1.1 Introduction
Aging is associated with muscle weakness and fatigue, a condition that has been termed sarcopenia [1–3]. The cause of sarcopenia is likely multifactorial and includes a variety of potential mechanisms including neuromuscular changes, decreased nutrition, hormonal changes, and inactivity [4–6]. Sarcopenia affects all elderly individuals to some extent because it is a consequence of normal aging [4]. In addition, sarcopenia has clinical relevance because the loss of muscle mass and strength in the aging musculature has functional consequences. Functional deficits have been well characterized in the limb musculature and include changes in balance and gait, increased risk of falls, and decreased independence [7, 8]. Recent data suggest that similar age-related changes occur in the cranial musculature involved in swallowing, and may be associated with the age-related changes seen in the swallow of elderly individuals [9–11]. Elderly individuals swallow more slowly and have increased residue in the pharyngeal cavity following the swallow, both of which may result from reduced strength in tongue and pharyngeal musculature [12, 13].
Due to the complex nature of sarcopenia, a variety of treatments have been suggested to decrease the functional consequences of this condition. Of the possible treatments proposed for limb musculature, resistance exercise appears to be the most promising [14–17]. In addition, resistance exercise training of the tongue musculature has been shown to have beneficial effects on tongue muscle strength and swallowing function in elderly individuals [18, 19]. Therefore, tongue exercise protocols are currently being used in clinical practice to strengthen the lingual musculature and to improve age-related declines in swallowing function [20, 21].
While evidence suggests that resistance exercise is a beneficial treatment for sarcopenia, the underlying mechanisms responsible for the increases in muscle strength and function associated with exercise are unknown. In addition to the changes in musculature with exercise, there is evidence to suggest that exercise has a neuroprotective component [22–24] mediated by neurotrophins in both the central and peripheral nervous systems [25]. Neurotrophins are a family of proteins that, when activated through binding with tropomysoin-related kinase (Trk) receptors, initiate signaling cascades that promote the development, survival, and function of neurons [26–28]. Given that the neurotrophin receptors TrkB and TrkC are decreased in the spinal motoneurons of aged rats [29], it appears that decreases in neurotrophin levels may also have a role in the limb deficits seen with aging. Results of previous studies in the brain and spinal cord support the hypothesis that neurotrophins act as a therapeutic agent in cases of neurodegenerative disease and nerve injury [30–32]. In addition, neurotrophins appear to be regulated in an exercise dependent manner. Vaynman and colleagues found that mRNA of brain-derived neurotrophic factor (BDNF) and its receptor TrkB was up regulated in the hippocampus of rats after 3 and 7 days of wheel running [33]. The same group also found that both mRNA and protein levels of BDNF were increased in the spinal cord following 5 days of wheel running exercise [34]. Other work has shown that BDNF is important for plasticity in respiratory regions of the spinal cord after intermittent hypoxia, which is used as a method of inducing long term facilitation in phrenic and hypoglossal motor outputs [35, 36]. Therefore, previous work has demonstrated a link between neurotrophins, aging, and activity-dependent neuroplasticity in the limb sensorimotor system.
No studies, however, have examined changes in neurotrophin levels in the cranial sensorimotor system with either age or exercise. Currently, our laboratory is using an animal model to study the underlying changes to the tongue musculature associated with progressive resistance exercise of the tongue [9, 10]. We have shown that tongue exercise induces changes in muscle fiber size and variability in the genioglossus muscle of aged rats that are associated with increased protrusive tongue forces. We found that there was a trend toward an increase in muscle fiber size with tongue exercise and a significant increase in muscle fiber size variability with tongue exercise [9]. In addition, we have shown that neuromuscular stimulation results in a reduction in age-related changes to the morphology of the neuromuscular junction in aged rats [10]. In this study we used our previously described animal model to examine changes in neurotrophin levels in the hypoglossal nucleus of rats with both age and exercise. We hypothesized that levels of BDNF and TrkB immunoreactivity would be decreased with age and increase with exercise. Therefore, the purpose of our study was to examine the levels of BDNF and TrkB in the hypoglossal nucleus of rats at different ages, in both control and exercise conditions, to determine the effect of age and exercise on BDNF and TrkB in the cranial nucleus that controls the tongue musculature involved in swallowing.
1.2 Methods
All procedures were performed in compliance with the NIH Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee at the University of Wisconsin. A total of 48 (16 young, 16 middle-aged, 16 old) male Fischer 344/Brown Norway (F344/BN) rats were obtained from the National Institute of Aging colony. At study completion, the animals were in one of three age groups: young (9–10 months), middle-aged (24–25 months), or old (32–33 months). The median life expectancy of F344/BN rats is 36 months [37]. Every effort was made to minimize the number of animals used and their suffering. Thus, tissues from these animals were assigned to more than one experiment [38]
1.2.1 Exercise
Animals were housed in pairs in standard polycarbonate cages on a 12:12 hour light-dark reversed light cycle. Rats were obtained 8 weeks prior to the start of the experiment to allow acclimation to the animal care facility, reversal of light cycle, water restriction, and familiarization to the tongue force operandum. Food was given ad libitum. Water was restricted to 3 hours per day to encourage the animals to press a disk for a water reward. Experimental methods for tongue press measurements in rats have been detailed previously [9, 39] but are discussed briefly below.
Throughout the experiment, animals were placed individually into a polycarbonate cage resembling the home cage, but equipped with a 1 × 1 centimeter (cm) aperture and force operandum that delivered aliquots of water based on tongue press behaviors.
After familiarization with the task, baseline tongue force measurements were obtained for the rats in the exercise group allowing for a measurement of baseline maximum force (g). Following baseline testing the animals in the exercise group underwent an 8-week training paradigm. Throughout the 8 weeks of training the force required for a water reward was increased to mimic a progressive resistance training program. For the first two weeks of training the animals were required to press at 50% of their estimated maximum press (EMP) force, which was established during baseline testing. During the second two weeks the force was increased to 60% of their EMP force, then 70%, and finally 80%. After the 8 weeks of training, post-exercise maximum force (g) values were obtained.
The control rats were placed on a water restriction protocol and light/dark cycle reversal identical to the exercise-treatment rats. However, they were not given access to the operandum enclosure and did not receive any exercise treatment. Instead, they were placed in an enclosure that resembled the operandum enclosure and were permitted to drink water ad libitum from a water dish for 3 hours.
1.2.2 Perfusion
Rats were anesthetized with isoflurane followed by sodium pentobarbital (120 mg/kg i.p.). Anesthetized rats were transcardially perfused with 200ml of heparinized saline (10,000 units/liter) followed by 400 mL of 4% paraformaldehyde and 0.1% glutaraldehyde in 0.1 M sodium phosphate buffer (PB) (pH 7.4). Brains were removed and postfixed for 1 hour at 4°C, then cryoprotected for 24–36 h at 4°C with 20% sucrose and 5% glycerol in 0.1M phosphate buffer. Sections were cut coronally (40µm) and stored in 0.1 M phosphate buffer containing 0.02% sodium azide at 4° C.
1.2.3 Immunocytochemistry
Two sections through the hypoglossal nucleus from each animal were immunochemically reacted for the presence of BDNF. A separate pair of adjacent sections were reacted for the presence of TrkB. Specifically, sections were selected from the junction between middle-caudal (to be known as caudal sections) and middle-rostral (to be known as rostral sections) of the hypoglossal nucleus in the medulla. A dilution series was conducted to identify the optimal dilution for each antibody. Sections were washed in Tris-buffered saline (TBS), then in 0.1% Triton X-100 in TBS (TBST). After 2 h in blocking solution (5% normal donkey serum in TBST), primary antibodies were applied for 24 h at room temperature in blocking solution. Primary antibodies were used at 1:50: anti-BDNF (Santa Cruz, sc-546, Santa Cruz, CA), and 1:200 anti-TrkB (Santa Cruz, sc-8316, Santa Cruz, CA). An Alexa-Fluor conjugated secondary antibody (594 donkey anti-rabbit IgG. Invitrogen, Eugene, OR) was used for both BDNF and TrkB staining at 1:500 in 5% normal donkey serum in TBST. All sections were reacted at the same time. Negative controls were reacted simultaneously with the omission of either the primary or secondary antibody. Sections were mounted and coverslipped with Vectashield Hard Set mounting medium for fluorescence (Vector Laboratories, Burlingame, CA). There were no labeled cells in negative control sections from all behavioral states.
1.2.4 Analysis
All images were acquired during the same session using SPOT (Advanced version) computer software and SPOT RT Slider camera (Diagnostic Instruments, Sterling Heights, MI, USA) attached to a Nikon Eclipse E600 microscope. In each section, one image was taken from each side of the ventral middle hypoglossal nucleus (Fig. 1A) for both BDNF and TrkB immunoreactivity using the 40X objective, resulting in 8 images from each animal (4 of BDNF, 4 of TrkB). Images were analyzed using ImageJ [40]. To separate signal from background an Otsu thresholding algorithm was applied to each image. The average fluorescent intensity of each image, captured in relative fluorescent units (RFU), was then measured and used for statistical analysis.
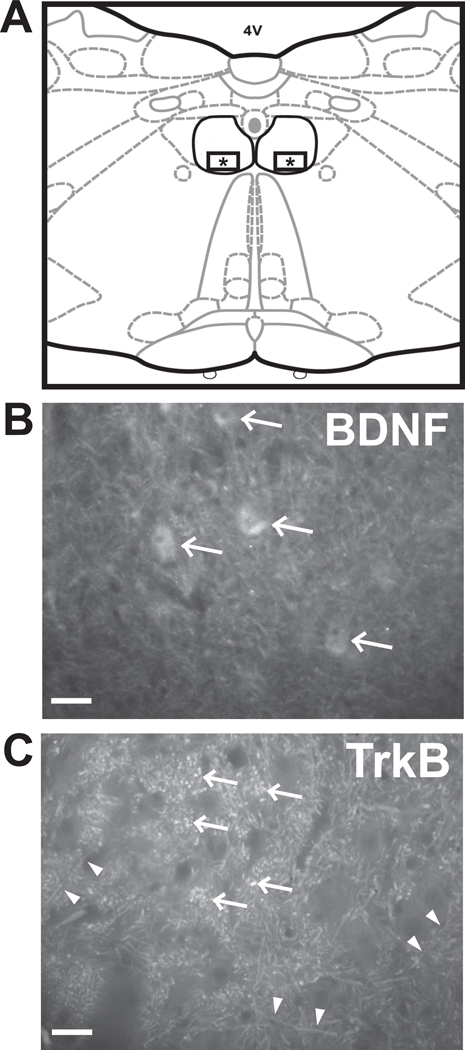
Fig 1.
(A) Diagram of a representative coronal section through the caudal medulla showing the location of images used for measurement of fluorescent signal for BDNF and TrkB. The hypoglossal nucleus is outlined in black. The squares containing asterisks within the ventral half of the nucleus depict the areas imaged. (B) Representative image of BDNF immunoreactivity showing diffuse staining throughout the nucleus. Staining was more intense in cell bodies (arrows). (C) Representative image of TrkB immunoreactivity showing punctuate staining of axons (double arrow heads) and synaptic boutons (arrows). Scale bar = 50 µm.
We used an analysis of variance (ANOVA) to compare tongue forces between age groups. The pre-to-post exercise change in force was assessed within each age group using a paired t-test. The impact of age, exercise, and region (caudal vs. rostral) on average fluorescent intensity within the specified area of the hypoglossal nucleus was examined using a mixed model analysis for both BDNF and TrkB independently. Age, exercise, and region were included as fixed variables, and the rat itself was included as a random variable to account for the multiple measures taken from each animal. Post-hoc testing was completed on all significant interactions found during the mixed model analysis using a Fisher’s LSD analysis to examine individual group differences. In addition, Spearman Correlation was used to examine the relationship between BDNF and TrkB immunoreactivity in the different age, exercise, and region groups. All analyses were performed using SAS statistical software version 9.1 (SAS Institute Inc., Cary, NC). P-values less than 0.05 were considered as significant.
1.3 Results
1.3.1 Force
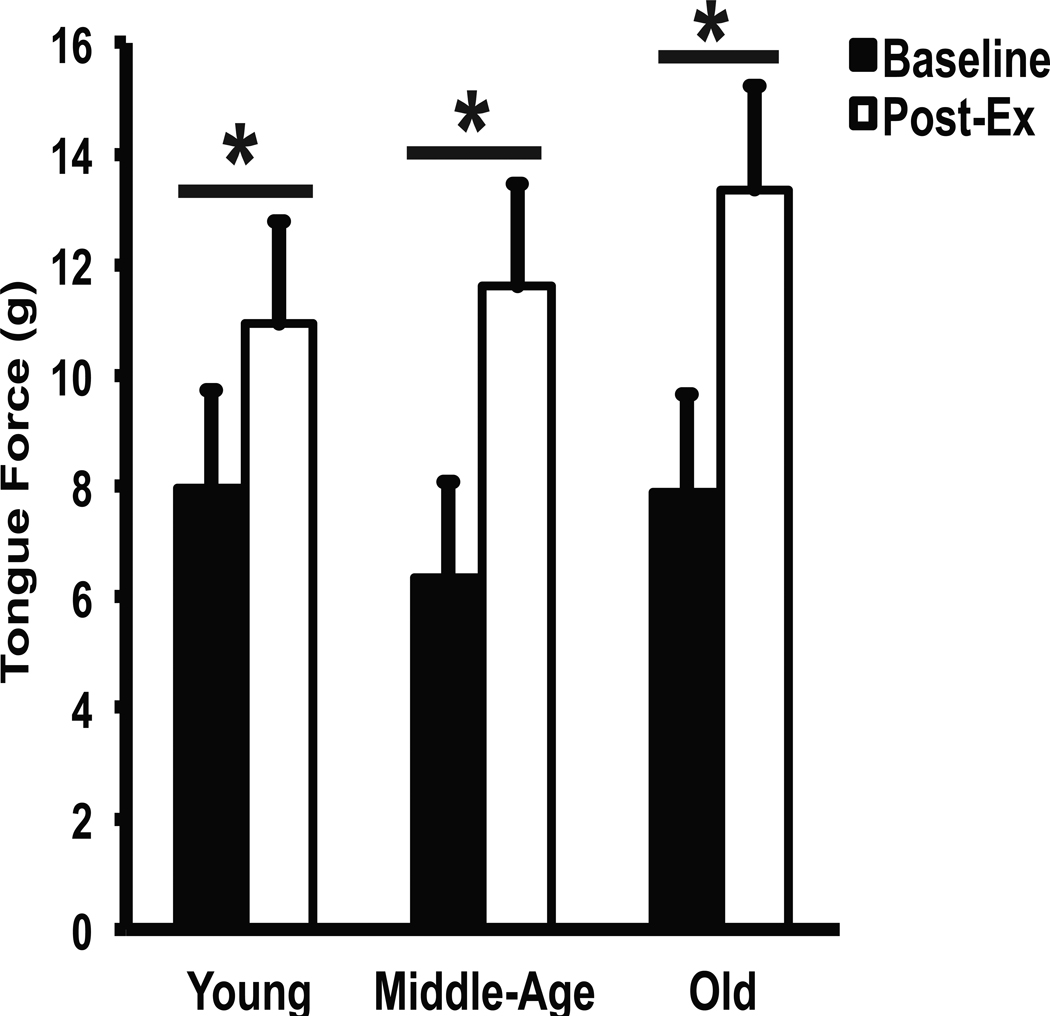
Tongue exercise was associated with increased maximum tongue force (g) at all ages compared with baseline values (Fig. 2; F2, 21=4.23, p=.03.) Posthoc testing revealed that middle aged and old rats had significantly greater gains in maximum tongue forces than young rats (p < .05). No difference in force gains was found between middle aged and old rats.
Fig 2.
Effect of tongue exercise on behavioral tongue forces at different ages. Age groups (young, middle-age, and old) are represented along the horizontal axis and tongue force (in grams) is represented along the vertical axis. Black bars represent baseline tongue forces and white bars represent tongue forces following 8-weeks of tongue exercise. A significant increase (p < 0.05) in tongue force was seen in all age groups. * denotes significant values; error bars represent standard error of the mean.
1.3.2 BDNF Immunoreactivity
BDNF immunoreactivity was found in both the rostral and caudal regions of the hypoglossal nucleus in all age groups. BDNF staining was diffuse throughout the ventral medial portion of the hypoglossal nucleus, with the areas of high intensity staining concentrated in cell bodies (arrows in Fig.1B).
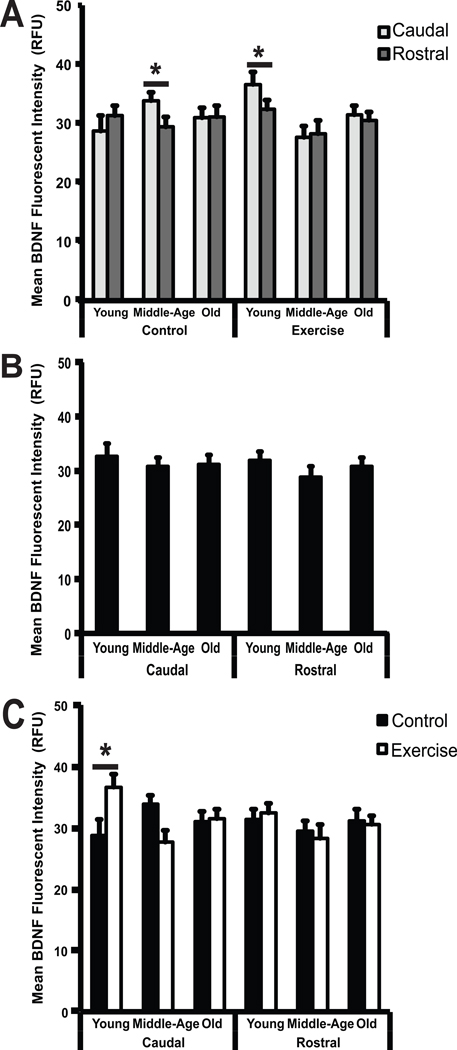
Significant regional differences in staining intensity were found (F (2, 42) = 9.71, p = 0003). Specifically, BDNF immunoreactivity was significantly higher in the caudal region than in the rostral region in both young exercised animals and in middle-aged control animals (Fig. 3A; p = 0.003, p = 0.002), respectively.
Fig 3.
(A–C) Changes in BDNF immunoreactivity by region (A), age (B), and exercise (C). (A) Mean BDNF fluorescent intensity, expressed in relative fluorescent units (RFU), is represented along the vertical axis. Age groups (young, middle-age, and old), divided into control and exercise conditions, are represented along the horizontal axis. BDNF immunoreactivity in the caudal portion of the hypoglossal nucleus is shown in light gray and BDNF immunoreactivity in the rostral portion of the hypoglossal nucleus is shown in dark gray. A significant increase (p < 0.05) in BDNF immunoreactivity was seen in the caudal portion of the hypoglossal nucleus compared to the rostral portion of the hypoglossal nucleus in middle-aged animals in the control group, and in young animals in the exercise group. (B) Mean BDNF fluorescent intensity, expressed relative fluorescent units (RFU) is represented along the vertical axis. Age groups (young, middle-age, and old), divided into control and exercise conditions, are represented along the horizontal axis. No significant differences (p < 0.05) in BDNF immunoreactivity were seen across age groups in either the caudal or rostral regions of the hypoglossal nucleus (C) Mean BDNF fluorescent intensity, expressed in relative fluorescent units (RFU) is represented along the vertical axis. Age groups (young, middle-age, and old), divided into control and exercise conditions, are represented along the horizontal axis. A significant increase (p < 0.05) in BDNF immunoreactivity was seen in the young age group in the caudal portion of the hypoglossal nucleus with exercise. * denotes significant values; error bars represent standard error of the mean.
1.3.3 Age and Exercise-associated changes in BDNF Immunoreactivity
BDNF levels in the ventral medial portion of the hypoglossal nucleus were not affected by age. BDNF immunoreactivity was similar in all age groups in both the caudal and rostral regions of the hypoglossal nucleus (Fig.3B). BDNF levels were, however, affected by exercise (F (2, 42) = 9.71, p = .0003), but this effect was only seen in the young rat group in the caudal portion of the hypoglossal nucleus (Fig.3C). Specifically, a significant increase was found in BDNF immunoreactivity with exercise in young animals in the caudal region (p = 0.03). However, no differences were found in any other age group or region.
1.3.4 TrkB Immunoreactivity
TrkB immunoreactivity was present in both the rostral and caudal regions of the hypoglossal nucleus in all age groups. In contrast to BDNF, TrkB staining was punctate and appeared to stain axons (double arrow heads in Fig.1C) and synaptic vesicles (arrows in Fig.1C).
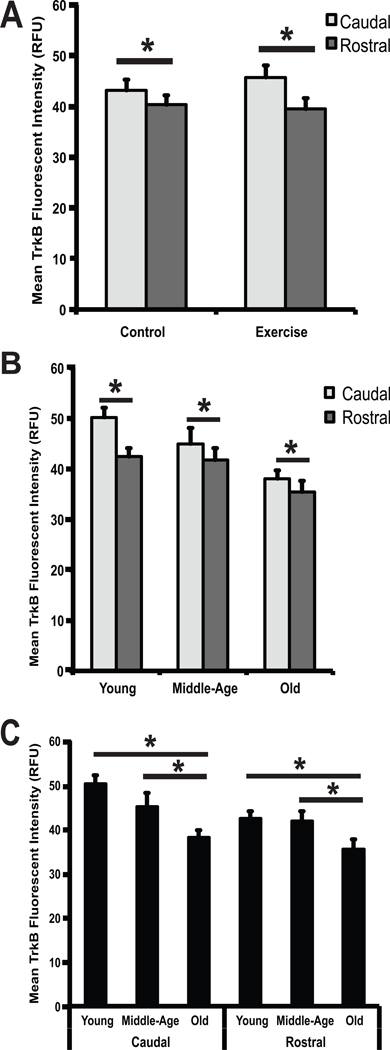
Significant regional differences in staining intensity were detected and were more widespread for TrkB than BDNF. Regional differences in TrkB immunoreactivity were present across conditions (F (1,38) = 5.32, p = 0.03) and age groups (F (2,38) = 5.11, p = 0.01). Specifically, TrkB immunoreactivity was greater in the caudal region than the rostral region for both the exercise group and the control group (Fig.4A; p < 0.0001, p = 0.0096) and in each of the three age groups (Fig.4B; p = <0.0001, p = 0.01, p = 0.05).
Fig 4.
(A–C) Changes in TrkB immunoreactivity by region (A), region and age (B) and age (C). (A) Mean TrkB fluorescent intensity, expressed in relative fluorescent units (RFU), is represented along the vertical axis. Control and exercise conditions are represented along the horizontal axis. TrkB immunoreactivity in the caudal portion of the hypoglossal nucleus is shown in light gray and TrkB immunoreactivity in the rostral portion of the hypoglossal nucleus is shown in dark gray. Significantly greater TrkB immunoreactivity (p < 0.05) was seen in the caudal portion of the hypoglossal nucleus compared to the rostral portion of the hypoglossal nucleus in both the control and exercise conditions. (B) Mean TrkB fluorescent intensity, expressed in relative fluorescent units (RFU) is represented along the vertical axis. Age groups (young, middle-age, and old) are represented along the horizontal axis. TrkB immunoreactivity in the caudal portion of the hypoglossal nucleus is shown in light gray and TrkB immunoreactivity in the rostral portion of the hypoglossal nucleus is shown in dark gray. Significantly greater TrkB immunoreactivity (p < 0.05) was seen in the caudal portion of the hypoglossal nucleus compared to the rostral portion of the hypoglossal nucleus in all age groups. (C) Mean TrkB fluorescent intensity, expressed in relative florescent units (RFU), is represented along the vertical axis. Age groups (young, middle-age, and old), divided into caudal and rostral regions, are represented along the horizontal axis. A significant decrease (p < 0.05) in TrkB immunoreactivity was seen in the old age group compared to the young and middle-age age groups in both the caudal and rostral portions of the hypoglossal nucleus. * denotes significant values; error bars represent standard error of the mean.
1.3.5 Age and Exercise-associated changes in TrkB Immunoreactivity
TrkB levels in the hypoglossal nucleus were affected by age (F (2,38) = 5.11, p = 0.01). Post-hoc testing revealed a significant decrease in TrkB immunoreactivity with age in both the caudal and rostral regions of the hypoglossal nucleus (Fig.4C). In the caudal region, there was a significant decrease in TrkB in old animals compared with young animals (p = 0.0001), and in old animals compared with middle-age animals (p = 0.02). No difference was found between middle-age animals and young animals. In the rostral region, there was a significant decrease in TrkB in the old animals compared with young animals (p = 0.02) and in old animals compared with middle-age animals (p = 0.03). TrkB levels in the hypoglossal nucleus were not affected by exercise at any age.
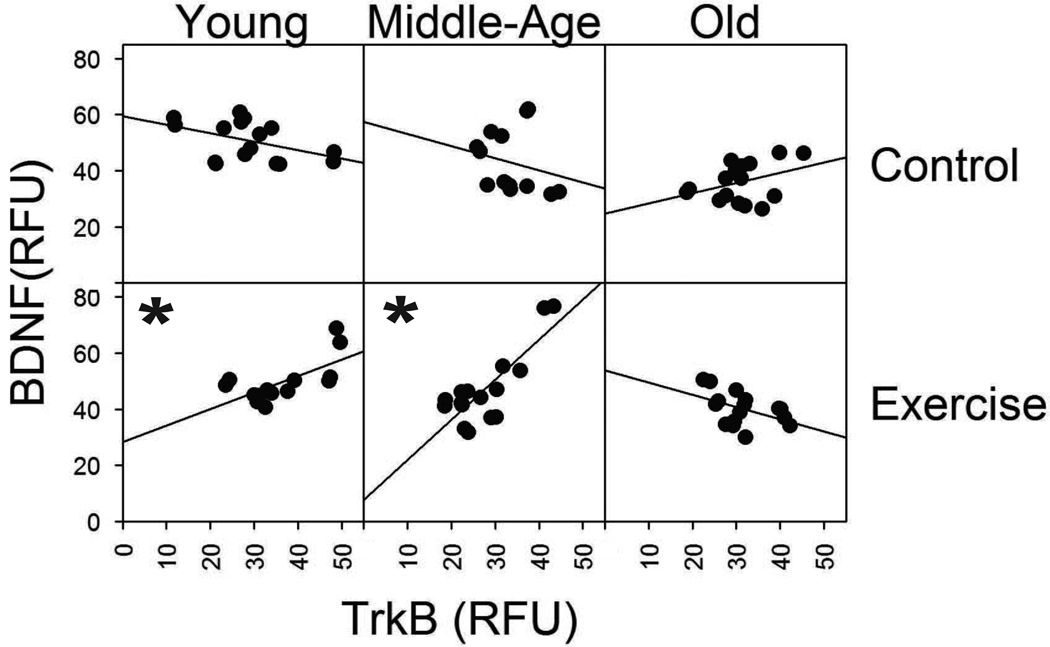
1.3.6 Relationship between BDNF and TrkB Immunoreactivity
Both BDNF and TrkB must be present to exert the downstream signaling effects that cause synaptic changes in the cell [28, 41, 42]. Thus, we examined the relationship between BDNF and TrkB immunoreactivity within age and exercise conditions. Spearman correlation analysis revealed that BDNF and TrkB immunoreactivity were positively correlated following exercise in both the caudal and rostral regions in young and middle-age animals (Fig.5; young caudal: rho = 0.59, p = 0.03; young rostral: rho = 0.62, p = 0.02; middle-age caudal rho = 0.58, p = 0.02; middle-age rostral rho = 0.52, p = 0.04). Negative or weak correlations were found in control animals at all ages. In addition, BDNF was negative or weakly correlated with TrkB immunoreactivity in old animals in both the exercise and control groups (Fig. 5).
Fig 5.
Correlation between BDNF and TrkB immunoreactivity with exercise in young and in age groups. Each square represents a different age group and condition. The control condition is represented in the first row and the exercise condition represented in the second row. Age groups (young, middle-age, and old) are represented across the three columns, respectively. Within each square, mean TrkB intensity expressed in relative fluorescent units (RFU), is represented along the horizontal axis and mean BDNF intensity along the vertical axis. Each data point represents one animal. With both caudal and rostral data combined for each age group and condition. The slope of the line indicates the correlation between BDNF and TrkB immunoreactivity. A significant positive correlation (p < 0.05) between TrkB and BDNF was seen in the young and middle-age groups with exercise. * denotes significant values.
1.4 Discussion
The hypothesis guiding this study was that the neurotrophin BDNF and its receptor TrkB would be decreased in the hypoglossal nucleus of rats in an age-dependent manner and would increase following a tongue exercise regime of eight weeks. We found that TrkB immunoreactivity was decreased with age in both the caudal and rostral regions of the hypoglossal nucleus and that BDNF was increased with exercise in caudal region of the hypoglossal nucleus in young rats. However, BDNF did not decrease with age, and exercise-induced changes in BDNF and TrkB were not found in the middle aged and old rats despite significant increases in tongue force.
Our results are similar to previous work that reported age-related reductions in TrkB in spinal cord motor and sensory neurons [29, 43, 44]. BDNF sparing in the presence of TrkB reductions with aging has also been found in the pituitary [45]. Because both the neurotrophin and its receptor must be present to allow downstream signaling cascades leading to synaptic changes within the neuron [28, 41, 42], age related changes to TrkB receptors alone may be sufficient to affect synaptic function. Therefore, reduced TrkB immunoreactivity with age in the hypoglossal nucleus suggests that there are age-related changes to the neurotrophin system that may interfere with normal synaptic function. It may not be necessary for both the neurotrophin and its receptor to manifest reductions with age for the entire system to be affected.
TrkB functions as a high affinity receptor for neurotrophins other than BDNF [28, 42, 46]. Specifically, TrkB is also a receptor for muscle-derived NT4/5 neurotrophin [41]. Accordingly, the lack of age-related decline in BDNF in this study may suggest that another neurotrophin, for instance NT4/5, may be reduced with age. In this study, only BDNF immunoreactivity was measured. Given that NT4/5 has been shown to be important for neuromuscular plasticity in the uninjured animal throughout the lifespan [29, 47], NT4/5 may serve as an important neurotrophin to focus on in future studies.
Our results indicated that BDNF immunoreactivity was increased with tongue exercise in the caudal region of young animals, and represents the first indicator that targeted tongue training can result in increased neurotrophin levels in the cranial sensorimotor system. Previous work in the limb sensorimotor system has shown that direct administration of BDNF into the spinal cord can promote growth and survival of damaged neurons [36, 48] and can lead to improved recovery after spinal cord injury [49]. In addition, BDNF and NT4/5 have been shown to improve synaptic transmission at the neuromusclular junction by binding with the TrkB receptors to increase release of synaptic vesicles [50]. Our results show that targeted tongue exercise may be a less invasive therapeutic method of increasing BDNF levels in the cranial motor system and further support the neuroprotective effects of exercise.
Additional support for the neuroprotective effects of exercise is provided by the results of our correlative data, in which we found a moderate positive correlation between TrkB and BDNF immunoreactivity in both sections of the hypoglossal nucleus in young and middle-age animals following exercise: the immunoreactivity levels of both BDNF and TrkB in young and middle-age animals increased following exercise. However, these increases were not large enough to lead to statistically significant changes, except in the caudal section of young animals. As stated previously, both the neurotrophin and receptor need to be present to initiate the down-stream signaling cascades that result in improved synaptic transmission [41]. Therefore exercise may serve as a method to regulate the levels of both BDNF and its receptor to promote more effective ligand to receptor binding. However, this does not appear to be the case in the old animals, as TrkB and BDNF immunoreactivity were weakly correlated in both the control and exercise conditions in old animals.
In a recent systematic review of the literature examining the effect of exercise on peripheral (serum or plasma) levels of BDNF in human subjects, it was shown that increases in BDNF levels following exercise were dependent on the type of exercise performed and appeared to be somewhat transient in nature [51]. In our study only one type of exercise (strength training) was evaluated, and there was a time delay between the end of our exercise protocol and our data collection. Therefore the lack of significant increases in BDNF and TrkB immunoreactivity observed in our study in middle age and old animals may be due to these factors. Future studies should examine the effect of different forms of exercise on levels of BDNF and TrkB in the cranial sensorimotor system, such as acute exercise vs. prolonged training, and should also measure neurotrophin levels immediately following the end of the exercise protocol in an attempt to capture more transient increases in neurotrophins and receptors following exercise.
Tongue forces increased following exercise in all age groups. Despite an increase in BDNF expression in young rats following exercise, there was not a concomitant increase in BDNF immunoreactivity in the middle aged and old rats. It is possible that different mechanisms and or neurotrophins are involved in the functional changes seen in older animals. For example, Ying and colleagues found increases in NT-3 mRNA and protein in the lumbar spinal cord after 7 days of treadmill running, and increases in TrkC mRNA in both lumbar spinal cord and soleus muscle after exercise. However, increases in NT-3 mRNA and not in protein were observed in the soleus muscle [52]. Similarly, Gomez-Pinilla and colleagues found that exercise has a differential effect on BDNF and NT-3 in both the spinal cord and muscle [34]. Based on the results of these studies it will be necessary to look at changes in mRNA and protein levels of several different neurotrophins and their receptors in both the hypoglossal nucleus and the tongue musculature before we can rule out the possibility that neurotrophins play a role in the increased tongue forces seen in old animals.
1.5 Conclusion
This study is the first to examine age and exercise-induced effects on neurotrophins in the cranial sensorimotor system. Our results show that the cranial system undergoes age related neurotrophic changes, specifically manifested as a decrease in TrkB receptors in the hypoglossal nucleus with age. In addition, our results show that exercise results in an increase in BDNF immunoreactivity in the hypoglossal nucleus of young animals. However the role of exercise induced neurotrophic up-regulation in older animals requires further study. Based on the results of this initial study it appears necessary to continue to explore age and exercise induced changes in neurotrophic factors in the tongue musculature and hypoglossal nucleus to further elucidate the neuroprotective role of exercise as it relates to disorders of the cranial sensorimotor system.
Acknowledgements
This study was supported by National Institute on Deafness and Other Communication Disorders Grants R01DC005935 and R01DC008149. We are grateful for the assistance of Glen Leverson, Aaron Johnson, John Russell, Michelle Ciucci, Heidi Kletzien, and Jaime Shier in the completion of this work.
References
- 1.Rosenberg IH. Sarcopenia: Origins and clinical relevance. J Nutr. 1997 May;127(5) Suppl:990S–991S. doi: 10.1093/jn/127.5.990S. [DOI] [PubMed] [Google Scholar]
- 2.Doherty TJ. Invited review: Aging and sarcopenia. J Appl Physiol. 2003 Oct;95(4):1717–1727. doi: 10.1152/japplphysiol.00347.2003. [DOI] [PubMed] [Google Scholar]
- 3.Janssen I. Evolution of sarcopenia research. Appl Physiol Nutr Metab. 2010 Oct;35(5):707–712. doi: 10.1139/H10-067. [DOI] [PubMed] [Google Scholar]
- 4.Roubenoff R. Origins and clinical relevance of sarcopenia. Can J Appl Physiol. 2001 Feb;26(1):78–89. doi: 10.1139/h01-006. [DOI] [PubMed] [Google Scholar]
- 5.Marcell TJ. Sarcopenia: Causes, consequences, and preventions. J Gerontol A Biol Sci Med Sci. 2003 Oct;58(10):M911–M916. doi: 10.1093/gerona/58.10.m911. [DOI] [PubMed] [Google Scholar]
- 6.Thomas DR. Sarcopenia. Clin Geriatr Med. 2010 May;26(2):331–346. doi: 10.1016/j.cger.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 7.Dutta C. Significance of sarcopenia in the elderly. J Nutr. 1997 May;127(5) Suppl:992S–993S. doi: 10.1093/jn/127.5.992S. [DOI] [PubMed] [Google Scholar]
- 8.Lang T, Streeper T, Cawthon P, Baldwin K, Taaffe DR, Harris TB. Sarcopenia: Etiology, clinical consequences, intervention, and assessment. Osteoporos Int. 2010 Apr;21(4):543–559. doi: 10.1007/s00198-009-1059-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Connor NP, Russell JA, Wang H, Jackson MA, Mann L, Kluender K. Effect of tongue exercise on protrusive force and muscle fiber area in aging rats. J Speech Lang Hear Res. 2009 Jun;52(3):732–744. doi: 10.1044/1092-4388(2008/08-0105). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson AM, Connor NP. Effects of electrical stimulation on neuromuscular junction morphology in the aging rat tongue. Muscle Nerve. 2011 Feb;43(2):203–211. doi: 10.1002/mus.21819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schaser AJ, Wang H, Volz LM, Connor NP. Biochemistry of the anterior, medial, and posterior genioglossus in the aged rat. Dysphagia. 2010 Aug 31; doi: 10.1007/s00455-010-9297-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cook IJ, Weltman MD, Wallace K, Shaw DW, McKay E, Smart RC, et al. Influence of aging on oral-pharyngeal bolus transit and clearance during swallowing: Scintigraphic study. Am J Physiol. 1994 Jun;266(6 Pt 1):G972–G977. doi: 10.1152/ajpgi.1994.266.6.G972. [DOI] [PubMed] [Google Scholar]
- 13.Nicosia MA, Hind JA, Roecker EB, Carnes M, Doyle J, Dengel GA, et al. Age effects on the temporal evolution of isometric and swallowing pressure. J Gerontol A Biol Sci Med Sci. 2000 Nov;55(11):M634–M640. doi: 10.1093/gerona/55.11.m634. [DOI] [PubMed] [Google Scholar]
- 14.Kirkendall DT, Garrett WE., Jr The effects of aging and training on skeletal muscle. Am J Sports Med. 1998 Jul–Aug;26(4):598–602. doi: 10.1177/03635465980260042401. [DOI] [PubMed] [Google Scholar]
- 15.Liu CJ, Latham NK. Progressive resistance strength training for improving physical function in older adults. Cochrane Database Syst Rev. 2009 Jul 8;3(3) doi: 10.1002/14651858.CD002759.pub2. CD002759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Waters DL, Baumgartner RN, Garry PJ, Vellas B. Advantages of dietary, exercise-related, and therapeutic interventions to prevent and treat sarcopenia in adult patients: An update. Clin Interv Aging. 2010 Sep 7;5:259–270. doi: 10.2147/cia.s6920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peterson MD, Sen A, Gordon PM. Influence of resistance exercise on lean body mass in aging adults: A meta-analysis. Med Sci Sports Exerc. 2011 Feb;43(2):249–258. doi: 10.1249/MSS.0b013e3181eb6265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robbins J, Gangnon RE, Theis SM, Kays SA, Hewitt AL, Hind JA. The effects of lingual exercise on swallowing in older adults. J Am Geriatr Soc. 2005;53(9):1483–1489. doi: 10.1111/j.1532-5415.2005.53467.x. [DOI] [PubMed] [Google Scholar]
- 19.Kays S, Robbins J. Effects of sensorimotor exercise on swallowing outcomes relative to age and age-related disease. Semin Speech Lang. 2006 Nov;27(4):245–259. doi: 10.1055/s-2006-955115. [DOI] [PubMed] [Google Scholar]
- 20.Logemann JA. The role of exercise programs for dysphagia patients. Dysphagia. 2005 Spring;20(2):139–140. doi: 10.1007/s00455-005-0005-1. [DOI] [PubMed] [Google Scholar]
- 21.Yeates EM, Molfenter SM, Steele CM. Improvements in tongue strength and pressure-generation precision following a tongue-pressure training protocol in older individuals with dysphagia: Three case reports. Clin Interv Aging. 2008;3(4):735–747. doi: 10.2147/cia.s3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mattson MP. Neuroprotective signaling and the aging brain: Take away my food and let me run. Brain Res. 2000 Dec 15;886(1–2):47–53. doi: 10.1016/s0006-8993(00)02790-6. [DOI] [PubMed] [Google Scholar]
- 23.Smith AD, Zigmond MJ. Can the brain be protected through exercise? lessons from an animal model of parkinsonism. Exp Neurol. 2003 Nov;184(1):31–39. doi: 10.1016/j.expneurol.2003.08.017. [DOI] [PubMed] [Google Scholar]
- 24.Grondard C, Biondi O, Armand AS, Lecolle S, Della Gaspera B, Pariset C, et al. Regular exercise prolongs survival in a type 2 spinal muscular atrophy model mouse. J Neurosci. 2005 Aug 17;25(33):7615–7622. doi: 10.1523/JNEUROSCI.1245-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ebadi M, Bashir RM, Heidrick ML, Hamada FM, Refaey HE, Hamed A, et al. Neurotrophins and their receptors in nerve injury and repair. Neurochem Int. 1997 Apr–May;30(4–5):347–374. doi: 10.1016/s0197-0186(96)00071-x. [DOI] [PubMed] [Google Scholar]
- 26.Schinder AF, Poo M. The neurotrophin hypothesis for synaptic plasticity. Trends Neurosci. 2000 Dec;23(12):639–645. doi: 10.1016/s0166-2236(00)01672-6. [DOI] [PubMed] [Google Scholar]
- 27.Poo MM. Neurotrophins as synaptic modulators. Nat Rev Neurosci. 2001 Jan;2(1):24–32. doi: 10.1038/35049004. [DOI] [PubMed] [Google Scholar]
- 28.Skaper SD. The biology of neurotrophins, signalling pathways, and functional peptide mimetics of neurotrophins and their receptors. CNS Neurol Disord Drug Targets. 2008 Feb;7(1):46–62. doi: 10.2174/187152708783885174. [DOI] [PubMed] [Google Scholar]
- 29.Johnson H, Hokfelt T, Ulfhake B. Decreased expression of TrkB and TrkC mRNAs in spinal motoneurons of aged rats. Eur J Neurosci. 1996 Mar;8(3):494–499. doi: 10.1111/j.1460-9568.1996.tb01233.x. [DOI] [PubMed] [Google Scholar]
- 30.Yuen EC, Mobley WC. Therapeutic applications of neurotrophic factors in disorders of motor neurons and peripheral nerves. Mol Med Today. 1995 Sep;1(6):278–286. doi: 10.1016/s1357-4310(95)91189-8. [DOI] [PubMed] [Google Scholar]
- 31.Lindsay RM. Therapeutic potential of the neurotrophins and neurotrophin-CNTF combinations in peripheral neuropathies and motor neuron diseases. Ciba Found Symp. 1996;196:39–48. discussion 48–53. [PubMed] [Google Scholar]
- 32.Yuen EC. The role of neurotrophic factors in disorders of peripheral nerves and motor neurons. Phys Med Rehabil Clin N Am. 2001 May;12(2):293–306. viii. [PubMed] [Google Scholar]
- 33.Vaynman S, Ying Z, Gomez-Pinilla F. Interplay between brain-derived neurotrophic factor and signal transduction modulators in the regulation of the effects of exercise on synaptic-plasticity. Neuroscience. 2003;122(3):647–657. doi: 10.1016/j.neuroscience.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 34.Gomez-Pinilla F, Ying Z, Opazo P, Roy RR, Edgerton VR. Differential regulation by exercise of BDNF and NT-3 in rat spinal cord and skeletal muscle. Eur J Neurosci. 2001 Mar;13(6):1078–1084. doi: 10.1046/j.0953-816x.2001.01484.x. [DOI] [PubMed] [Google Scholar]
- 35.Baker-Herman TL, Fuller DD, Bavis RW, Zabka AG, Golder FJ, Doperalski NJ, et al. BDNF is necessary and sufficient for spinal respiratory plasticity following intermittent hypoxia. Nat Neurosci. 2004 Jan;7(1):48–55. doi: 10.1038/nn1166. [DOI] [PubMed] [Google Scholar]
- 36.Wilkerson JE, Mitchell GS. Daily intermittent hypoxia augments spinal BDNF levels, ERK phosphorylation and respiratory long-term facilitation. Exp Neurol. 2009 May;217(1):116–123. doi: 10.1016/j.expneurol.2009.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Turturro A, Witt WW, Lewis S, Hass BS, Lipman RD, Hart RW. Growth curves and survival characteristics of the animals used in the biomarkers of aging program. J Gerontol A Biol Sci Med Sci. 1999 Nov;54(11):B492–B501. doi: 10.1093/gerona/54.11.b492. [DOI] [PubMed] [Google Scholar]
- 38.Behan M, Moeser AE, Thomas CF, Russell JA, Wang H, Leverson G, Connor NP. The effect of tongue exercise on serotonergic input to the hypoglossal nucleus in young and old rats. Soc. Neurosci. Abstr; Presented at the annual meeting of the Society for Neuroscience; October 17, 2009; Chicago; p. 82.7. [Google Scholar]
- 39.Ciucci MR, Connor NP. Dopaminergic influence on rat tongue function and limb movement initiation. Exp Brain Res. 2009 Apr;194(4):587–596. doi: 10.1007/s00221-009-1736-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abramoff MD, Magelhaes PJ, Ram SJ. Image processing with ImageJ. Biophotonics International. 2004;11(7):36–42. [Google Scholar]
- 41.Bothwell M. Functional interactions of neurotrophins and neurotrophin receptors. Annu Rev Neurosci. 1995;18:223–253. doi: 10.1146/annurev.ne.18.030195.001255. [DOI] [PubMed] [Google Scholar]
- 42.Barbacid M. Neurotrophic factors and their receptors. Curr Opin Cell Biol. 1995 Apr;7(2):148–155. doi: 10.1016/0955-0674(95)80022-0. [DOI] [PubMed] [Google Scholar]
- 43.Bergman E, Johnson H, Zhang X, Hokfelt T, Ulfhake B. Neuropeptides and neurotrophin receptor mRNAs in primary sensory neurons of aged rats. J Comp Neurol. 1996 Nov 11;375(2):303–319. doi: 10.1002/(SICI)1096-9861(19961111)375:2<303::AID-CNE9>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 44.Johnson H, Hokfelt T, Ulfhake B. Expression of p75(NTR), trkB and trkC in nonmanipulated and axotomized motoneurons of aged rats. Brain Res Mol Brain Res. 1999 May 21;69(1):21–34. doi: 10.1016/s0169-328x(99)00068-6. [DOI] [PubMed] [Google Scholar]
- 45.Rage F, Silhol M, Biname F, Arancibia S, Tapia-Arancibia L. Effect of aging on the expression of BDNF and TrkB isoforms in rat pituitary. Neurobiol Aging. 2007 Jul;28(7):1088–1098. doi: 10.1016/j.neurobiolaging.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 46.Patapoutian A, Reichardt LF. Trk receptors: Mediators of neurotrophin action. Curr Opin Neurobiol. 2001 Jun;11(3):272–280. doi: 10.1016/s0959-4388(00)00208-7. [DOI] [PubMed] [Google Scholar]
- 47.Funakoshi H, Belluardo N, Arenas E, Yamamoto Y, Casabona A, Persson H, et al. Muscle-derived neurotrophin-4 as an activity-dependent trophic signal for adult motor neurons. Science. 1995 Jun 9;268(5216):1495–1499. doi: 10.1126/science.7770776. [DOI] [PubMed] [Google Scholar]
- 48.Friedman B, Kleinfeld D, Ip NY, Verge VM, Moulton R, Boland P, et al. BDNF and NT-4/5 exert neurotrophic influences on injured adult spinal motor neurons. J Neurosci. 1995 Feb;15(2):1044–1056. doi: 10.1523/JNEUROSCI.15-02-01044.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jakeman LB, Wei P, Guan Z, Stokes BT. Brain-derived neurotrophic factor stimulates hindlimb stepping and sprouting of cholinergic fibers after spinal cord injury. Exp Neurol. 1998 Nov;154(1):170–184. doi: 10.1006/exnr.1998.6924. [DOI] [PubMed] [Google Scholar]
- 50.Zhan WZ, Mantilla CB, Sieck GC. Regulation of neuromuscular transmission by neurotrophins. Sheng Li Xue Bao. 2003 Dec 25;55(6):617–624. [PubMed] [Google Scholar]
- 51.Knaepen K, Goekint M, Heyman EM, Meeusen R. Neuroplasticity - exercise-induced response of peripheral brain-derived neurotrophic factor: A systematic review of experimental studies in human subjects. Sports Med. 2010 Sep 1;40(9):765–801. doi: 10.2165/11534530-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 52.Ying Z, Roy RR, Edgerton VR, Gomez-Pinilla F. Voluntary exercise increases neurotrophin-3 and its receptor TrkC in the spinal cord. Brain Res. 2003 Oct 10;987(1):93–99. doi: 10.1016/s0006-8993(03)03258-x. [DOI] [PubMed] [Google Scholar]