Abstract
Increased consumption of cruciferous vegetables such as broccoli may reduce the risk of various cancers. Myrosinase is required to convert dietary glucosinolates from broccoli into bioactive isothiocyanates. We evaluated isothiocyanate excretion profiles in healthy subjects who consumed broccoli sprouts or broccoli supplement (no myrosinase) with equivalent glucosinolate content. Urinary metabolites of two major isothiocyanates, sulforaphane and erucin, were measured by liquid chromatography coupled with tandem mass spectrometry. Peak excretion of sulforaphane and erucin was higher and occurred sooner in subjects who consumed broccoli sprouts compared to subjects who consumed the supplement. A subject-dependent shift in the ratio of urinary sulforaphane to erucin metabolites was observed in both groups indicating conversion of sulforaphane to erucin. Lower histone deacetylase activity was observed in the peripheral blood mononuclear cells only in subjects consuming sprouts. In conclusion, fresh broccoli sprouts differ from broccoli supplements in regards to excretion of isothiocyanates and bioactivity in human subjects.
Keywords: isothiocyanate, sulforphane, erucin, histone deacetylase, broccoli sprouts, supplement
Introduction
Epidemiological data indicates that dietary consumption of cruciferous vegetables may reduce the risk of prostate, breast, lung and colorectal cancers (1). Cruciferous vegetables contain high levels of glucosinolates, a class of phytochemicals not found in any other vegetable. Glucosinolates are stable compounds that require enzymatic hydrolysis by myrosinase, a β-thioglucosidase present only in the plant and in gut microbial flora, to form many different metabolites. Myrosinase cleaves the glucosinolate forming glucose, hydrogen sulfate and either a thiocyanate, nitrile, or an isothiocyanate (ITC) depending on the starting glucosinolate, reaction pH, and availability of transition metal ions (2). ITCs are the putative bioactive constituents of cruciferous vegetables. Once consumed, ITCs are metabolized through the mercapturic acid pathway and ultimately excreted in the urine predominately as the N-acetylcysteine conjugates. Intense interest surrounds the use of ITCs to inhibit cancer development and growth at many stages in cancer progression. Sulforaphane (SFN) is an aliphatic ITC that has shown promise as a chemopreventive agent both in vitro and in vivo (3). The closely related aliphatic ITC erucin (ERN) has also received attention due to its similar structure and activity to SFN. The classic chemoprevention mechanism for SFN involves induction of phase 2 enzymes thereby facilitating detoxification of carcinogens and other genotoxic stresses. Another chemopreventive mechanism by which SFN is reported to work is through inhibition of histone deacetylases (HDACs) which causes an increase in histone acetylation at the promoters of aberrantly silenced tumor suppressor genes, reactivating the tumor suppressor and ultimately inducing cell cycle arrest and/or apoptosis (4–7). Broccoli sprouts contains high amounts of glucoraphanin and glucoerucin, the glucosinolate precursors to SFN and ERN, respectively (8). Although total glucoraphanin content increases as the broccoli plant matures, the concentration of glucoraphanin per plant weight is highest in the seeds followed by the sprouts (9). The amount of pre-clinical and clinical data for the chemopreventive effects of broccoli and ITCs is strong and growing (10).
Many cancer patients in the United States use dietary supplements which represents a large proportion of complementary and alternative medicine (11). A meta-analysis of vitamin and mineral supplement use in US adults after cancer diagnosis reported that between 64% and 81 % of cancer survivors (combined cancer site data) reported using a supplement (11). dietary constituents is evident there are limited data regarding in vivo efficacy of whole foods versus supplements. Broccoli supplements contain glucosinolates rather than their cognate ITCs because glucosinolates are more stable than ITCs. It has been speculated that consuming broccoli supplements will not produce high plasma and urinary concentrations of the bioactive ITCs (12). Currently little is known regarding the metabolism of glucosinolates in supplement form. One of the main issues regarding the utility of broccoli supplements is the efficacious conversion of the glucosinolates to ITCs because many supplements do not contain active myrosinase enzymes. A small number of studies in animals and humans have found that inactivation of myrosinase decreases availability of ITCs (12–18). Importantly, none of these studies profiled all of the major ITC metabolites by liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS). Several studies used the cyclocondensation reaction which does not distinguish between different ITCs or between their various metabolites, while other studies used LC-MS/MS but only measured the mercapturic acid conjugate in the urine. Furthermore, a majority of the research has focused on the pharmacokinetics and bioactivity of SFN while only one human study has looked into kinetics of ERN excretion (19). The current study was undertaken to assess differences in the metabolism and excretion of glucoraphanin and glucoerucin metabolites in subjects consuming broccoli sprouts versus broccoli supplement and test if consumption of an ITC-rich food will alter HDAC activity in healthy human subjects.
Materials and Methods
Participants
Twelve men and 12 women aged 19–50 y were recruited in and around Corvallis, Oregon. For most analyses one man and one woman were excluded due to issues with protocol compliance. See each table and graph for the number of subjects used. The study was conducted in the Nutrition and Exercise Sciences department's metabolic kitchen and clinical collection lab at Oregon State University. Exclusion criteria included: smokers, vegetarians, anemic, engaged in vigorous activity for more than 6 h per week, or history of viral diseases, high blood pressure, high blood cholesterol, abnormal blood chemistries or urinary tract problems. All subjects gave written, informed consent to participate in the study. The study protocol was reviewed and approved by the Institutional Review Boards at Oregon State University and the Ohio State University.
Interventions
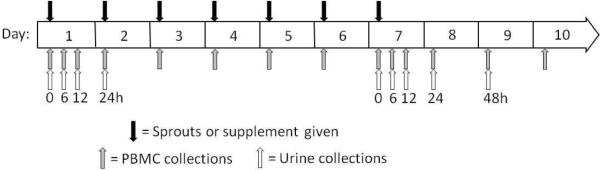
Subjects were randomized into two groups; either broccoli sprouts or BroccoMax® a commercially available broccoli supplement. A single lot of broccoli sprouts were obtained from Sprouters Northwest, Inc (Kent, WA) and BroccoMax® was obtained from Jarrow Formulas (Los Angeles, CA). Subjects avoided eating foods that contain glucosinolates or isothiocyanates for 24 h prior to the beginning of the study and throughout the duration of the study. Subjects participated in a pre-study meeting in which the protocol was explained and subjects were taught how to accurately keep dietary records by a registered dietician (RD). The RD was available throughout the study to assist with diet records. Subjects kept dietary records for the first 7 days of the study. Subjects fasted every morning prior to sprouts or supplement and blood draws. Subjects consumed either 68 g of broccoli sprouts or 6 BroccoMax® pills (~3 g of freeze dried broccoli sprouts in 6 pills) with a breakfast consisting of bagels with cream cheese and orange juice every morning for 7 days (Figure 1). On days 8–10 the same breakfast was served except without sprouts or supplement.
Figure 1. Research design and timeline.
Black arrows indicate when subjects received sprouts or supplement, grey arrows indicate when blood was drawn and PBMCs were isolated and white arrows indicate when a complete urine collection was sampled.
Study protocol
Complete urine and whole blood were collected at different times in the study (Figure 1). On day 1 urine was collected prior to consumption of sprouts or supplement and total urine was collected during the following time blocks: 0–6, 6–12, and 12–24 h after sprout or supplement. On day 7, urine was collected prior to consumption of sprouts or supplement and total urine was collected during the following time blocks: 0–6, 6–12, 12–24, and 24–48 h post sprout or supplement. Urine volume was recorded and aliquots were frozen at −80°C until analysis. Ten mL of whole blood were collected by venipuncture into a vacutainer containing EDTA at the same time points urine samples were collected. In addition whole blood was also collected on the mornings of days 3, 4, 5, 6 and 10 (Figure 1). Phlebotomy was performed in the Nutrition and Exercise Sciences Department Clinical Collection Lab by a trained phlebotomist.
Peripheral blood mononuclear cell (PBMC) isolation
Approximately 10 mL of whole blood were carefully layered on 10 mL of room temperature Histopaque® 1077 (Sigma-Aldrich, St. Louis, MO). The blood was then centrifuged at 400 × g for 30 minutes at room temperature. The PBMCs form a cloudy band at the interface between the Histopaque and the plasma. The PBMCs were collected and washed with PBS. Following the wash the PBMCs were centrifuged again at 300 RCF for 10 minutes at room temperature. To ensure no red cell contamination in the PBMCs, the cell pellet was resuspended in 1 mL of red cell lysis buffer (0.15 M NH4Cl, 1.0 mM KHCO3 and 0.1 mM EDTA) and allowed to sit on ice for 5 minutes. The cells were washed 2 more times with PBS. Cells were then lysed in IP lysis buffer with protease inhibitors and proteins were quantified using RC DC™ protein assay (Bio-Rad, Hercules, CA).
Glucosinolate analysis
Glucoiberin, glucoraphanin and glucoerucin were purchased from The Royal Veterinary School of Denmark. The glucosinolate quantitative method as described by Tian et al. (8) was used with some modification. Broccoli sprouts were frozen fresh in liquid nitrogen and stored at −80°C until analysis. Prior to extraction, previously flash-frozen sprouts (liquid nitrogen) were steamed in a strainer above a pot of boiling water with a lid for five minutes to destroy endogenous myrosinase activity. Steamed sprouts were chopped finely and 5 g was extracted three times with 20 mL of 70 % (v/v) methanol/water, each time for 10 min at room temperature in a bath sonicator. A portion of the pooled extract was centrifuged, filtered through a 0.22 μm nylon syringe filter and diluted twenty fold with 0.1 % (v/v) formic acid in water prior to ultra-high performance LC-MS/MS (UPLC-MS/MS) analysis as described by Tian et al. (8). Broccomax supplement capsules (0.7 g) were extracted with 5 mL of 70% aqueous methanol three times. HPLC was conducted on an Acquity system (Waters Corp., Milford, MA) and eluent interfaced with a triple quadrupole mass spectrometer (Quattro Ultima, Micromass, UK) for MS/MS analysis.
Preparation of isothiocyanate standards
SFN and ERN were purchased from LKT laboratories Inc (St. Paul, MN). SFN-cysteinylglycine (SFN-CG), ERN-glutathione (ERN-GSH), ERN-cysteinylglycine (ERN-CG), ERN-cysteine (ERN-Cys) and ERN-N-acetylcysteine (ERN-NAC) were prepared following methods described by Vermeulen et al. (20) in which isothiocyanates and their respective conjugate groups were reacted, to generate the various metabolites, e.g. SFN + CG for SFN−CG. The reaction mixtures were purified by semi-preparative reversed phase chromatography (10 × 250mm, 5 μm C18 Bondapak, Waters Corp, Milford, MA) with a water/acetonitrile mobile phase. ACN in metabolite fractions was removed by Rotovap and the remaining aqueous phase freeze-dried to achieve a powder. Powders were weighed and extinction coefficients at appropriate wavelengths determined as the average of triplicate determinations. Each metabolite displayed spectra with two characteristic UV features at 250 and 270 nm. HPLC was performed and compounds were shown to be spectrally pure (all >95%). The molar extinction coefficients at 270 nm in methanol, in M−1·cm−1, were 7551 SFN-Cys, 4796 SFN-CG, 7334 SFN-GSH, 6832 SFN-NAC, 2302 ERN-CG, 1609 ERN-Cys, 1653 ERN-GSH, 3391 ERN-NAC.
Analysis of isothiocyanates
For sample preparation, urine was diluted 1:10 with 0.1 % (v/v) formic acid in water. Plasma processing was slightly modified from that of Janobi et al. (21). Cold trifluoroacetic acid (TFA) (0°C) was added at 10 % (v/v) to plasma that had been pre-chilled on ice. After 5 minutes on ice the cloudy suspension was centrifuged at 16,000 × g for five min to pellet the proteins and recover metabolites in the supernatant. Supernatant was injected directly (10 uL) for quantitative analysis.
UPLC chromatography was as follows: Acquity BEH C18 (2.1 × 100mm, 1.7 μm) with a mobile phase of 0.1 % (v/v) formic acid in water versus 0.1 % (v/v) formic acid in acetonitrile at 0.45 mL/min at 40°C. Five μL was injected onto the column. Initially the mobile phase was 0% B increased linearly to 10% B at 1 min, 33.3% B at 2.5 min, 72% B at 4 min (curve 7) and returned to 0% B by 6 min. HPLC eluent was interfaced without flow splitting to a triple quadrupole mass spectrometer (Quattro Ultima, Micromass, UK) via an electrospray probe operated in positive mode. Selected reaction monitoring (SRM) MS/MS transitions were developed for each of nine analytes using collision induced dissociation (CID) – sulforaphane (178>114), SFN-GSH (485>136), SFN-CG (356>136), SFN-Cys (299>136), SFN-NAC (341>114), ERN-ERN (469>179), ERN-CG (340>103), ERN-Cys (283>103), and ERN-NAC (325>164) with dwell times of 80–150 ms. Source parameters included capillary 3.2 kV, desolvation temperature 450°C, cone voltage 35 V, RF1 12.5 V, collision energy (10–18 eV), and CID argon pressure (3 × 10−3 mBar). Reproducible chromatography and MS response could not be achieved with free ERN and thus it was omitted from the analysis.
HDAC activity assay
HDAC activity was assayed using the 386 well format Fluor-de-Lys HDAC activity kit (Upstate) as described in (6). Approximately 5 μg of total PBMC lysate were added to each well and the assay was performed according to the manufacturer's instructions. Fluorescence was measured and recorded as arbitrary fluorescence units using a Spectra Max Gemini XS fluorescent plate reader (Molecular Devices, Sunnyvale, CA).
Statistical analysis
Repeated measures two-way ANOVA and Student's t-test were performed using GraphPad Prism version 5.00 for Windows, GraphPad Software, San Diego California USA, www.graphpad.com as indicated in the applicable tables and graphs.
Results
Subjects' gender, age and body mass index (BMI) are shown in Table 1. No significant differences were found in these subject characteristics between dietary groups. One male and one female subject were not included in further biochemical analyses due to issues with protocol compliance. Diet records indicate that the total intake and percent of required intake for calories, protein, carbohydrates and fats were similar between the broccoli sprout and broccoli supplement groups during the study period, although the percent of intake for protein was significantly higher in the sprout group (Table 2).
Table 1.
Demographics of subjectsa
| Sprouts | Supplement | |||
|---|---|---|---|---|
| Age | BMI | Age | BMI | |
| Male(n=11) | 27.8 ± 3.1 | 25.4 ± 1.6 | 28.8 ± 2.1 | 29.5 ± 1.5 |
| Female (n=12) | 31.5 ± 5.7 | 21.7 ± 1.3 | 29.8 ± 3.9 | 22.5 ± 1.0 |
Mean ± SEM.
Table 2.
Total caloric intake and macronutrient intake from food records (days 1–7)a
| Sprouts (n=12) | Supplement (n=11) | |||
|---|---|---|---|---|
| Total intake | % required | Total intake | % required | |
| Caloric (kcal) | 2552 ± 172 | 102.1 ± 6.8 | 2484 ± 174 | 91.6 ± 5.3 |
| Protein (g) | 96.2 ± 7.1 | 171.9 ± 15.1 | 86.0 ± 7.7 | 131.3 ± 9.2b |
| Carbohydrate (g) | 365.1 ± 21.3 | 106.3 ± 6.2 | 344.9 ± 23.1 | 93.0 ± 5.8 |
| Fat(g) | 77.3 ± 7.6 | 99.0 ± 9.0 | 85.2 ± 7.9 | 95.2 ± 11.8 |
Mean ± SEM
p<0.05, Student's t-test between percent required for sprouts and supplement groups
Three glucosinolates, glucoraphanin, glucoerucin and glucoiberin were quantified in the sprouts and supplement. Table 3 shows the total μmol of each glucosinolate that was consumed per 68 g of sprouts or 6 pills of supplement (i.e. per day). Total glucosinolate content was equal between the broccoli sprout and Broccomax supplement intervention groups. Interestingly, even though the amounts of glucosinolates administered to each group was the same, there was a 5-fold lower amount of SFN metabolites and an 8-fold lower amount of ERN metabolites excreted in the urine during 24 hours after consumption in the supplement consumers compared to the sprout consumers (Table 4). There was no change or accumulation in the amount of metabolites excreted after repeated 7 day consumption of sprouts or supplement (Table 4), which is consistent with previous reports (22). Although plasma samples were collected during this study we were unaware of the need to acidify the plasma in order to stabilize the metabolites as per Al Janobi et al (21). Nevertheless we expect all samples were similarly impacted and we observed that the relative quantity of the metabolites in the plasma was much lower in the supplement group as compared to the sprout group (data not shown). This suggests that differences in urinary metabolite excretion levels between sprout and supplement groups were due to differences in the amount of bioavailable ITCs rather than retention.
Table 3.
Total μmol of glucosinolates per consumption of broccoli sprouts or broccoli supplementa
| Glucosinolate | Sprouts (μmol/68g FWb) | Supplement (μmol/6 pills) | t-testc p-value |
|---|---|---|---|
| Glucoraphanin | 218.4 ± 24.4 | 220.3 ± 3.3 | 0.964 |
| Glucoerucin | 67.1 ± 8.5 | 75.9 ± 0.8 | 0.553 |
| Glucoiberin | 33.8 ± 4.0 | 31.7 ± 0.4 | 0.764 |
Mean ± SEM
Fresh weight
Student's t-test between sprouts and supplement groups for each glucosinolate
Table 4.
Total μmol and percent of SFN compounds and ERN compounds excreted in urine during 24 h after consumption of broccoli sprouts or broccoli supplementa
| Sprouts | Supplement | |||
|---|---|---|---|---|
| Day 1: single dose (n=10b) | Day 7: repeated dose (n=10) | Day 1: single dose (n=11) | Day 7: repeated dose (n=11) | |
| SFN | ||||
| Total μmolc | 192 ± 23 | 217 ± 27 | 41.3 ± 14.8 | 40.8 ± 10.6 |
| % excreted | 96 ± 9 | 91 ± 11 | 19 ± 7 | 19 ± 5 |
| ERN | ||||
| Total μmold | 125 ± 10 | 134 ± 19 | 13.1 ± 1.4 | 18.5 ± 3.2 |
| % excreted | 187 ± 12 | 182 ± 27 | 17 ± 2 | 24 ± 4 |
Mean ± SEM. No significant dose number x treatment group interactions (repeated measures two-way ANOVA).
n=10 because one subject did not have an entire urine collection at one time point and therefore could not be included in the two-way ANOVA.
Significant treatment group, p<0.0001 (repeated measures two-way ANOVA). Subject matching, p<0.0001
Significant treatment group, p<0.0001 (repeated measures two-way ANOVA). Subject matching, p=0.0015
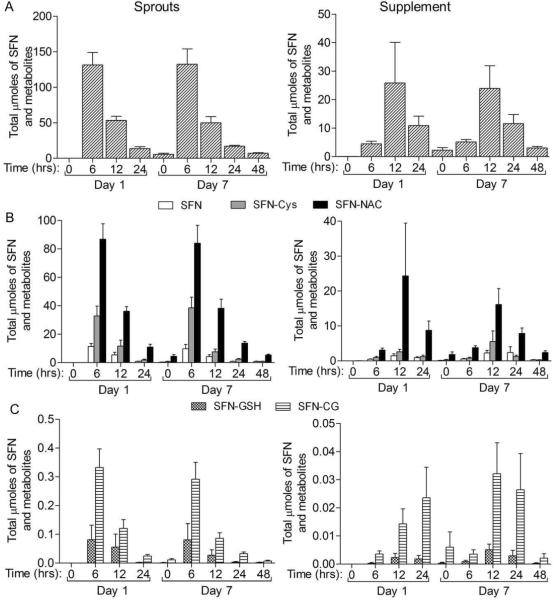
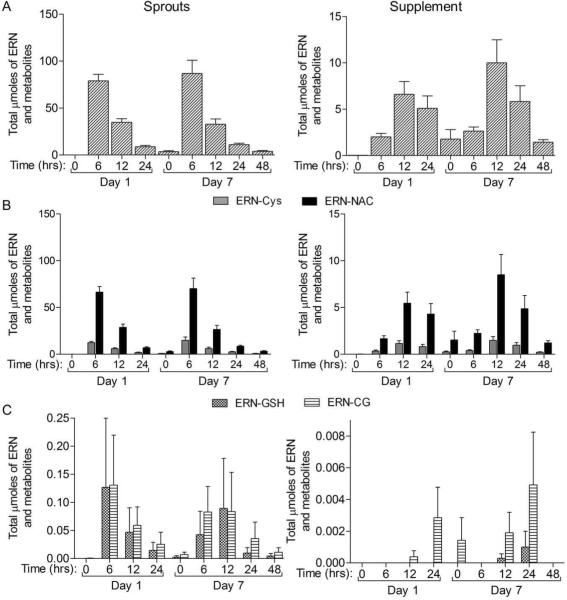
The timing of metabolite excretion was also different between sprout and supplement groups. The peak SFN and ERN metabolite excretion was between 0–6 h post consumption for the sprout group, but at 6−12 h post consumption in the supplement group (Figure 2). This peak in excretion can also be observed when looking at the total μmoles of each metabolite over all time points collected (Figure 3). As expected SFN-NAC was the major metabolite followed by SFN-Cys, free SFN, SFN-CG and then SFN-GSH (Table 5 and Figure 2). Similarly, ERN-NAC was the most abundant of the ERN compounds followed be ERN-Cys, ERN-CG and then ERN-GSH (Table 5 and Figure 4). Although striking differences were observed in the total amounts and timing of SFN and ERN metabolites, the relative percent of each ITC metabolite within its respective group of metabolites was not drastically different between the two groups. For the ERN metabolites there was no difference in the percent of each metabolite between the two treatment groups (Table 5). Similar results were observed among the SFN metabolites although a small but significant shift in metabolite ratios was observed between the treatment groups, with a higher percent of SFN-Cys and lower percent of SFN-NAC observed in the sprout group compared to the supplement group (Table 5).
Figure 2. SFN and ERN metabolite excretion rates over first 24h of study.
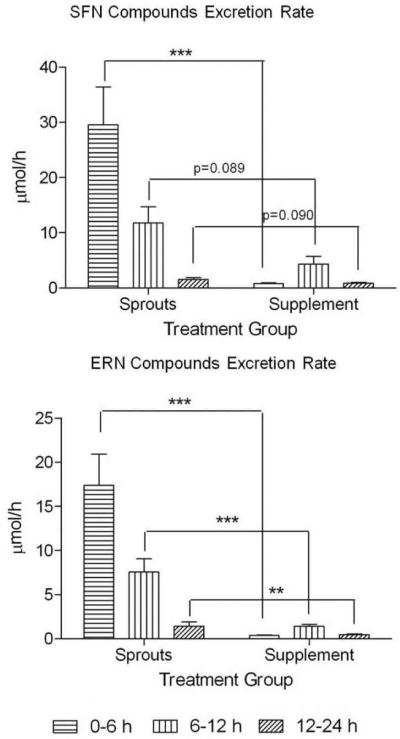
The peak in ERN and SFN metabolite excretion occurred during the 0–6 h time block in subjects consuming sprouts and during the 6–12 h time block in subjects consuming supplement. Data in bar graphs represent mean ± SEM (n=11). Statistical significance: **p<0.01, ***p<0.001 or p-value indicated using Student's t-test.
Figure 3. The μmoles of SFN and SFN metabolites excreted in the urine over the course of the study.
The time indicated is the time since last consumption of sprouts or supplement on either 1st or 7th day of consumption. Complete urine was collected from the previous time point to the time point indicated. A) All SFN compounds summed for each time block. B) The major metabolites in the urine, SFN-NAC and SFN-Cys and free SFN. C) The minor metabolites in the urine, SFN-CG and SFN-GSH. Data in bar graphs represent mean ± SEM (n=11).
Table 5.
Percent each SFN compound and each ERN compound represent within each group of compounds excreted during 24 h (Day 1 and Day 7) after consumption of broccoli sprouts or broccoli supplementa
| Sprouts | Supplement | ||
|---|---|---|---|
| %b | % | P-valuec | |
| SFN compounds | |||
| SFN | 7.8 ± 3.2 | 9.4 ± 4.3 | 0.334 |
| SFN-GSH | 0.09 ± 0.23 | 0.01 ± 0.01 | 0.291 |
| SFN-CG | 0.25 ± 0.20 | 0.13 ± 0.09 | 0.077 |
| SFN-Cys | 23.2 ± 7.2 | 16.1 ± 6.4 | 0.023 |
| SFN-NAC | 68.7 ± 6.8 | 74.4 ± 5.7 | 0.044 |
| ERN compounds | |||
| ERN-GSH | 0.15 ± 0.47 | 0.02 ± 0.04 | 0.391 |
| ERN-CG | 0.19 ± 0.42 | 0.001 ± 0.006 | 0.164 |
| ERN-Cys | 18.4 ± 4.7 | 17.1 ± 6.7 | 0.624 |
| ERN-NAC | 81.3 ± 4.6 | 82.8 ± 6.7 | 0.536 |
Mean ± SEM.
Percentage each compound represents within the its respective group of compounds (i.e. SFN compounds and ERN compounds)
Student's t-test between sprouts and supplement groups for percent of each metabolite
Figure 4. The μmoles of ERN metabolites excreted in the urine over the course of the study.
The time indicated is the time since last consumption of sprouts or supplement on either 1st or 7th day of consumption. Complete urine was collected from the previous time point to the time point indicated. A) All ERN compounds summed for each time block. B) The major metabolites in the urine, ERN-NAC and ERN-Cys. C) The minor metabolites in the urine, ERNCG and ERN-GSH. Data in bar graphs represent mean ± SEM (n=11).
We also looked at the ratio of ERN-NAC to SFN-NAC that was excreted in the urine and found that, although the starting ratio of glucoerucin to glucoraphanin was approximately 1:3, all but two subjects had an increase in this ratio indicating that some SFN may have converted to ERN (Figure 5A). Within each subject the ratio was consistent at all time points of the study (see error bars in Figure 5A) and each subject had a unique ratio ranging from a dominance of SFN-NAC in one subject to a dominance of ERN-NAC in another subject, with a majority of subjects (n=18) having more SFN-NAC than ERN-NAC which was expected since dietary glucoraphanin was in 3:1 excess of glucoerucin. There was no difference in the ratios between the treatment groups indicating that the source or absorption site (upper GI tract versus lower GI) of the glucosinolates did not affect the conversion of SFN to ERN (Figure 5B). As evidence that in vivo conversion is occurring rather than differential absorption of the two ITCs, we observed recovered well over 100% of the ERN metabolites (Table 4). Several groups have reported this interconversion between SFN and ERN (13, 19, 23) and here we provide further evidence for this possibility.
Figure 5. ERN-NAC to SFN-NAC ratios in subjects.
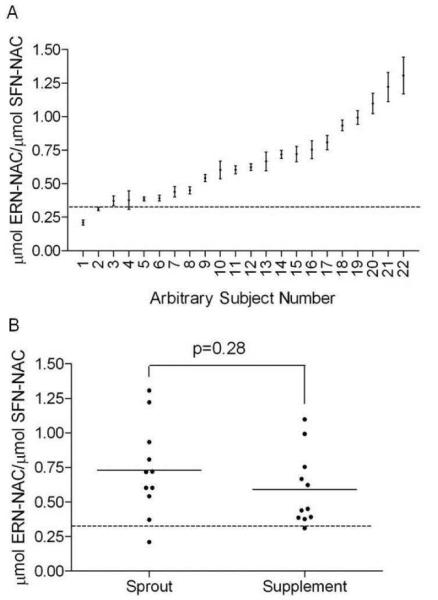
The dotted line in each graph represents the ratio of glucoerucin to glucoraphanin (in μmoles) each subject received in the sprouts or supplement (0.324 ± 0.009). A) The ratio of μmoles of ERN-NAC over μmoles of SFN-NAC for every subject over the entire study period. Each data point represents one subject and the subjects were ordered from lowest to highest ratio. Error bars represent SEM of all nine time points for that subject. B) Ratios for each subject, separated by sprout and supplement consumers. Each data point represents all nine time points for one subject. No significant difference between treatment groups as determined by Student's t-test.
HDAC activity in PBMCs was measured throughout the course of the study. Using repeated measures two-way ANOVA both treatment group and time have significant effects on HDAC activity (Figure 6). The level of HDAC activity in the supplement group was at or above baseline activity indicating that the lower in vivo concentrations had no inhibitory effect. In contrast the HDAC activity in the sprout group was below the baseline at most time points. At every time point tested the sprout group had lower HDAC activity than the supplement group although the difference was only statistically significant 12 and 48 h after the final dose of sprouts or supplement. These data provide further evidence that SFN can lower HDAC activity in vivo.
Figure 6. Histone deacetylase (HDAC) activity in peripheral blood mononuclear cells (PBMC) following sprout or supplement consumption.
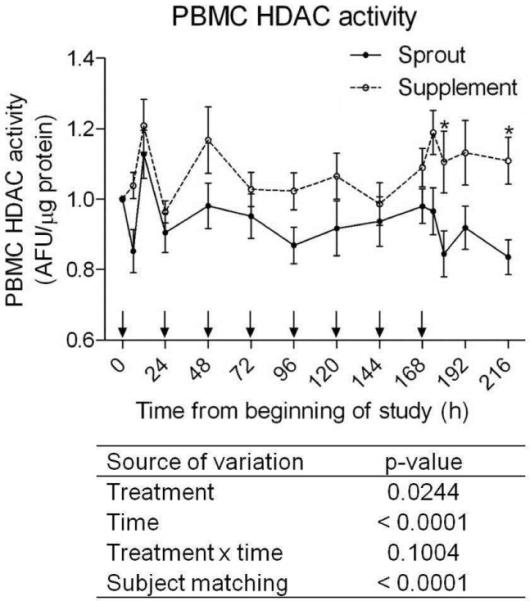
Each subject was normalized to their baseline HDAC activity (time zero). Solid circles and lines represent the sprout group and open circle and dotted lines represent the supplement group. Arrows along the x-axis indicate when sprouts or supplement were consumed. Each data point represent mean ± SEM (n=11). The data were analyzed by two-way ANOVA and p-values are shown below the graph.
Discussion
The current study shows that subjects who consumed a broccoli supplement, which does not contain active myrosinase, have 5 times lower excretion amounts of SFN metabolites, 8 fold lower excretion amounts of ERN metabolites and a delayed peak excretion compared to subjects that consumed fresh broccoli sprouts (Table 4 and Figure 2). Although several studies have looked at the effect of inactive myrosinase on ITC bioavailability and excretion (12–18), this is the first study to directly compare the excretion of ITCs, as measured by LC-MS/MS analysis of nearly all major and minor SFN and ERN metabolites formed in mercapturic acid metabolism, and bioactivity in human subjects who repeatedly consumed fresh broccoli sprouts to subjects who repeatedly consumed a broccoli supplement.
Broccoli supplements do not have active myrosinase and therefore conversion of the glucosinolates to the isothiocyanates in the subjects from that group was primarily dependent on the myrosinase activity from their gut microbial flora. This conversion was not as effective as the conversion from the plant endogenous myrosinase but was sufficient to produce as much as 25 μmoles of SFN metabolites at the excretion peak (Figure 3A). A recent study showed that rats given glucoraphanin either by gavage or in the diet only recovered 27.6% and 23.8% of the dose, respectively, in the urine after 24 h. In contrast, recovery in rats fed a broccoli floret diet, which contained myrosinase was 62.5%. Interestingly, hepatic NQO1 activity was only induced in rats fed the floret diet, not those fed glucosinolates without myrosinase, indicating that the induction of phase 2 enzymes, one bioactivity attributed to the ITCs, was lost when myrosinase was not present (12). Cooking broccoli also inactivates the myrosinase enzymes and it has been observed that the bioavailability of SFN is three to ten times greater from fresh broccoli compared to cooked broccoli (14, 15). Similar to our supplement group data, there was a delay in peak SFN plasma concentration with heat-inactivation of myrosinase in cooked broccoli (15). Another study showed that ITCs are six times more bioavailable than glucosinolates and chewing sprouts thoroughly to release the plant myrosinases rather than swallowing them intact increased the ITC excretion approximately two fold (16). Together these data confirm the importance of active myrosinases in increasing bioavailability of ITCs in vivo.
This is the first report to show detailed ERN metabolite data in human subjects using UPLC-MS/MS analysis. The metabolism and urinary excretion of ERN metabolites were similar to SFN metabolites with respect to peak excretion times and a predominant N-acetylcysteine metabolite. One difference between ERN and SFN was in the percentage of each metabolite that was excreted (Table 5). There was no change in the percentage of the various ERN metabolites between the treatment groups whereas subjects who consumed broccoli sprouts had an increase in the percent of SFN-Cys and a decrease in the percent of SFN-NAC metabolites excreted in the urine during 24 h post consumption as compared to the subjects who consumed the supplement (Table 5). This is consistent with the report by Gasper et al in which the treatment group that had the higher amount of SFN metabolites excreted in the urine had a slight and significant increase in the percentage of SFN-Cys and a slight but non-significant decrease in the percentage of SFN-NAC (24). In that study, the authors found that subjects who consumed three times more SFN metabolites (50 μmoles compared to 16 μmoles) had approximately three times higher peak plasma concentrations (7.3 μmol/L compared to 2.3 μmol/L). Although we could not accurately determine the plasma ITC metabolite concentrations in our study, it is conceivable that the subjects who consumed broccoli sprouts could have 2–4 times higher plasma concentrations of SFN metabolites than subjects who consumed 50 μmoles from Gasper et al since the subjects in our study consumed >300 μmoles of glucosinolates every day for 7 days, with approximately 220 μmoles of those glucosinolates being glucoraphanin, the precursor to SFN. Importantly, a phase I placebo controlled, double-blind, randomized clinical trial to assess toxicities of either glucosinolate (300 μmoles/day) or isothiocyanate (75 μmoles/day) preparations showed no toxicities or abnormal events occurred with any of the test extracts (25). Consistent with this, no adverse events were reported in our study indicating that the high dose of glucosinolates was well tolerated in our subjects.
Herein we provide further evidence for the possible interconversion between SFN and ERN and for the first time report that this interconversion favors conversion of SFN to ERN when human subjects consume either fresh broccoli sprouts or a broccoli supplement. This conversion is consistent within a single subject but variable between subjects through a period of 10 days. The starting ratio of glucoraphanin to glucoerucin was 3:1, or 75% glucoraphanin and 25% glucoerucin, and the ratio in the urine ranged from 43% to 80% of ITC metabolites as SFN metabolites, indicating that some of the SFN was converted to ERN in vivo. As further evidence that SFN is converted to ERN we observed an approximately 180% recovery of ERN metabolites in the urine from the subjects who consumed sprouts. Currently it is not known what dictates the in vivo balance between the reduced thioether ERN and its oxidized sulfoxide SFN, although one report indicates that ERN can be converted to SFN after reaction with hydroperoxides (26). In this study, since glucoraphanin, the source of SFN, was in large excess to ERN, it seems reasonable to expect the conversion to tend towards ERN metabolites. Currently much less is known about the mechanism and bioactivity of ERN compared to SFN but due to their structural similarities it is expected that they act in a similar manner. There have been several reports that have looked into the bioactivity of ERN and found that indeed the bioactivity of these two compounds is similar but not identical. Multiple reports have indicated similar induction of phase II enzymes by ERN and SFN in rat lung (27), duodenum and urinary bladder (28), although some enzyme and tissue specificity for each compound was noted. Another study showed that both ERN and SFN induced phase III detoxification via induction of multidrug resistance pump 1 and 2 (MRP1 and MRP2, respectively), although SFN was substantially more potent than ERN (29). Another report indicated that ERN was able to inhibit proliferation and modulate p53 and p21 protein expression in human lung cancer A549 cells, but again to a lesser degree than observed after SFN treatment (30). In contrast to these results, a study performed in Caco-2 colon cancer cells showed that ERN was substantially more effective at inducing G2/M cell cycle arrest, cell death, phase II enzymes and MRP2 (31). This may indicate that the potency of each compound may be dependent on the endpoint and/or cell line of interest. It has also been suggested that ERN can directly scavenge certain reactive oxygen species (ROS) and be converted to SFN (26). This possibility is intriguing because it would indicate that a higher amount of ERN would favor a modest induction of phase 2 enzymes by ERN itself as well as direct scavenging of ROS thereby forming SFN which is a more potent inducer of phase 2 enzymes. More research is required to determine what underlies the consistent interconversion of SFN and ERN within each individual and whether this interconversion is important for the chemopreventive effects of cruciferous vegetables.
Previously our group reported a preliminary human trial showing that consumption of broccoli sprouts can inhibit HDAC activity in PBMCs (7). Here we provide further evidence for this by showing that subjects consuming sprouts had higher urinary ITC levels and evidence of HDAC inhibition whereas subjects consuming the supplement had lower urinary ITC levels and did not demonstrate HDAC inhibition (Figure 6). Concerns have been raised regarding the impact of consuming dietary HDAC inhibitors on healthy individuals and a discussion regarding dietary HDAC inhibitors and their relative potencies compared to pharmacological HDAC inhibitors has been published (32). Importantly, here we show that the difference in HDAC activity between the groups was never greater than 30% and the decrease from baseline was never greater than 20%. This likely reflects the fact that the dietary HDAC inhibitors are weak ligands for the HDAC enzymes relative to pharmacological HDAC inhibitors. It still remains to be determined whether this change in human PBMC HDAC activity translates to changes in HDAC activity in specific tissues and whether it can influence chemoprevention.
In conclusion, we show that consumption of a broccoli supplement results in significantly lower amounts of ITC metabolites that are excreted in the urine which is likely due to the lack of myrosinase enzymes in the supplement. This finding has significant implications for people who consume broccoli supplements for the chemopreventive benefits and believe they are getting equivalent amounts of the bioactive ITCs as if they were consuming fresh broccoli sprouts. We also conclude that the conversion of SFN metabolites to ERN metabolites is a consistent occurrence. Overall, these data provide further information regarding the metabolism and bioactivity of SFN and ERN in human subjects.
Acknowledgements
We gratefully acknowledge Karin Hardin for performing the phlebotomy and assistance with sample processing. This work was supported by NIH grants CA122906, Oregon AES (OR00735), and the Environmental Health Science Center at Oregon State University (NIEHS P30 ES00210).
Support by funding: NIH grant CA122906
Abbreviations
- (BMI)
body mass index
- (CID)
collision induced dissociation
- (ERN)
erucin
- (ERN-CG)
ERN-cysteinylglycine
- (ERN-Cys)
ERN-cysteine
- (ERN-GSH)
ERN-glutathione
- (ERN-NAC)
ERN-N-acetylcysteine
- (HDACs)
histone deacetylases
- (ITC)
isothiocyanate
- (LC-MS/MS)
liquid chromatography coupled to tandem mass spectrometry
- (MRP1)
multidrug resistance pump 1
- (MRP2)
multidrug resistance pump 2
- (PBMC)
peripheral blood mononuclear cell
- (ROS)
reactive oxygen species
- (RD)
registered dietician
- (SRM)
selected reaction monitoring
- (SFN)
sulforaphane
- (SFN-CG)
SFN-cysteinylglycine
- (SFN-Cys)
SFN-Cysteine
- (SFN-GSH)
SFN-glutathione
- (SFN-NAC)
SFN-N-acetylcysteine
- (TFA)
trifluoroacetic acid
- (UPLC-MS/MS)
ultra-igh performance LC-MS/MS
Literature Cited
- 1.Higdon JV, Delage B, Williams DE, Dashwood RH. Cruciferous vegetables and human cancer risk: epidemiologic evidence and mechanistic basis. Pharmacol Res. 2007;55(3):224–36. doi: 10.1016/j.phrs.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bones AM, Rossiter JT. The myrosinase-glucosinolate system, its organisation and biochemistry. Physiologia Plantarum. 1996;97:194–208. [Google Scholar]
- 3.Clarke JD, Dashwood RH, Ho E. Multi-targeted prevention of cancer by sulforaphane. Cancer Letters. 2008;269(2):291–304. doi: 10.1016/j.canlet.2008.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Myzak MC, Dashwood WM, Orner GA, Ho E, Dashwood RH. Sulforaphane inhibits histone deacetylase in vivo and suppresses tumorigenesis in Apc-minus mice. Faseb J. 2006;20(3):506–8. doi: 10.1096/fj.05-4785fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Myzak MC, Hardin K, Wang R, Dashwood RH, Ho E. Sulforaphane inhibits histone deacetylase activity in BPH-1, LnCaP and PC-3 prostate epithelial cells. Carcinogenesis. 2006;27(4):811–819. doi: 10.1093/carcin/bgi265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Myzak MC, Karplus PA, Chung FL, Dashwood RH. A novel mechanism of chemoprotection by sulforaphane: inhibition of histone deacetylase. Cancer Research. 2004;64(16):5767–74. doi: 10.1158/0008-5472.CAN-04-1326. [DOI] [PubMed] [Google Scholar]
- 7.Myzak MC, Tong P, Dashwood WM, Dashwood RH, Ho E. Sulforaphane retards the growth of human PC-3 xenografts and inhibits HDAC activity in human subjects. Experimental Biology and Medicine. 2007;232(2):227–34. [PMC free article] [PubMed] [Google Scholar]
- 8.Tian Q, Rosselot RA, Schwartz SJ. Quantitative determination of intact glucosinolates in broccoli, broccoli sprouts, Brussels sprouts, and cauliflower by high-performance liquid chromatography-electrospray ionization-tandem mass spectrometry. Analytical Biochemistry. 2005;343(1):93–99. doi: 10.1016/j.ab.2005.04.045. [DOI] [PubMed] [Google Scholar]
- 9.Rangkadilok N, Nicolas ME, Bennett RN, Premier RR, Eagling DR, Taylor PWJ. Developmental changes of sinigrin and glucoraphanin in three Brassica species (Brassica nigra, Brassica juncea and Brassica oleracea var. italica) Scientia Horticulturae. 2002;96(1–4):11–26. [Google Scholar]
- 10.Jeffery EH, Keck AS. Translating knowledge generated by epidemiological and in vitro studies into dietary cancer prevention. Mol Nutr Food Res. 2008;52(Suppl 1):S7–17. doi: 10.1002/mnfr.200700226. [DOI] [PubMed] [Google Scholar]
- 11.Velicer CM, Ulrich CM. Vitamin and mineral supplement use among US adults after cancer diagnosis: a systematic review. J Clin Oncol. 2008;26(4):665–73. doi: 10.1200/JCO.2007.13.5905. [DOI] [PubMed] [Google Scholar]
- 12.Zhu N, Soendergaard M, Jeffery EH, Lai RH. The impact of loss of myrosinase on the bioactivity of broccoli products in F344 rats. J Agric Food Chem. 2010;58(3):1558–63. doi: 10.1021/jf9034817. [DOI] [PubMed] [Google Scholar]
- 13.Bheemreddy RM, Jeffery EH. The Metabolic Fate of Purified Glucoraphanin in F344 Rats. J. Agric. Food Chem. 2007;55(8):2861–2866. doi: 10.1021/jf0633544. [DOI] [PubMed] [Google Scholar]
- 14.Conaway CC, Getahun SM, Liebes LL, Pusateri DJ, Topham DKW, Botero-Omary M, Chung F-L. Disposition of Glucosinolates and Sulforaphane in Humans After Ingestion of Steamed and Fresh Broccoli. Nutrition and Cancer. 2000;38(2):168–178. doi: 10.1207/S15327914NC382_5. [DOI] [PubMed] [Google Scholar]
- 15.Vermeulen M, Klopping-Ketelaars IW, van den Berg R, Vaes WH. Bioavailability and kinetics of sulforaphane in humans after consumption of cooked versus raw broccoli. Journal of agricultural and food chemistry. 2008;56(22):10505–9. doi: 10.1021/jf801989e. [DOI] [PubMed] [Google Scholar]
- 16.Shapiro TA, Fahey JW, Wade KL, Stephenson KK, Talalay P. Chemoprotective Glucosinolates and Isothiocyanates of Broccoli Sprouts: Metabolism and Excretion in Humans. Cancer Epidemiol Biomarkers Prev. 2001;10(5):501–508. [PubMed] [Google Scholar]
- 17.Rungapamestry V, Duncan AJ, Fuller Z, Ratcliffe B. Effect of meal composition and cooking duration on the fate of sulforaphane following consumption of broccoli by healthy human subjects. Br J Nutr. 2007;97(4):644–52. doi: 10.1017/S0007114507381403. [DOI] [PubMed] [Google Scholar]
- 18.Cramer JM, Jeffery EH. Sulforaphane Absorption and Excretion Following Ingestion of a Semi-Purified Broccoli Powder Rich in Glucoraphanin and Broccoli Sprouts in Healthy Men. Nutrition and Cancer. 2011;63(2):196–201. doi: 10.1080/01635581.2011.523495. [DOI] [PubMed] [Google Scholar]
- 19.Vermeulen M, van den Berg R, Freidig AP, van Bladeren PJ, Vaes WH. Association between consumption of cruciferous vegetables and condiments and excretion in urine of isothiocyanate mercapturic acids. Journal of Agricultural and Food Chemistry. 2006;54(15):5350–8. doi: 10.1021/jf060723n. [DOI] [PubMed] [Google Scholar]
- 20.Vermeulen M, Zwanenburg B, Chittenden GJ, Verhagen H. Synthesis of isothiocyanate-derived mercapturic acids. European journal of medicinal chemistry. 2003;38(7–8):729–37. doi: 10.1016/s0223-5234(03)00141-7. [DOI] [PubMed] [Google Scholar]
- 21.Al Janobi AA, Mithen RF, Gasper AV, Shaw PN, Middleton RJ, Ortori CA, Barrett DA. Quantitative measurement of sulforaphane, iberin and their mercapturic acid pathway metabolites in human plasma and urine using liquid chromatography-tandem electrospray ionisation mass spectrometry. Journal of chromatography. 2006;844(2):223–34. doi: 10.1016/j.jchromb.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 22.Hanlon N, Coldham N, Gielbert A, Sauer MJ, Ioannides C. Repeated intake of broccoli does not lead to higher plasma levels of sulforaphane in human volunteers. Cancer Lett. 2009;284(1):15–20. doi: 10.1016/j.canlet.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 23.Kassahun K, Davis M, Hu P, Martin B, Baillie T. Biotransformation of the Naturally Occurring Isothiocyanate Sulforaphane in the Rat: Identification of Phase I Metabolites and Glutathione Conjugates. Chem. Res. Toxicol. 1997;10(11):1228–1233. doi: 10.1021/tx970080t. [DOI] [PubMed] [Google Scholar]
- 24.Gasper AV, Al-Janobi A, Smith JA, Bacon JR, Fortun P, Atherton C, Taylor MA, Hawkey CJ, Barrett DA, Mithen RF. Glutathione S-transferase M1 polymorphism and metabolism of sulforaphane from standard and high-glucosinolate broccoli. American Journal of Clinical Nutrition. 2005;82(6):1283–91. doi: 10.1093/ajcn/82.6.1283. [DOI] [PubMed] [Google Scholar]
- 25.Shapiro TA, Fahey JW, Dinkova-Kostova AT, Holtzclaw WD, Stephenson KK, Wade KL, Ye L, Talalay P. Safety, tolerance, and metabolism of broccoli sprout glucosinolates and isothiocyanates: a clinical phase I study. Nutrition and Cancer. 2006;55(1):53–62. doi: 10.1207/s15327914nc5501_7. [DOI] [PubMed] [Google Scholar]
- 26.Barillari J, Canistro D, Paolini M, Ferroni F, Pedulli GF, Iori R, Valgimigli L. Direct antioxidant activity of purified glucoerucin, the dietary secondary metabolite contained in rocket (Eruca sativa Mill.) seeds and sprouts. J Agric Food Chem. 2005;53(7):2475–82. doi: 10.1021/jf047945a. [DOI] [PubMed] [Google Scholar]
- 27.Hanlon N, Coldham N, Sauer MJ, Ioannides C. Modulation of rat pulmonary carcinogen-metabolising enzyme systems by the isothiocyanates erucin and sulforaphane. Chem Biol Interact. 2009;177(2):115–20. doi: 10.1016/j.cbi.2008.08.015. [DOI] [PubMed] [Google Scholar]
- 28.Munday R, Munday CM. Induction of phase II detoxification enzymes in rats by plant-derived isothiocyanates: comparison of allyl isothiocyanate with sulforaphane and related compounds. Journal of agricultural and food chemistry. 2004;52(7):1867–71. doi: 10.1021/jf030549s. [DOI] [PubMed] [Google Scholar]
- 29.Harris KE, Jeffery EH. Sulforaphane and erucin increase MRP1 and MRP2 in human carcinoma cell lines. J Nutr Biochem. 2008;19(4):246–54. doi: 10.1016/j.jnutbio.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 30.Melchini A, Costa C, Traka M, Miceli N, Mithen R, De Pasquale R, Trovato A. Erucin, a new promising cancer chemopreventive agent from rocket salads, shows anti-proliferative activity on human lung carcinoma A549 cells. Food Chem Toxicol. 2009;47(7):1430–6. doi: 10.1016/j.fct.2009.03.024. [DOI] [PubMed] [Google Scholar]
- 31.Jakubikova J, Sedlak J, Mithen R, Bao Y. Role of PI3K/Akt and MEK/ERK signaling pathways in sulforaphane- and erucin-induced phase II enzymes and MRP2 transcription, G2/M arrest and cell death in Caco-2 cells. Biochemical Pharmacology. 2005;69(11):1543–52. doi: 10.1016/j.bcp.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 32.Dashwood RH, Myzak MC, Ho E. Dietary HDAC inhibitors: time to rethink weak ligands in cancer chemoprevention? Carcinogenesis. 2006;27(2):344–9. doi: 10.1093/carcin/bgi253. [DOI] [PMC free article] [PubMed] [Google Scholar]