Abstract
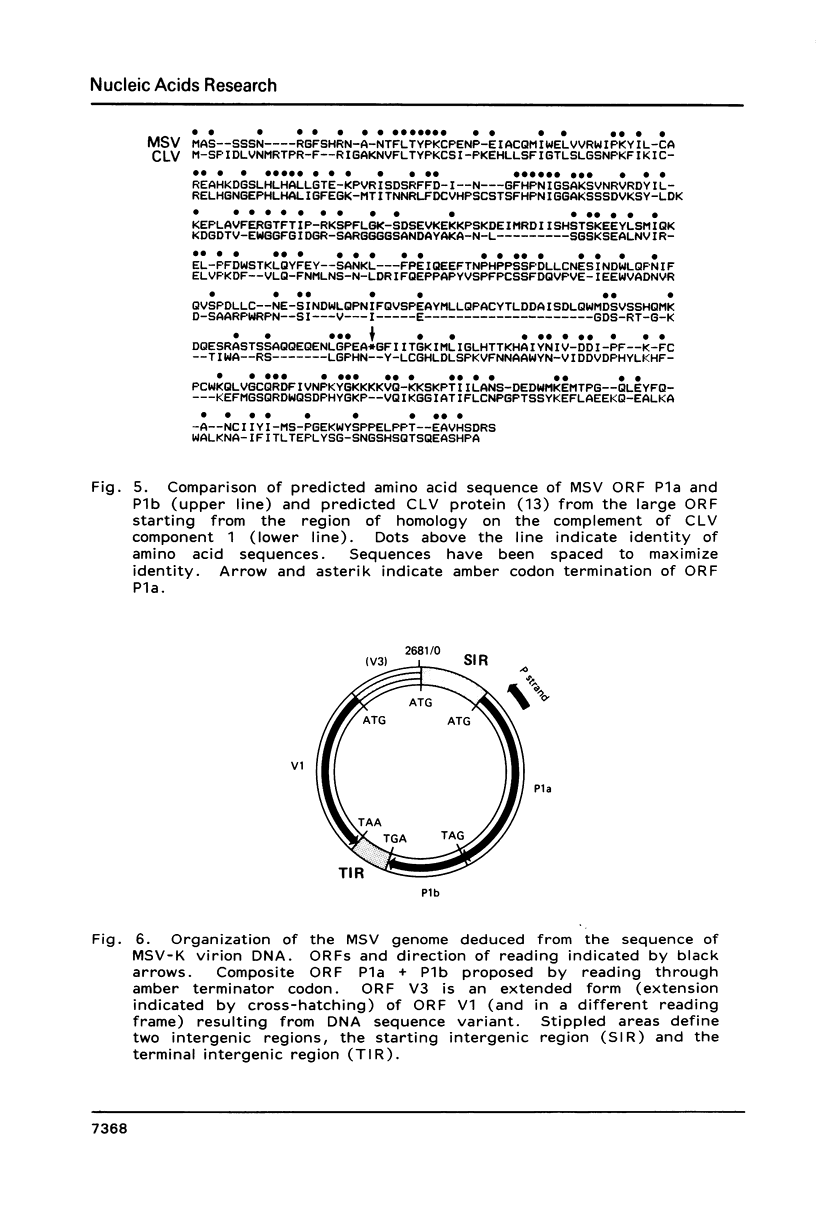
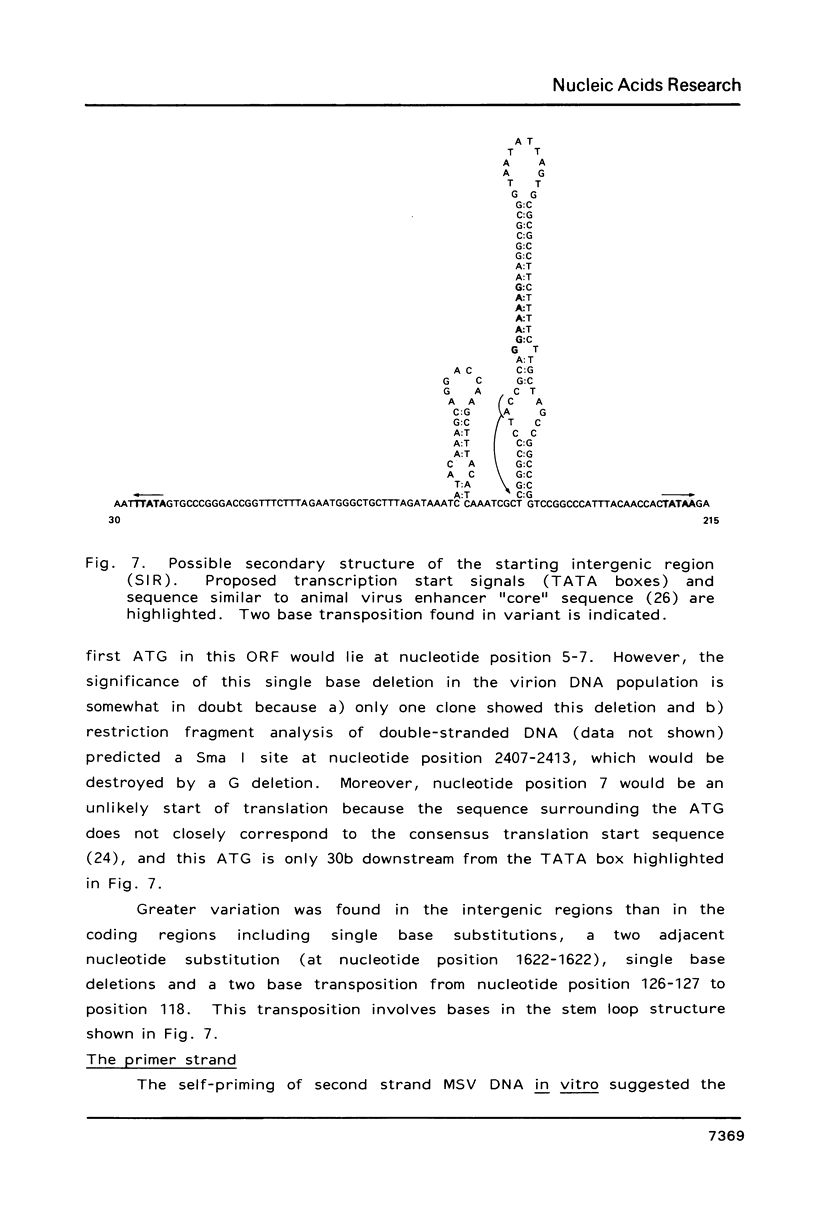
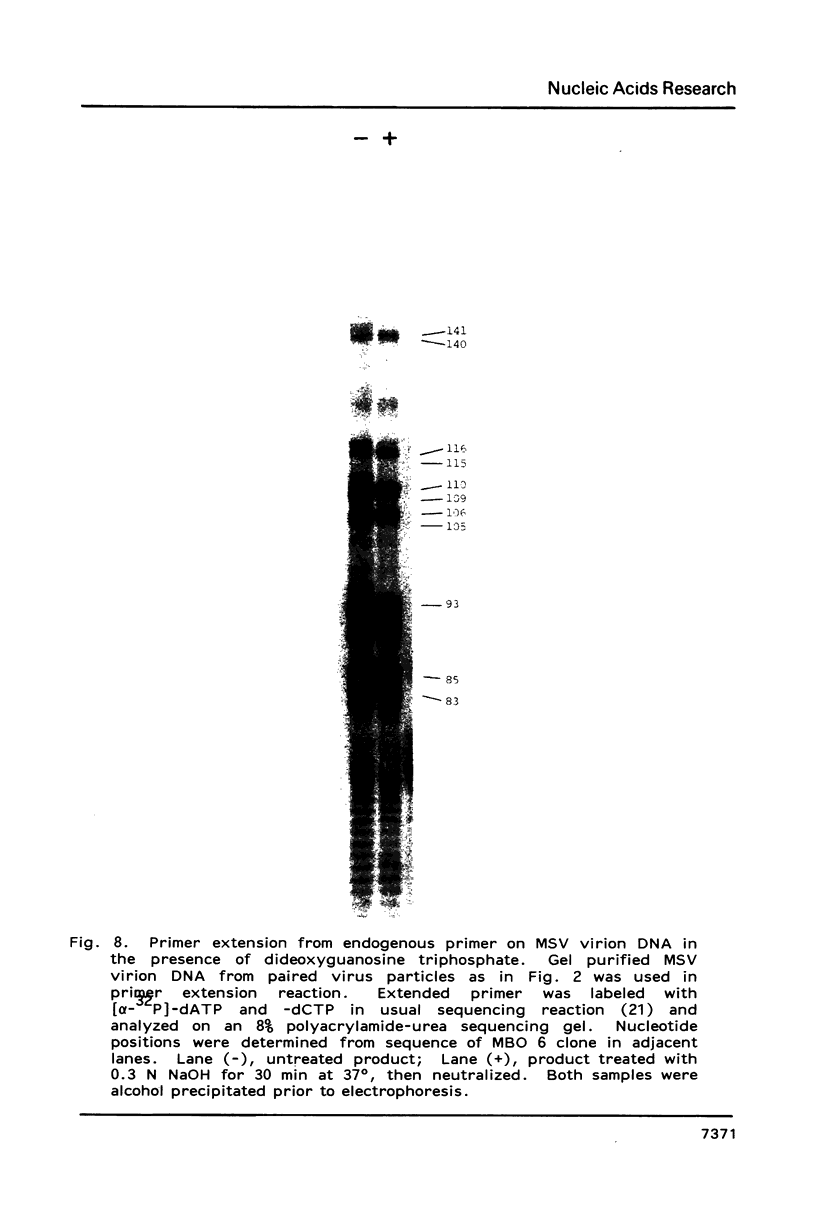
The structure of the maize streak virus genome (Kenyan isolate, MSV-K), as determined from the sequence of clones obtained from DNA isolated from virus particles, is composed of one major DNA component of about 2.6 kb. MSV virion DNA is partially double-stranded, composed of a full-length virion (V) strand and a short (70-80b) primer (P) strand. The primer strand has a fixed 5'-end capped with alkaline labile material, presumably 1-2 ribonucleotides. The MSV genome has two major coding regions oriented on opposite strands and flanked by two small intergenic regions. The coding region on the P strand is composed of two major open reading frames (ORFs), arranged in tandem and in the same reading frame. Because the predicted protein derived from a composite of these two ORFs closely corresponds to the product from a single ORF in the cassava latent virus genome, it is likely that this region encodes two proteins with common amino-termini, one a read-through product of the amber codon terminator in the first ORF. The intergenic regions contain potential transcription start and stop signals oriented in the direction of the two opposing coding regions. Considerable DNA sequence heterogeneity was observed, mostly silent or conservative third base substitutions in coding regions and base substitutions, small insertions and small, close-range transpositions in intergenic regions.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bisaro D. M., Hamilton W. D., Coutts R. H., Buck K. W. Molecular cloning and characterisation of the two DNA components of tomato golden mosaic virus. Nucleic Acids Res. 1982 Aug 25;10(16):4913–4922. doi: 10.1093/nar/10.16.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock K. R., Guthrie E. J., Meredith G., Barker H. RNA and protein components of maize streak and cassava latent viruses. Ann Appl Biol. 1977 Mar;85(2):305–308. doi: 10.1111/j.1744-7348.1977.tb01804.x. [DOI] [PubMed] [Google Scholar]
- Gay N. J., Walker J. E. Homology between human bladder carcinoma oncogene product and mitochondrial ATP-synthase. Nature. 1983 Jan 20;301(5897):262–264. doi: 10.1038/301262a0. [DOI] [PubMed] [Google Scholar]
- Goelet P., Lomonossoff G. P., Butler P. J., Akam M. E., Gait M. J., Karn J. Nucleotide sequence of tobacco mosaic virus RNA. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5818–5822. doi: 10.1073/pnas.79.19.5818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilley H., Richards K. E., Jonard G. Observations concerning the discontinuous DNAs of cauliflower mosaic virus. EMBO J. 1983;2(2):277–282. doi: 10.1002/j.1460-2075.1983.tb01417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton W. D., Bisaro D. M., Buck K. W. Identification of novel DNA forms in tomato golden mosaic virus infected tissue. Evidence for a two component viral genome. Nucleic Acids Res. 1982 Aug 25;10(16):4901–4912. doi: 10.1093/nar/10.16.4901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton W. D., Bisaro D. M., Coutts R. H., Buck K. W. Demonstration of the bipartite nature of the genome of a single-stranded DNA plant virus by infection with the cloned DNA components. Nucleic Acids Res. 1983 Nov 11;11(21):7387–7396. doi: 10.1093/nar/11.21.7387. [DOI] [PMC free article] [PubMed] [Google Scholar]
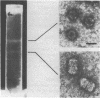
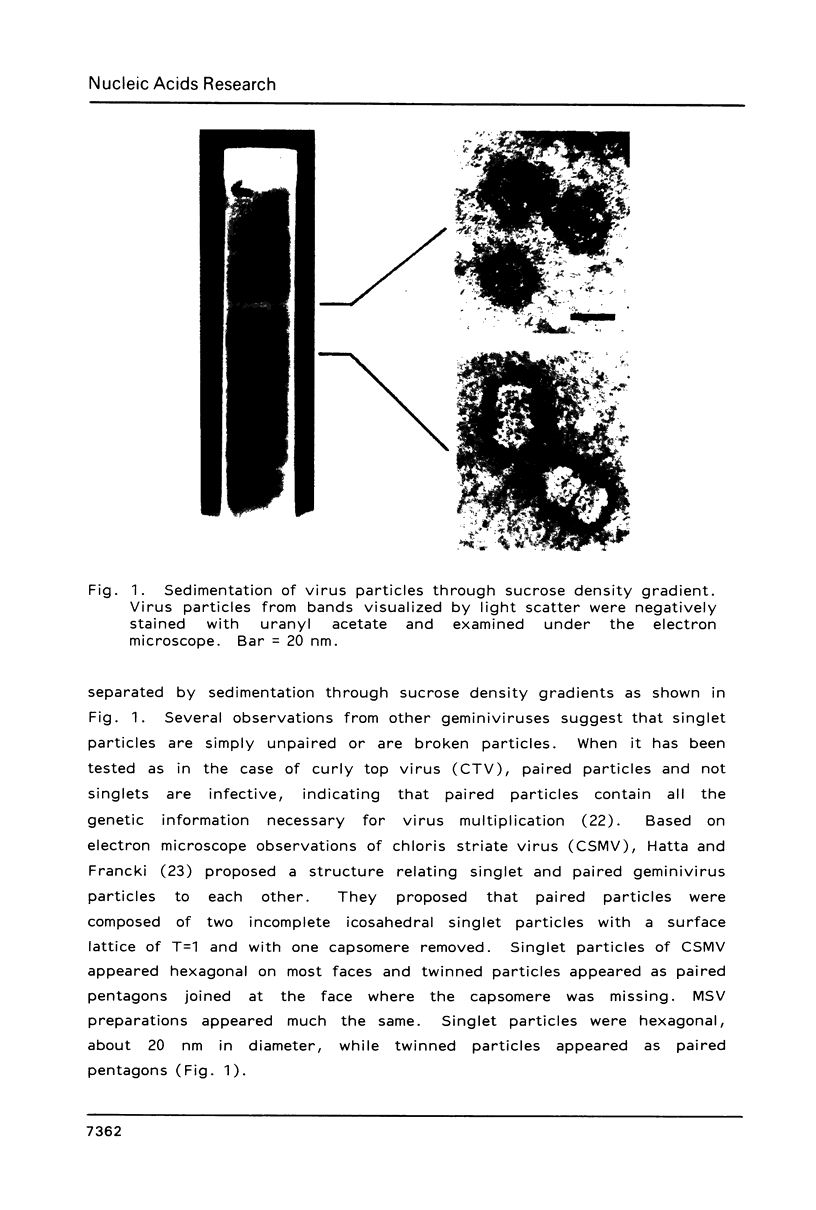
- Hatta T., Francki R. I. The fine structure of chloris striate mosaic virus. Virology. 1979 Jan 30;92(2):428–435. doi: 10.1016/0042-6822(79)90147-8. [DOI] [PubMed] [Google Scholar]
- Hull R., Covey S. N. Characterisation of cauliflower mosaic virus DNA forms isolated from infected turnip leaves. Nucleic Acids Res. 1983 Mar 25;11(6):1881–1895. doi: 10.1093/nar/11.6.1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull R., Davies J. W. Genetic engineering with plant viruses, and their potential as vectors. Adv Virus Res. 1983;28:1–33. doi: 10.1016/s0065-3527(08)60720-4. [DOI] [PubMed] [Google Scholar]
- Ikegami M., Haber S., Goodman R. M. Isolation and characterization of virus-specific double-stranded DNA from tissues infected by bean golden mosaic virus. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4102–4106. doi: 10.1073/pnas.78.7.4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohli J., Grosjean H. Usage of the three termination codons: compilation and analysis of the known eukaryotic and prokaryotic translation termination sequences. Mol Gen Genet. 1981;182(3):430–439. doi: 10.1007/BF00293932. [DOI] [PubMed] [Google Scholar]
- Kozak M. Point mutations close to the AUG initiator codon affect the efficiency of translation of rat preproinsulin in vivo. Nature. 1984 Mar 15;308(5956):241–246. doi: 10.1038/308241a0. [DOI] [PubMed] [Google Scholar]
- Marco Y., Howell S. H. Intracellular forms of viral DNA consistent with a model of reverse transcriptional replication of the cauliflower mosaic virus genome. Nucleic Acids Res. 1984 Feb 10;12(3):1517–1528. doi: 10.1093/nar/12.3.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews R. E. Third report of the International Committee on Taxonomy of Viruses. Classification and nomenclature of viruses. Intervirology. 1979;12(3-5):129–296. doi: 10.1159/000149081. [DOI] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Richards K. E., Guilley H., Jonard G. Further characterization of the discontinuities in cauliflower mosaic virus DNA. FEBS Lett. 1981 Nov 2;134(1):67–70. doi: 10.1016/0014-5793(81)80552-2. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunter G., Coutts R. H., Buck K. W. Negatively supercoiled DNA from plants infected with a single-stranded DNA virus. Biochem Biophys Res Commun. 1984 Feb 14;118(3):747–752. doi: 10.1016/0006-291x(84)91458-x. [DOI] [PubMed] [Google Scholar]
- Tiollais P., Charnay P., Vyas G. N. Biology of hepatitis B virus. Science. 1981 Jul 24;213(4506):406–411. doi: 10.1126/science.6264599. [DOI] [PubMed] [Google Scholar]
- Weiher H., König M., Gruss P. Multiple point mutations affecting the simian virus 40 enhancer. Science. 1983 Feb 11;219(4585):626–631. doi: 10.1126/science.6297005. [DOI] [PubMed] [Google Scholar]