rabbit, 1:10,000, Chemicon).
For isolation of axons, DRG explants were cultured on a Transwell tissue culture insert containing a polyethylene terephthalate (PET) membrane with 3 μm pores (BD Biosciences) (Wu et al., 2005). After 7 days, the membrane was washed with PBS, and cell bodies were isolated from the upper membrane by gentle scraping with a rubber cell scraper. The lower membrane surface was scraped in a similar manner to isolate axons.
For biotinylation of cell surface proteins, E14.5 sensory neurons were cultured in 6-well plates for 2 days. Neurons were serum starved for 16 hours prior to the addition of vehicle alone (control) or 5 μg/ml recombinant mouse ErbB4-Fc for 2 hours. Biotinylation of cell surface proteins used 0.5 mg/ml EZ-Link Sulfo-NHS-LC-biotin (Pierce) in PBS, as described previously (Bouchard et al., 2008). Biotinylated cells were washed with PBS, lysed and incubated with streptavidin-agarose beads (Pierce) for 1 hour at 4°C to precipitate biotinylated proteins, which were then separated by electrophoresis on 10% NuPAGE gels. Detection and band intensity quantification were performed using IRDye infrared secondary antibodies (LI-COR Biosciences) and the Odyssey Infrared Imaging System (version 2.1; LI-COR Biosciences).
RESULTS
Selective DRG neuron death in type III Nrg1–/– mice
We initially examined type III Nrg1 expression patterns. Type III Nrg1 is expressed in DRG neurons as early as E10.5 and throughout embryogenesis (Hippenmeyer et al., 2002; Meyer et al., 1997), but its expression profile in subpopulations of sensory neurons has not been determined. Using a type III Nrg1-specific antibody (Yang et al., 1998), we examined which subpopulations of sensory neurons express type III Nrg1 in wild-type embryos. The antibody against type III Nrg1 was specific, as immunolabeling was not apparent in DRG neurons from type III Nrg1–/– mice (supplementary material Fig. S1). At E17.5, expression of type III Nrg1 was seen throughout the population of cells that was labeled with markers of the Ngf-dependent class of neurons: TrkA or calcitonin gene-related peptide (CGRP, Calca) (Fig. 1A). Type III Nrg1 expression was also seen in neurons labeled for TrkB (Ntrk2), consistent with labeling of mechanoreceptive neurons, and in a subset of neurons labeled for NF200 (Nefh), a marker of sensory neurons that become myelinated (Lawson and Waddell, 1991). Together, these findings indicate that during embryogenesis type III Nrg1 is expressed by most sensory neurons.
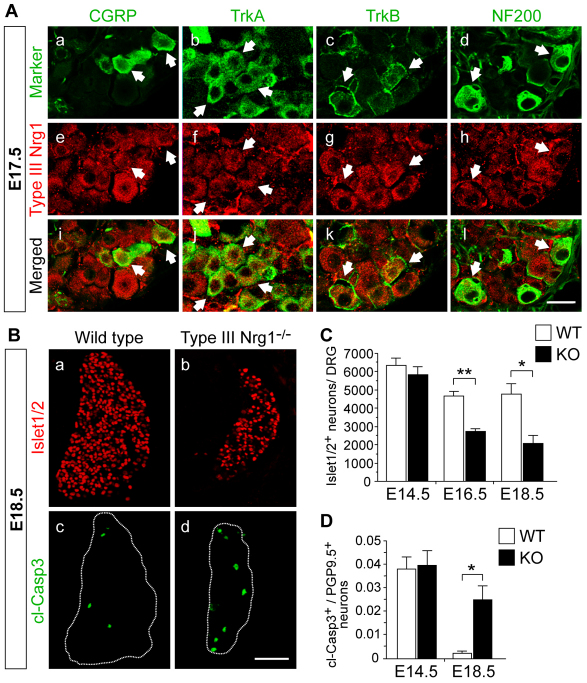
Fig. 1.
Type III Nrg1 is required for sensory neuron survival. (A) Type III Nrg1 is expressed in subsets of dorsal root ganglion (DRG) neurons. E17.5 DRG sections were co-labeled for type III Nrg1 and CGRP (a,e,i), TrkA (b,f,j), TrkB (c,g,k) or NF200 (d,h,l). Arrows indicate double-labeled neurons. See also supplementary material Fig. S1. (B) DRG sections from wild-type (WT) or type III Nrg1–/– mouse embryos were labeled for Islet1/2 (a,b) or cleaved caspase 3 (cl-Casp3; c,d). (C) Quantification of Islet1/2+ neurons in WT and mutant DRG at E14.5, E16.5 and E18.5. (D) Quantification of cl-Casp3+ neurons in WT and type III Nrg1–/– (KO) DRG sections at E14.5 and E18.5. DRG were co-labeled for PGP9.5 (Uchl1) to identify sensory neurons, and numbers represent the fraction of double-labeled neurons. Mean ± s.e.m. *, P<0.01; **, P<0.001 (ANOVA with post-hoc Fisher’s PLSD test). Scale bars: 25 μm in A; 100 μm in B.
Type III Nrg1–/– embryos exhibit a 60% loss in sensory neurons by E18.5, indicating that type III Nrg1 signaling is essential for the survival of many sensory neurons (Wolpowitz et al., 2000). To determine when sensory neurons depend on type III Nrg1 for survival, we quantified the number of sensory neurons in DRG from wild-type versus type III Nrg1–/– embryos at different stages of development. To identify the total population of sensory neurons, DRG were labeled with an antibody against the homeobox proteins Islet1 and Islet2 (Islet1/2+). At E14.5, there were equivalent numbers of Islet1/2+ neurons in wild-type and type III Nrg1–/– DRG. At E16.5 and E18.5, Islet1/2+ neuron numbers were reduced in mutants by 42% and 57%, respectively (Fig. 1B,C; supplementary material Table S1). The reduction in sensory neuron numbers in mutant DRG was accompanied by an increase in neurons undergoing apoptosis. At E14.5, there was no difference in the number of apoptotic neurons in wild-type versus mutant DRG, but mutant DRG contained ∼10-fold more apoptotic neurons than wild-type DRG by E18.5 (2.5±0.6% versus 0.2±0.01%, P<0.01), indicating that sensory neuron survival is dependent on type III Nrg1 in late embryogenesis (Fig. 1B,D).
We next determined which sensory neurons depend on type III Nrg1 for survival. At E14.5, wild-type and type III Nrg1–/– DRG contained equivalent numbers of sensory neurons expressing peripherin, TrkA, TrkB or parvalbumin, a marker of proprioceptive neurons (Arber et al., 2000; Carr et al., 1989) (Fig. 2A). At E18.5, there was a loss of peripherin+, TrkA+, TrkB+ and substance P+ (Tac1) neurons in type III Nrg1–/– DRG, whereas no loss of parvalbumin+ neurons occurred in mutant DRG, indicating that the survival of proprioceptive sensory neurons is independent of type III Nrg1 expression (Fig. 2A,B; supplementary material Table S2). Although the total number of parvalbumin+ neurons did not change, type III Nrg1 deficiency resulted in a higher percentage of parvalbumin+ neurons per DRG (supplementary material Fig. S2A). Thus, the survival requirement for type III Nrg1 was selective for cutaneous sensory neurons.
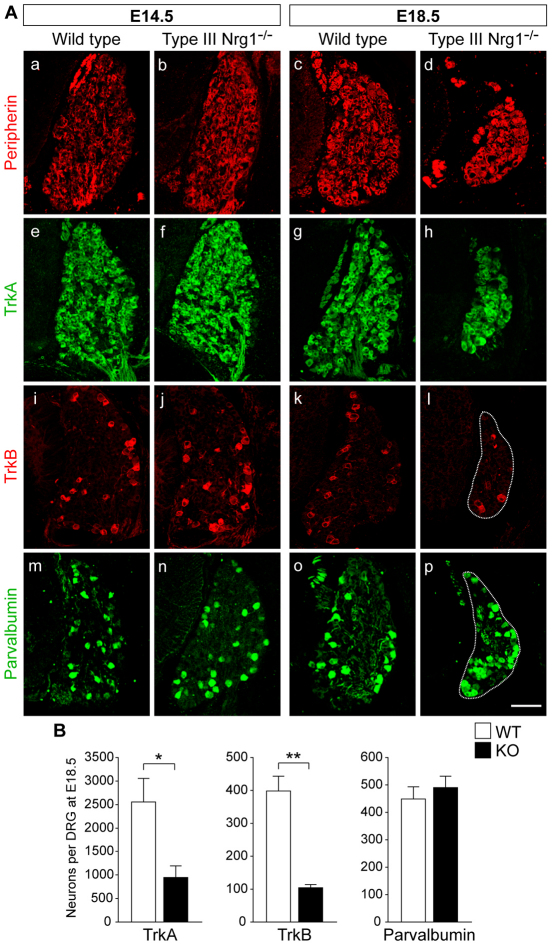
Fig. 2.
Type III Nrg1 is required for the survival of cutaneous sensory neurons. (A) DRG sections from WT or type III Nrg1–/– mouse embryos at E14.5 or E18.5 were labeled for peripherin (a-d), TrkA (e-h), TrkB (i-l) or parvalbumin (m-p). At E18.5, type III Nrg1 is essential for the survival of peripherin+, TrkA+ and TrkB+ neurons. Scale bar: 100 μm. (B) Quantification of TrkA+, TrkB+ or parvalbumin+ neurons in WT and mutant DRG at E18.5. Mean ± s.e.m. *, P<0.02; **, P<0.001 (Student’s t-test).
Given that Nrg1 signaling via its intracellular domain (back-signaling) reduces apoptosis in hippocampal neurons (Bao et al., 2003), we performed a survival assay using cultured DRG neurons to determine whether type III Nrg1 signaling in a cell-autonomous manner can promote sensory neuron survival. In vitro, survival of DRG neurons from wild-type or type III Nrg1–/– mice can be sustained for extended periods by relatively high concentrations of serum and Ngf (Taveggia et al., 2005), and removal of these factors results in a rapid decrease in neuronal survival, which was detected by cleaved caspase 3 immunolabeling (supplementary material Fig. S2B). When wild-type neurons were cultured in serum-free media supplemented with exogenous ErbB4 extracellular domain (B4-ECD) for 16 hours, there was a reduction in cell death compared with control neurons. In type III Nrg1–/– neurons, however, B4-ECD treatment did not increase cell survival, consistent with the idea that the neuronal loss observed in vivo reflects, at least in part, a role for type III Nrg1 back-signaling in sensory neurons.
Defective central and peripheral projections in type III Nrg1–/– embryos
To investigate whether the type III Nrg1 mutants have axon pathfinding abnormalities in either central or peripheral targets, we first compared the central projections of wild-type and type III Nrg1–/– embryos at different stages of development. TrkA+ axons begin to penetrate the lateral-most region of the dorsal horn at E14.5 (Ozaki and Snider, 1997). In type III Nrg1–/– embryos, TrkA+ axons projected normally through the dorsal root and entered the dorsal funiculus by E12.5 (data not shown). At E14.5, however, many of the mutant TrkA+ axons that entered the dorsal horn projected past the appropriate target zones of laminae I and II, some as far as the intermediate and deep dorsal laminae and the ventral horn (Fig. 3A). Comparable phenotypes were also seen later at E16.5 and E18.5, including disorganized axon trajectories and some axons extending abnormally into ventral regions of the spinal cord (Fig. 3A-C; supplementary material Fig. S4). Aberrant TrkA+ axon projections into intermediate and ventral regions were never detected in either wild-type or type III Nrg1+/– embryos.
Fig. 3.
Type III Nrg1 is required for appropriate targeting of TrkA+ fibers to the dorsal horn. (A) Spinal cord sections from WT or type III Nrg1–/– mouse embryos at E14.5 (a,b), E16.5 (c,d) and E18.5 (e-g) were labeled for TrkA. Several TrkA+ axons extended beyond the superficial dorsal horn and innervated the intermediate and deep dorsal laminae and ventral horn in mutants (arrows) but not in WT. (B) Summary plots of TrkA+ fiber endings in lateral (gray) or medial (black) regions of the spinal cord along the dorsoventral axis, represented as the dorsal termination index (DTI), of WT (n=16; blue dots) and type III Nrg1–/– (n=17; red dots) embryos (combined ages: E14.5, E16.5 and E18.5). (C) Summary plots of individual TrkA+ fiber endings within the spinal cord of E14.5, E16.5 and E18.5 WT (blue dots) and type III Nrg1–/– (red dots) embryos. Numbers of embryos used are indicated in parentheses. (D) WT or type III Nrg1–/– spinal cord sections were labeled for MAP2 (a,b), Sema3A (c,d) or parvalbumin (e-h). At E14.5, MAP2+ neurons were concentrated in the dorsal horn in WT (a) and mutant (b) spinal cords. At E15.5, Sema3A was expressed in the ventral spinal cord in WT (c) and mutant (d) embryos. Parvalbumin+ axons innervated the spinal cord of WT and mutant embryos at E14.5 (e,f) and E18.5 (g,h). (a,b) Dotted line indicates the midline and bracket indicates dorsal horn. Scale bars: 50 μm in Ae,f and Da,b; 100 μm in Aa-d,g; 200 μm in Dc-h.
The TrkA+ central projection defect in type III Nrg1–/– embryos appeared to reflect a requirement for type III Nrg1 for normal penetration and/or guidance of axons within the dorsal horn and was not due to defects in the initial bifurcation and extension of central afferents, as the ascending and descending axons appeared normal (supplementary material Fig. S3). The defect was not associated with the loss or disorganization of target neurons within the dorsal horn; MAP2+ (Mtap2) neurons were similar in density and distribution within wild-type and mutant dorsal horns at E14.5 and E17.5 (Fig. 3D). Likewise, there were no differences in Sema3A expression (Fig. 3D) or in apoptotic cell number (data not shown) in mutant versus wild-type spinal cord. Additionally, defects in sensory projections were selective for TrkA+ neurons, as the pattern and extent of proprioceptive axons in the spinal cord were indistinguishable in wild-type and type III Nrg1–/– embryos (Fig. 3D), in agreement with a previous report (Hippenmeyer et al., 2002).
We also assessed the effect of loss of type III Nrg1 on the maintenance of peripheral sensory projections, focusing on cutaneous targets. Previously, we found that type III Nrg1 is essential for peripheral innervation during embryonic development (Wolpowitz et al., 2000). To assess when defects in peripheral innervation occur relative to sensory neuron death or central projection defects, we examined axons labeled for TrkA in hindlimb epithelium from wild-type or type III Nrg1–/– embryos at different developmental stages. In the mouse, peripheral sensory projections first approach cutaneous target fields of the proximal hindlimb between E11.5 and E13.5 (White et al., 1996). We found that TrkA+ axons innervated the proximal hindlimb in both wild-type and type III Nrg1–/– embryos at E12.5, but axonal projections appeared highly disorganized in the mutant embryos, with a 27% increase in epidermal nerve endings (Fig. 4A,B). At E14.5 and E16.5, TrkA+ axons projecting to the proximal hindlimb epithelium appeared more diffuse and disorganized in mutant embryos and were reduced in number by 43% and 52%, respectively, compared with wild-type littermates. Two days later, at E18.5, there was a 76% reduction in TrkA+ nerve endings, and axons were difficult to discern (Fig. 4A-C). Similar results were seen using antibodies to peripherin to visualize cutaneous axons (supplementary material Fig. S5). In contrast to the defects in TrkA+ peripheral axons, parvalbumin+ axon innervation at muscle spindles is not affected by loss of type III Nrg1 (Hippenmeyer et al., 2002), indicating that the dependence on type III Nrg1 signaling in peripheral target innervation is selective for TrkA+ neurons.
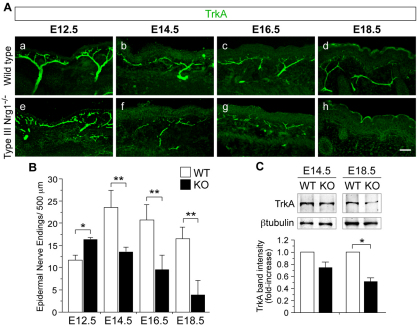
Fig. 4.
Innervation of cutaneous targets is not maintained in type III Nrg1–/– mouse embryos during late embryogenesis. (A) Hindlimb sections of WT (a-d) or type III Nrg1–/– (e-h) embryos were labeled for TrkA. At E12.5, TrkA+ axons innervated the proximal hindlimb epithelium in WT (a) and type III Nrg1–/– (e) embryos, but axons appeared disorganized in mutants. TrkA+ axon innervation was reduced in mutants at E14.5 (f), E16.5 (g) and E18.5 (h). Scale bar: 50 μm. (B) Quantification of TrkA+ nerve endings within the hindlimb upper dermis and epidermis (n≥2 embryos per genotype). (C) Immunoblot of TrkA expression in hindlimb epithelium from WT or type III Nrg1–/– embryos at E14.5 and E18.5. β-tubulin was used as a lysate loading control. TrkA band intensity is shown relative to WT (n≥3 embryos per genotype). Mean ± s.e.m. *, P<0.05; **, P<0.01 (ANOVA with post-hoc Fisher’s PLSD test).
Defects in pathfinding persist after Bax rescue of sensory neuron survival
The axon pathfinding phenotype in type III Nrg1–/– mice is unlikely to be entirely secondary to the survival phenotype because pathfinding defects are first seen at an earlier developmental stage, as described above. For further evidence, we used a strategy that has been described previously to distinguish the survival and pathfinding effects of other cues, which involves genetic elimination of Bax, a Bcl2 family member required for apoptosis in response to survival signal withdrawal (Honma et al., 2010; Patel et al., 2000; Patel et al., 2003). To examine the role of type III Nrg1 in target innervation, independent of changes in sensory neuron number, we crossed type III Nrg1 and Bax heterozygous mice to generate Bax–/–; type III Nrg1–/– mice. At E18.5, no differences were seen in the number of Islet1/2+ and TrkA+ neurons in DRG from Bax–/–; type III Nrg1–/– embryos when compared with Bax–/–; type III Nrg1+/+ littermates (Fig. 5A; supplementary material Table S3). Although elimination of Bax rescued neuron loss, the axon defect in Bax–/–; type III Nrg1–/– embryos persisted throughout development, with pronounced abnormalities of TrkA+ axon trajectories, including projection beyond laminae I and II to the intermediate and deep dorsal laminae and ventral horn at E18.5 (Fig. 5B,C).
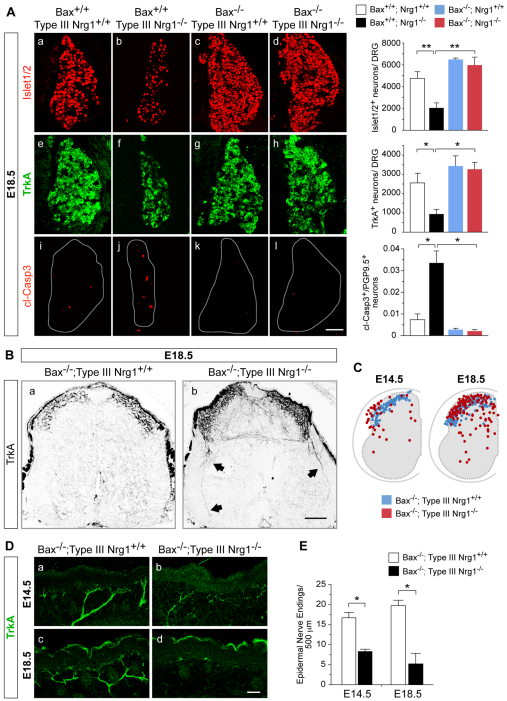
Fig. 5.
TrkA+ central projections and cutaneous innervation are aberrant in Bax–/–; type III Nrg1–/– mutants. (A) Sections of lumbar DRG from mouse embryos of the indicated genotypes at E18.5 were labeled for Islet1/2 (a-d), TrkA (e-h) or cl-Casp3 (i-l). Bar charts show quantification of Islet1/2+, TrkA+ and cl-Casp3+ neurons in DRG at E18.5. (B) Spinal cord sections from Bax–/–; type III Nrg1+/+ (a) or Bax–/–; type III Nrg1–/– embryos (b) at E18.5 were labeled for TrkA. Aberrant TrkA+ axons were detected in the double-null spinal cord at E18.5 (arrows). (C) Summary plots of individual TrkA+ axon termination points within Bax–/–; type III Nrg1+/+ (blue dots) or Bax–/–; type III Nrg1–/– (red dots) spinal cords at E14.5 and E18.5 (n=3 spinal cords per genotype). (D) Sections of hindlimb epithelium from Bax–/–; type III Nrg1+/+ (a,c) or Bax–/–; type III Nrg1–/– (b,d) embryos at E14.5 or E18.5 were labeled for TrkA. In double-mutant embryos, TrkA+ axons appeared thin and disorganized at E14.5 (b), and by E18.5 (d) axon innervation was reduced. (E) Quantification of TrkA+ nerve endings in hindlimb epithelium (n≥2 embryos per genotype). Mean ± s.e.m. *, P<0.02; **, P<0.005 (ANOVA with post-hoc Fisher’s PLSD test). Scale bars: 100 μm in A,B; 50 μm in D.
We also examined whether cutaneous innervation was rescued in the Bax–/–; type III Nrg1–/– embryos. At E14.5, TrkA+ axons innervated the hindlimb epithelium in both Bax–/–; type III Nrg1+/+ and Bax–/–; type III Nrg1–/– embryos, but cutaneous nerve bundles were reduced in number by 53% and 74% at E14.5 and E18.5, respectively (Fig. 5D,E). These results provide evidence that the defective central and peripheral innervation seen in the type III Nrg1 mutants was not a secondary effect of the loss of sensory neurons, but rather reflects a requirement for type III Nrg1 signaling for sensory axon pathfinding and maintenance.
We next asked whether the TrkA+ projection abnormalities reflect a defect in Ngf/TrkA signaling. Since Ngf/TrkA signaling is essential for the synthesis of nociceptive peptides in developing sensory neurons (Patel et al., 2000), we assessed whether sensory neurons are able to differentiate into a peptidergic state in the absence of type III Nrg1 signaling. We detected the neuropeptide substance P in DRG neurons and in the hindlimb epithelium of Bax–/–; type III Nrg1–/– embryos, indicating that Ngf/TrkA signaling occurs normally in the absence of type III Nrg1 (supplementary material Fig. S6).
Together, our findings indicate that, in addition to its effects on neuron survival, type III Nrg1 is required for proper TrkA+ axon pathfinding and maintenance in the spinal cord and periphery.
Type III Nrg1–/– sensory axons show impaired response to Sema3A
The axon pathfinding phenotype of type III Nrg1–/– embryos, with abnormally deep penetration of TrkA+ axons into the ventral spinal cord, is reminiscent of that seen in Sema3a and Nrp1 null embryos (Behar et al., 1996; Gu et al., 2003), suggesting that type III Nrg1 signaling might regulate aspects of Sema3A responsiveness. Sema3A is expressed in the ventral horn and selectively repels Ngf-responsive axons, helping to prevent them from projecting into the ventral spinal cord during development (Fitzgerald et al., 1993; Fu et al., 2000; Messersmith et al., 1995; Puschel et al., 1996). We therefore directly tested whether type III Nrg1 regulates Sema3A responsiveness in isolated sensory neurons in culture.
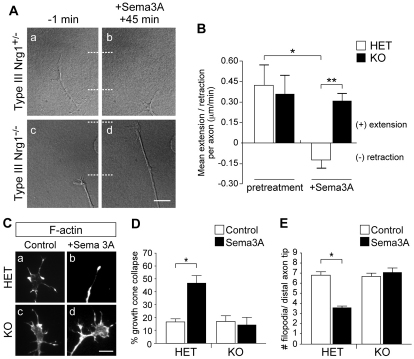
We imaged growth cones from DRG explants cultured in media containing Ngf, which selectively promotes the growth of TrkA+ axons (Hory-Lee et al., 1993). We initially tested the effect of different conditions on rates of axon extension. In the absence of added Sema3A, no difference was seen in the advancement of axons from type III Nrg1+/– versus type III Nrg1–/– DRG explants [0.43±0.15 (n=29) versus 0.36±0.14 (n=28) μm/minute, respectively; no differences were observed between wild-type and type III Nrg1+/– neurons, and therefore data from heterozygous neurons were used as control]. As expected, in response to Sema3A treatment (45 minutes), type III Nrg1+/– axons retracted (–0.13±0.06 μm/minute, n=31). By contrast, when type III Nrg1–/– axons were treated with Sema3A they showed no retraction response, and instead continued to extend (0.31±0.06 μm/minute, n=37) (Fig. 6A,B). We also assayed growth cone collapse in response to Sema3A. In type III Nrg1+/– sensory neurons, Sema3A treatment induced growth cone collapse, whereas growth cones of type III Nrg1–/– sensory axons showed no detectable collapse response to Sema3A (Fig. 6C-E). Thus, the type III Nrg1 mutation has a strong effect on the ability of sensory neurons to respond to Sema3A in vitro.
Fig. 6.
Type III Nrg1–/– sensory neurons do not respond to the chemorepellent cue Sema3A. (A) DRG explants from E14.5 type III Nrg1+/– (a,b) or type III Nrg1–/– (c,d) mouse embryos were imaged by time-lapse microscopy to measure Sema3A responsiveness. Whereas type III Nrg1+/– axons retracted in response to Sema3A after 45 minutes (b), type III Nrg1–/– axons continued to extend (d). Dotted lines indicate distal tip of axon. (B) Quantification of axon extension or retraction in response to Sema3A (n=3 explants per genotype). *, P<0.02; **, P<0.01 (ANOVA with post-hoc Fisher’s PLSD test). HET, type III Nrg1+/–. (C) Type III Nrg1+/– (a,b) or type III Nrg1–/– (c,d) DRG explants were assayed for growth cone collapse in response to Sema3A (1 hour). F-actin was labeled with phalloidin. (D) Quantification of growth cone collapse (n>4 explants per condition). *, P<0.05 (Student’s t-test). (E) Quantification of the number of filopodia per distal axon tip (n>4 explants per condition). *, P<0.01 (Student’s t-test). Mean ± s.e.m.
Regulation of Sema3A receptor expression by type III Nrg1
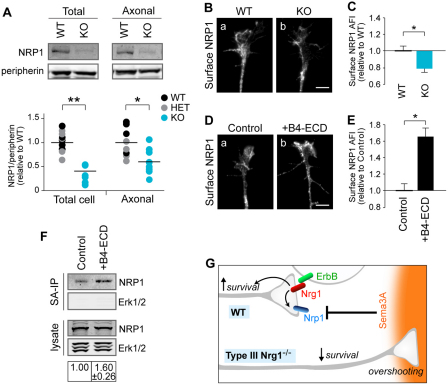
In considering potential molecular mechanisms that might account for the impaired Sema3A responsiveness of type III Nrg1–/– neurons, one possibility is a reduction in the expression of Sema3A receptors. The Sema3A receptor on sensory neurons is a complex that contains both Nrp1 and plexin A4 (He and Tessier-Lavigne, 1997; Kolodkin et al., 1997; Yaron et al., 2005). Type III Nrg1, Nrp1 and plexin A4 are expressed in sensory axons (supplementary material Fig. S7). To determine whether type III Nrg1 regulates Sema3A receptor expression, we measured Nrp1 levels in sensory neurons and in sensory axons from wild-type, type III Nrg1+/– and type III Nrg1–/– embryos by immunoblot (Fig. 7A). In type III Nrg1–/– neurons, Nrp1 was reduced by 58% compared with control neurons. Additionally, we detected a 61% reduction in plexin A4 (supplementary material Fig. S7).
Fig. 7.
Type III Nrg1 signaling regulates axonal Nrp1 expression. (A) DRG explants from WT, type III Nrg1+/– or type III Nrg1–/– mouse embryos were cultured on Transwell membranes, and protein extracts from the upper (total cell) and lower (axons only) membranes were collected and analyzed by immunoblotting. Nrp1 levels were quantified and normalized to peripherin levels. WT and type III Nrg1+/– ratios were pooled and set to 1, and type III Nrg1–/– values are relative to this. Data points are presented in a scatter plot, with bars representing the mean (n≥4 pups per genotype for littermate comparisons). *, P<0.02; **, P<0.0001 (Student’s t-test). (B) Growth cones of WT (a) or type III Nrg1–/– (b) sensory neurons were immunolabeled for surface Nrp1. (C) Quantification of surface Nrp1 in WT and mutant growth cones. Results are measures of the average fluorescence intensity (AFI) per unit area to normalize for differences in growth cone size (n≥6 explants per genotype). *, P<0.05 (Student’s t-test). (D) WT sensory neurons were treated with control conditions (a) or B4-ECD (b) for 2 hours and immunolabeled for surface Nrp1. (E) Quantification of surface Nrp1 in growth cones treated with control conditions or B4-ECD for 2 hours, as described in C (n≥8 explants per condition). *, P<0.0001 (Student’s t-test). (F) Sensory neurons were treated with B4-ECD or control conditions for 2 hours. Surface proteins were biotinylated and isolated using streptavidin (SA)-agarose beads, and surface Nrp1 levels were assessed by immunoblotting. Erk1/2 (Mapk3/1) was not detected in the SA-IP samples, indicating that only surface proteins were biotinylated. (G) Summary of type III Nrg1 signaling. In WT axons, type III Nrg1 (red) binds to ErbB receptors (green) expressed by a neighboring cell, which stimulates an increase in cell surface Nrp1 as well as survival in the type III Nrg1-expressing neuron. In the absence of type III Nrg1, sensory axons have reduced cell surface Nrp1 and aberrantly project to Sema3A-expressing regions, and, ultimately, neurons have reduced survival. Scale bars: 10 μm.
Since guidance responses to Sema3A occur within the axon, we also tested receptor protein levels specifically within axons. DRG neurons were grown in Transwell chambers on a filter with a pore size large enough to permit penetration of axons but not cell bodies, and the axons were then harvested (supplementary material Fig. S7). Nrp1 protein levels were found to be reduced in type III Nrg1–/– axons (Fig. 7A). Although there was also a trend toward reduction in plexin A4 levels in the Nrg1–/– axons, this did not reach statistical significance (supplementary material Fig. S7). To further assess the effect of the type III Nrg1 mutation on the expression of Sema3A receptors, growth cones were examined by immunolocalization to detect receptor on the cell surface. In this set of experiments, Nrp1 was tested but not plexin A4, because the plexin A4 antibodies did not produce a detectable immunofluorescence signal in non-permeabilized neurons. The results showed that loss of type III Nrg1 resulted in a reduction in Nrp1 receptor at the cell surface (Fig. 7B,C).
Finally, we tested whether stimulation of Nrg1 signaling influences the surface expression of Nrp1. Stimulation of Nrg1 back-signaling by addition of B4-ECD for 2 hours was found to increase the cell surface immunofluorescence of Nrp1 at the growth cone (Fig. 7D,E). To obtain further evidence, cell surface proteins in cultured sensory neurons were biotinylated, isolated, and the level of surface Nrp1 assessed by immunoblot (Fig. 7F). When neurons were treated with B4-ECD for 2 hours, as compared with neurons under control conditions, the amount of surface Nrp1 was increased, confirming that type III Nrg1 back-signaling can regulate the neuronal cell surface levels of Nrp1.
DISCUSSION
This study reveals a novel role for type III Nrg1 signaling in sensory axon pathfinding independent of its effect on neuron survival: in the proper targeting of TrkA+ central projections as well as in the pathfinding and maintenance of cutaneous peripheral projections. In characterizing downstream molecular mechanisms that might contribute to the central projection phenotype, we found that type III Nrg1 is required for neurons to respond normally to Sema3A and that it could regulate expression of the corresponding receptors, providing a new molecular pathway to regulate responsiveness to an axon guidance cue.
Type III Nrg1 in sensory neuron survival and axon pathfinding
Although type III Nrg1 deficiency was known to result in sensory neuron death, it had not been determined whether type III Nrg1 has selective functions in distinct sensory neuron populations, or whether it might have roles in axon pathfinding. In this study, we found that type III Nrg1 is required for survival and target innervation by TrkA+ neurons, a subpopulation that expresses high levels of type III Nrg1. Type III Nrg1 is expressed along sensory axons and is known to provide support for the migration and survival of nearby Schwann cells and their precursors, which express the Nrg1 receptors ErbB2 and ErbB3 (Hippenmeyer et al., 2002; Meyer et al., 1997; Michailov et al., 2004; Taveggia et al., 2005; Wolpowitz et al., 2000).
Schwann cells and their precursors are a source of trophic support for DRG neurons, especially prior to neuronal dependence on target-derived neurotrophins (Buj-Bello et al., 1995; Chen et al., 2003; Davies, 1998; Ernfors, 2001; Thoenen et al., 1988). Therefore, part of the survival effect on sensory neurons seen in type III Nrg1 mutant mice might, in principle, be secondary to a reduction of Schwann cell support. However, several observations indicate that the phenotypes seen here in TrkA+ neurons are unlikely to be entirely explained by insufficient neurotrophin support from Schwann cells. Loss of ErbB3 was previously found to result in the total absence of Schwann cells along peripheral nerves and in a subsequent 70% loss of both small and large diameter neurons within the DRG as early as E14.5 (Riethmacher et al., 1997). By contrast, we found that loss of type III Nrg1 did not result in sensory neuron loss until E16.5 (when there was a 42% reduction), and that the survival of large diameter proprioceptive neurons was unaffected, indicating that some Schwann cell precursor-derived support is present in type III Nrg1–/– embryos. Consistent with this, although Schwann cells and their precursors are reduced in number in type III Nrg1–/– embryos, they are present along ventral and dorsal roots in mutants at E18.5 (Wolpowitz et al., 2000). Finally, our in vitro studies directly showed effects of type III Nrg1 back-signaling on sensory neuron survival, indicating a cell-autonomous role for type III Nrg1 in cultured sensory neurons.
Coordination of central and peripheral axon targeting
Sensory neurons convey information from the periphery to the central nervous system. Therefore, it is essential that pathfinding and survival mechanisms correctly coordinate connections of these neurons with both central and peripheral targets. If type III Nrg1 signaling, like Ngf/TrkA signaling, is involved in sensory neuron pathfinding and survival, why would a neuron require both type III Nrg1 and TrkA signaling? The findings reported here indicate that TrkA and type III Nrg1 signaling regulate target innervation and survival of sensory neurons in distinct and complementary ways. In previous studies, Ngf/TrkA signaling was found to be essential for peripheral target innervation by TrkA+ axons, but not for central projections in the spinal cord (Patel et al., 2000). By contrast, we found that type III Nrg1 is required for normal trajectories of TrkA+ axons in the spinal cord, as well as for the innervation and maintenance of axons in the periphery. Differences in the expression patterns of Ngf and ErbB proteins are also consistent with neuronal TrkA and type III Nrg1 having distinct functions: Ngf is abundantly expressed in the epidermis but not in the spinal cord (White et al., 1996), whereas ErbB proteins are expressed in both the epidermis and the dorsal horn (Kokai et al., 1987; Meyer et al., 1997; Pearson and Carroll, 2004).
These results support a model in which TrkA and type III Nrg1 have overlapping but distinct functions, with Ngf/TrkA being essential for the development of peripheral axons (Patel et al., 2000), whereas ErbB/type III Nrg1 is required for both correct target innervation by central axons and target innervation and maintenance of axons in the periphery. Such effects on pathfinding and survival could act coordinately in both central and peripheral targets to help establish the necessary circuitry for the relay of incoming sensory information to the central nervous system.
Role of type III Nrg1 in Sema3A responsiveness
In addition to guidance cues that can steer the growth cone, axon pathfinding involves extracellular signals that regulate whether an axon reacts to a guidance cue with an attractant response, a repellent response, or no response (Butler and Tear, 2007; Chen et al., 2008; He et al., 2002; Law et al., 2008; Parra and Zou, 2009; Tessier-Lavigne and Goodman, 1996). Sema3A is a diffusible repellent guidance cue expressed in the ventral horn and periphery that selectively repels axons of Ngf-dependent sensory neurons, preventing them from overshooting their normal targets in the dorsal horn (Fu et al., 2000; Messersmith et al., 1995; Pond et al., 2002; Puschel et al., 1996; Wright et al., 1995). The overlap of mutant phenotypes in the spinal cord for Sema3a, Nrp1 (Behar et al., 1996; Gu et al., 2003; Huettl et al., 2011) and type III Nrg1 supports a model in which type III Nrg1 regulates aspects of Sema3A responsiveness. In the spinal cord of type III Nrg1 mutants, as well as Sema3a and Nrp1 mutants, numerous Ngf-dependent sensory axons extend beyond their dorsal horn targets, sometimes projecting as deep as the ventral horn (Gu et al., 2003). The phenotypic overlap between the type III Nrg1 mutants and those defective in Sema3A signaling is not complete, however, particularly in the periphery. In type III Nrg1–/– mice, peripheral axons in the hindlimb epithelium appeared defasciculated and disorganized at E12.5, and then a gradual loss of cutaneous innervation was apparent by E14.5. By contrast, loss of Nrp1 or Sema3A signaling did not result in a loss of peripheral innervation; instead, axons were highly defasciculated and exuberantly extended in peripheral target fields (Gu et al., 2003; Huettl et al., 2011; Kitsukawa et al., 1997; White and Behar, 2000; Yaron et al., 2005). Therefore, the peripheral phenotype observed in the type III Nrg1 mutant embryos is likely to reflect additional Sema3A-independent functions of type III Nrg1; for example, one interesting possibility is that it might regulate the levels of other receptors in addition to Nrp1.
Our characterization of mechanisms by which type III Nrg1 can regulate Sema3A responsiveness revealed that cultured sensory neurons from type III Nrg1–/– mice express reduced levels of Sema3A receptors in the axon. Moreover, stimulation of type III Nrg1 back-signaling increased the surface expression of Nrp1 at the growth cone. Although further work would be required to delineate the entire molecular pathway involved, it is intriguing that the intracellular domain of type III Nrg1 interacts with LIM kinase 1 (Wang et al., 1998), a cytoplasmic protein with roles in growth cone motility and axon guidance (Endo et al., 2007; Phan et al., 2010). Taken together with previous reports that type III Nrg1 back-signaling regulates gene expression and neurotransmitter receptor expression at the synapse (Bao et al., 2004; Bao et al., 2003; Hancock et al., 2008; Zhong et al., 2008), our findings support a general model in which type III Nrg1 back-signaling regulates the expression and/or trafficking of signaling components, including various cell surface receptors.
Why might it be useful to regulate axon responsiveness to Sema3A? A potential explanation comes from previous observations on Sema3A. As sensory axons begin their extension from the DRG to the spinal cord, they grow through regions containing Sema3A expressed at high levels by mesodermal tissue surrounding the DRG and dorsal root entry zone, prior to their penetration of the gray matter, raising the question of why the axons are not repelled by Sema3A present along the early part of their trajectory (Wright et al., 1995)? Likewise, sensory axons extending towards the periphery must pass through Sema3A-expressing regions in the limb bud and dermis en route to their targets. Thus, although at later stages of development Sema3A helps to prevent sensory axons from extending out of the dorsal roots into inappropriate regions, at earlier stages axons must initially be able to penetrate through regions expressing Sema3A (Wright et al., 1995). Taken together with our results, this leads to the following model: the migrating axon at early stages of its trajectory would have low levels of Sema3A receptors, allowing it to pass through tissues that express Sema3A on the way to its target; then, after reaching the target, upregulation of Sema3A receptors triggered by type III Nrg1 back-signaling would sensitize the axon to Sema3A repulsion, helping to prevent the axon from subsequently straying outside its correct target region (Fig. 7G). The net effect of this model would be a switch in axon responsiveness that would allow axons to first reach, and then be retained within, their correct final targets. This model is analogous to the switch models previously proposed to occur at axon intermediate targets (Butler and Tear, 2007; Chen et al., 2008; He et al., 2002; Law et al., 2008; Nawabi et al., 2010; Parra and Zou, 2009; Tessier-Lavigne and Goodman, 1996), but in this case the switch in responsiveness would occur at the final target.
The results reported here identify functions of type III Nrg1 in mediating sensory axon pathfinding, in addition to its effects on neuron survival. We also identify a novel mechanism whereby type III Nrg1 can regulate responsiveness to a guidance cue, Sema3A. By regulating axon pathfinding and survival in a specific subset of sensory neurons, type III Nrg1 helps to selectively ensure the correct connectivity of sensory neurons with both central and peripheral targets.
Supplementary Material
Acknowledgments
We thank M. Tessier-Lavigne and N. Preitner for comments on the manuscript; T. Jessell for the Islet1/2 antibody; A. Kolodkin for the neuropilin 1 antibody; L. Reichardt for the TrkA antibody; B. Sherman, S. Gebre and J. Matulonis for mouse breeding and genotyping; the Nikon Imaging Facility at Harvard Medical School; and J. Tcherkezian for helpful discussions.
Footnotes
Funding
This work was funded by National Institutes of Health grants [HD29417 to J.G.F., NS029071 to L.W.R. and D.A.T., and T32-NS007484 and F32-NS073307 to M.L.H.]; and by the Sidney Baer Foundation/National Alliance for Research on Schizophrenia and Depression (NARSAD) (L.W.R.). Deposited in PMC for release after 12 months.
Competing interests statement
The authors declare no competing financial interests.
Supplementary material
Supplementary material available online at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.072306/-/DC1
References
- Arber S., Ladle D. R., Lin J. H., Frank E., Jessell T. M. (2000). ETS gene Er81 controls the formation of functional connections between group Ia sensory afferents and motor neurons. Cell 101, 485–498 [DOI] [PubMed] [Google Scholar]
- Bao J., Wolpowitz D., Role L. W., Talmage D. A. (2003). Back signaling by the Nrg-1 intracellular domain. J. Cell Biol. 161, 1133–1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao J., Lin H., Ouyang Y., Lei D., Osman A., Kim T. W., Mei L., Dai P., Ohlemiller K. K., Ambron R. T. (2004). Activity-dependent transcription regulation of PSD-95 by neuregulin-1 and Eos. Nat. Neurosci. 7, 1250–1258 [DOI] [PubMed] [Google Scholar]
- Behar O., Golden J. A., Mashimo H., Schoen F. J., Fishman M. C. (1996). Semaphorin III is needed for normal patterning and growth of nerves, bones and heart. Nature 383, 525–528 [DOI] [PubMed] [Google Scholar]
- Bermingham-McDonogh O., Xu Y. T., Marchionni M. A., Scherer S. S. (1997). Neuregulin expression in PNS neurons: isoforms and regulation by target interactions. Mol. Cell. Neurosci. 10, 184–195 [DOI] [PubMed] [Google Scholar]
- Birchmeier C., Nave K. A. (2008). Neuregulin-1, a key axonal signal that drives Schwann cell growth and differentiation. Glia 56, 1491–1497 [DOI] [PubMed] [Google Scholar]
- Bouchard J. F., Horn K. E., Stroh T., Kennedy T. E. (2008). Depolarization recruits DCC to the plasma membrane of embryonic cortical neurons and enhances axon extension in response to netrin-1. J. Neurochem. 107, 398–417 [DOI] [PubMed] [Google Scholar]
- Buj-Bello A., Buchman V. L., Horton A., Rosenthal A., Davies A. M. (1995). GDNF is an age-specific survival factor for sensory and autonomic neurons. Neuron 15, 821–828 [DOI] [PubMed] [Google Scholar]
- Butler S. J., Tear G. (2007). Getting axons onto the right path: the role of transcription factors in axon guidance. Development 134, 439–448 [DOI] [PubMed] [Google Scholar]
- Carr P. A., Yamamoto T., Karmy G., Baimbridge K. G., Nagy J. I. (1989). Analysis of parvalbumin and calbindin D28k-immunoreactive neurons in dorsal root ganglia of rat in relation to their cytochrome oxidase and carbonic anhydrase content. Neuroscience 33, 363–371 [DOI] [PubMed] [Google Scholar]
- Chen S., Rio C., Ji R. R., Dikkes P., Coggeshall R. E., Woolf C. J., Corfas G. (2003). Disruption of ErbB receptor signaling in adult non-myelinating Schwann cells causes progressive sensory loss. Nat. Neurosci. 6, 1186–1193 [DOI] [PubMed] [Google Scholar]
- Chen S., Velardez M. O., Warot X., Yu Z. X., Miller S. J., Cros D., Corfas G. (2006). Neuregulin 1-erbB signaling is necessary for normal myelination and sensory function. J. Neurosci. 26, 3079–3086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Hancock M. L., Role L. W., Talmage D. A. (2010). Intramembranous valine linked to schizophrenia is required for neuregulin 1 regulation of the morphological development of cortical neurons. J. Neurosci. 30, 9199–9208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Gore B. B., Long H., Ma L., Tessier-Lavigne M. (2008). Alternative splicing of the Robo3 axon guidance receptor governs the midline switch from attraction to repulsion. Neuron 58, 325–332 [DOI] [PubMed] [Google Scholar]
- Davies A. M. (1998). Neuronal survival: early dependence on Schwann cells. Curr. Biol. 8, R15–R18 [DOI] [PubMed] [Google Scholar]
- Davies A. M., Lumsden A. (1990). Ontogeny of the somatosensory system: origins and early development of primary sensory neurons. Annu. Rev. Neurosci. 13, 61–73 [DOI] [PubMed] [Google Scholar]
- Deckwerth T. L., Elliott J. L., Knudson C. M., Johnson E. M., Jr, Snider W. D., Korsmeyer S. J. (1996). BAX is required for neuronal death after trophic factor deprivation and during development. Neuron 17, 401–411 [DOI] [PubMed] [Google Scholar]
- Endo M., Ohashi K., Mizuno K. (2007). LIM kinase and slingshot are critical for neurite extension. J. Biol. Chem. 282, 13692–13702 [DOI] [PubMed] [Google Scholar]
- Ernfors P. (2001). Local and target-derived actions of neurotrophins during peripheral nervous system development. Cell. Mol. Life Sci. 58, 1036–1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falls D. L. (2003). Neuregulins: functions, forms, and signaling strategies. Exp.Cell Res. 284, 14–30 [DOI] [PubMed] [Google Scholar]
- Fitzgerald M., Kwiat G. C., Middleton J., Pini A. (1993). Ventral spinal cord inhibition of neurite outgrowth from embryonic rat dorsal root ganglia. Development 117, 1377–1384 [DOI] [PubMed] [Google Scholar]
- Fu S. Y., Sharma K., Luo Y., Raper J. A., Frank E. (2000). SEMA3A regulates developing sensory projections in the chicken spinal cord. J. Neurobiol. 45, 227–236 [PubMed] [Google Scholar]
- Gassmann M., Casagranda F., Orioli D., Simon H., Lai C., Klein R., Lemke G. (1995). Aberrant neural and cardiac development in mice lacking the ErbB4 neuregulin receptor. Nature 378, 390–394 [DOI] [PubMed] [Google Scholar]
- Gu C., Rodriguez E. R., Reimert D. V., Shu T., Fritzsch B., Richards L. J., Kolodkin A. L., Ginty D. D. (2003). Neuropilin-1 conveys semaphorin and VEGF signaling during neural and cardiovascular development. Dev. Cell 5, 45–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock M. L., Canetta S. E., Role L. W., Talmage D. A. (2008). Presynaptic type III neuregulin1-ErbB signaling targets {alpha}7 nicotinic acetylcholine receptors to axons. J. Cell Biol. 181, 511–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Z., Tessier-Lavigne M. (1997). Neuropilin is a receptor for the axonal chemorepellent Semaphorin III. Cell 90, 739–751 [DOI] [PubMed] [Google Scholar]
- He Z., Wang K. C., Koprivica V., Ming G., Song H. J. (2002). Knowing how to navigate: mechanisms of semaphorin signaling in the nervous system. Sci. STKE 2002, re1 [DOI] [PubMed] [Google Scholar]
- Hippenmeyer S., Shneider N. A., Birchmeier C., Burden S. J., Jessell T. M., Arber S. (2002). A role for neuregulin1 signaling in muscle spindle differentiation. Neuron 36, 1035–1049 [DOI] [PubMed] [Google Scholar]
- Ho W. H., Armanini M. P., Nuijens A., Phillips H. S., Osheroff P. L. (1995). Sensory and motor neuron-derived factor. A novel heregulin variant highly expressed in sensory and motor neurons. J. Biol. Chem. 270, 26722 [PubMed] [Google Scholar]
- Honma Y., Kawano M., Kohsaka S., Ogawa M. (2010). Axonal projections of mechanoreceptive dorsal root ganglion neurons depend on Ret. Development 137, 2319–2328 [DOI] [PubMed] [Google Scholar]
- Hory-Lee F., Russell M., Lindsay R. M., Frank E. (1993). Neurotrophin 3 supports the survival of developing muscle sensory neurons in culture. Proc. Natl. Acad. Sci. USA 90, 2613–2617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huettl R. E., Soellner H., Bianchi E., Novitch B. G., Huber A. B. (2011). Npn-1 contributes to axon-axon interactions that differentially control sensory and motor innervation of the limb. PLoS Biol. 9, e1001020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitsukawa T., Shimizu M., Sanbo M., Hirata T., Taniguchi M., Bekku Y., Yagi T., Fujisawa H. (1997). Neuropilin-semaphorin III/D-mediated chemorepulsive signals play a crucial role in peripheral nerve projection in mice. Neuron 19, 995–1005 [DOI] [PubMed] [Google Scholar]
- Kokai Y., Cohen J. A., Drebin J. A., Greene M. I. (1987). Stage- and tissue-specific expression of the neu oncogene in rat development. Proc. Natl. Acad. Sci. USA 84, 8498–8501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodkin A. L., Levengood D. V., Rowe E. G., Tai Y. T., Giger R. J., Ginty D. D. (1997). Neuropilin is a semaphorin III receptor. Cell 90, 753–762 [DOI] [PubMed] [Google Scholar]
- Kramer R., Bucay N., Kane D. J., Martin L. E., Tarpley J. E., Theill L. E. (1996). Neuregulins with an Ig-like domain are essential for mouse myocardial and neuronal development. Proc. Natl. Acad. Sci. USA 93, 4833–4838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law C. O., Kirby R. J., Aghamohammadzadeh S., Furley A. J. (2008). The neural adhesion molecule TAG-1 modulates responses of sensory axons to diffusible guidance signals. Development 135, 2361–2371 [DOI] [PubMed] [Google Scholar]
- Lawson S. N., Waddell P. J. (1991). Soma neurofilament immunoreactivity is related to cell size and fibre conduction velocity in rat primary sensory neurons. J. Physiol. 435, 41–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemke G. (2006). Neuregulin-1 and myelination. Sci. STKE 2006, pe11 [DOI] [PubMed] [Google Scholar]
- Messersmith E. K., Leonardo E. D., Shatz C. J., Tessier-Lavigne M., Goodman C. S., Kolodkin A. L. (1995). Semaphorin III can function as a selective chemorepellent to pattern sensory projections in the spinal cord. Neuron 14, 949–959 [DOI] [PubMed] [Google Scholar]
- Meyer D., Birchmeier C. (1995). Multiple essential functions of neuregulin in development. Nature 378, 386–90 [DOI] [PubMed] [Google Scholar]
- Meyer D., Yamaai T., Garratt A., Riethmacher-Sonnenberg E., Kane D., Theill L. E., Birchmeier C. (1997). Isoform-specific expression and function of neuregulin. Development 124, 3575–3586 [DOI] [PubMed] [Google Scholar]
- Michailov G. V., Sereda M. W., Brinkmann B. G., Fischer T. M., Haug B., Birchmeier C., Role L., Lai C., Schwab M. H., Nave K. A. (2004). Axonal neuregulin-1 regulates myelin sheath thickness. Science 304, 700–703 [DOI] [PubMed] [Google Scholar]
- Morris J. K., Lin W., Hauser C., Marchuk Y., Getman D., Lee K. F. (1999). Rescue of the cardiac defect in ErbB2 mutant mice reveals essential roles of ErbB2 in peripheral nervous system development. Neuron 23, 273–283 [DOI] [PubMed] [Google Scholar]
- Nawabi H., Briancon-Marjollet A., Clark C., Sanyas I., Takamatsu H., Okuno T., Kumanogoh A., Bozon M., Takeshima K., Yoshida Y., et al. (2010). A midline switch of receptor processing regulates commissural axon guidance in vertebrates. Genes Dev. 24, 396–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley R. A., Garner A. S., Large T. H., Frank E. (1995). Muscle sensory neurons require neurotrophin-3 from peripheral tissues during the period of normal cell death. Development 121, 1341–1350 [DOI] [PubMed] [Google Scholar]
- Ozaki S., Snider W. D. (1997). Initial trajectories of sensory axons toward laminar targets in the developing mouse spinal cord. J. Comp. Neurol. 380, 215–229 [PubMed] [Google Scholar]
- Parra L. M., Zou Y. (2009). Sonic hedgehog induces response of commissural axons to Semaphorin repulsion during midline crossing. Nat. Neurosci. 13, 29–35 [DOI] [PubMed] [Google Scholar]
- Patel T. D., Jackman A., Rice F. L., Kucera J., Snider W. D. (2000). Development of sensory neurons in the absence of NGF/TrkA signaling in vivo. Neuron 25, 345–357 [DOI] [PubMed] [Google Scholar]
- Patel T. D., Kramer I., Kucera J., Niederkofler V., Jessell T. M., Arber S., Snider W. D. (2003). Peripheral NT3 signaling is required for ETS protein expression and central patterning of proprioceptive sensory afferents. Neuron 38, 403–416 [DOI] [PubMed] [Google Scholar]
- Pearson R. J., Jr, Carroll S. L. (2004). ErbB transmembrane tyrosine kinase receptors are expressed by sensory and motor neurons projecting into sciatic nerve. J. Histochem. Cytochem. 52, 1299–1311 [DOI] [PubMed] [Google Scholar]
- Phan K. D., Hazen V. M., Frendo M., Jia Z., Butler S. J. (2010). The bone morphogenetic protein roof plate chemorepellent regulates the rate of commissural axonal growth. J. Neurosci. 30, 15430–15440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pond A., Roche F. K., Letourneau P. C. (2002). Temporal regulation of neuropilin-1 expression and sensitivity to semaphorin 3A in NGF- and NT3-responsive chick sensory neurons. J. Neurobiol. 51, 43–53 [DOI] [PubMed] [Google Scholar]
- Puschel A. W., Adams R. H., Betz H. (1996). The sensory innervation of the mouse spinal cord may be patterned by differential expression of and differential responsiveness to semaphorins. Mol. Cell. Neurosci. 7, 419–431 [DOI] [PubMed] [Google Scholar]
- Riethmacher D., Sonnenberg-Riethmacher E., Brinkmann V., Yamaai T., Lewin G. R., Birchmeier C. (1997). Severe neuropathies in mice with targeted mutations in the ErbB3 receptor. Nature 389, 725–730 [DOI] [PubMed] [Google Scholar]
- Snider W. D., Silos-Santiago I. (1996). Dorsal root ganglion neurons require functional neurotrophin receptors for survival during development. Philos. Trans. R. Soc. Lond. B Biol. Sci. 351, 395–403 [DOI] [PubMed] [Google Scholar]
- Taveggia C., Zanazzi G., Petrylak A., Yano H., Rosenbluth J., Einheber S., Xu X., Esper R. M., Loeb J. A., Shrager P., et al. (2005). Neuregulin-1 type III determines the ensheathment fate of axons. Neuron 47, 681–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessier-Lavigne M., Goodman C. S. (1996). The molecular biology of axon guidance. Science 274, 1123–1133 [DOI] [PubMed] [Google Scholar]
- Thoenen H., Bandtlow C., Heumann R., Lindholm D., Meyer M., Rohrer H. (1988). Nerve growth factor: cellular localization and regulation of synthesis. Cell. Mol. Neurobiol. 8, 35–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. Y., Frenzel K. E., Wen D., Falls D. L. (1998). Transmembrane neuregulins interact with LIM kinase 1, a cytoplasmic protein kinase implicated in development of visuospatial cognition. J. Biol. Chem. 273, 20525–20534 [DOI] [PubMed] [Google Scholar]
- Wang J. Y., Miller S. J., Falls D. L. (2001). The N-terminal region of neuregulin isoforms determines the accumulation of cell surface and released neuregulin ectodomain. J. Biol. Chem. 276, 2841–2851 [DOI] [PubMed] [Google Scholar]
- White F. A., Behar O. (2000). The development and subsequent elimination of aberrant peripheral axon projections in Semaphorin3A null mutant mice. Dev. Biol. 225, 79–86 [DOI] [PubMed] [Google Scholar]
- White F. A., Silos-Santiago I., Molliver D. C., Nishimura M., Phillips H., Barbacid M., Snider W. D. (1996). Synchronous onset of NGF and TrkA survival dependence in developing dorsal root ganglia. J. Neurosci. 16, 4662–4672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woldeyesus M. T., Britsch S., Riethmacher D., Xu L., Sonnenberg-Riethmacher E., Abou-Rebyeh F., Harvey R., Caroni P., Birchmeier C. (1999). Peripheral nervous system defects in erbB2 mutants following genetic rescue of heart development. Genes Dev. 13, 2538–2548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpowitz D., Mason T. B., Dietrich P., Mendelsohn M., Talmage D. A., Role L. W. (2000). Cysteine-rich domain isoforms of the neuregulin-1 gene are required for maintenance of peripheral synapses. Neuron 25, 79–91 [DOI] [PubMed] [Google Scholar]
- Wright D. E., White F. A., Gerfen R. W., Silos-Santiago I., Snider W. D. (1995). The guidance molecule semaphorin III is expressed in regions of spinal cord and periphery avoided by growing sensory axons. J. Comp. Neurol. 361, 321–333 [DOI] [PubMed] [Google Scholar]
- Wu K. Y., Hengst U., Cox L. J., Macosko E. Z., Jeromin A., Urquhart E. R., Jaffrey S. R. (2005). Local translation of RhoA regulates growth cone collapse. Nature 436, 1020–1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Kuo Y., Devay P., Yu C., Role L. (1998). A cysteine-rich isoform of neuregulin controls the level of expression of neuronal nicotinic receptor channels during synaptogenesis. Neuron 20, 255–270 [DOI] [PubMed] [Google Scholar]
- Yaron A., Huang P. H., Cheng H. J., Tessier-Lavigne M. (2005). Differential requirement for Plexin-A3 and -A4 in mediating responses of sensory and sympathetic neurons to distinct class 3 Semaphorins. Neuron 45, 513–523 [DOI] [PubMed] [Google Scholar]
- Zhong C., Du C., Hancock M., Mertz M., Talmage D. A., Role L. W. (2008). Presynaptic type III neuregulin 1 is required for sustained enhancement of hippocampal transmission by nicotine and for axonal targeting of alpha7 nicotinic acetylcholine receptors. J. Neurosci. 28, 9111–9116 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.