Abstract
Despite considerable evidence for potential effects of estrogen on emotional processing, several studies of postmenopausal women who began hormone therapy (HT) remote from menopause report no effects of HT on emotional measures. As early HT initiation may preserve brain mechanisms, we examined effects of HT on emotional processing in postmenopausal women who started HT early after menopause. We performed a cross-sectional comparison of 52 postmenopausal women 66±5 years old, including 15 users of conjugated equine estrogen, 20 users of conjugated equine estrogen plus medroxyprogesterone acetate, and 17 who never used hormones (NT). All hormone users started therapy within two years of menopause, and received at least 10 years of continuous therapy. Outcomes were fMRI-detected brain activity and behavioral measures during an emotional processing picture rating task. During processing of positive pictures, NT women had greater activation than estrogen treated women in medial prefrontal cortex extending to the anterior cingulate, and more activation than estrogen plus progestin treated women in the insula. During processing of negative pictures, estrogen treated women had higher activation than NT women in the entorhinal cortex. Current compared to past HT users showed greater activation in the hippocampus and higher emotion recognition accuracy of neutral stimuli. Estrogen plus progestin treated women had slower response time than NT women when rating all pictures. In conclusion, hormone use was associated with differences in brain functional responses during emotional processing. These fMRI effects were more prominent than those observed for behavioral measures and involved brain regions implicated in cognitive-emotional integration.
Keywords: Estrogen, Progesterone, Hormone Therapy, Postmenopause, Functional Magnetic Resonance Imaging, Emotion
1. INTRODUCTION
It has been proposed that gonadal hormones, especially estrogen, may affect mood and affective regulation[1–5]. In fact, women’s vulnerability to mood disorders begins post puberty and during reproductive age years women are twice as likely to experience major depression compared to men[2]. More specifically, hormonal shifts and/or low gonadal hormone levels have been primarily linked to negative affective states and depression symptomatology[2, 4]. Activation of the stress response circuitry is dependent on the gonadal hormone environment[6] and hypoactivation of the stress response circuitry in women with Major Depressive Disorder in remission is associated with gonadal hormone dysregulation[7]. Furthermore, estrogen therapy in women with depression may result in a full or partial therapeutic response (see review in Ancelin 2007[5]) and may accelerate or enhance the response to selective-serotonin reuptake inhibitors (SSRI)[1, 3]. Thus, postmenopausal hormone therapy (HT) is likely to have effects on emotional processing.
Several mechanisms by which estrogen may affect brain emotional processing circuits have been identified. These include effects on serotonin, dopamine, noradrenaline, and GABA systems, and increases in dendritic spines and synapses in stress and emotion regulatory regions, including the hippocampus and prefrontal cortex (PFC)[4, 8, 9]. Although evidence suggests that postmenopausal estrogen therapy might contribute to the preservation of brain emotional processing mechanisms and mood stabilization, progesterone is thought to have opposite effects, increasing monoamine oxidase (MAO - the enzyme that catabolizes serotonin, dopamine and norepinephrine)[10] and preventing estrogen induced dendritic spine formation in cultured hippocampal neurons[11]. Detrimental effects of progestins have also been corroborated in human studies[12–15].
Despite considerable evidence for the potential effects of estrogen and progestin on emotional processing, several studies report no effects of postmenopausal HT on measures such as emotional functioning, positive or negative affect, depressive symptoms, mood, and quality of life [16–20]. These studies, which collected mainly behavioral data, involved women who started HT several years after menopause. In contrast, studies of cognitive function suggest that estrogen treatment may be most effective during perimenopause or early postmenopause, prior to significant neuronal loss [9, 21–23]. This window may also be applicable to emotional health, however this has not been directly explored. Although Pruis et al[24] found only minor effects of HT on emotion-induced brain activation and no effects on emotional recognition or valence ratings in a group of 16 HT users of whom 13 started HT during menopause, the study was not designed or analyzed to examine this specific question, nor did it differentiate between the effects of different types of HT.
As part of a comprehensive assessment of hormone effects on brain function in menopause[25], our study examined the effects of long-term HT (estrogen therapy, ET; or estrogen plus progestin therapy, EPT) on emotional processing, using blood-oxygen-level dependent functional Magnetic Resonance Imaging (BOLD fMRI) in postmenopausal women who started HT within 2 years of menopause. We hypothesized that fMRI activation patterns would reflect greater preservation of neural emotional processing mechanisms in ET users compared to EPT or never users of HT.
2. METHODS
2.1 Participants
Fifty-two healthy, right-handed non-smoking postmenopausal women (mean age 66±5, age range 60–81) were recruited. All women on HT used the same dose and preparation of estrogen: conjugated equine estrogens 0.625 mg/day (Premarin®, Wyeth Pharmaceuticals, Philadelphia, PA), with or without cyclic or continuous medroxyprogesterone acetate (Provera, Pfizer, New York, NY or Prempro, Wyeth Pharmaceuticals, Philadelphia, PA). All hormone users started therapy within two years of menopause (defined as the absence of menses for one year, onset of severe symptoms after hysterectomy, or at the time of hysterectomy with bilateral salpingo-oophorectomy), and received at least 10 years of uninterrupted therapy. Participants included 15 ET users, 20 EPT users and 17 women who had never used hormone therapy (NT). Five of 15 ET (33%) and 6 of 20 EPT participants (30%) were still taking hormones at the time of the study. For the others, mean time since hormone use ended was only 2±1 years, and it did not significantly differ between the two HT groups.
Participants underwent an initial phone screen followed by a complete medical, psychiatric and neurologic history and physical exam. Screening laboratory tests included electrolytes, glucose, complete blood count, thyrotropin, and estradiol. Exclusion criteria included having psychiatric illness, neurologic illness, history of head injury, substance abuse, cardiac disease, pulmonary disease, renal disease, liver disease, diabetes, cancer or other acute or uncorrected illness; using centrally acting medications, intermittent estrogen, or phytoestrogen supplements; smoking within the last 5 years; being unable to tolerate the scanning procedures and having contraindications to MRI. Women with hypertension or hypercholesterolemia were included. A neuropsychological battery of tests was also administered, including the Mini-Mental State Examination (MMSE)[26] to exclude the presence of dementia, and the Geriatric Depression Rating Scale (GDS)[27] to assess emotional state and exclude the presence of depression. All procedures were approved by University of Michigan Institutional Review Board.
2.2 Experimental Task
To examine brain activation during emotional processing, subjects viewed emotional pictures from the International Affective Picture System (IAPS)[28] and rated each picture while in the MRI scanner as positive (pleasant), neutral, or negative (unpleasant) using a button press[29]. Subjects viewed sixty-four pictures from each emotional category, chosen from this set of pictures based on their affective rating by a normative female sample. Examples of positive images included pictures of puppies, sunsets, and babies, whereas negative images included pictures of guns, crying people, and cemeteries. Neutral pictures included light switches, abstract art, and buildings. Using a commercial software package (E-Prime, Psychology Software Tools Inc., Pittsburg, PA), each picture was presented for 3.5 seconds with a 1.5 seconds inter-stimulus interval. Pictures were presented in a block design across 4 runs (4–5 minutes each), with 12 pictures/block and 4 blocks/run. Prior to fMRI data collection, participants practiced the task on a computer outside the scanner until comfortable with the procedures. Response times and accuracy scores were collected.
2.3 fMRI acquisition and processing
Scans were acquired using a 3T scanner. Anatomical MRI scans were acquired axially with a spoiled gradient recall (SPGR) three-dimensional volumetric acquisition (repetition time (TR) 9.6 ms, echo time (TE) 3.3 ms, inversion recovery preparation (IR prep) 200 ms, flip angle (FA) 17°, 24-cm field of view (FOV), 1.5-mm slice thickness, 106 – 110 slices, 256 × 256 matrix, two excitations) for anatomical localization and coregistration to standardized stereotactic coordinates. Functional MRI was acquired using a single-shot spiral pulse sequence, with 32 oblique-axial slices approximately parallel to the anterior commissure-posterior commissure line (spiral gradient echo (GRE), TE 25 ms, TR 2000 ms, FOV 60°, 4-mm-thick contiguous slices, 24-cm FOV, 64 × 64 image matrix).
Image reconstruction included processing steps to remove distortions caused by magnetic field inhomogeneity and other sources of misalignment to structural data[30]. Data were sinc-interpolated in time, slice-by-slice, to correct for the staggered sequence of slice acquisition[31]. The first four functional volumes of each run were discarded to remove magnetic saturation effects. The remaining functional images were realigned to the first volume to eliminate movement artifacts[32]. Anatomical and functional images were coregistered and then spatially normalized into the MDT2 template, derived from T1-weighted MR images of 25 normal elderly females[33]. Finally, a three-dimensional Gaussian smoothing kernel set at 8 mm full-width at half-maximum was applied to functional data, to improve signal-to-noise ratios.
2.4 Behavioral data analysis
Accuracy score was defined as the percentage of pictures labeled by a participant under the same emotional category as originally rated by a normative sample. Response time (RT) was defined as the time in milliseconds from picture presentation to rating of the picture. Mean RT for each emotional category was calculated using RT for all the pictures which belonged to that emotional category based on rating by the normative sample. Emotional positive bias was calculated as the sum of the percentage of neutral pictures incorrectly rated as positive and the percentage of negative pictures incorrectly rated as either neutral or positive. Emotional negative bias was calculated as the sum of the percentage of neutral pictures incorrectly rated as negative and the percentage of positive pictures incorrectly rated as either neutral or negative. Group differences for demographic and behavioral measures were analyzed using t-tests and ANOVA. Demographic and behavioral measures between past and current hormone users were compared using two-sample t-test.
2.5 fMRI data analysis
All fMRI data were analyzed using SPM (Wellcome Department of Cognitive Neurology, London, UK). For the first-level analyses, contrast images were generated for each subject to assess differences in activation between viewing pictures with affective and pictures with neutral content. At the second level analysis, we examined the brain regions involved in emotional processing in the entire sample (task main effects) using one sample t-test. Correlations between behavioral measures and regional activation were analyzed using simple linear regression performed on a voxel by voxel base. Treatment (group) effects were identified by pair-wise group comparisons using general linear models. When comparing ET to EPT, education, HT duration and age at HT initiation were entered as covariates, because they were significantly different between these two groups (see results). Since the HT groups included both current and past hormone users, to differentiate between effects of long-term and current usage of HT, we compared brain activation of past users (combined from both HT groups) and current users (combined from both HT groups), using two-sample t-tests.
Task main effects and correlation results were considered significant at cluster-level p ≤0.05 corrected for multiple comparisons (familywise error rate (FWE) method), with 10 voxels minimum cluster size. For the treatment/group effects and the difference between current and past users, the same significance criteria were applied; we also included clusters located in regions whose activation was significantly increased by the task main effects if, after small volume correction, they were significant at p ≤0.05 FWE corrected for multiple comparisons.
3. RESULTS
3.1 Demographic and behavioral data
Using ANOVA, the three groups did not differ significantly in age, years of education, GDS or MMSE scores, rating accuracy for positive, negative or neutral pictures, or positive or negative emotional bias (Table 1). Post hoc comparisons revealed that the ET and EPT groups differed significantly in years of education (t=−2.32, p=0.03), HT duration (t=3.19, p=0.004) and age at HT initiation (t=2.74, p=0.01). A significant effect of treatment on RT during picture rating was observed using ANOVA for positive (F=3.53, p=0.04), negative (F=4.0, p=0.02) and neutral (F=4.25, p=0.02) pictures. Post-hoc comparisons showed that EPT users had longer RTs than never users for positive, negative and neutral pictures (p=0.03, p=0.02, and p=0.02, respectively). There were no differences between past and current hormone users except in accuracy of rating neutral pictures, where current users were more accurate (t=−2.05, p=0.05).
Table 1.
Demographic and behavioral data of the three menopausal groups.
| Group ET (n=15) | Group EPT (n=20) | T Test (P)a | Group NT (n=17) | ANOVA (p)b | |
|---|---|---|---|---|---|
| Age | 67.3 ± 5.6 | 64.6 ± 4.9 | n.s. | 65.5 ± 4.8 | n.s. |
| Years of education | 15.4 ± 2.5 | 17.4 ± 2.6 | 0.03 | 16.8 ± 2.9 | n.s. |
| Years of HT | 19.3 ± 6.2 | 13.4 ± 4.1 | 0.004 | - | - |
| Age at HT initiation | 45.7 ± 4.2 | 49.4 ± 3.3 | 0.01 | - | - |
| % current HT users | 0.33 | 0.30 | - | - | - |
| Time (years) since hormone cessation for past users | 1.84 | 2.27 | n.s. | - | - |
| MMSE | 28.3 ± 1.6 | 28.7 ± 1.3 | n.s. | 28.8 ± 1.9 | n.s. |
| GDS | 1.0 ± 1.2 | 0.5 ± 0.8 | n.s. | 0.6 ± 0.8 | n.s. |
| Accuracy for positive pictures | 0.92±0.05 | 0.85±0.13 | 0.05 | 0.90±0.07 | n.s. |
| Accuracy for negative pictures | 0.89±0.08 | 0.85±0.09 | n.s. | 0.86±0.09 | n.s. |
| Accuracy for neutral pictures | 0.47±0.17 | 0.54±0.19 | n.s. | 0.50±0.22 | n.s. |
| % trials correct | 76.01±5.4 | 75.42±8.60 | n.s. | 76.39±8.5 | n.s. |
| % trials positive bias | 13.01±4.23 | 12.88±4.76 | n.s. | 14.31±8.46 | n.s. |
| % trials negative bias | 10.99±5.87 | 11.70±6.91 | n.s. | 9.3±2.91 | n.s. |
| RT for positive pictures | 1.46±0.29 | 1.61±0.46 | n.s. | 1.31±0.21 | 0.04 |
| RT for negative pictures | 1.70±0.29 | 1.83±0.47 | n.s. | 1.50±0.22 | 0.02 |
| RT for neutral pictures | 2.02±0.37 | 2.05±0.37 | n.s. | 1.75±0.24 | 0.02 |
Data are mean±SD.
HT, hormone therapy; ET, estrogen therapy; EPT, estrogen + progestin therapy; NT, never treated; ANOVA, analysis of variance; n.s., not significant.
Comparison between the ET and EPT groups.
ANOVA comparison between all three treatment groups.
3.2 Brain activation
3.2.1 Task effects and their correlations with behavioral measures
Task main effects for the entire sample are summarized in Table 2 and the correlations with behavioral measures are shown in Table 3. During the processing of positive stimuli, negative bias was negatively correlated with posterior cingulate activation, while RT was positively correlated with activation in frontal and occipito-temporal regions. During negative stimuli presentation, RT was negatively correlated with activation in precentral and frontal areas.
Table 2.
Task Effects: Brain regions activated across the entire sample
| Region (Brodmann Area) | Coordinatesc |
Z score | Cluster sized | ||
|---|---|---|---|---|---|
| X | Y | Z | |||
| Regions activated during viewing of positive picturesa | |||||
| Bilateral medial frontal gyrus and anterior cingulate(10, 32) | 2 | 56 | 6 | 5.67 | 876 |
| Bilateral precuneus (31) extending to: | 0 | −58 | 28 | 6.59 | 3958 |
| Right precentral gyrus (4) | 24 | −26 | 66 | 5.36 | |
| Bilateral posterior cingulate (31) | 2 | −44 | 36 | 7.27 | |
| L superior temporal gyrus (22) | −64 | −38 | 8 | 4.81 | 212 |
| Right occipital-temporal junction (37,39) extending to: | 52 | −66 | 10 | 5.67 | 2475 |
| Right superior temporal gyrus (42) | 66 | −28 | 8 | 5.21 | |
| Right transverse temporal gyrus (21) | 54 | −6 | −6 | 5.02 | |
| Left insula extending to: | −42 | −20 | −4 | 5.55 | 1237 |
| Left medial temporal gyrus (21) | −56 | −8 | −10 | 4.68 | |
| Left medial occipital gyrus (39,19) | −50 | −72 | 8 | 5.15 | 394 |
| Regions activated during viewing of negative picturesb | |||||
| Bilateral anterior cingulate/superior frontal gyrus (32, 10) | 12 | 52 | 12 | 4.44 | 112 |
| Right medial temporal gyrus and hippocampus (21) | 58 | −6 | −16 | 4.44 | 213 |
| Bilateral posterior cingulate and precuneus (31/7) | 0 | −52 | 30 | 6.52 | 1486 |
| Right medial occipito-temporal gyrus/junction (39/19) | 54 | −68 | 8 | >10 | 2380 |
| Left inferior occipito-temporal gyrus/junction (37/19) | −48 | −76 | 4 | >10 | 2148 |
Positive-Nuetral contrast.
Negative-Neutral contrast.
International Consortium for Brain Mapping coordinates where regions were centered.
Cluster size is based on SPM display threshold of P = 0.0001.
Table 3.
Correlations between regional activations and behavioral measures
| Region (Brodmann) | Coordinatesc |
Z score | Cluster sized | ||
|---|---|---|---|---|---|
| X | Y | Z | |||
| Region whose activation during viewing of positive picturesa was negatively correlated with negative bias: | |||||
| Bilateral posterior cingulate/postcentral gyrus (7) | 16 | −46 | 48 | 4.05 | 2759 |
| Regions whose activation during viewing of positive picturesa were positively correlated with RT: | |||||
| Right middle frontal gyrus (45) | 56 | 20 | 30 | 3.58 | 1356 |
| Left superior frontal gyrus (8) extending to: | 0 | 22 | 44 | 4.12 | 2917 |
| Left middle frontal gyrus (6) | −46 | 4 | 42 | 3.83 | |
| Right occipito-temporal gyrus extending to: | 18 | −50 | −20 | 3.89 | 2374 |
| Cerebellum | |||||
| Left occipito-temporal gyrus (21/37/18) | −56 | −48 | −8 | 4.23 | 2072 |
| Regions whose activation during viewing of negative picturesb were negatively correlated with RT: | |||||
| Right paracentral gyrus (1,2,3/7) extending to: | 24 | −34 | 64 | 3.75 | 1225 |
| Left superior frontal gyrus (5) | −4 | −30 | 52 | 3.46 | |
RT, response time.
Positive-Nuetral contrast.
Negative-Neutral contrast.
International Consortium for Brain Mapping coordinates where regions were centered.
Cluster size is based on SPM display threshold of P = 0.01.
3.2.2 Hormone treatment effects
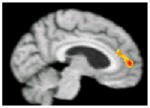
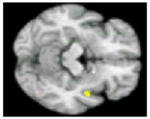
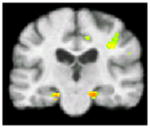
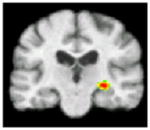
No significant differences in brain activation were found between the NT group and the entire HT sample during processing of positive or negative pictures. To assess specific effects of ET and EPT, these groups were compared to the NT group and to each other (Table 4). During processing of positive pictures, the NT group showed greater activation than the ET group in the left medial frontal gyrus (BA 10) and the left anterior cingulate (BA 32/24), and greater activation than the EPT group in the right posterior insula. No regions showed significantly greater activity in either HT group compared to the NT group. During negative pictures processing, no areas of greater activation were detected within the NT when compared to the ET or EPT groups. However, the ET sample showed greater activation than the NT participants in the right entorhinal cortex. Comparing the ET and EPT groups revealed no significant differences in brain activation during processing of positive or negative stimuli. All current compared to all past hormone users had greater activation in the right hippocampal region during processing of positive pictures (Table 4).
Table 4.
Areas of activation with significant differences between hormone use groups during the emotional processing task
| Experimental Condition | Positive-Neutral NT > ET |
Positive-Neutral NT > EPT |
Negative-Neutral ET > NT |
Positive-Neutral Current > Past HT users |
|---|---|---|---|---|
| Region of significant difference in BOLD response | Left medial frontal gyrus (BA 10), and anterior cingulate (BA 32/24) | Right posterior insula | Right entorhinal cortex | Right hippocampus |
| Brain activationa
|
|
|
|
|
| Coordinates (x y z)b | −4 54 8 | 48 −2 −10 | 22 −20 −18 | 32 −20 −6 |
| Z score | 3.11 | 2.7 | 3.02 | 3.66 |
| PFWE-corr | 0.01 | 0.028 | 0.013 | 0.004 |
| Cluster sizec | 200 | 327 | 59 | 115 |
NT, Never users; ET, Estrogen Therapy; EPT, Estrogen plus Progestin Therapy; PFWE-corr, P value corrected for multiple comparisons using the familywise error rate method.
Color coded bar indicates the t-test values for the significance of BOLD response.
International Consortium for Brain Mapping coordinates where regions were centered.
Cluster size is based on the original size of the cluster (before applying small volume correction) using SPM display threshold of P = 0.01.
No significant differences in brain activation were observed for all other possible group comparisons which are not presented in the table.
4. DISCUSSION
This study assessed the effects of long-term, early initiated HT on emotional processing in healthy, non-depressed, postmenopausal women using behavioral and brain functional measures. Brain activation patterns were significantly different between the three groups and varied based on affective valence. During processing of positive pictures, the NT group had significantly greater activation than the ET group in the medial prefrontal cortex and anterior cingulate, and significantly more activation than the EPT group in the right insula. Conversely, during processing of negative stimuli, the ET women had significantly greater activation than the NT women in the entorhinal cortex. In addition, current compared to past users had higher activation in the right hippocampal region. Behaviorally, EPT users had significantly slower RT when rating positive and negative pictures compared to never users, and current users were more accurate than past users when rating neutral pictures.
When interpreting brain activation patterns, less BOLD response is sometimes interpreted as more efficient task performance, while other times it is viewed as poor brain function. Aging is accompanied by brain structural and functional changes that affect cognitive and emotional processing, and previous animal and human studies suggest that estrogen administration during menopause might defer some of those changes. Thus, to adequately interpret our results we compare the differences that we found between the HT groups to the behavioral and brain differences found between young and elderly during emotional processing tasks. The interpretation of our results is based on the interpretation for these differences as portrayed in the aging literature. During functional neuroimaging employing emotional tasks, and when compared to young adults, elderly samples show increased activation in the prefrontal cortex (PFC) [34–36], and diminished activation in the amygdala and surrounding temporo-limbic regions[37, 38]. Such changes in brain activation associated with emotional tasks are detected in women already in their menopausal years[39]. Behaviorally, the elderly demonstrate a tendency towards an emotional positive bias, expressed as higher attention to, and better recognition and memory of positive stimuli [40–45], and higher frequency of positive bias during emotional stimuli ratings[34, 46]. With aging there is also a tendency for a decrease in the experience of negative affect and a slight increase in the experience of positive affect[47], as well as increases in RT[34–36]. Except for effects on RT, no significant differences in emotional bias in picture classification accuracy were observed in the present study among the different HT groups, possibly secondary to the relatively small sample sizes and lower sensitivity of this behavioral test compared to brain imaging measures.
Two models that integrate behavioral and brain changes in aging have been suggested. Some researchers maintain that with aging, amygdala and hippocampal degeneration reduce the physiological responses to negative stimuli and consequently reduce the perception and emotional experience of those stimuli, which result in positive bias[35, 37, 48, 49]. The reduction in limbic activation is suggested to cause, as a compensation mechanism, an increased use of frontal cortex[36]. Others maintain that the emotional positive bias in elderly derives from an intentional focus on positive affect and stimuli, as a result of awareness of approaching end-of-life (the socioemotional selectivity theory[50]), and that emotional regulation processes, originating in the PFC during processing of negative stimuli, reduce activation in the amygdala, through top-down control mechanisms[38, 51, 52].
Based on the model of amygdala and hippocampus degeneration with age, the activation differences between the ET and NT groups in the PFC and entorhinal cortex, as well as current HT users’ increased activation in the hippocampus, appear consistent with estrogen affecting age-related changes in emotional processing circuitry. The hippocampus is traditionally involved in the typical neurodegenerative changes that take place during senescence, and shows reduced activity in elderly compared to young adults[37]. The increased activation we found in the right hippocampus in current compared to past HT users indicates that HT might have contributed to the preservation of this region, as was also suggested by Lord[53], who found increased right hippocampus volume in postmenopausal women who used estrogen therapy compared to non-users. The behavioral finding of higher accuracy while processing neutral stimuli (which are the most difficult to categorize) among the current compared to the past users, indicates better emotional processing functioning, which further supports the idea that higher hippocampal activation may indicate structural preservation. The entorhinal cortex, the main interface between the neocortex and hippocampus[54, 55], is an area rich with estrogen receptors[56], and normally associated with age-related decreases in activation[35]. Our finding of higher entorhinal cortex activation during emotional processing of negative pictures in ET compared to NT participants is also consistent with its structural and functional preservation.
In addition, in contrast to Lord’s findings which suggested that the neuroprotective effects of ET on hippocampus volume are diminished once the women stop using hormones[53], we identified lasting effects on limbic region function after treatment cessation. In our ET group the majority of women (67%) were past users. Yet, the ET and NT groups showed regional brain activation differences. The notion of ET contributing to preservation of limbic regions is further supported by our finding of greater activation in the NT compared to the ET group in the medial PFC and anterior cingulate during processing of positive pictures, similar to the greater activation in these regions observed in elderly adults[34]. This difference in activation was not observed between the NT and EPT groups, supporting the hypothesis that addition of progestin might reduce the protective effects of estrogen treatment in areas involved in emotional processing.
Preservation of emotional processing regions by ET would predict increased activation in the amygdala in the ET compared to the NT group, and greater activation in PFC and enhanced positive bias in the NT compared to the ET group during processing of negative stimuli. We did not observe such results possibly because of emotional regulation by the ET group, as suggested by the socioemotional selectivity theory[50]. Emotional regulation could reduce the perceived negativity of the stimuli by reducing amygdala activation through a top down control mechanism originating in the PFC, and consequently reduce differences between the ET and NT groups in affective rating and brain activation within these regions. Alternatively, the neuroprotective effects of ET may be region specific for the hippocampus and its surroundings. Absence of ET effects on amygdala preservation was also noted by Pruis[24] as well as Lord[53], who in contrast to increased hippocampal volume, did not find ET effects on amygdala volume, and suggested that this difference may derived from differences in estrogen receptors in the two regions, and/or from the effects of estrogen on other growth factors which promote neurogenesis in the hippocampus.
The lower activation level in the right posterior insula during emotional processing of positive stimuli by the EPT compared to the NT group is more difficult to interpret in this context. The insula was differentially activated in a study examining differences in emotional processing between the follicular and luteal phases of the menstrual cycle, indicating that the relative levels of estrogen and progesterone may affect brain activation in this region[57]. The insula has a role in interoception[58], integrating internal somatic feelings with external cues, and guiding behavioral decisions based on the emotional significance of the stimulus[59, 60]. Thus, the reduced insula activation in the EPT users might have contributed to their longer RT due to the reduced efficiency of perceiving and rating emotion.
Limitations of this study include the cross-sectional design, which allows for biases in subject study entry. However, randomization to long-term hormone use was unfeasible, and this weakness was addressed by keeping demographic and hormone-use characteristics similar among groups, and controlling in the analyses for factors that differ among the groups. Another limitation was a relatively small sample size which did not provide enough power to demonstrate significant differences in most behavioral measures. The sample size, however, was adequate for fMRI analysis which was the main objective of this study. As a comparison group of women who started HT well after menopause was not included in the design, this study can not separate the effects of early HT initiation from the effects of long-term use of hormones. Lastly, each HT group included both past and current users, preventing a more accurate evaluation of the effect of current hormone use of each type of HT. The study was performed at a time when due to new HT guidelines many long-term hormone users stopped taking hormones, making recruitment of current users more challenging. We minimized this limitation by comparing current users combined from both HT groups to past users combined from both groups.
5. CONCLUSIONS
The results acquired in this cross-sectional study support the notion that early-initiated postmenopausal HT has influences on emotion processing circuitry that may underlie the potential for estrogens to modulate emotion and mood regulatory systems, as has been suggested in clinical trials[61]. Differences in brain activation patterns between groups during picture rating may indicate preservation of some emotional processing mechanisms in estrogen users. These differences, however, had no measurable behavioral consequences for the estrogen users, possibly as a consequence of greater variability in behavioral measures, compared to the biologically more proximal neuroimaging data. In contrast, combination therapy was associated with reduced emotional processing speed, but no difference in accuracy or mood. Future randomized studies are needed to examine the effect of selective estrogen receptor agonists on these mechanisms.
Research Highlights.
Long-term early-initiated hormone therapy effects an emotional task in postmenopause.
Estrogen therapy seemed to preserve brain emotional processing mechanisms.
Brain differences had no measurable behavioral consequences for the estrogen users.
Estrogen plus progestin therapy appeared less neuroprotective than estrogen therapy.
Current compared to past hormone users showed greater activation in the hippocampus.
Acknowledgments
We thank the Michigan Clinical Research Unit, University of Michigan fMRI laboratory, and Anne Tkaczyk, M.S. (study coordinator) for their assistance. We especially thank the participants of our study.
Financial Support
This work was supported by the National Center for Research Resources (K23 RR17043 and UL1RR024896), and for investigator support, by the National Institute for Child Health and Human Development (5T32HD007048), National Institute on Aging and the Office for Research on Women’s Health (RO1AG027675), the University of Michigan’s Postdoctoral Translational Scholars Program award and the Phil F. Jenkins Depression Research Fund
ABBREVIATIONS
- HT
Hormone Therapy
- ET
Estrogen Therapy
- EPT
Estrogen plus Progestin Therapy
- NT
No hormone Therapy use
Footnotes
Conflicts of Interest & Disclosures
Tal Shafir served on the board of Teva Pharmaceutical USA during 2009. Jon-Kar Zubieta served as a consultant for Johnson & Johnson, Merck, Abbott and Eli Lilly in the last 3-year period, and in the Lecture Bureau of Eli Lilly. The rest of the authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Westlund Tam L, Parry BL. Does estrogen enhance the antidepressant effects of fluoxetine? Journal of Affective Disorders. 2003;77:87–92. doi: 10.1016/s0165-0327(02)00357-9. [DOI] [PubMed] [Google Scholar]
- 2.Steiner M, Dunn E, Born L. Hormones and mood: from menarche to menopause and beyond. Journal of Affective Disorders. 2003;74:67–83. doi: 10.1016/s0165-0327(02)00432-9. [DOI] [PubMed] [Google Scholar]
- 3.Rasgon NL, Dunkin J, Fairbanks L, Altshuler LL, Troung C, Elman S, et al. Estrogen and response to sertraline in postmenopausal women with major depressive disorder: A pilot study. Journal of Psychiatric Research. 2007;41:338–43. doi: 10.1016/j.jpsychires.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 4.Morrison JH, Brinton RD, Schmidt PJ, Gore AC. Estrogen, Menopause, and the Aging Brain: How Basic Neuroscience Can Inform Hormone Therapy in Women. J Neurosci. 2006;26:10332–48. doi: 10.1523/JNEUROSCI.3369-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ancelin M-L, Scali J, Ritchie K. Hormonal therapy and depression: Are we overlooking an important therapeutic alternative? Journal of Psychosomatic Research. 2007;62:473–85. doi: 10.1016/j.jpsychores.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 6.Goldstein JM, Jerram M, Abbs B, Whitfield-Gabrieli S, Makris N. Sex Differences in Stress Response Circuitry Activation Dependent on Female Hormonal Cycle. The Journal of Neuroscience. 30:431–8. doi: 10.1523/JNEUROSCI.3021-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holsen LM, Spaeth SB, Lee J-H, Ogden LA, Klibanski A, Whitfield-Gabrieli S, et al. Stress response circuitry hypoactivation related to hormonal dysfunction in women with major depression. Journal of Affective Disorders. doi: 10.1016/j.jad.2010.11.024. In Press, Corrected Proof. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Halbreich U, Kahn LS. Role of Estrogen in the Aetiology and Treatment of Mood Disorders. CNS Drugs. 2001;15:797–817. doi: 10.2165/00023210-200115100-00005. [DOI] [PubMed] [Google Scholar]
- 9.Thakur M, Sharma P. Aging of Brain: Role of Estrogen. Neurochemical Research. 2006;31:1389–98. doi: 10.1007/s11064-006-9191-y. [DOI] [PubMed] [Google Scholar]
- 10.Sherwin BB. Progestogens used in menopause. Side effects, mood and quality of life. Journal of reproductive medicine. 1999;44:227–32. [PubMed] [Google Scholar]
- 11.Murphy DD, Segal M. Progesterone Prevents Estradiol-Induced Dendritic Spine Formation in Cultured Hippocampal Neurons. Neuroendocrinology. 2000;72:133–43. doi: 10.1159/000054580. [DOI] [PubMed] [Google Scholar]
- 12.Sherwin BB. The impact of different doses of estrogen and progestin on mood and sexual behavior in postmenopausal women. J Clin Endocrinol Metab. 1991;72:336–43. doi: 10.1210/jcem-72-2-336. [DOI] [PubMed] [Google Scholar]
- 13.Björn I, Bixo M, Nöjd KS, Nyberg S, Bäckström T. Negative mood changes during hormone replacement therapy: A comparison between two progestogens. American Journal of Obstetrics and Gynecology. 2000;183:1419–26. doi: 10.1067/mob.2000.107781. [DOI] [PubMed] [Google Scholar]
- 14.Whooley M, Grady D, Cauley J. Postmenopausal estrogen therapy and depressive symptoms in older women. Journal of General Internal Medicine. 2000;15:535–41. doi: 10.1046/j.1525-1497.2000.04029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andreen L, Bixo M, Nyberg S, Sundstrom-Poromaa I, Backstrom T. Progesterone effects during sequential hormone replacement therapy. Eur J Endocrinol. 2003;148:571–7. doi: 10.1530/eje.0.1480571. [DOI] [PubMed] [Google Scholar]
- 16.Hays J, Ockene JK, Brunner RL, Kotchen JM, Manson JE, Patterson RE, et al. Effects of Estrogen plus Progestin on Health-Related Quality of Life. New England Journal of Medicine. 2003;348:1839–54. doi: 10.1056/NEJMoa030311. [DOI] [PubMed] [Google Scholar]
- 17.Resnick SM, Maki PM, Rapp SR, Espeland MA, Brunner R, Coker LH, et al. Effects of Combination Estrogen Plus Progestin Hormone Treatment on Cognition and Affect. J Clin Endocrinol Metab. 2006;91:1802–10. doi: 10.1210/jc.2005-2097. [DOI] [PubMed] [Google Scholar]
- 18.Morrison MF, Kallan MJ, Ten Have T, Katz I, Tweedy K, Battistini M. Lack of efficacy of estradiol for depression in postmenopausal women: a randomized, controlled trial. Biological Psychiatry. 2004;55:406–12. doi: 10.1016/j.biopsych.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 19.Heinrich AB, Wolf OT. Investigating the Effects of Estradiol or Estradiol/Progesterone Treatment on Mood, Depressive Symptoms, Menopausal Symptoms and Subjective Sleep Quality in Older Healthy Hysterectomized Women: A Questionnaire Study. Neuropsychobiology. 2005;52:17–23. doi: 10.1159/000086173. [DOI] [PubMed] [Google Scholar]
- 20.Resnick SM, Espeland MA, An Y, Maki PM, Coker LH, Jackson R, et al. Effects of Conjugated Equine Estrogens on Cognition and Affect in Postmenopausal Women with Prior Hysterectomy. J Clin Endocrinol Metab. 2009;94:4152–61. doi: 10.1210/jc.2009-1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robert B, Gibbs RG. Estrogen and cognition: Applying preclinical findings to clinical perspectives. Journal of Neuroscience Research. 2003;74:637–43. doi: 10.1002/jnr.10811. [DOI] [PubMed] [Google Scholar]
- 22.Pauline MM. A Systematic Review of Clinical Trials of Hormone Therapy on Cognitive Function: Effects of Age at Initiation and Progestin Use. Annals of the New York Academy of Sciences. 2005;1052:182–97. doi: 10.1196/annals.1347.012. [DOI] [PubMed] [Google Scholar]
- 23.Sherwin BB. The Critical Period Hypothesis: Can It Explain Discrepancies in the Oestrogen-Cognition Literature? Journal of Neuroendocrinology. 2007;19:77–81. doi: 10.1111/j.1365-2826.2006.01508.x. [DOI] [PubMed] [Google Scholar]
- 24.Pruis TA, Roalf DR, Janowsky JS. Hormone therapy does not modify emotion-induced brain activity in older women. Hormones and Behavior. 2009;56:539–47. doi: 10.1016/j.yhbeh.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berent-Spillson A, Persad CC, Love T, Tkaczyk A, Wang H, Reame NK, et al. Early menopausal hormone use influences brain regions used for visual working memory. Menopause. 2010;17:692–9. doi: 10.1097/gme.0b013e3181cc49e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of psychiatric research. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 27.Yesavage JA, Rose TLDL. Validity of the geriatric depression scale in subjects with senile dementia. Palo Alto: Clinical Diagnostic and Rehabilitation Unit, Veterans Administrative Medical Center; 1981. [Google Scholar]
- 28.Lang PJ, Bradley MM, Cuthbert BN. International affective picture system (IAPS): Technical manual and affective ratings. Gainsville: University of Florida, Center for Research in Psychology; 1997. [Google Scholar]
- 29.Love T, Smith YR, Persad CC, Tkaczyk A, Zubieta J-K. Short-term hormone treatment modulates emotion response circuitry in postmenopausal women. Fertility and Sterility. 93:1929–37. doi: 10.1016/j.fertnstert.2008.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Noll D, Stenger V, Vasquez A, Peltier S. Spiral scanning in functional MRI. Heidelberg: Springer-Verlag; 1999. [Google Scholar]
- 31.Acquirre C, D’Esposito M. Experimental design for brain fMRI. Heidelberg: Springer-Verlag; 1999. [Google Scholar]
- 32.Friston K, Ashburner J, Frith C, Poline J-B, Heather J, Frackowiak R. Spatial registration and normalization of images. Human Brain Mapping. 1995;2:165 – 89. [Google Scholar]
- 33.Sun FT, Schriber RA, Greenia JM, He J, Gitcho A, Jagust WJ. Automated Template-based PET Region of Interest Analyses in the Aging Brain. Neuroimage. 2007;34:608–17. doi: 10.1016/j.neuroimage.2006.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leclerc CM, Kensinger EA. Age-related differences in medial prefrontal activation in response to emotional images. Cognitive, Affective, & Behavioral Neuroscience. 2008;8:153–64. doi: 10.3758/cabn.8.2.153. [DOI] [PubMed] [Google Scholar]
- 35.Gunning-Dixon FM, Gur RC, Perkins AC, Schroeder L, Turner T, Turetsky BI, et al. Age-related differences in brain activation during emotional face processing. Neurobiology of Aging. 2003;24:285–95. doi: 10.1016/s0197-4580(02)00099-4. [DOI] [PubMed] [Google Scholar]
- 36.Tessitore A, Hariri AR, Fera F, Smith WG, Das S, Weinberger DR, et al. Functional changes in the activity of brain regions underlying emotion processing in the elderly. Psychiatry Research: Neuroimaging. 2005;139:9–18. doi: 10.1016/j.pscychresns.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 37.Iidaka T, Okada T, Murata T, Omori M, Kosaka H, Sadato N, et al. Age-related differences in the medial temporal lobe responses to emotional faces as revealed by fMRI. Hippocampus. 2002;12:352–62. doi: 10.1002/hipo.1113. [DOI] [PubMed] [Google Scholar]
- 38.Mather M, Canli T, English T, Whitfield S, Wais P, Ochsner K, et al. Amygdala Responses to Emotionally Valenced Stimuli in Older and Younger Adults. Psychological Science. 2004;15:259–63. doi: 10.1111/j.0956-7976.2004.00662.x. [DOI] [PubMed] [Google Scholar]
- 39.Frey BN, Hall GB, Attard S, Yucel K, Skelin I, Steiner M, et al. Shift in the brain network of emotional regulation in midlife women: is the menopausal transition the turning point? Menopause. 17:840–5. doi: 10.1097/gme.0b013e3181df840f. [DOI] [PubMed] [Google Scholar]
- 40.Isaacowitz DM, Löckenhoff CE, Lane RD, Wright R, Sechrest L, Riedel R, et al. Age differences in recognition of emotion in lexical stimuli and facial expressions. Psychology and Aging. 2007;22:147–59. doi: 10.1037/0882-7974.22.1.147. [DOI] [PubMed] [Google Scholar]
- 41.Mather M, Carstensen LL. Aging and Attentional Biases for Emotional Faces. Psychological Science. 2003;14:409–15. doi: 10.1111/1467-9280.01455. [DOI] [PubMed] [Google Scholar]
- 42.Isaacowitz DM, Wadlinger HA, Goren D, Wilson HR. Is there an age-related positivity effect in visual attention? A comparison of two methodologies. Emotion. 2006;6:511–6. doi: 10.1037/1528-3542.6.3.511. [DOI] [PubMed] [Google Scholar]
- 43.Charles ST, Mather M, Carstensen LL. Aging and emotional memory: The forgettable nature of negative images for older adults. Journal of Experimental Psychology: General. 2003;132:310–24. doi: 10.1037/0096-3445.132.2.310. [DOI] [PubMed] [Google Scholar]
- 44.Leigland LA, Schulz LE, Janowsky JS. Age related changes in emotional memory. Neurobiology of Aging. 2004;25:1117–24. doi: 10.1016/j.neurobiolaging.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 45.Mather M, Knight M. Goal-directed memory: The role of cognitive control in older adults’ emotional memory. Psychology and Aging. 2005;20:554–70. doi: 10.1037/0882-7974.20.4.554. [DOI] [PubMed] [Google Scholar]
- 46.Neiss MB, Leigland LA, Carlson NE, Janowsky JS. Age differences in perception and awareness of emotion. Neurobiology of Aging. 2009;30:1305–13. doi: 10.1016/j.neurobiolaging.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mroczek DK, Kolarz CM. The effect of age on positive and negative affect: A developmental perspective on happiness. Journal of Personality and Social Psychology. 1998;75:1333–49. doi: 10.1037//0022-3514.75.5.1333. [DOI] [PubMed] [Google Scholar]
- 48.Ruffman T, Henry JD, Livingstone V, Phillips LH. A meta-analytic review of emotion recognition and aging: Implications for neuropsychological models of aging. Neuroscience & Biobehavioral Reviews. 2008;32:863–81. doi: 10.1016/j.neubiorev.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 49.Calder AJ, Keane J, Manly T, Sprengelmeyer R, Scott S, Nimmo-Smith I, et al. Facial expression recognition across the adult life span. Neuropsychologia. 2003;41:195–202. doi: 10.1016/s0028-3932(02)00149-5. [DOI] [PubMed] [Google Scholar]
- 50.Carstensen LL, Isaacowitz DM, Charles ST. Taking time seriously: A theory of socioemotional selectivity. American Psychologist. 1999;54:165–81. doi: 10.1037//0003-066x.54.3.165. [DOI] [PubMed] [Google Scholar]
- 51.Mather M, Carstensen LL. Aging and motivated cognition: the positivity effect in attention and memory. Trends in Cognitive Sciences. 2005;9:496–502. doi: 10.1016/j.tics.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 52.Roalf DR, Pruis TA, Stevens AA, Janowsky JS. More is less: Emotion induced prefrontal cortex activity habituates in aging. Neurobiology of Aging. doi: 10.1016/j.neurobiolaging.2009.10.007. In Press, Corrected Proof. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lord C, Buss C, Lupien SJ, Pruessner JC. Hippocampal volumes are larger in postmenopausal women using estrogen therapy compared to past users, never users and men: A possible window of opportunity effect. Neurobiology of Aging. 2008;29:95–101. doi: 10.1016/j.neurobiolaging.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 54.Muñoz M, Insausti R. Cortical efferents of the entorhinal cortex and the adjacent parahippocampal region in the monkey (Macaca fascicularis) European Journal of Neuroscience. 2005;22:1368–88. doi: 10.1111/j.1460-9568.2005.04299.x. [DOI] [PubMed] [Google Scholar]
- 55.Insausti R, Amaral DG. Entorhinal cortex of the monkey: IV. Topographical and laminar organization of cortical afferents. The Journal of Comparative Neurology. 2008;509:608–41. doi: 10.1002/cne.21753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Österlund MK, Hurd YL. Estrogen receptors in the human forebrain and the relation to neuropsychiatric disorders. Progress in Neurobiology. 2001;64:251–67. doi: 10.1016/s0301-0082(00)00059-9. [DOI] [PubMed] [Google Scholar]
- 57.Protopopescu X, Pan H, Altemus M, Tuescher O, Polanecsky M, McEwen B, et al. Orbitofrontal cortex activity related to emotional processing changes across the menstrual cycle. PNAS. 2005;102:16060–5. doi: 10.1073/pnas.0502818102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Craig AD. How do you feel [mdash] now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- 59.Phan KL, Wager TD, Taylor SF, Liberzon I. Functional neuroimaging studies of human emotions. CNS Spectrum. 2004;9:258–66. doi: 10.1017/s1092852900009196. [DOI] [PubMed] [Google Scholar]
- 60.Damasio AR. The feeling of what happens : body and emotion in the making of consciousness. New York: Harcourt Brace; 1999. [Google Scholar]
- 61.Nilsson S, Gustafsson JA. Estrogen Receptors: Therapies Targeted to Receptor Subtypes. Clin Pharmacol Ther. 89:44–55. doi: 10.1038/clpt.2010.226. [DOI] [PubMed] [Google Scholar]


