Abstract
Rational
Transplantation of human CD34+ stem cells to ischemic tissues has been associated with reduced angina, improved exercise time and reduced amputation rates in phase 2 clinical trials and has been shown to induce neo-vascularization in pre-clinical models. Previous studies have suggested that paracrine factors secreted by these pro-angiogenic cells are responsible, at least in part, for the angiogenic effects induced by CD34+ cell transplantation.
Objective
Our objective was to investigate the mechanism of CD34+ stem cell induced pro-angiogenic paracrine effects and to examine if exosomes, a component of paracrine secretion, are involved.
Methods and Results
Exosomes collected from the conditioned media of mobilized human CD34+ cells had the characteristic size (40–90 nm; determined via dynamic light scattering), cup-shaped morphology (electron microscopy), expressed exosome-marker proteins CD63, phosphatidylserine (flow cytometry) and TSG101 (immunoblotting), besides expressing CD34+ cell lineage marker protein, CD34. In vitro, CD34+ exosomes replicated the angiogenic activity of CD34+ cells by increasing endothelial cell viability, proliferation and tube formation on Matrigel. In vivo, the CD34+ exosomes stimulated angiogenesis in Matrigel plug and corneal assays. Interestingly, exosomes from CD34+ cells, but not from CD34+ cell-depleted mononuclear cells had angiogenic activity.
Conclusions
Our data demonstrate that human CD34+ cells secrete exosomes that have independent angiogenic activity both in vitro and in vivo. CD34+ exosomes may represent a significant component of the paracrine effect of progenitor-cell transplantation for therapeutic angiogenesis.
Keywords: CD34+ cells, paracrine factor, exosomes, angiogenesis
Introduction
Clinical studies have provided evidence that locally transplanted autologous CD34+ stem cells reduce angina and improve exercise capacity in patients with refractory angina1 and lower amputation rates in patients with critical limb ischemia.2 Preclinical studies indicate that the benefit of human CD34+ cell transplantation after ischemic injury occurs through increases in neovascularization3; however, the mechanisms of new blood vessel formation have not been completely characterized. Incorporation of CD34+ cells into the growing vasculature has been documented in multiple studies4 however the magnitude of structural contribution of transplanted cells has typically seemed modest compared to the significant overall physiological impact. This discrepancy has led to the assumption that paracrine factors secreted by CD34+ cells contribute significantly to the therapeutic angiogenesis induced by the cells.5
Exosomes, a component of paracrine secretion are extracellular, membrane-bound nano-vessicles that originate intracellularly in multivesicular bodies (MVBs) and are secreted out when the MVBs fuse with the plasma membrane.6 They often carry proteins, RNAs, and/or microRNAs and mediate some aspects of cell-to-cell signaling.6 Here, we investigated the potential role of exosomes in CD34+ cell-induced neovascularization by determining whether CD34+ stem cells secrete exosomes, and if so, whether these exosomes can induce angiogenic activity in the absence of CD34+ cells.
Methods
All experimental protocols were approved by the Northwestern University Animal Care and Use Committee. Both CD34+ cells3 and CD34+ cell-depleted mononuclear cells (MNCs)3 were cultured, and exosomes from the conditioned media were obtained as described previously.7 Electron microscopy, dynamic light scattering (DLS), flow cytometry, and immunoblotting analyses were performed according to established protocols.7 The angiogenic activity of cultured human umbilical-vein endothelial cells (HUVECs) was evaluated via the Matrigel tube-formation assay, proliferation was evaluated via 5-bromo-2-deoxyuridine incorporation, and viability was assessed via the MTS assay. In vivo angiogenesis was evaluated in nude (nu/J) mice via the Matrigel-plug and corneal angiogenesis assays. Detailed methods are provided in the Supplemental Methods. Quantified results are presented as mean±SD; comparisons between groups were evaluated with the Student’s t test; P<0.05 was considered significant.
Results
CD34+ cells secrete exosomes
To investigate the role of exosomes in CD34+ cell-induced neo-vascularization, we examined if the CD34+ cells produce and secrete exosomes. Electron micrographs identified several MVBs in the cytoplasm of CD34+ cells, carrying bilipidic membrane-bound exosome-like vesicles. The MVB membrane invaginated inward initiating the biogenesis of exosomes as previously shown6; MVB fused to the plasma membrane and released the exosome-like vesicles to the media (Figure 1a). Because CD34+ cells appear to be significantly more potent for inducing angiogenesis in ischemic tissue than unselected MNCs,3 experiments were performed with exosomes isolated both from CD34+ cells (CD34+ exosomes) and from CD34+-depleted MNCs (MNC exosomes). Exosomes isolated from the conditioned media (CM) of both CD34+ cells and MNCs were similar to previous descriptions of exosomes in size (40- to 90-nm in diameter)7, cup-shaped morphology (Figure 1b), and in their unique flotation density (1.127 g/cm3, floated on 30% sucrose-D2O solution). DLS analysis confirmed the purity (100%) and mean hydrodynamic radius (CD34+: 50±7.8 nm; MNC: 75±0.4 nm) of the exosomes in each preparation (Figure 1c). Exosomes from both CD34+ cells and MNCs displayed the exosomal surface marker proteins CD63 and phosphatidylserine (Figures 2a, b) and contained the exosomal luminal protein TSG101 (Figure 2c). Further, CD34 protein was present on the surface of exosomes from CD34+ cells but not on exosomes from MNCs (Figure 2d), which is consistent with previous reports that exosomes carry the same marker proteins that are specific for the secreting cell.6 Collectively, these observations confirm that both CD34+ cells and MNCs secrete exosomes, and that the exosomes secreted by each cell population are biochemically distinct.
Figure 1. Morphological analyses of exosomes from human CD34+ cells and MNCs.
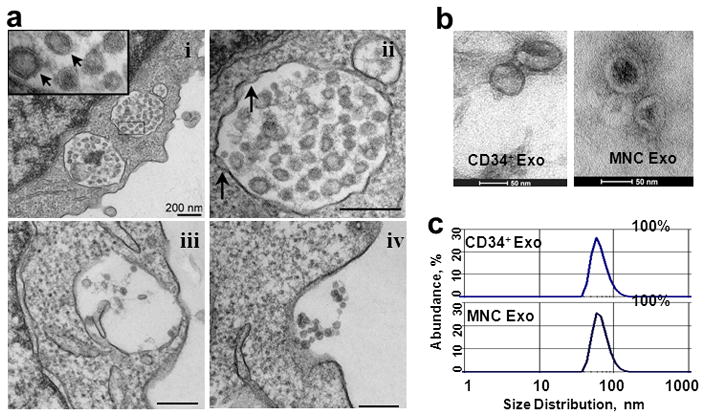
(a) Transmission electron micrograph of CD34+ cell (i) cytoplasm with MVBs enclosing numerous bilipidic layer-bound exosomes (Exo) (inset, arrows), (ii) inward invagination (arrows) in the MVB membrane indicate the beginning of exosome biogenesis, (iii) MVB fusing with cell membrane, (iv) Exosomes are secreted out from the cell (b) Electron microscopy of exosomes purified from CD34+ cell and MNC CM (c) Representative DLS number distribution measurement of isolated exosome population demonstrates a single peak (40–90 nm diameter) indicating they are free of contamination.
Figure 2. Biochemical analyses of exosomes from human CD34+cells and MNCs.

Representative flow cytometry dot-plots showing detection of exosomal surface proteins (a) CD63 and (b) Annexin V bound to exposed phosphatidylserine, (c) Immunoblot showing exosomal luminal protein TSG101. (d) Flow cytometry dot-blot analysis for CD34 surface protein. The isolated exosomes were conjugated to 4-μm latex beads and stained for all flow cytometry analyses, Control represents non-specific antibody binding to the beads; the numbers inside the box represents the % of positive beads.
CD34+ exosomes induce angiogenic activity in endothelial cells in vitro
To determine whether CD34+ exosomes induce angiogenic activity in vitro, tube formation was evaluated in HUVECs that had been cultured for 8 hours with PBS, CD34+ cells, CD34+ cell-CM, CD34+ exosomes, or the exosome-depleted CM (Figure 3a). Tube length was significantly greater in HUVECs incubated with the CD34+ cell-CM or with CD34+ exosomes than in HUVECs incubated with PBS, but was unchanged in HUVECs incubated with the exosome-depleted CM (Figure 3b). This suggests that CD34+ exosomes mediate the in vitro angiogenic activity from the CD34+ cell-CM. Interestingly, CD34+ exosomes, similar to CD34+ cells, induced longer-lasting tubes in HUVECs (Supplemental Figure 1). Tube formation was less pronounced at lower exosome concentrations (Figure 3c). Both CD34+ exosomes and CD34+ cells significantly enhanced HUVEC viability (Figure 3d) and proliferation (Figure 3e). Thus, most of the in vitro angiogenic activity associated with CD34+ cells appears to be mediated by exosomes. HUVECs incubated with MNCs or MNC exosomes did not differ significantly from saline-treated cells in any functional parameter (Figures 3d–e, and Supplemental Figures 1–2). The superior efficacy of CD34+ exosomes compared to MNC exosomes is consistent with prior in vivo studies documenting the enhanced angiogenic activity of CD34+ cells vs. MNC for therapeutic angiogenesis.3 Although the mechanisms that mediate the enhanced potency of CD34+ cells vs. MNC have not been completely clarified, preliminary data show that the pro-angiogenic miRNAs 126 and 130a8 are highly expressed in CD34+ exosomes compared to MNC.(Supplemental Figure 3)
Figure 3. In-vitro assays.

(a) HUVECs (2.5×104) were treated with PBS, 2.0×104 CD34+ cells, or with conditioned media (CM), exosomes (Exo), or exosome-depleted CM from 2.0×104 CD34+ cells, and plated on Matrigel; tube length was measured 8 hours later and (b) expressed as percentage of saline-treated HUVECs; n= 6–9 (c) A representative dose-response of CD34+ exosome tube formation, evaluated in HUVECs incubated with exosomes from 1.5×105 CD34+ cells and serially diluted with saline to the indicated ratios, (d) Viability (e) Proliferation of HUVECs (1×104) in response to PBS, 2.5×103 cells, or exosomes from 2.5×103 cells, measured 20 hours later and expressed as percentage of PBS-treated HUVECs; n=3–6. *P<0.001 versus PBS, †P<0.05 versus Exo-depleted CM, ‡P<0.05 versus MNCs or MNC exosomes.
CD34+ exosomes induce angiogenesis in vivo
The angiogenic potency of CD34+ exosomes was evaluated in vivo by performing the Matrigel-plug and corneal angiogenesis assays in mice. Both CD34+ cells and CD34+ exosomes induced the formation of vessel-like endothelial structures (Figure 4a) and significantly increased the proportion of endothelial cells (Figure 4b) in the Matrigel plug. In the corneal angiogenesis assay, pellets containing CD34+ exosomes, but not MNC exosomes, were associated with significantly greater vessel growth (Figure 4c–d); the effect of CD34+ cells on corneal angiogenesis could not be evaluated, because the pellets could not be prepared with viable cells.
Figure 4. In vivo assays.
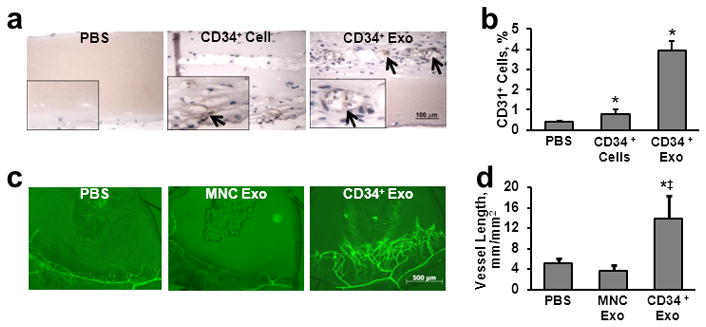
(a–b) Matrigel plug assay: Matrigel containing PBS, 5×105 CD34+ cells, or exosomes from 5×105 CD34+ cells was subcutaneously injected into mice, and the plugs were harvested 7 days later. (a) Sections from the plug were stained with isolectin to identify endothelial cells (brown) and vessel-like endothelial structures (arrows). (b) The plug was digested and CD31+ endothelial cells were quantified via flow cytometry; n=3–6. (c–d) Corneal angiogenesis assay: pellets containing PBS or exosomes from 1×106 MNCs or CD34+ cells were implanted in the corneas of mice; corneas were harvested 7 days later, (c) stained with fluorescently labeled lectin identifying vascular structures, and (d) the extent of vessel growth was quantified; n=4. *P<0.05 versus PBS, ‡P<0.01 versus MNC exosomes.
Discussion
CD34+ cells have been shown to form a structural component of the neovasculature in ischemic tissue4 and secrete paracrine factors that also stimulate neovascularization.5 Here, we demonstrate that a significant component of the pro-angiogenic paracrine activity associated with CD34+ cells is mediated by exosomes. The exosomes secreted by CD34+ cells were morphologically similar in size and shape to exosomes described in previous reports, carried known exosomal protein markers, and potently induced angiogenic activity both in vitro and in vivo.
The cell-culture medium was supplemented with growth factors and may have contained soluble proteins secreted directly from the cells, which could, in principle, have contributed to the angiogenic effects associated with CD34+ exosomes. However, the MNC exosomes were derived from MNCs cultured with the same growth factors, and the exosome-depleted conditioned media would have contained both the supplemental growth factors and any secreted soluble proteins. Since none of these treatments stimulated angiogenic activity, our findings indicate that the CD34+ exosomes are the key paracrine component of CD34+ cell–induced vessel growth.
Exosomes can stimulate both receptor-mediated and genetic mechanisms by transferring proteins, RNA, or microRNA directly into the cytoplasm of target cells.9 We have presented data demonstrating that CD34+ exosomes are enriched with pro-angiogenic microRNAs; the extent to which these microRNAs are transferred and induce any molecular changes in the recipient cells will be clarified in ongoing studies. Indeed the repertoire of specific molecules transported by CD34+ exosomes remains to be fully characterized, but they are likely to be more stable than molecules secreted directly into the extracellular matrix, because the exosomal membrane protects the contents of the exosome from degradation.6, 9 Furthermore, the exosomes used in our investigation were sufficiently durable to remain intact and biologically active throughout the isolation procedure, which suggests that the functional radius of CD34+ exosomes could extend beyond the immediate vicinity of the secreting cell. The observation that in some of the in vitro and in vivo assays the exosomes from CD34+ cells appeared more potent than the cells themselves is interesting and might also be a by-product of the durability of the exosome in culture providing the ability to deliver a high dose of exosomes via collection from culture medium in which exosomes are secreted over a period of time.
In summary, our observations demonstrate, for the first time, that adult human CD34+ stem cells secrete exosomes, and that these exosomes induce angiogenic activity in isolated endothelial cells and in murine models of vessel growth. Thus, the benefit of CD34+ cell therapy on functional recovery after ischemic injury could be induced primarily through the exosome-mediated transfer of angiogenic factors to surrounding cells. Novel therapies designed to exploit this previously unidentified mechanism of paracrine signaling may enhance recovery from ischemic disease or injury.
Supplementary Material
Novelty and Significance.
What is known?
CD34+ cells have been shown to stimulate therapeutic neovascularization in pre-clinical studies and in phase I and II human clinical trials.
The potency of CD34+ cells is greater than unselected mononuclear cells (MNC).
The mechanisms by which CD34+ cells induce neovascularization appear to include both direct participation in vessel formation and undefined “paracrine” effects.
Exosomes are small membrane bound vesicles secrected from various cells that contain protein and nucleic acids and are increasingly being shown to mediate cell-to-cell signaling.
What new information does this article contribute?
CD34+ cells secrete exosomes that independently induce angiogenesis in vitro and in vivo
The pro-angiogenic activity of CD34+ exosomes is significantly greater than CD34-depleted MNC exosomes.
The exosomes of CD34+ cells contain higher levels of pro-angiogenic miRNA’s
The clinical potential of CD34+ cells for therapeutic neovascularization of ischemic tissue is being evaluated in a series of completed and ongoing clinical trials. Hence, the mechanisms by which CD34+ cells mediate these effects are of high scientific and clinical importance. While paracrine effects have been assumed to be responsible for a significant proportion of the effects of endothelial progenitor cell (EPC) based therapies in general, the precise nature of the paracrine phenomena have not been defined. Our data show that CD34+ cells secrete exosomes that appear to be responsible for much, if not most, of their paracrine activity. Specifically, the conditioned medium (CM) from CD34+ cells exerts pro-angiogenic effects that are abolished when the exosomes are removed, while the exosomes alone, without any of the soluble material from the conditioned medium, exhibit the full potentcy of the CM. Complete characterization of the exosome content of EPCs could provide new insights permitting enhancement of their therapeutic potency.
Acknowledgments
The authors would like to thank Baxter Healthcare for providing the CD34+ cells, Dr. CS Thaxton for providing the DLS machine, J. Marvin for assistance with flow cytometry measurements, and L. Renolds for assistance with electron microscopy.
Sources of Funding:
This work was supported by grants from the National Institutes of Health: 2R01HL053354, 5R01HL095874, 5R01HL077428, 1P01HL108795.
Non-standard abbreviations and acronyms
- CM
conditioned media
- DLS
dynamic light scattering
- Exo
exosomes
- HUVEC
human umbilical-vein endothelial cells
- MNC
mononuclear cells
- MVB
multivesicular bodies
- PBS
phosphate buffered saline
Footnotes
Disclosures
None.
References
- 1.Losordo DW, Henry TD, Davidson C, Lee JS, Costa MA, Bass T, Mendelsohn F, Fortuin FD, Pepine CJ, Traverse JH, Amrani D, Ewenstein BM, Riedel N, Story K, Barker K, Povsic TJ, Harrington RA, Schatz RA. Intramyocardial, autologous cd34+ cell therapy for refractory angina. Circ Res. 2011 doi: 10.1161/CIRCRESAHA.111.245993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Losordo D. Randomized double-blind, placebo controlled trial of autologous cd34+ cell therapy for critical limb ischemia: 1 year results. Circulation. 2010;122:A16920. [Google Scholar]
- 3.Kawamoto A, Iwasaki H, Kusano K, Murayama T, Oyamada A, Silver M, Hulbert C, Gavin M, Hanley A, Ma H, Kearney M, Zak V, Asahara T, Losordo DW. Cd34-positive cells exhibit increased potency and safety for therapeutic neovascularization after myocardial infarction compared with total mononuclear cells. Circulation. 2006;114:2163–2169. doi: 10.1161/CIRCULATIONAHA.106.644518. [DOI] [PubMed] [Google Scholar]
- 4.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 5.Kumar AH, Caplice NM. Clinical potential of adult vascular progenitor cells. Arterioscler Thromb Vasc Biol. 2010;30:1080–1087. doi: 10.1161/ATVBAHA.109.198895. [DOI] [PubMed] [Google Scholar]
- 6.Chaput N, Thery C. Exosomes: Immune properties and potential clinical implementations. Semin Immunopathol. 2010 doi: 10.1007/s00281-010-0233-9. [DOI] [PubMed] [Google Scholar]
- 7.Thery C, Amigorena S, Raposo G, Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol. 2006;Chapter 3(Unit 3):22. doi: 10.1002/0471143030.cb0322s30. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Q, Kandic I, Kutryk MJ. Dysregulation of angiogenesis-related micrornas in endothelial progenitor cells from patients with coronary artery disease. Biochem Biophys Res Commun. 2011;405:42–46. doi: 10.1016/j.bbrc.2010.12.119. [DOI] [PubMed] [Google Scholar]
- 9.Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mrnas and micrornas is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


