Abstract
Background
Disquieting reports of increased complication and death rates after transfusions of red-cell concentrates stored for more than 14 days prompted us to perform an observational retrospective cohort study of mortality in relation to storage time.
Study design and methods
We conducted a cohort study utilizing data on all recipients of at least one red-cell transfusion in Sweden and Denmark between 1995 and 2002, as recorded in the Scandinavian donations and Transfusions (SCANDAT) database. Relative risks of death in relation to storage time were estimated using Cox regression, adjusted for several possible confounding factors.
Results
After various exclusions, 402,874 transfusion episodes remained for analysis. The 7 day risk of death was similar in all exposure groups, but a tendency for a higher risk emerged among recipients of blood stored for 30-42 days (hazard ratio, 1.05; 95% CI, 0.97-1.12), as compared to recipients of blood stored for 10-19 days. With 2-year follow-up, this excess remained at the same level (hazard ratio, 1.05; 95% CI, 1.02-1.08). No dose-response pattern was revealed and no differential effect was seen when the analyses were restricted to recipients of leukocyte depleted units only.
Conclusion
Although a small excess mortality was noted in recipients of the oldest red-cell concentrates, the risk pattern was more consistent with weak confounding than with an effect of the momentary exposure to stored red-cell concentrates. It seems, thus, that any excess mortality conferred by older red-cells in the combined Swedish and Danish transfusion recipient population is likely less than 5%, which is considerably smaller than in the hitherto largest investigation.
Introduction
It is well established that when stored, red blood cells undergo several changes,1, 2 collectively referred to as a “storage lesion,” including loss of deformability,3 morphological changes,4 and depletion of adenosine triphosphate and 2,3-diphosphoglycerate.4, 5 These changes decrease oxygen transporting capacity and impair capillary passage.2, 6 Yet, the clinical consequences of prolonged red-cell concentrate storage remain poorly understood. With few exceptions,7-12 previous studies have found suggestive evidence of various adverse consequences such as increased mortality and increased length of hospital stay as well as higher rates of infectious complications, thrombosis and multi-organ failure.13-27 However, studies have generally been small or have failed to control important sources of error.1, 28, 29 While two are underway,1 no large randomized trial of fresh vs. stored red-cell concentrates has been completed. Because the tolerance for risks in transfusion medicine is low,30 very large studies are needed to rule out small but still clinically meaningful effects. If inconclusiveness prevails, large and well-designed observational studies may be crucial to inform policy decisions.
To investigate the effect of prolonged storage of red-cell concentrates on patient survival we performed a large, retrospective cohort study using a population-based database of blood transfusion recipients in Sweden and Denmark.
Material and Methods
Setting
Blood services in Sweden and Denmark are part of the public health care system. Component production and transfusion practice follow very similar national guidelines, but maximum storage time for red-cell concentrates in SAGMAN (saline-adenosine-glucose-mannitol) solution has been 42 days in Sweden and 35 days in Denmark. Units that were leukocyte-depleted through filtering or other means were used to a varying but generally increasing extent throughout the entire study period.
Data sources
The first computerized transfusion register in Sweden was initiated in 1966.31 Similar registration began in Denmark at the same time,32 but was not made permanent until the 1980's. All individuals in these registers are uniquely identifiable through their national registration numbers, which are assigned to all residents of both countries and are used in all population and health registers. Therefore, records on individuals can be electronically linked across registers with little or no error.
Available computerized data from transfusion registers in Sweden and Denmark were retrieved and entered into two databases, one for each country. Electronic linkages with continuously updated national population and migration registers as well as with complete national registers for death and inpatient care, provided dates of emigration, dates and causes of death, and information on hospitalizations (including date and diagnoses at discharge, along with codes for surgical or anesthesiological procedures). Finally, the Swedish and Danish databases were merged to form the Scandinavian Donations and Transfusions (SCANDAT) database.33 Data from the SCANDAT database has been used to address several issues regarding long-term safety of blood donations and transfusions.33-38
Study design
We performed a retrospective cohort study with exposure defined by transfusion register data, information on disease or indication and length of stay obtained from national registers of in-hospital care, and follow-up via registers of death and migration. We identified all patients in the SCANDAT database who had at least one red-cell concentrate transfusion recorded between January, 1995 and December, 2002. The investigation was limited to patients aged 15 to 90 years with known blood type, who had not received any transfusions in the two years preceding the index transfusion. We excluded data from hospitals where fewer than 5,000 patients were transfused during the 7-year study period to allow adjustment for hospital of transfusion in the statistical analyses. All restrictions/exclusions were pre-planned with the purpose of minimizing ambiguous data and maximizing our ability to adjust for possible confounding.
Transfusions were grouped into episodes containing an exposure period and a follow-up period, both of which were varied to assess short- and long-term survival in relation to red-cell concentrate storage time. Patients could contribute to more than one transfusion episode as long as two years without any transfusions had passed before the next index transfusion. We excluded episodes where autologous units, ABO non-identical units, units from unknown donors, or units of unknown storage time were transfused during the exposure period.
We assessed short-term survival using a 1-day exposure period and an overlapping 7-day follow-up period (i.e., from day 1 through 7). Long-term survival was assessed using a 7-day exposure period with follow-up from day 8 through 730 (i.e. 2 years). Otherwise, follow-up was terminated upon death, emigration, or end of registration in the SCANDAT database (31st of December, 2002). We classified exposure similarly as in previous investigations,22 with discrete exposure categories where patients had exclusively received units of a certain age (i.e. 0-9, 10-19, 20-29, or 30-42 days old). We also established a mixed category, with recipients of multiple units that fell into more than one age class. Since freshly prepared units may be used for special indications, e.g. when units are prepared for patients with irregular red cell antibodies, recipients of 10-19 days old units were used as the reference group.
Statistical analysis
The relative risks of death, expressed as hazard ratios, were estimated using Cox proportional hazards regression. In addition to a variable denoting the storage time of red-cell concentrates, models included variables for recipient's age, calendar period, weekday (weekday or weekend), season (winter, spring, summer, or fall), and hospital (35 Swedish and 17 Danish), all as defined at the start of the exposure period, as well as number of transfusions during the exposure period (red-cell, plasma and platelet transfusions considered as separate variables), recipient's sex and ABO-Rh blood type. Storage time of non-red-cell units was not considered. We also adjusted for transfusion indication, defined by diagnosis and procedure codes from the inpatient registers.38 Six indication groups were established according to a sequential algorithm: 1. trauma; 2. non-trauma, cardiovascular surgery; 3. non-trauma, non-cardiovascular surgery for malignant disease; 4. non-trauma, non-cardiovascular, non-malignancy, major surgery; 5. other care for hematologic malignancy; or 6. other hospital care. In analyses confined to Swedish patients, for whom data was available, we further adjusted for planned versus emergency admission, but since this adjustment had no effect on the studied associations, this variable was dropped. All variables were treated as categorical except age, calendar period and number of red-cell concentrate transfusions, the effects of which were modeled as restricted cubic splines. These piecewise polynomials provide a flexible way to model non-linear trends smoothly, thus achieving tight adjustment for confounding.
In addition to analyses of all-cause mortality, we also performed analyses of cause-specific risks of death, divided according to the chapters of revisions 9 and 10 of the International Classification of Diseases (ICD). For simplicity, these analyses compared recipients of units stored for 30-42 days to recipients of units stored for 10-19 days. We also modeled the effect of maximum storage time of any red-cell unit given during the exposure period as a restricted cubic spline to create a smooth graphical representation of the association with mortality. Moreover, an analysis was conducted for episodes where only leukocyte-depleted units were used. Finally, as a test of dose-response, we performed an analysis where the main exposure was the number of transfusions of red-cell concentrates stored for 30-42 days, regardless of whether they were given together with concentrates of other ages. Covariates and parameterizations were the same as in the main analyses.
Possible statistical interactions between the main exposure and country, sex, age, calendar time and number of transfusions were explored with likelihood ratio tests. We also limited the analyses to possible high-risk groups defined a priori (age ≥70 years; coronary artery by-pass graft surgery; or extracorporeal circulation for other reasons).22 As a sizable number of patient episodes were excluded from the analyses based on the strict inclusion criteria, the main analyses were repeated including also patients transfused in smaller hospitals. Due to the large number of smaller hospitals, it was not possible to adjust for individual hospitals. Therefore, we adjusted instead for the number of transfusions administered in that hospital that year categorized into deciles as a proxy for hospital size. In these extended analyses we also included recipients of ABO non-identical units. The proportional hazards assumption was evaluated using Schoenfeld residuals from the main models and by exploring the effect of red-cell concentrate storage time across follow-up, split into three time windows (1-30, 31-90 and 91-730 days).
All statistical analyses were conducted using Stata 10. Since some patients experienced more than one transfusion episode, robust variance estimates were used.39 All p-values were two-sided. The creation of the SCANDAT database and the conduct of this study were approved by relevant scientific ethics committees in both countries.
Results
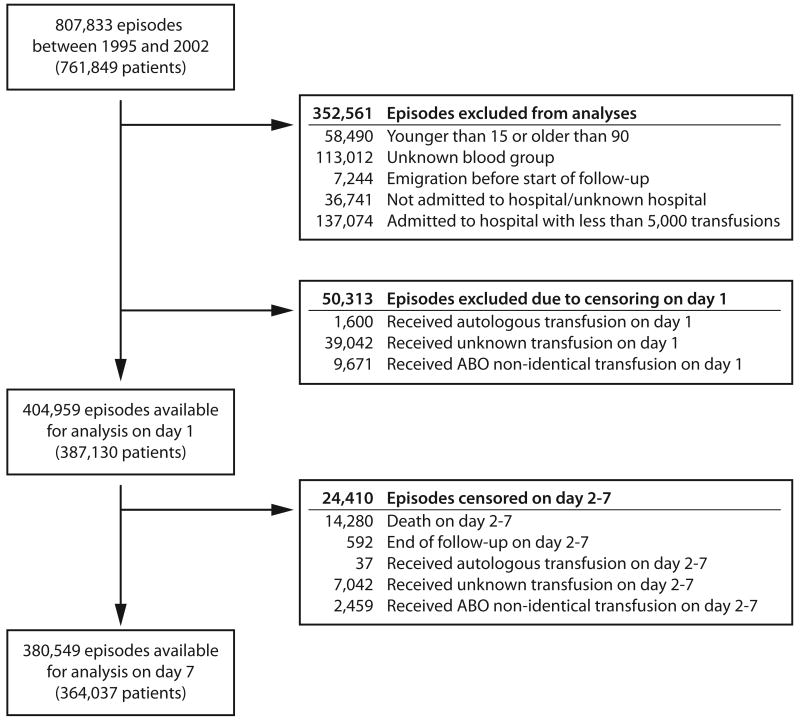
We identified 761,849 patients who received at least one red-cell concentrate transfusion during 807,833 transfusion episodes. After exclusion of 402,874 transfusion episodes (374,719 patients), a total of 404,959 episodes (387,130 patients) remained for analysis (Figure 1). For the long-term analyses, 380,549 episodes (364,037 patients) remained (Figure 1).
Figure 1.
Reasons for exclusion, and number of patients excluded from the study population.
Table 1 presents characteristics of the patient population. In the short-term analyses the exclusive use of concentrates stored for 0-9 days occurred in 103,402 episodes, exclusively 10-19 days in 140,619 episodes, 20-29 days in 63,729 episodes, and 30-42 days in 32,855 episodes. The median storage time ranged from 7 days in the 0-9 days category to 34 days in the 30-42 days category, and was 17 days in the mixed exposure category. Units of mixed ages were used in 64,354 episodes. The median age of patients was similar in all exposure categories, ranging from 71.6 to 73.2 years. With the exception of the mixed category, where a higher median number of transfusions was evident, similar numbers of transfusions were administered. As expected, proportions of the rarer blood groups (AB and B) increased with longer storage. The duration of hospital stay and distributions of indications was similar in the four main exposure categories, somewhat allaying concerns about differential use of fresh and old red-cell concentrates among patients with different prognosis.
Table 1. Characteristics of blood transfusion episodes.
| Exposure category (storage age of transfused red-cell concentrates) | |||||
|---|---|---|---|---|---|
| 0-9 days | 10-19 days | 20-29 days | 30-42 days | Mixed age | |
| No. transfusion episodes available for analysis at day 1 (% of total) | 103,402 (25.5%) | 140,619 (34.7%) | 63,729 (15.7%) | 32,855 (8.1%) | 64,354 (15.9%) |
| No. transfusion episodes available for analysis at day 7 (% of total)* | 83,828 (22.0%) | 113,953 (29.9%) | 49,845 (13.1%) | 25,897 (6.8%) | 107,026 (28.1%) |
| Median storage time (IQR) | 7 (5-8) | 14 (12-16) | 23 (21-26) | 34 (31-37) | 17 (11-22) |
| Female sex, No. (% of category) | 58,142 (56.2%) | 79,285 (56.4%) | 35,561 (55.8%) | 18,608 (56.6%) | 34,101 (53.0%) |
| Swedish, No. (% of category) | 87,412 (84.5%) | 113,720 (80.9%) | 51,899 (81.4%) | 28,507 (86.8%) | 55,722 (86.6%) |
| Patient age, median (IQR) | 71.7 (57.8-79.6) | 72.3 (59.1-80.1) | 72.6 (60.3-80.2) | 73.2 (61.1-80.6) | 71.6 (58.0-79.3) |
| Number of transfusions on day 1, No. (% of category) | |||||
| 1-5 | 96,536 (93.4%) | 133,195 (94.7%) | 61,035 (95.8%) | 31,677 (96.4%) | 49,897 (77.5%) |
| 6-10 | 4,793 (4.6%) | 5,476 (3.9%) | 2,108 (3.3%) | 957 (2.9%) | 8,315 (12.9%) |
| >10 | 2,073 (2.0%) | 1,948 (1.4%) | 586 (0.9%) | 221 (0.7%) | 6,142 (9.5%) |
| Median number (IQR) | 2 (2-2) | 2 (2-2) | 2 (1-2) | 2 (1-2) | 3 (2-5) |
| Number of transfusions on day 1-7, No. (% of category)† | |||||
| 1-5 | 74,585 (89.0%) | 103,590 (90.9%) | 46,302 (92.9%) | 24,097 (93.0%) | 69,847 (65.3%) |
| 6-10 | 6,265 (7.5%) | 7,385 (6.5%) | 2,646 (5.3%) | 1,366 (5.3%) | 21,758 (20.3%) |
| >10 | 2,978 (3.6%) | 2,978 (2.6%) | 897 (1.8%) | 434 (1.7%) | 15,421 (14.4%) |
| Median number (IQR) | 2 (2-4) | 2 (2-3) | 2 (2-3) | 2 (2-3) | 4 (3-7) |
| Median length of hospital stay (IQR)** | 13 (7-26) | 12 (7-27) | 12 (7-25) | 12 (7-23) | 13 (7-26) |
| Blood group, No. (% of category) | |||||
| A | 56,928 (55.1%) | 67,772 (48.2%) | 25,854 (40.6%) | 9,828 (29.9%) | 26,178 (40.7%) |
| AB | 2,444 (2.4%) | 2,979 (2.1%) | 3,163 (5.0%) | 4,536 (13.8%) | 4,234 (6.6%) |
| B | 8,807 (8.5%) | 10,114 (7.2%) | 7,161 (11.2%) | 5,643 (17.2%) | 8,279 (12.9%) |
| O | 35,223 (34.1%) | 59,754 (42.5%) | 27,551 (43.2%) | 12,848 (39.1%) | 25,663 (39.9%) |
| Indication for transfusion, No. (% of category) | |||||
| 1. Trauma | 14,065 (13.6%) | 21,654 (15.4%) | 9,400 (14.7%) | 5,344 (16.3%) | 9,090 (14.1%) |
| 2. Non-trauma, CVD surgery | 12,895 (12.5%) | 15,980 (11.4%) | 7,465 (11.7%) | 3,657 (11.1%) | 9,196 (14.3%) |
| 3. Non-trauma, non-CVD surgery for malignant disease | 9,677 (9.4%) | 13,247 (9.4%) | 6,624 (10.4%) | 3,285 (10.0%) | 7,166 (11.1%) |
| 4. Non-trauma, non-CVD, non-malignant surgery | 22,387 (21.7%) | 32,136 (22.9%) | 15,226 (23.9%) | 7,402 (22.5%) | 14,897 (23.1%) |
| 5. Non-trauma, non-surgery, hematologic malignancy | 3,893 (3.8%) | 3,338 (2.4%) | 1,238 (1.9%) | 645 (2.0%) | 1,412 (2.2%) |
| 6. Other indication | 40,485 (39.2%) | 54,264 (38.6%) | 23,776 (37.3%) | 12,522 (38.1%) | 22,593 (35.1%) |
IQR denotes interquartile range; CVD denotes cardiovascular disease.
The number of patients available for analysis at day 7 decreased in all but the Mixed category due to deaths occurring during the first 7 days and due to patients receiving units of another exposure category, non-identical units or autologous units on days 2-7.
Considering only patients available for analysis on day 8.
During first 30 days following transfusion.
Table 2 presents short- and long-term relative risks of death in relation to storage time for all patients and for the selected high-risk groups. The relative risk of death in days 1-7 was similar in all exposure groups, albeit with a statistically non-significant 5 percent risk elevation among recipients of units stored for 30-42 days (hazard ratio, 1.05; 95 percent confidence interval, 0.97-1.12). During the two year follow-up the relative risk in the 30-42 day category remained slightly elevated (hazard ratio, 1.05; 95 percent confidence interval, 1.02-1.08). Results from the analyses confined to possible high-risk patients showed that the risk pattern among the elderly did not differ notably from the overall analyses. Although based on small numbers and not achieving statistical significance, hazard ratios tended to be somewhat higher, both in the short- and long-term follow-up, among coronary artery by-pass surgery patients who exclusively received concentrates stored for 30-42 days, compared to patients who only received units that were 10-19 days old. No obvious association between red-cell storage and mortality was observed among patients on extracorporeal circulation.
Table 2.
Short- and long-term relative risk of death following blood transfusion in relation to storage time of administered red-cell concentrates, presented overall, for the elderly and for patients undergoing coronary artery by-pass graft surgery or extracorporeal circulation.
| All patients | ||||
|---|---|---|---|---|
| Day 1 through 7 | Day 8 through 730 | |||
| Storage age | Events/Person-years | Hazard ratio (95% CI)* | Events/Person-years | Hazard ratio (95% CI)* |
| 0-9 days | 3,338/1,951 | 0.96 (0.91-1.00) | 23,218/115,369 | 1.01 (0.99-1.02) |
| 10-19 days | 4,932/2,647 | 1.00 (ref) | 31,610/154,141 | 1.00 (ref) |
| 20-29 days | 2,099/1,201 | 1.01 (0.96-1.06) | 13,495/67,103 | 0.99 (0.97-1.01) |
| 30-42 days | 1,101/619 | 1.05 (0.97-1.12) | 7,322/34,292 | 1.05 (1.02-1.08) |
| Mixed age | 2,808/1,204 | 0.98 (0.93-1.04) | 29,626/146,751 | 1.03 (1.02-1.05) |
|
| ||||
| Patients aged 70 or older | ||||
| Day 1 through 7 | Day 8 through 730 | |||
|
|
|
|||
| Storage age | Events/Person-years | Hazard ratio (95% CI)* | Events/Person-years | Hazard ratio (95% CI)* |
| 0-9 days | 2,191/1,053 | 0.97 (0.92-1.03) | 8,531/57,368 | 1.01 (0.98-1.03) |
| 10-19 days | 3,265/1,477 | 1.00 (ref) | 12,451/79,984 | 1.00 (ref) |
| 20-29 days | 1,411/681 | 1.01 (0.94-1.08) | 5,377/35,678 | 0.98 (0.96-1.00) |
| 30-42 days | 748/363 | 1.03 (0.94-1.12) | 3,029/18,966 | 1.04 (1.00-1.07) |
| Mixed age | 1,779/648 | 0.99 (0.93-1.06) | 10,394/76,597 | 1.03 (1.00-1.05) |
|
| ||||
| Patients undergoing coronary artery by-pass graft surgery | ||||
| Day 1 through 7 | Day 8 through 730 | |||
|
|
|
|||
| Storage age | Events/ Person-years | Hazard ratio (95% CI)* | Events/ Person-years | Hazard ratio (95% CI)* |
| 0-9 days | 139/135 | 0.94 (0.72-1.16) | 200/9,479 | 1.09 (0.94-1.28) |
| 10-19 days | 167/160 | 1.00 (ref) | 196/11,186 | 1.00 (ref) |
| 20-29 days | 74/72 | 1.11 (0.84-1.46) | 73/4,629 | 1.00 (0.82-1.22) |
| 30-42 days | 29/36 | 1.08 (0.71-1.64) | 45/2,332 | 1.10 (0.85-1.42) |
| Mixed age | 190/81 | 1.06 (0.85-1.33) | 308/12,164 | 1.02 (0.88-1.18) |
|
| ||||
| Patients requiring extracorporeal circulation | ||||
| Day 1 through 7 | Day 8 through 730 | |||
|
|
|
|||
| Storage age | Events/Person-years | Hazard ratio (95% CI)* | Events/Person-years | Hazard ratio (95% CI)* |
| 0-9 days | 179/151 | 1.01 (0.81-1.26) | 224/10,450 | 1.06 (0.91-1.23) |
| 10-19 days | 194/166 | 1.00 (ref) | 206/11,389 | 1.00 (ref) |
| 20-29 days | 87/72 | 1.13 (0.88-1.46) | 67/4,573 | 0.92 (0.75-1.12) |
| 30-42 days | 34/37 | 1.03 (0.70-1.50) | 40/2,338 | 0.92 (0.70-1.20) |
| Mixed age | 256/91 | 0.98 (0.80-1.20) | 345/13,618 | 1.00 (0.87-1.14) |
CI denotes confidence interval.
Estimated using Cox proportional hazards regression, adjusted for number of transfusions, age, sex, blood group, calendar period, season, weekday, hospital and indication.
A restriction to all episodes where only leukocyte depleted units were used did not change risk estimates (Table 3). There was a notable lack of variation of the slightly elevated hazard ratios among recipients of 30-42 day old red-cell concentrates across follow-up time; the point estimates for days 1-30, 31-90, and 91-730 were 1.05, 1.05, and 1.06, respectively. In analyses of cause-specific mortality, no single category of cause of death was conspicuously overrepresented (Table 4).
Table 3.
Short- and long-term relative risk of death following blood transfusion in relation to storage age of administered red-cell concentrates, among recipients of leukocyte depleted units only.
| Day 1 through 7 | Day 8 through 730 | |||
|---|---|---|---|---|
| Storage age | Events/ Person yrs | Hazard ratio (95% CI)* | Events/ Person yrs | Hazard ratio (95% CI)* |
| 0-9 days | 768/504 | 0.88 (0.79-0.97) | 2,691/26,778 | 0.98 (0.94-1.02) |
| 10-19 days | 750/473 | 1.00 (ref) | 2,379/20,152 | 1.00 (ref) |
| 20-29 days | 280/189 | 0.94 (0.82-1.08) | 847/6,768 | 0.96 (0.90-1.02) |
| 30-42 days | 182/105 | 1.10 (0.93-1.31) | 481/3,587 | 1.00 (0.93-1.08) |
| Mixed age | 421/194 | 0.96 (0.84-1.09) | 1,706/17,836 | 1.00 (0.95-1.04) |
CI denotes confidence interval.
Estimated using Cox proportional hazards regression, adjusted for number of transfusions, age, sex, blood group, calendar period, season, weekday, hospital and indication.
Table 4.
Cause-specific relative risk of death, according to ICD chapter, following blood transfusion comparing recipients of old to recipients of fresh red-cell concentrates.
| Day 1 through 7 | Day 8 through 730 | |||
|---|---|---|---|---|
| Cause of death (Chapter) | No. deaths in recipients of old/recipients of fresh units* | Hazard ratio (95% CI)† | No. deaths in recipients of old/recipients of fresh units* | Hazard ratio (95% CI)† |
| Infectious diseases (I) | 26/108 | 1.06 (0.66-1.69) | 35/215 | 0.95 (0.65-1.39) |
| Neoplasms (II) | 262/1267 | 1.01 (0.88-1.17) | 1496/7916 | 1.01 (0.96-1.08) |
| Endocrine and nutritional diseases (III) | 6/39 | 0.84 (0.33-2.12) | 24/108 | 0.87 (0.54-1.40) |
| Diseases of the blood and blood-forming organs (IV) | 22/81 | 1.17 (0.70-1.96) | 96/411 | 1.26 (0.99-1.60) |
| Mental and behavioural disorders (V) | 4/42 | ** | 101/415 | 1.25 (0.99-1.57) |
| Diseases of the central nervous system and sensory organs (VI) | 9/43 | 0.87 (0.40-1.88) | 61/265 | 1.17 (0.86-1.58) |
| Diseases of the circulatory system (VII) | 376/1917 | 1.08 (0.96-1.21) | 1295/6648 | 1.04 (0.98-1.11) |
| Diseases of the respiratory system (VIII) | 48/248 | 0.94 (0.67-1.30) | 190/1123 | 0.91 (0.77-1.07) |
| Diseases of the digestive system (IX) | 159/777 | 1.13 (0.94-1.36) | 137/751 | 1.11 (0.91-1.35) |
| Diseases of the genitourinary system (X) | 10/65 | 0.61 (0.30-1.24) | 73/287 | 1.30 (0.98-1.71) |
| Diseases of the pregnancy and childbirth (XI) | 0/2 | ** | 0/0 | ** |
| Diseases of the skin and subcutaneous tissue (XII) | 0/17 | ** | 8/38 | 0.99 (0.43-2.27) |
| Diseases of the musculoskeletal system and connective tissue (XIII) | 9/51 | 0.86 (0.41-1.84) | 29/145 | 1.05 (0.68-1.61) |
| Other diseases (XVI) | 2/30 | 0.54 (0.12-2.38) | 36/236 | 0.93 (0.64-1.36) |
| External causes of mortality (XVII) | 88/482 | 1.09 (0.85-1.39) | 144/771 | 1.15 (0.95-1.39) |
CI denotes confidence interval; ICD denotes international classification of disease.
The numerator on each row represents the number of deaths from that cause among recipients of old units and the denominator the number of deaths among recipients of old units. A total of 32,855 and 140,619 patients were available for analysis on short term among recipients of old and fresh units, respectively, and for long term a total of 25,897 and 113,953 for recipients of old and fresh units, respectively.
Estimated using Cox proportional hazards regression, adjusted for number of transfusions, age, sex, blood group, calendar period, season, weekday, hospital and indication. Comparing recipients of “old units” (30-42 days) to recipients of “fresh units” (10-19 days).
Models not run.
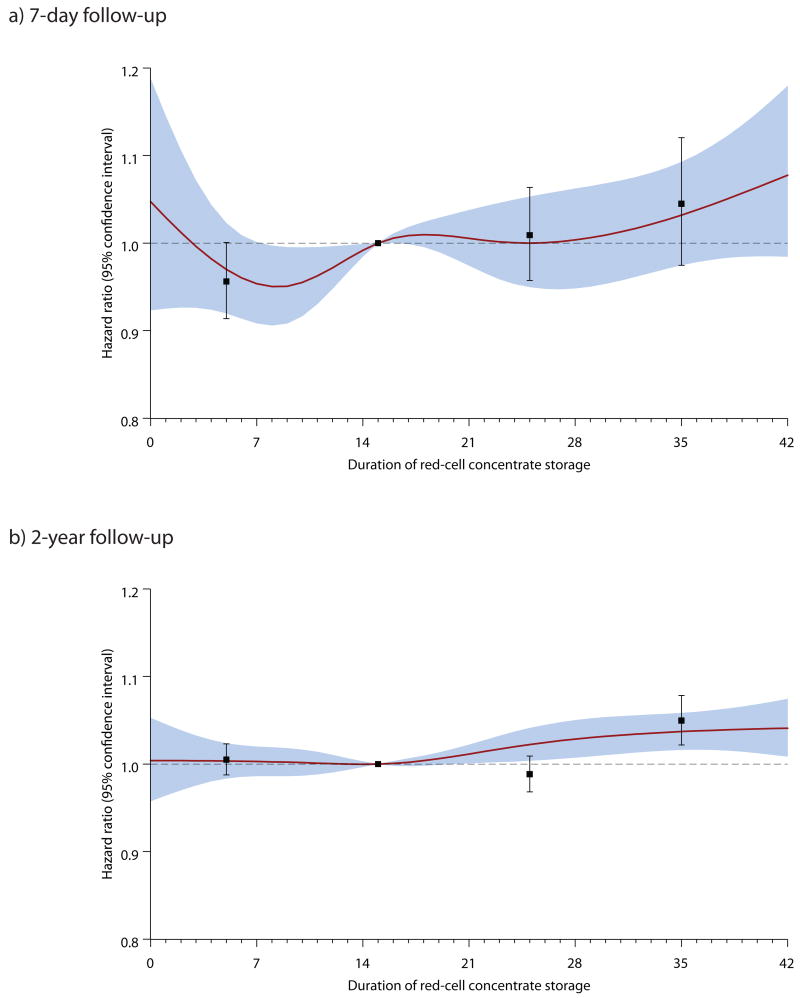
Figure 2 presents the relative risk estimates from the short- and long-term analyses together with results from the analyses where the effect of maximum storage time was modeled as a restricted cubic spline. The association between red-cell storage and mortality followed essentially the same pattern in both analyses, with a trend to higher mortality with older storage time that was significant only in the long-term analysis.
Figure 2.
Graphical comparison of short- and long-term relative risks of death with 95 percent confidence intervals, estimated from categorical analyses of exclusive exposure categories (boxes with error bars) and from cubic spline analyses (solid lines with shaded confidence bands). Panel A shows results from short-term analyses with a 1-day exposure period and an overlapping 7-day follow-up. Panel B shows results from long-term analyses with a 7-day exposure period and 2-year follow-up period.
Table 5 shows short- and long-term relative risks of death in relation to the number of transfused 30-42 day old units among all red-cell transfusion recipients. The adjusted point estimates for 7-day mortality tended to go up with the number of such units; compared to recipients of no such units, risks for those who received 1-2, 3-4, and ≥5 units were, respectively, 1.04, 1.10, and 1,14, but none of these estimates attained statistical significance and the trend test was also non-significant (p=0.074). In the long-term analysis, there was a slightly increased risk in recipients exposed to 1-2 units stored for 30-42 days, but there was no upward trend with increasing number of old units.
Table 5.
Short- and long-term relative risk of death following blood transfusion in relation to number of transfusions of red-cell concentrates that were stored for 30-42 days.
| Day 1 through 7 | Day 8 through 730 | |||
|---|---|---|---|---|
| No. units stored 30-42 days | Events/ Person-years | Hazard ratio (95% CI)* | Events/ Person-years | Hazard ratio (95% CI)* |
| No units | 11,939/6,469 | 1.00 (ref) | 89,464/441,256 | 1.00 (ref) |
| 1-2 units | 1,766/914 | 1.04 (0.99-1.10) | 12,734/60,945 | 1.03 (1.01-1.05) |
| 3-4 units | 330/176 | 1.10 (0.96-1.26) | 2,317/11,505 | 1.04 (0.99-1.08) |
| ≥5 units | 243/63 | 1.14 (0.95-1.37) | 756/3,951 | 1.00 (0.92-1.07) |
CI denotes confidence interval.
Estimated using Cox proportional hazards regression, adjusted for number of transfusions, age, sex, blood group, calendar period, season, weekday, hospital and indication.
Addition of interaction terms in the short- and long-term models did not reveal any important effect modification by country (Pshort=0.89, Plong =0.07), sex (Pshort =0.21, Plong =0.75), calendar time (Pshort =0.93, Plong =0.37), or number of transfusions (Pshort =0.64, Plong=0.33), but there was a weakly significant interaction between storage time and patient age in the long-term analysis (Pshort =0.10, Plong =0.04). Stratified analyses, however, showed no important differences between strata (data not shown). Upon including also patients transfused in smaller hospitals or with ABO non-identical units 544,203 transfusion episodes were available for short-term and 514,265 transfusion episodes for long-term analyses. Again, the risk estimates for the recipients of the oldest units was similar to the overall analyses, with relative risks of 1.06 (95 percent confidence interval, 1.00-1.12) on short term and 1.03 (95 percent confidence interval, 1.01-1.05) on long term.
Discussion
Here we present the results from a large-scale, population-based observational retrospective cohort study comparing transfusion recipient survival after exposure to red-cell concentrates stored for periods in the upper range (30-42 days) with that experienced by recipients who exclusively received moderately fresh red-cell concentrates (10-19 days). We found transfusion of blood stored for more than 30 days to be associated with a modest 5 percent excess mortality in both short- and long-term analyses. Analyses restricted to supposedly more susceptible sub-populations, including the elderly, patients undergoing coronary artery by-pass graft surgery and patients requiring extracorporeal circulation for other reasons, revealed no noteworthy deviations from the general pattern.
In the hitherto largest investigation of red-cell concentrate storage,22 criticized for insufficient attention to possible confounding,28, 29 in-hospital mortality was increased by 65 percent when comparing recipients of red-cell concentrates stored >14 days to those who received units stored ≤14 days. In our study, we did indeed find a slight excess mortality, but this excess was much smaller and was confined exclusively to recipients of units stored for more than 30 days. This small excess mortality followed no apparent dose-response pattern in the comparison of the different exposure categories. However there was a suggestion of a weak and statistically non-significant dose-response when the number of old units was considered with 7-day follow-up, but not with 2-year follow-up. An exploration of causes of death revealed no indication that any excess risks would be attributable to any specific disease mechanism.
Although variation in the relative risk over time would be expected after a momentary insult, such variation was lacking in our data. Instead, we observed a more or less constant, risk elevation suggesting that the excess observed among recipients of the oldest red-cell concentrates was due to an increased baseline risk rather than to an effect of red-cell storage. In turn, this implies that aged red-cell units might have been differentially distributed among patients at low and high risk of dying. Careful adjustments for number and types of transfusions, hospital, calendar period, season, weekday, and blood group should have taken care of most unintended links between prognosis and age of the allocated concentrates. Further, adjustment for broad categories of indications is likely to have reduced – albeit not eliminated – confounding by indication. We were unable to adjust directly for disease severity because we did not have access to the same richness of clinical variables as in some previous investigations.8, 22 However, the current and historical practice of primarily choosing the oldest available unit of the appropriate blood group, leaves little room for a positive association between disease severity and the age of the transfused units. This is well demonstrated by the lack of associations between storage time and most clinical variables in previous investigations.8, 22 Even so, our data supports the presence of some residual confounding, the nature of which is purely conjectural. Therefore, we believe that any excess mortality truly caused by the transfusion of 30-42 day-old red-cell concentrates, if at all existing, was inflated by undocumented confounding by indication. Consequently, any true effect of red-cell storage on mortality is likely to be less than 5 percent in Sweden and Denmark.
Although we did conduct sub-analyses in some special patient groups, the patients in our overall analyses may not be entirely comparable to those investigated in previous studies, which were all conducted either in the critically ill,9-11, 14-21, 27 or in other special patient populations,7, 8, 12, 22-27 and for the most part in the United States or Canada. While the inclusion of the majority of transfusions in two countries over 7 years strengthens the external validity of our study, it remains difficult to make too detailed comparisons between our findings and those of previous investigators. That said, however, it seems clear that whereas the majority of previous investigators have found associations between storage time and adverse outcomes,13-27 we see only a weakly increased mortality in our total patient population, and no significantly raised mortality in the special patient subgroups we examined. Furthermore, the small overall risk was evident only among the recipients of the units stored the longest, and seemed more consistent with confounding rather than a true effect.
With more than 400,000 transfusion episodes and 100,000 events the material allows a finer categorization of storage time, permits elaborate multivariable modeling with adjustment for essentially all of the potential confounding claimed to have distorted the hitherto largest investigation,1, 22, 29 and offers unprecedented statistical precision with prevailing generalizability. Nonetheless, statistical power was insufficient to exclude weaker associations in small groups undergoing specialized treatments. As the study was based on mortality alone, we may have overlooked associations with non-fatal clinical outcomes. However, essentially identical hospital stay in all exposure groups makes this less likely.
A key strength of this study is the unbiased exposure and outcome ascertainment. We chose to establish four mutually exclusive exposure categories, whereby a sizable number of patients were excluded due to having received units of mixed storage time. Although inefficient from a statistical viewpoint, this scheme is robust as storage time is invariably successively higher in each category. We therefore based the main analyses on this system. Nevertheless, it is reassuring that sensitivity analyses with alternative exposure classification confirmed our results, and that similar results were obtained in supplementary analyses including also patients from the smallest hospitals and recipients of ABO non-identical units produced very similar results. Admittedly, the study was by definition retrospective, but it must be emphasized that all the data was prospectively collected and computerized, and the study is consequentially less susceptible to the types of bias commonly associated with retrospective studies.
Clearly, the confidence in causal inference based on observational study results is limited, despite tight adjustments for the majority of previously implicated confounding factors.1, 29 While a randomized trial is the preferred study design to assess an intervention, an effect as small (but clinically important) as the 5% we have observed suggests that very large trials will be needed to provide convincing evidence.
In conclusion, this large study showed a 5 percent excess mortality confined exclusively to recipients of red-cell concentrates stored for 30 days or more. The observed excess in this group, which remained constant across follow-up but attained statistical significance only in the long-term follow-up, seemed more compatible with a higher baseline risk among the recipients of the very oldest units than with an actual deleterious effect of the old units per se. Thus, confounding may have inflated any true effect of older red-cells, and the true effect, if any, in the combined Swedish and Danish transfusion recipient population is likely to be less than what was observed in this investigation. At any rate, the excess is considerably smaller than in the hitherto largest investigation,22 and seems more compatible with recently published reviews articles which have concluded that the evidence supporting an association between longer storage and adverse clinical outcomes is scarce.1, 13, 29
Acknowledgments
We are greatly indebted to the personnel at all blood banks and transfusion medicine clinics in Sweden and Denmark who contributed data to the SCANDAT database.
No co-author has reported possible conflicts of interest, other sources of financial support, corporate involvement or patent holdings that are relevant to this work. Dr Edgren had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
This study was funded by the National Heart, Lung, and Blood Institute of the U.S. National Institutes of Health (N01-HB-47174). The creation of the SCANDAT database was funded by the National Heart, Lung, and Blood Institute and the National Cancer Institute of the US National Institutes of Health (N01-CP-21175). Dr Edgren has received funding through a post-doctoral stipend from Svenska Sällskapet för Medicinsk Forskning (SSMF). Drs Kamper-Jørgensen and Hjalgrim have received funding from the Danish Medical Research Council (271-08-0831). Dr. Murphy has received a career development award (K24-HL-75036) from the U.S. National Heart, Lung and Blood Institute. The funding organization did not participate in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Footnotes
Conflicts of interest: We declare no conflicts of interest
Author Contributions: Gustaf Edgren obtained funding, contributed to the study design, data procurement, data management, data analysis, data interpretation, and drafting, revision, and final approval of the manuscript. Mads Kamper-Jørgensen contributed to the study design, data management, data interpretation and writing of the manuscript. Sandra Eloranta contributed to the study design, performed the statistical analyses and participated in the revision of the manuscript. Klaus Rostgaard contributed to the study design, data procurement, data management, data interpretation, and drafting of the manuscript. Brian Custer contributed to the study design, interpretation of data and with the drafting of the manuscript. Henrik Ullum contributed to the data procurement, data interpretation, and drafting of the manuscript. Edward Murphy obtained funding and contributed to the study design, interpretation of data as well as with the drafting of the manuscript. Michael Busch obtained funding and contributed to the study design, interpretation of data as well as with the drafting of the manuscript. Marie Reilly contributed to the data procurement, data interpretation, and drafting and review of the manuscript. Mads Melbye participated in the procurement of data, data interpretation and revision as well as final approval of the manuscript. Henrik Hjalgrim participated in the procurement of data, contributed to the study design, data management, data interpretation and revision as well as final approval of the manuscript. Olof Nyrén contributed to the study design and participated in the procurement of data, data interpretation and revision as well as final approval of the manuscript.
References
- 1.Zimrin AB, Hess JR. Current issues relating to the transfusion of stored red blood cells. Vox Sang. 2009;96:93–103. doi: 10.1111/j.1423-0410.2008.01117.x. [DOI] [PubMed] [Google Scholar]
- 2.Tinmouth A, Fergusson D, Yee IC, Hebert PC. Clinical consequences of red cell storage in the critically ill. Transfusion. 2006;46:2014–27. doi: 10.1111/j.1537-2995.2006.01026.x. [DOI] [PubMed] [Google Scholar]
- 3.Card RT. Red cell membrane changes during storage. Transfus Med Rev. 1988;2:40–7. doi: 10.1016/s0887-7963(88)70030-9. [DOI] [PubMed] [Google Scholar]
- 4.Nakao M, Nakao T, Yamazoe S. Adenosine triphosphate and maintenance of shape of the human red cells. Nature. 1960;187:945–6. doi: 10.1038/187945a0. [DOI] [PubMed] [Google Scholar]
- 5.Valeri CR, Hirsch NM. Restoration in vivo of erythrocyte adenosine triphosphate, 2,3-diphosphoglycerate, potassium ion, and sodium ion concentrations following the transfusion of acid-citrate-dextrose-stored human red blood cells. J Lab Clin Med. 1969;73:722–33. [PubMed] [Google Scholar]
- 6.Ho J, Sibbald WJ, Chin-Yee IH. Effects of storage on efficacy of red cell transfusion: when is it not safe? Crit Care Med. 2003;31:S687–97. doi: 10.1097/01.CCM.0000099349.17094.A3. [DOI] [PubMed] [Google Scholar]
- 7.Vamvakas EC, Carven JH. Length of storage of transfused red cells and postoperative morbidity in patients undergoing coronary artery bypass graft surgery. Transfusion. 2000;40:101–9. doi: 10.1046/j.1537-2995.2000.40010101.x. [DOI] [PubMed] [Google Scholar]
- 8.van de Watering L, Lorinser J, Versteegh M, Westendord R, Brand A. Effects of storage time of red blood cell transfusions on the prognosis of coronary artery bypass graft patients. Transfusion. 2006;46:1712–8. doi: 10.1111/j.1537-2995.2006.00958.x. [DOI] [PubMed] [Google Scholar]
- 9.Hebert PC, Chin-Yee I, Fergusson D, et al. A pilot trial evaluating the clinical effects of prolonged storage of red cells. Anesth Analg. 2005;100:1433–8. doi: 10.1213/01.ANE.0000148690.48803.27. [DOI] [PubMed] [Google Scholar]
- 10.Schulman CI, Nathe K, Brown M, Cohn SM. Impact of age of transfused blood in the trauma patient. J Trauma. 2002;52:1224–5. doi: 10.1097/00005373-200206000-00036. [DOI] [PubMed] [Google Scholar]
- 11.Walsh TS, McArdle F, McLellan SA, et al. Does the storage time of transfused red blood cells influence regional or global indexes of tissue oxygenation in anemic critically ill patients? Crit Care Med. 2004;32:364–71. doi: 10.1097/01.CCM.0000108878.23703.E0. [DOI] [PubMed] [Google Scholar]
- 12.Yap CH, Lau L, Krishnaswamy M, Gaskell M, Yii M. Age of transfused red cells and early outcomes after cardiac surgery. Ann Thorac Surg. 2008;86:554–9. doi: 10.1016/j.athoracsur.2008.04.040. [DOI] [PubMed] [Google Scholar]
- 13.Lelubre C, Piagnerelli M, Vincent JL. Association between duration of storage of transfused red blood cells and morbidity and mortality in adult patients: myth or reality? Transfusion. 2009;49:1384–94. doi: 10.1111/j.1537-2995.2009.02211.x. [DOI] [PubMed] [Google Scholar]
- 14.Martin CM, Sibbald WJ, Lu X, Hebert P. Age of transfused red blood cells is associated with ICU length of stay. Clin Invest Med. 1994;17(Suppl 4) Abstr. [Google Scholar]
- 15.Purdy FR, Tweeddale MG, Merrick PM. Association of mortality with age of blood transfused in septic ICU patients. Can J Anaesth. 1997;44:1256–61. doi: 10.1007/BF03012772. [DOI] [PubMed] [Google Scholar]
- 16.Zallen G, Offner PJ, Moore EE, et al. Age of transfused blood is an independent risk factor for postinjury multiple organ failure. Am J Surg. 1999;178:570–2. doi: 10.1016/s0002-9610(99)00239-1. [DOI] [PubMed] [Google Scholar]
- 17.Keller ME, Jean R, LaMorte WW, Millham F, Hirsch E. Effects of age of transfused blood on length of stay in trauma patients: a preliminary report. J Trauma. 2002;53:1023–5. doi: 10.1097/00005373-200211000-00037. [DOI] [PubMed] [Google Scholar]
- 18.Offner PJ, Moore EE, Biffl WL, Johnson JL, Silliman CC. Increased rate of infection associated with transfusion of old blood after severe injury. Arch Surg. 2002;137:711–6. doi: 10.1001/archsurg.137.6.711. discussion 6-7. [DOI] [PubMed] [Google Scholar]
- 19.Murrell Z, Haukoos JS, Putnam B, Klein SR. The effect of older blood on mortality, need for ICU care, and the length of ICU stay after major trauma. Am Surg. 2005;71:781–5. doi: 10.1177/000313480507100918. [DOI] [PubMed] [Google Scholar]
- 20.Smith MJ, Stiefel MF, Magge S, et al. Packed red blood cell transfusion increases local cerebral oxygenation. Crit Care Med. 2005;33:1104–8. doi: 10.1097/01.ccm.0000162685.60609.49. [DOI] [PubMed] [Google Scholar]
- 21.Weinberg JA, McGwin G, Jr, Griffin RL, et al. Age of transfused blood: an independent predictor of mortality despite universal leukoreduction. J Trauma. 2008;65:279–82. doi: 10.1097/TA.0b013e31817c9687. [DOI] [PubMed] [Google Scholar]
- 22.Koch CG, Li L, Sessler DI, et al. Duration of red-cell storage and complications after cardiac surgery. N Engl J Med. 2008;358:1229–39. doi: 10.1056/NEJMoa070403. [DOI] [PubMed] [Google Scholar]
- 23.Basran S, Frumento RJ, Cohen A, et al. The association between duration of storage of transfused red blood cells and morbidity and mortality after reoperative cardiac surgery. Anesth Analg. 2006;103:15–20. doi: 10.1213/01.ane.0000221167.58135.3d. table of contents. [DOI] [PubMed] [Google Scholar]
- 24.Mynster T, Nielsen HJ. Storage time of transfused blood and disease recurrence after colorectal cancer surgery. Dis Colon Rectum. 2001;44:955–64. doi: 10.1007/BF02235483. [DOI] [PubMed] [Google Scholar]
- 25.Leal-Noval SR, Jara-Lopez I, Garcia-Garmendia JL, et al. Influence of erythrocyte concentrate storage time on postsurgical morbidity in cardiac surgery patients. Anesthesiology. 2003;98:815–22. doi: 10.1097/00000542-200304000-00005. [DOI] [PubMed] [Google Scholar]
- 26.Vamvakas EC, Carven JH. Transfusion and postoperative pneumonia in coronary artery bypass graft surgery: effect of the length of storage of transfused red cells. Transfusion. 1999;39:701–10. doi: 10.1046/j.1537-2995.1999.39070701.x. [DOI] [PubMed] [Google Scholar]
- 27.Spinella PC, Carroll CL, Staff I, et al. Duration of red blood cell storage is associated with increased incidence of deep vein thrombosis and in hospital mortality in patients with traumatic injuries. Crit Care. 2009;13:R151. doi: 10.1186/cc8050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Benjamin RJ, Dodd RY, Hall SW, et al. Red-Cell Storage and Complications of Cardiac Surgery. N Engl J Med. 2008;358:2840–2. doi: 10.1056/NEJMc080874. [DOI] [PubMed] [Google Scholar]
- 29.Dzik W. Fresh blood for everyone? Balancing availability and quality of stored RBCs. Transfus Med. 2008;18:260–5. doi: 10.1111/j.1365-3148.2008.00870.x. [DOI] [PubMed] [Google Scholar]
- 30.Alter HJ, Klein HG. The hazards of blood transfusion in historical perspective. Blood. 2008;112:2617–26. doi: 10.1182/blood-2008-07-077370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Högman CF, Ramgren O. Computer system for blood transfusion service. Transfusion. 1970;10:121–32. doi: 10.1111/j.1537-2995.1970.tb00718.x. [DOI] [PubMed] [Google Scholar]
- 32.Freiesleben E, Jensen KG, Tamborg O. Donorregistrering og elektronisk databehandling. Ugeskr Laeger. 1965;127:1171–6. [PubMed] [Google Scholar]
- 33.Edgren G, Hjalgrim H, Tran TN, et al. A population-based binational register for monitoring long-term outcome and possible disease concordance among blood donors and recipients. Vox Sang. 2006;91:316–23. doi: 10.1111/j.1423-0410.2006.00827.x. [DOI] [PubMed] [Google Scholar]
- 34.Edgren G, Hjalgrim H, Reilly M, et al. Risk of cancer after blood transfusion from donors with subclinical cancer: a retrospective cohort study. Lancet. 2007;369:1724–30. doi: 10.1016/S0140-6736(07)60779-X. [DOI] [PubMed] [Google Scholar]
- 35.Hjalgrim H, Edgren G, Rostgaard K, et al. Cancer incidence in blood transfusion recipients. J Natl Cancer Inst. 2007;99:1864–74. doi: 10.1093/jnci/djm248. [DOI] [PubMed] [Google Scholar]
- 36.Edgren G, Reilly M, Hjalgrim H, et al. Donation frequency, iron loss, and risk of cancer among blood donors. J Natl Cancer Inst. 2008;100:572–9. doi: 10.1093/jnci/djn084. [DOI] [PubMed] [Google Scholar]
- 37.Kamper-Jorgensen M, Ahlgren M, Rostgaard K, et al. Survival after blood transfusion. Transfusion. 2008;48:2577–84. doi: 10.1111/j.1537-2995.2008.01881.x. [DOI] [PubMed] [Google Scholar]
- 38.Shanwell A, Andersson TM, Rostgaard K, et al. Post-transfusion mortality among recipients of ABO-compatible but non-identical plasma. Vox Sang. 2009;96:316–23. doi: 10.1111/j.1423-0410.2009.01167.x. [DOI] [PubMed] [Google Scholar]
- 39.Lin DY, Wei LJ. The Robust Inference for the Proportional Hazards Model. Journal of the American Statistical Association. 1989;84:1074–8. [Google Scholar]