Abstract
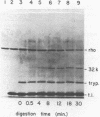
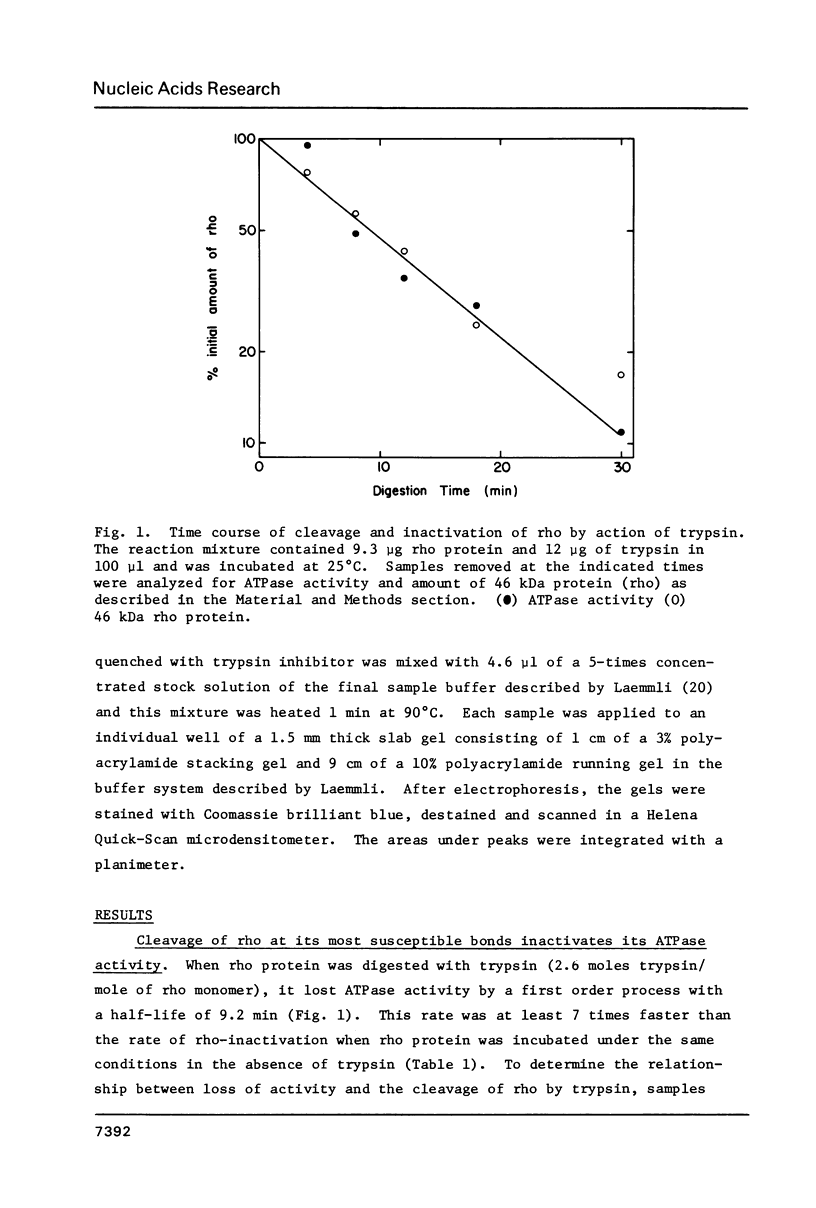
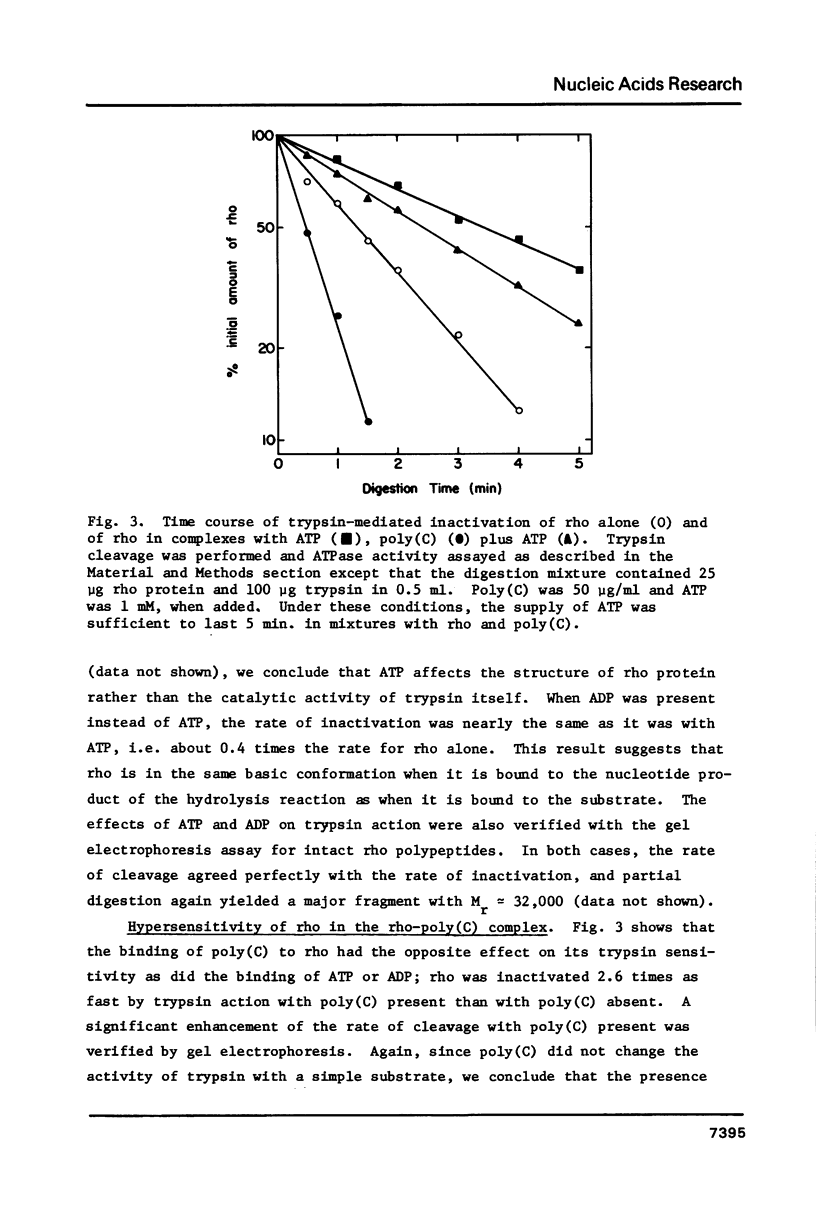
Transcription termination protein rho from Escherichia coli possesses an RNA-dependent ATP hydrolysis activity necessary for expression of its termination function. We have used the rate of trypsin-mediated inactivation of ATPase activity as a conformational probe to test for ligand binding-induced conformational changes in the rho polypeptide. When present in molar excess over rho polypeptide, trypsin inactivates rho ATPase by a first order process that correlates well with the loss of intact rho polypeptide. When rho protein binds poly(C) or poly(dC), its susceptible bonds become more accessible to trypsin action. On the other hand, when rho binds either ATP or ADP those bonds become less accessible. These results suggest that rho protein assumes an altered conformation when an RNA cofactor is bound and that is assumes a distinctly different conformation when a nucleotide substrate or product is bound. A special change in the accessibility of trypsin-susceptible bonds is also detected when rho in its complex with poly(C) is catalyzing the hydrolysis of ATP.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adhya S., Gottesman M. Control of transcription termination. Annu Rev Biochem. 1978;47:967–996. doi: 10.1146/annurev.bi.47.070178.004535. [DOI] [PubMed] [Google Scholar]
- BREMER H., KONRAD M. W. A COMPLEX OF ENZYMATICALLY SYNTHESIZED RNA AND TEMPLATE DNA. Proc Natl Acad Sci U S A. 1964 May;51:801–808. doi: 10.1073/pnas.51.5.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglass J., Blumenthal T. Conformational transition of protein synthesis elongation factor Tu induced by guanine nucleotides. Modulation by kirromycin and elongation factor Ts. J Biol Chem. 1979 Jun 25;254(12):5383–5387. [PubMed] [Google Scholar]
- Finger L. R., Richardson J. P. Procedure for purification of Escherichia coli ribonucleic acid synthesis termination protein rho. Biochemistry. 1981 Mar 17;20(6):1640–1645. doi: 10.1021/bi00509a036. [DOI] [PubMed] [Google Scholar]
- Finger L. R., Richardson J. P. Stabilization of the hexameric form of Escherichia coli protein rho under ATP hydrolysis conditions. J Mol Biol. 1982 Mar 25;156(1):203–219. doi: 10.1016/0022-2836(82)90467-3. [DOI] [PubMed] [Google Scholar]
- Galluppi G. R., Richardson J. P. ATP-induced changes in the binding of RNA synthesis termination protein Rho to RNA. J Mol Biol. 1980 Apr 15;138(3):513–539. doi: 10.1016/s0022-2836(80)80016-7. [DOI] [PubMed] [Google Scholar]
- Howard B. H., de Crombrugghe B. ATPase activity required for termination of transcription by the Escherichia coli protein factor rho. J Biol Chem. 1976 Apr 25;251(8):2520–2524. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lau L. F., Roberts J. W., Wu R. RNA polymerase pausing and transcript release at the lambda tR1 terminator in vitro. J Biol Chem. 1983 Aug 10;258(15):9391–9397. [PubMed] [Google Scholar]
- Lowery-Goldhammer C., Richardson J. P. An RNA-dependent nucleoside triphosphate phosphohydrolase (ATPase) associated with rho termination factor. Proc Natl Acad Sci U S A. 1974 May;71(5):2003–2007. doi: 10.1073/pnas.71.5.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowery C., Richardson J. P. Characterization of the nucleoside triphosphate phosphohydrolase (ATPase) activity of RNA synthesi termination factor p. I. Enzymatic properties and effects of inhibitors. J Biol Chem. 1977 Feb 25;252(4):1375–1380. [PubMed] [Google Scholar]
- Naito S., Ishihama A. Isolation and properties of the transcription complex of Escherichia coli RNA polymerase. Biochim Biophys Acta. 1975 Aug 6;402(1):88–104. doi: 10.1016/0005-2787(75)90373-1. [DOI] [PubMed] [Google Scholar]
- Pinkham J. L., Platt T. The nucleotide sequence of the rho gene of E. coli K-12. Nucleic Acids Res. 1983 Jun 11;11(11):3531–3545. doi: 10.1093/nar/11.11.3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt T. Termination of transcription and its regulation in the tryptophan operon of E. coli. Cell. 1981 Apr;24(1):10–23. doi: 10.1016/0092-8674(81)90496-7. [DOI] [PubMed] [Google Scholar]
- Richardson J. P. Activation of rho protein ATPase requires simultaneous interaction at two kinds of nucleic acid-binding sites. J Biol Chem. 1982 May 25;257(10):5760–5766. [PubMed] [Google Scholar]
- Richardson J. P., Conaway R. Ribonucleic acid release activity of transcription termination protein rho is dependent on the hydrolysis of nucleoside triphosphates. Biochemistry. 1980 Sep 2;19(18):4293–4299. doi: 10.1021/bi00559a022. [DOI] [PubMed] [Google Scholar]
- Richardson J. P. Enzymic synthesis of RNA from T7 DNA. J Mol Biol. 1966 Oct 28;21(1):115–127. doi: 10.1016/0022-2836(66)90083-0. [DOI] [PubMed] [Google Scholar]
- Roberts J. W. Termination factor for RNA synthesis. Nature. 1969 Dec 20;224(5225):1168–1174. doi: 10.1038/2241168a0. [DOI] [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- Schendel P. F., Wells R. D. The synthesis and purification of (gamma-32P)-adenosine triphosphate with high specific activity. J Biol Chem. 1973 Dec 10;248(23):8319–8321. [PubMed] [Google Scholar]
- Shigesada K., Wu C. W. Studies of RNA release reaction catalyzed by E. coli transcription termination factor rho using isolated ternary transcription complexes. Nucleic Acids Res. 1980 Aug 11;8(15):3355–3369. doi: 10.1093/nar/8.15.3355. [DOI] [PMC free article] [PubMed] [Google Scholar]