Abstract
Background
Despite having fewer risk factors for atrial fibrillation (AF), white patients have a greater prevalence of AF in the community than black patients, and a genetic basis has been postulated. However, it is unknown whether occurrence of new-onset AF after cardiac surgery is different in white versus black patients, and secondarily, other non-Caucasian patients.
Methods and Results
From 1995 through 2005, 20,282 white, 1,323 black, and 1,919 other non-Caucasian patients in sinus rhythm underwent coronary artery bypass grafting (CABG) with or without valve surgery. To adjust for clinical and socioeconomic confounders, we performed propensity-adjusted analyses. 7,093 white patients (35%) developed postoperative AF, compared to 255 (22%) black patients, and 550 (29%) other non-Caucasians (p <0.0001). Whites were older than black patients, had higher socioeconomic position, and greater left atrial size, but were less likely to have hypertension or congestive heart failure. In 847 propensity-matched patient pairs postoperative AF occurred more frequently in white than black patients (OR 1.74, 95% CI 1.7-1.78). Other than higher occurrence of bradycardia requiring pacing and re-intubation in white patients, occurrence of other postoperative complications, hospital mortality, and length of postoperative stay were similar. Age and valvular surgery were the strongest predictors of AF irrespective of race.
Conclusions
White patients had a markedly higher risk of postoperative AF than black and other non-Caucasian patients. The cause for racial differences of arrhythmic risk is unknown, but a genetic predisposition is plausible. Our results have implications for risk stratification and mechanistic understanding of postoperative AF.
Keywords: atrial fibrillation, race/ethnicity, cardiac surgery, epidemiology, risk factors
Atrial fibrillation (AF) after cardiac surgery affects up to 50% of patients and, similar to AF outside the peri-operative period, is associated with increased morbidity, mortality and costs.1, 2 A substantial and consistent body of evidence demonstrates a low prevalence of non-surgical AF in black patients despite their possessing multiple arrhythmic risk factors.3-6 However, these studies could be confounded by differences in access to medical care and lower socioeconomic position (SEP) that might reduce detection of AF.7, 8 Recently, a lower occurrence of AF was observed in black patients undergoing CABG, a time when AF detection may be less biased by medical access issues.9 Objectives of this study were (1) to compare the occurrence of postoperative AF after cardiac surgery in black patients with that of propensity-matched white patients, (2) to further define adjusted risk for postoperative AF in Asians, Hispanics and Native Americans, (3) to compare postoperative arrhythmic and non-arrhythmic complications, such as heart block, ventricular arrhythmia, renal failure, myocardial infarction, stroke, hospital mortality and length of hospital stay of matched white and black patients, and (4) to identify predictors of postoperative AF in black patients. To fulfill these aims, we studied a large prospectively followed patient cohort and utilized propensity matching with adjustment for important socioeconomic differences between patient groups.
Methods
Patients
From 1/1995 to 12/2005, 27,765 patients underwent coronary artery bypass grafting (CABG), valve surgery or both at Cleveland Clinic. We excluded patients with multiple cardiac operations during their hospital stay. Race was self-reported in the categories White, Black, Hispanic, Asian, Native American or other. Although the category of other race is not further defined, a large proportion of this group represents patients from the Middle East. Patients whose race was unknown were excluded from the analysis, as were patients with incomplete socioeconomic position data, incomplete preoperative medication data, and those not in sinus rhythm at time of surgery. Thus 21,458 individuals were available for propensity-adjusted analyses, with 20,282 white, 1,176 black, and and 23,625 white, 1,323 black and 1,919 non-Caucasians for unadjusted analyses (patients without SEP data included, Figure 1). Because the two most common races represented in the study were White and Black, we focused adjusted analyses on these racial groups.
Figure 1.
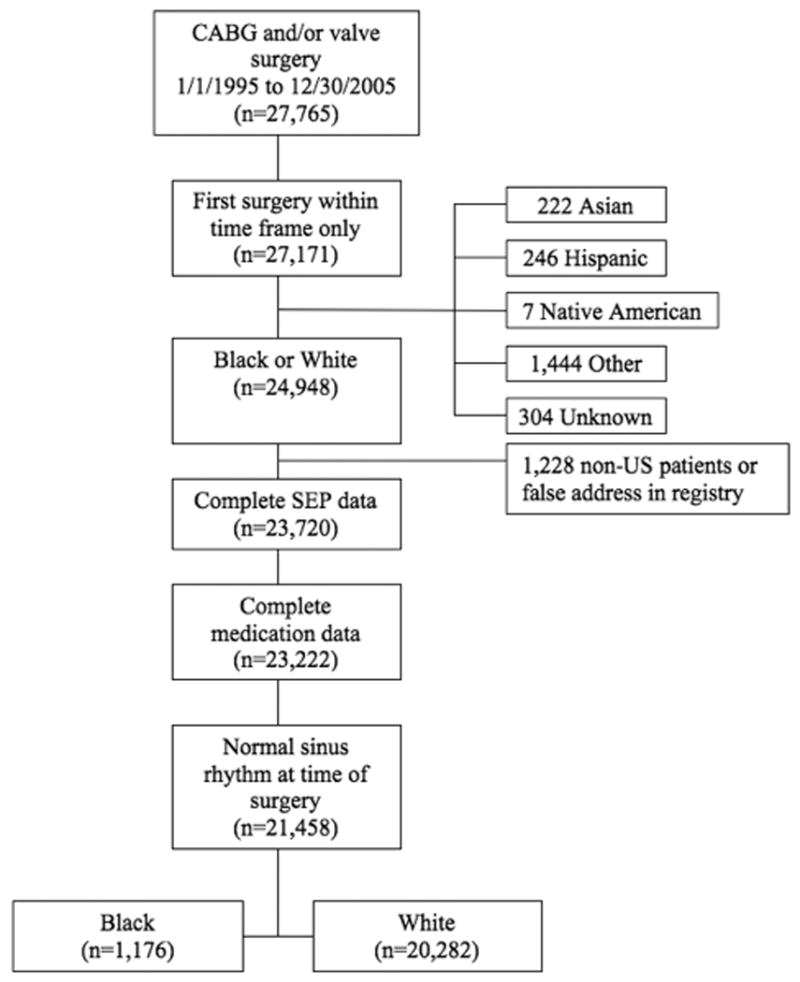
Patient flow diagram: CABG indicates coronary artery bypass graft surgery; SEP, socioeconomic position;
Patients were predominantly male (71%) and white (88%) and most frequently underwent isolated CABG (68%). There were clinically important differences in the clinical characteristics of white and black patients, many of which are established risk factors for AF and would favor increased occurrence of AF in blacks (Table 1). There was no prophylactic amiodarone use.
Table 1. Patient characteristics.
| Variable | White (n=20,282) No. (%) or Mean ± SD |
Black (n=1,176) No. (%) or Mean ± SD |
P value before matching | P value after matching | |
|---|---|---|---|---|---|
| Demographics | |||||
| Age (years) | 64 ± 12 | 61 ± 12 | <.0001 | 0.6 | |
| Body mass index (kg•m-2) | 28 ± 5 | 29 ± 6 | <.0001 | 0.9 | |
| Female gender | 6,202 (31) | 537 (46) | <.0001 | 0.8 | |
| Proportion with high school diploma [%] | 86 ± 9.4 | 75 ± 14 | <.0001 | 0.7 | |
| Z-Proportion with high school diploma* | 0.57 (0.15, 0.92) | -0.24 (-0.86, 0.48) | <.0001 | 0.7 | |
| Proportion with college degree [%] | 26 ± 18 | 15 ± 16 | <.0001 | 0.5 | |
| Z-Proportion with college degree* | -0.019 (-0.54, 0.89) | -0.67 (-0.94, -0.1) | <.0001 | 0.5 | |
| Proportion in executive position [%] | 35 ± 15 | 26 ± 15 | <.0001 | 0.6 | |
| Z-Proportion in executive position* | 0.15 (-0.45, 0.93) | -0.48 (-0.94, 0.12) | <.0001 | 0.6 | |
| Proportion of dividend paying properties [%] | 45 ± 16 | 23 ± 16 | <.0001 | 0.9 | |
| Z-Proportion of dividend paying properties* | 0.63 (0.01, 1.26) | -0.96 (-1.32, -0.12) | <.0001 | 0.9 | |
| Median household income [dollars] | 50,861 ± 23,294 | 34,054 ± 18,517 | <.0001 | 0.7 | |
| Z-Median household income* | 0.35 (-0.16, 0.93) | -0.56 (-1.25, 0.16) | <.0001 | 0.7 | |
| Median value of housing unit* [dollars] | 113,400 (76,300, 175,400) | 70,950 (51,300, 104,300) | <.0001 | 0.7 | |
| Z-Median value of housing unit* | 0.23 (-0.23, 0.69) | -0.49 (-0.79, -0.033) | <.0001 | 0.7 | |
| Cardiac comorbidity | |||||
| History of atrial arrhythmia | 2,036 (10) | 78 (6.6) | 0.0001 | 0.9 | |
| History of myocardial infarction | 8,682 (43) | 596 (51) | <.0001 | 0.6 | |
| Heart failure | 6,135 (30) | 552 (47) | <.0001 | 0.6 | |
| Aortic valve stenosis2 | 4,378 (22) | 135 (12) | <.0001 | 0.7 | |
| Aortic valve regurgitation† | 5,989 (30) | 308 (27) | 0.01 | 0.5 | |
| Mitral valve stenosis† | 633 (3.3) | 34 (3) | 0.6 | 0.6 | |
| LV ejection fraction (%) | 50 ± 29 | 47 ± 13 | <.0001 | 0.8 | |
| Left atrial volume (mL) | 49 ± 29 | 45 ± 27 | 0.0005 | 1 | |
| LV posterior wall thickness (cm) | 1.17 ± 0.23 | 1.23 ± 0.24 | <.0001 | 0.8 | |
| Non-cardiac comorbidity | |||||
| Smoking | 12,011 (59) | 810 (69) | <.0001 | 0.8 | |
| COPD | 3,854 (23) | 304 (30) | <.0001 | 0.6 | |
| Stroke | 1,493 (7.4) | 149 (13) | <.0001 | 0.2 | |
| Diabetes mellitus | 4,472 (23) | 391 (34) | <.0001 | 0.3 | |
| Hypertension | 13,277 (67) | 988 (85) | <.0001 | 0.4 | |
| Peripheral arterial disease | 7,666 (38) | 436 (37) | 0.6 | 0.8 | |
| Renal disease | 977 (4.8) | 142 (12) | <.0001 | 0.3 | |
| Preoperative medication use | |||||
| Angiotensin blockade‡ | 7,896 (39) | 570 (52) | <.0001 | 0.4 | |
| Beta blockers | 8,853 (44) | 616 (64) | 0.0002 | 0.6 | |
| Calcium channel blockers | 3,475 (17) | 286 (24) | <.0001 | 0.8 | |
| Inotropes | 377 (1.9) | 31 (2.6) | 0.06 | 0.6 | |
| Procedure | |||||
| CABG | 13,824 (68) | 862 (73) | 0.0002 | 0.6 | |
| Aortic valve replacement | 5,109 (25) | 216 (18) | <.0001 | 0.4 | |
| Mitral valve replacement | 4,488 (22) | 207 (18) | 0.0003 | 0.7 | |
| Mitral valve repair | 2,426 (19) | 101 (15) | 0.0003 | 0.9 | |
| CPB time (min) | 101 ± 40 | 101 ± 45 | 0.6 | 0.7 | |
| Postoperative RBC transfusion (units) 0 | 11,506 (57) | 554 (47) | <.0001 | 0.3 | |
| 1 | 2290 (11) | 155 (13) | |||
| 2 | 2606 (13) | 173 (15) | |||
| >2 | 3880 (19) | 294 (25) | |||
50th (25th, 75th) percentile.
Any grade of valvular stenosis or regurgitation.
Preoperative ACE inhibitor or Angiotensin II receptor blocker use.
KEY: AF indicates atrial fibrillation; CABG, coronary artery bypass grafting; COPD, chronic obstructive pulmonary disease; CPB, cardiopulmonary bypass; LV, left ventricular; RBC, red blood cell; SD, standard deviation.
Patient Data
Epidemiologic, cardiac and non-cardiac co-morbidity data and procedural data were obtained from the Cleveland Clinic Cardiovascular Information Registry, a prospective cardiovascular database, and our echocardiography database (E-Appendix A). Our Institutional Review Board approved use of these data for research with requirement for individual written patient consent waived.
Socioeconomic Position Data
We obtained socioeconomic position (SEP) data from the 2000 U.S. census,10 which provides block data from approximately 1000 residents to estimate individual SEP. Census blocks typically follow street or natural border lines and do not cross county borders. Six measures of SEP were considered: median household income, median value of housing unit, proportion of households receiving interest, dividend or rental income, proportion of adults ≥25 years who graduated from high school, proportion of adults ≥25 years who completed college, and proportion of adults ≥16 years with an executive or managerial occupation. A Z-score for each measure was calculated by subtracting U.S. population mean and then dividing it by its standard deviation; these scores were used for analyses.
Definition and Ascertainment of Postoperative AF
Postoperative AF was defined as any episode of AF or atrial flutter requiring medical treatment or electrical cardioversion after surgery and before hospital discharge. This definition is consistent with that of the Society of Thoracic Surgeons national cardiac database, with the exception that patients with history of AF but in sinus rhythm at time of surgery were included in the study. (http://www.ctsnet.org/file/rptDataSpecifications252_1_ForVendorsPGS.pdf, page 126). AF was detected by continuous telemetry monitoring throughout the hospital stay.
Postoperative Complications
We compared occurrence of the following adverse events after cardiac surgery in the propensity-matched cohort: renal failure, defined as rise of creatinine level >2mg/dl and/or a 2-fold increase over preoperative levels, development of pulmonary edema from heart failure, re-intubation due to respiratory failure, pacemaker-dependent bradycardia or complete heart block, ventricular arrhythmias, cardiac arrest, stroke, hospital death, and length of stay.
Statistical Analysis
Propensity Score Modeling
Propensity score adjusted analyses can create comparison groups with balanced covariates and therefore randomized trial-like conditions in observational studies.11 Such analyses are specifically helpful for situations in which exposure cannot be randomized, such as race. The caveat and major difference with randomized experiments is that only measured and closely related unmeasured variables are adjusted for. We calculated the propensity of black race group membership as a score between 0 and 1 from a saturated logistic regression model with 66 variables (E-Appendix A). Non-linear relationships of continuous patient variables with black race were relaxed using restricted cubic spline transformation. To identify the most influential predictors of race, stepwise variable selection with 1000-fold bagging (i.e. variables are selected from 1000 bootstrap samples of the original data)12 for improved model stability was employed (E-Appendix B). Because of the apparent co-linearity of SEP measures, we conducted a cluster analysis, which chose the most common constellation of six SEP measures (E-Appendix B). The propensity model had a C-statistic of 0.91, indicating excellent model fit to the data. Hosmer-Lemeshow goodness-of-fit test indicated model fit (p-value of 0.4). The propensity score was logit-transformed and used for propensity matching and propensity-adjusted logistic regression.
Adjusted Analyses
Patients with similar propensity scores (60% of the standard deviation)13 underwent 1:1 greedy matching (i.e. patients with most similar propensity scores are matched first) without replacement. Propensity score distribution of 20,282 white and 1,176 black patients allowed for 847 white patients to match with 847 similar black patients. To evaluate covariate balance between racial groups, we compared standardized differences of those variables that most accurately discriminated between white and black race, before and after propensity matching (Figure 2). A hypothetical confounder, which increased the P-value of the association between white race and occurrence of postoperative AF beyond 0.05, was investigated in the propensity-adjusted cohort (i.e. sensitivity analysis). Matched black patients showed substantial differences compared to unmatched black patients (E-Appendix C). To avoid selection bias by exclusion of poorly matched patients, we also conducted propensity-adjusted logistic regression in all 21,458 patients.14
Figure 2.
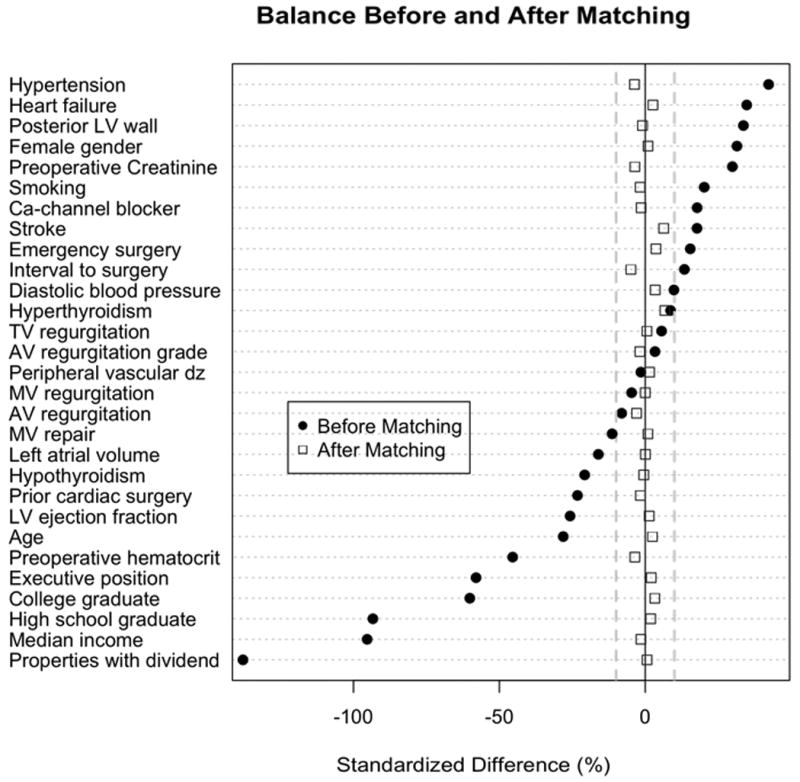
Standardized differences of black vs. white patients: Variables most intimately linked to black race are shown. The further the standardized difference deviates from 0%, the greater the difference between comparison groups. Key: AV indicates aortic valve; Ca, calcium; dz, disease; LV, left ventricle; MV, mitral valve; TV, tricuspid valve;
Racial Distribution of Postoperative AF
Occurrence of postoperative AF in Hispanic, Asian and Native American patients was compared with that of white and black patients and relative risk reported. Odds ratios for all racial/ethnic subgroups were adjusted for independent predictors of AF from our study.
χ2 statistics were used to compare occurrence of postoperative AF and postoperative complications in propensity-matched analyses.
Predictors of Postoperative AF in Black Patients
To study risk factors for postoperative AF in 1,176 black patients we applied stepwise logistic regression with 1000-fold bagging. Predictors, which were retained in greater than 50% of bootstrap models were selected for the final model.12 For comparison, we identified risk factors in the predominantly white cohort with identical methods (E-Appendix D). We studied the interactions between race, age and left atrial size in a conditional (i.e. stratified) plot for the probability of postoperative AF.
Presentation
Continuous variables are summarized by means and standard deviations or median and 25th and 75th percentile if skewed and compared with two-tailed student's t-test or Whitney-Mann-U-test, respectively. Categorical variables are summarized by frequencies and percentages and compared with χ2 statistics (Table 1).
SAS version 9.2 (©SAS Institute Inc., Cary, NC) and R version 2.9.0 (©The R Foundation of Statistical Computing, www.r-project.org) were used for statistical analyses.
Results
Of 20,282 white patients, 7093 (35%) developed AF after surgery, compared to 255 (22%) of 1,176 black patients, and 550 (29%) other non-Caucasians (p<.0001). Occurrence of postoperative AF varied with surgery complexity. Patients undergoing mitral valve and combined surgeries had the highest occurrence of AF, while those undergoing isolated bypass surgery the lowest. Black patients had a lower occurrence of postoperative AF in all surgical subgroups (Figure 3). Among all racial/ethnic groups, white patients experienced by far the most new-onset postoperative AF, and other races had similar substantially lower prevalence (Table 2). Multivariable adjustment identified only white and black race as independent racial predictors of AF (Figure 4).
Figure 3.
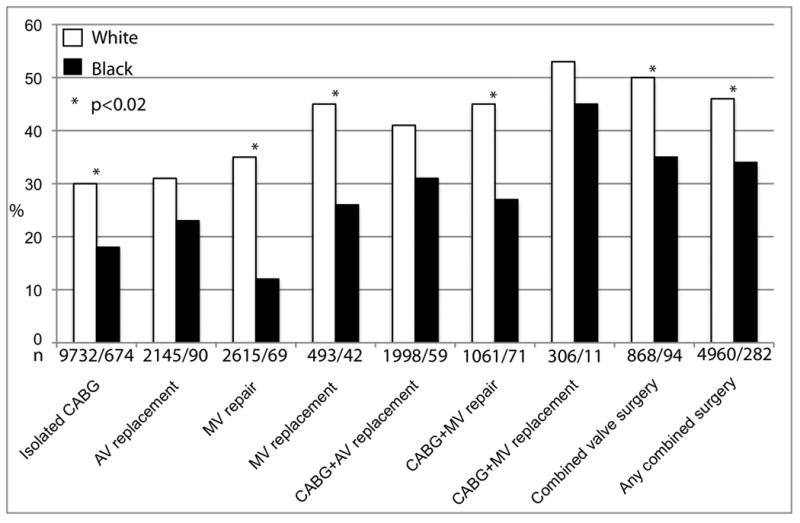
Occurrence of postoperative atrial fibrillation by surgery: Patients undergoing mitral valve and combined surgeries had highest and those undergoing isolated CABG lowest occurrence of AF. N describes number of white/black patients in the surgical subgroup. Key: AV indicates aortic valve; CABG, coronary artery bypass surgery; MV, mitral valve.
Table 2. Unadjusted Comparison of Postoperative AF between Racial/Ethnic Groups.
| Race/Ethnic Group | Total | AF (%) | RR (vs. white) |
P-value (vs. white) |
RR (vs. black) |
P-value (vs. black) |
|---|---|---|---|---|---|---|
| White | 23,625 | 9,022 (38%) | NA | NA | 1.56 | <.0001 |
| Black | 1,323 | 324 (24%) | 0.64 | <.0001 | NA | NA |
| Hispanic | 246 | 71 (29%) | 0.76 | 0.006 | 1.18 | 0.1 |
| Asian | 222 | 65 (29%) | 0.77 | 0.02 | 1.2 | 0.1 |
| Native American | 7 | 1 (14%) | 0.37 | 0.2 | 0.58 | 1* |
| Other | 1,444 | 413 (29%) | 0.75 | <.0001 | 1.17 | 0.01 |
Fisher's exact test
KEY: AF indicates atrial fibrillation; RR, relative risk.
Figure 4.
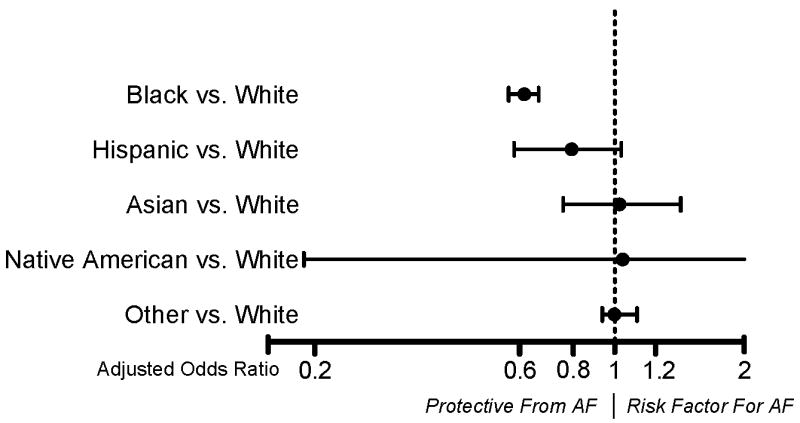
Multivariable adjusted odds ratios for postoperative AF of racial/ethnic subgroups in comparison to the risk in Whites: Only black race was independently associated with postoperative AF. There was a trend for Hispanics having lower occurrence of AF. Adjustment variables were: Age, gender, body mass index, preoperative creatinine level, preoperative beta blocker use, history of AF, mitral stenosis, tricuspid regurgitation, left atrial volume aortic and mitral valve replacement/repair, internal thoracic artery bypass grafting, perioperative blood transfusions, cardiopulmonary bypass time, surgery date in registry, intravenous nitroglycerin
Of 847 propensity-matched white patients, 229 (27%) developed postoperative AF compared to 188 (22%) of 847 black patients (odds ratio [OR] 1.74, 95% confidence interval [CI] 1.7-1.78). Propensity-adjusted logistic regression of all patients confirmed this result (OR 1.46, CI 1.23-1.72).
To explain the increased risk of postoperative AF in white patients, sensitivity analysis demonstrated that a hidden confounder had to change the odds of AF by 42%.
Among all racial/ethnic groups, white patients experienced by far the most new-onset postoperative AF, and other races had similar substantially lower prevalence (Table 2). Multivariable adjustment identified only white and black race as independent racial predictors of AF (Figure 4).
Postoperative Complications
Propensity-matched black and white patients had similar rates of occurrence of postoperative ventricular tachycardia, renal failure, pulmonary emboli, cardiac arrest, stroke and in-hospital death. Postoperative bradycardia requiring temporary or permanent pacing was more common in white patients, independent of AF occurrence. There was a higher rate of venous thromboses in black patient with AF, but in those without AF. However, length of stay was similar in white and black patients but as expected differed significantly between patients with vs. without AF (Table 3).
Table 3. Propensity-matched Postoperative Outcomes of White vs. Black Patients with or without AF [n (%)].
| AF (n=417) | No AF (n=1,277) | |||||||
|---|---|---|---|---|---|---|---|---|
| Outcome | White (n=229) |
Black (n=188) |
P | White (n=618) |
Black (n=659) |
P | OR (All White vs. Black) |
95% CI (All White vs. Black) |
| Renal failure* | 8 (3.5) | 7 (3.7) | 0.9 | 34 (5.5) | 43 (6.5) | 0.4 | 0.95 | 0.66-1.36 |
| Venous thrombosis | 3 (1.3) | 11 (5.9) | 0.01 | 9 (1.5) | 10 (1.5) | 0.9 | 0.57 | 0.28-1.16 |
| Myocardial infarction | 3 (1.3) | 0 | 0.1 | 1 (0.2) | 1 (0.2) | 1 | 4 | 0.44-36 |
| Pulmonary edema | 9 (3.9) | 8 (4.3) | 0.9 | 17 (2.8) | 13 (2) | 0.3 | 1.24 | 0.7-2.2 |
| Respiratory failure with re-intubation | 8 (3.5) | 7 (3.7) | 0.9 | 14 (2.3) | 12 (1.8) | 0.6 | 1.16 | 0.63-1.88 |
| Bradycardia requiring pacemaker† | 32 (14) | 15 (8) | 0.05 | 39 (6.3) | 25 (3.8) | 0.04 | 1.91 | 1.26-3 |
| Complete heart block | 4 (1.7) | 2 (1.1) | 0.7 | 10 (1.6) | 7 (1.1) | 0.4 | 1.28 | 0.68-3.6 |
| Stroke | 7 (3.1) | 7 (3.7) | 0.9 | 9 (1.5) | 10 (1.5) | 0.9 | 1.06 | 0.53-2.12 |
| Ventricular tachycardia | 15 (6.6) | 12 (6.4) | 0.9 | 20 (3.2) | 27 (4.1) | 0.4 | 0.9 | 0.57-1.42 |
| Ventricular fibrillation | 2 (0.9) | 2 (1.1) | 1 | 7 (1.1) | 6 (0.9) | 0.7 | 1.07 | 0.44-2.92 |
| Cardiac arrest | 3 (1.3) | 6 (3.2) | 0.3 | 3 (0.5) | 9 (1.4) | 0.2 | 0.4 | 0.16-1.03 |
| Hospital death | 9 (3.9) | 7 (3.7) | 1 | 11 (1.8) | 7 (1.1) | 0.3 | 1.43 | 0.72-2.05 |
| Length of ICU stay‡ | 2 (1, 4) | 2 (1, 4) | 0.2 | 1 (1, 2) | 1 (1, 2) | 0.2 | ||
| Length of operative stay‡ | 8 (6, 12) | 8 (7, 13) | 0.4 | 6 (5, 8) | 7 (5, 8) | 0.3 | ||
| Length of hospital stay‡ | 10 (7, 16) | 12 (8, 20) | 0.1 | 8 (6, 13) | 9 (7,13) | 0.5 | ||
Defined as new Creatinine level >2mg/dl or increase of Creatinine >40% of baseline.
Temporary or permanent pacing of new onset bradycardia.
50th (25th, 75th) percentile
KEY: CI indicates confidence interval; OR, odds ratio
Predictors of Postoperative AF in Black Patients
We identified older age, aortic or mitral valve replacement, more perioperative red blood cell transfusions, more remote surgery date in the registry and preoperative use of intravenous nitroglycerin as independent predictors of postoperative AF in black patients (Table 4). With the exception of intravenous nitroglycerin use, all of these variables were also predictors of AF in the predominantly white overall cohort (E-appendix D). Figure 5 demonstrates that the probability of postoperative AF increases with age regardless of patients' race or left atrial volume. The curves are shifted in a near parallel fashion in black and white patients, indicating that interactions between atrial size and race do not seem to be clinically meaningful.
Table 4. Predictors of Postoperative Atrial Fibrillation in Black Patients.
| Outcome | Odds Ratio† | 95% CI† | Reliability‡ |
|---|---|---|---|
| Older age* | 2.1 | 1.94-2.29 | 100% |
| Aortic valve replacement | 1.12 | 1.04-1.2 | 51% |
| Mitral valve replacement | 2 | 1.79-2.24 | 65% |
| More perioperative RBC transfusions* | 1.22 | 1.15-1.29 | 50% |
| Earlier surgery in registry | 1.14 | 1.05-1.24 | 60% |
| Preoperative i.v. nitroglycerin | 0.91 | 0.82-0.99 | 60% |
Restricted cubic spline transformed.
Comparison of 25th with 75th percentile of continuous variables.
Percent of bootstrap models in which predictor was selected.
KEY: CI indicates confidence interval; i.v., intravenous; RBC, red blood cells;
Figure 5.
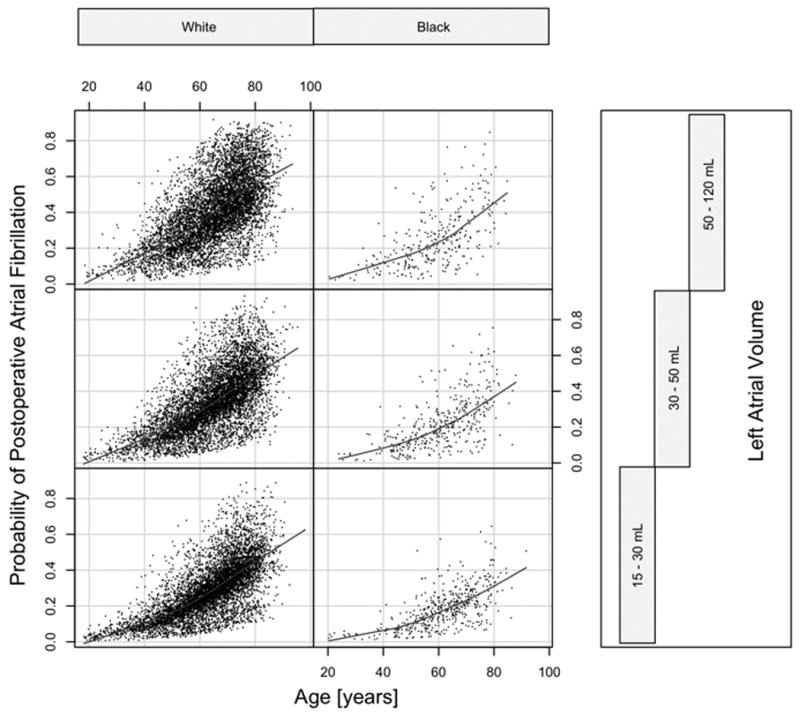
Conditional plot of probability for postoperative atrial fibrillation grouped by race and equal-count tertiles of left atrial size. Risk of postoperative atrial fibrillation increases with age in all panels and is shifted in a near-parallel fashion regardless of left atrial size, indicating only small interactions between race and left atrial volume.
Discussion
Using a propensity score adjusted analytic approach, we observed a markedly higher occurrence of postoperative atrial fibrillation in white compared to black patients. With this largest study of racial differences in postoperative AF, we add to the growing body of evidence that suggests a predisposition to AF among white patients compared to other races. Occurrence of postoperative complications, including hospital mortality, as well as clinical and procedural risk factors for postoperative AF appear to be similar in white and black patients, with older age and valve surgery being the most important predictors of AF.
Racial Differences of AF Occurrence
Racial differences in the occurrence of AF after cardiac surgery are incompletely studied and poorly understood. However, a series of observational studies has shown lower prevalence of non-surgical AF in black patients. Afzal and colleagues3 and Ruo and colleagues4 described approximately two-fold higher prevalence of AF in white heart failure patients. Borzecki5 and Go6 made similar observations in 664,754 veterans and 1.89 million enrollees of a large health insurance provider, respectively. These studies specifically excluded postoperative AF.6 In the study of Nazari and colleagues9 racial/ethnic subgroups of non-whites undergoing CABG showed a similar risk for postoperative AF, although individual patient numbers in those groups were small and their results were not adjusted for procedural or clinical confounders.
Such observations are counter-intuitive given the high prevalence of known clinical risk factors for AF in black patients, such as hypertension, obesity and heart failure15, 16 Some have argued that ascertainment bias from poor access to health care8 and low awareness of AF17 may contribute to false-low prevalence of AF in black subjects; such bias plays less of a role in our study. Interestingly, black patients also have higher rates of stroke. However, Singh and colleagues18 showed that black patients had more lacunar and fewer thromboembolic strokes, which could be an indicator of more prevalent or severe hypertension, rather than more AF in black patients.
Genetic Determinants and Racial Differences in AF
Several rare familial forms of AF19, 20 and single nucleotide polymorphisms identified by genome wide association studies in lone AF have been identified21-23, suggesting that a genetic predisposition for non-surgical AF is plausible. Body and colleagues demonstrated that two single nucleotide polymorphisms (SNP), located on gene 4q25 predict postoperative AF after CABG with or without valve surgery.24 Although the functional impact of these SNPs is unknown, they are in close proximity to PITX2, a gene responsible for the development of the pulmonary veins, a frequent source of AF triggers. Thus far, these studies have largely been conducted in white patient populations.
Recently, in a meta-analysis of 1,747 ancestry informative markers from patients enrolled in the Atherosclerosis Risk in Communities (ARIC) study and the Cardiovascular Health Study (CHS), Marcus and colleagues25 demonstrated that European ancestry is an independent risk factor for prevalent AF in black outpatients. The adjusted hazard ratio was 1.17 (95% CI 1.07-1.29) for each 10% increase in European ancestry informative markers. These findings demonstrate a genetic basis for the observed differences in AF prevalence and are consistent with our observations on the occurrence of postoperative AF.
Other Potential Mediators for Racial Differences in AF
Left atrial size is an established risk factor for postoperative and non-surgical AF.26, 27 A recent study suggested that smaller atrial size in black patients may contribute to a lower AF prevalence.28 In our study, atrial size was smaller in black subjects despite a higher prevalence of hypertension and heart failure (Table 1). Their left atria may be less likely to increase in size as response to elevated pressure than those of white subjects. However, because AF was less frequent in black subjects despite almost identical atrial size in propensity-matched white subjects, and because the interaction between race and atrial size was small (Figure 4), differences in atrial size alone cannot explain our results.
Racial Differences of Postoperative Complications
Surgical complication event rates were low in our study, limiting the power to detect differences. However, complication rates appeared to be similar in black and white patients. The occurrence of less bradycardia in black patients, but equal occurrence of complete heart block might indicate a greater degree of sinus node dysfunction or a higher vagal tone in white patients, both known markers of non-surgical AF. A greater occurrence of cardiac arrest in black patients did not affect overall hospital mortality, which may be in part related to fewer respiratory complications in our study. Interestingly, despite lower occurrence of postoperative AF and other complications, length of stay was similar in black and white patients, which may be due to non-health related (e.g. social) factors. However, length of stay was longer in patients who developed AF compared to those who did not, irrespective of race.
Risk factors of Postoperative AF in Black Patients
Our study demonstrates that, although occurrence of AF after cardiac surgery is less common in black patients compared to white patients, clinical risk factors for AF appear to be comparable (Table 4). Older age is associated with higher degree of fibrosis and left atrial remodeling, leading to the electrophysiological substrate leading to AF.29 The protective effect of preoperative intravenous nitroglycerin infusion against postoperative AF may be related to a reduction of pulmonary capillary wedge pressure,30 decreased reperfusion injury or to anti-inflammatory effects.31, 32 Mitral and aortic valve replacement were strong predictors of postoperative AF in Blacks, likely due to left atrial fibrosis and remodeling from valve disease.33, 34 Inflammation caused by direct manipulation during surgery must be considered as well. The association of peri-operative blood transfusions with postoperative AF has been previously established 35, and may be related to a triggered inflammatory response.
Limitations
Although we adjusted for many clinical and procedural characteristics, hidden confounders cannot be excluded. Our study results represent the experience from a single tertiary referral center. The proportion of black patients was relatively small and patients may be somewhat selected. However, socioeconomic position data showed expected disparities and we controlled for these. Because reoperations were excluded, event rate of secondary surgical complications may have been lower than expected and power to detect differences between black and white patients may have been limited despite the large patient number included in our study.
Conclusion
In propensity-adjusted analyses, white patients had significantly higher occurrence of AF after cardiac surgery compared to black patients and non-Caucasian races/ethnicities. Although the causes of this difference in postoperative AF are unclear from our results, genetic predisposition for the development of postoperative AF should be considered and further studied. Racial differences in arrhythmic risk may also influence decision-making about application of prophylactic interventions against postoperative AF.
Supplementary Material
Postoperative atrial fibrillation (AF) is the most common arrhythmic complication after cardiac surgery and shares many risk factors with AF in the community. Recently, white race has been associated with increased risk for AF. Therefore we tested the hypothesis that AF occurs more frequently in white compared to black patients undergoing coronary bypass grafting, valve surgery or a combination of both. We found multiple clinical and socioeconomic differences between race groups, many of which can confound unadjusted estimates of arrhythmic risk. After controlling for these in propensity-adjusted analysis of 20,282 white and 1,176 black patients, we demonstrate 46% greater odds of developing AF in white compared to black patients. Other postoperative complications and length of hospital stay were similar in white and black patients. Interestingly, with the exception of intravenous nitroglycerin use prior to surgery, all multivariable-adjusted predictors for postoperative AF in black patient were also associated with postoperative AF in white patients. In conclusion, our results suggest that race should be considered when assessing the risk of postoperative AF. Additionally, effects of prophylactic measures and therapies against postoperative AF should be reported within racial subgroups. Studies investigating genetic determinants of postoperative AF in patients from different race or ethnic backgrounds may provide an explanation for our results.
Acknowledgments
Funding Sources: This publication, in part, was made possible by Grant Number RR024990 (Dr. Rader) from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Information on NCRR is available at http://www.ncrr.nih.gov.
This work was also supported by grants from the NIH to Dr. Ellinor (HL092577, DA027021, HL104156, HL105780) and Dr. Chung (NIH-HL090620), and by a grant to Drs. Van Wagoner and Chung from the Fondation Leducq (European–North American Atrial Fibrillation Research Alliance, ENAFRA, 07/CVD-03).
Footnotes
Conflict of Interest Disclosures: None
References
- 1.Mathew JP, Fontes ML, Tudor IC, Ramsay J, Duke P, Mazer CD, Barash PG, Hsu PH, Mangano DT Investigators of the Ischemia Research and Education Foundation, Multicenter Study of Perioperative Ischemia Research Group. A multicenter risk index for atrial fibrillation after cardiac surgery. JAMA. 2004;291:1720–1729. doi: 10.1001/jama.291.14.1720. [DOI] [PubMed] [Google Scholar]
- 2.Aranki SF, Shaw DP, Adams DH, Rizzo RJ, Couper GS, VanderVliet M, Collins JJ, Jr, Cohn LH, Burstin HR. Predictors of atrial fibrillation after coronary artery surgery. Current trends and impact on hospital resources. Circulation. 1996;94:390–397. doi: 10.1161/01.cir.94.3.390. [DOI] [PubMed] [Google Scholar]
- 3.Afzal A, Ananthasubramaniam K, Sharma N, al-Malki Q, Ali AS, Jacobsen G, Jafri SM. Racial differences in patients with heart failure. Clin Cardiol. 1999;22:791–794. doi: 10.1002/clc.4960221207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ruo B, Capra AM, Jensvold NG, Go AS. Racial variation in the prevalence of atrial fibrillation among patients with heart failure: the Epidemiology, Practice, Outcomes, and Costs of Heart Failure (EPOCH) study. J Am Coll Cardiol. 2004;43:429–435. doi: 10.1016/j.jacc.2003.09.035. [DOI] [PubMed] [Google Scholar]
- 5.Borzecki AM, Bridgers DK, Liebschutz JM, Kader B, Kazis LE, Berlowitz DR. Racial differences in the prevalence of atrial fibrillation among males. J Natl Med Assoc. 2008;100:237–245. doi: 10.1016/s0027-9684(15)31212-8. [DOI] [PubMed] [Google Scholar]
- 6.Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, Singer DE. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370–2375. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 7.Havranek EP, Masoudi FA. What we're talking about when we talk about race. J Am Coll Cardiol. 2004;43:436–437. doi: 10.1016/j.jacc.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 8.Soliman EZ, Alonso A, Goff DC., Jr Atrial fibrillation and ethnicity: the known, the unknown and the paradox. Future Cardiol. 2009;5:547–556. doi: 10.2217/fca.09.49. [DOI] [PubMed] [Google Scholar]
- 9.Nazeri A, Razavi M, Elayda MA, Lee VV, Massumi A, Wilson JM. Race/ethnicity and the incidence of new-onset atrial fibrillation after isolated coronary artery bypass surgery. Heart Rhythm. 2010;7:1458–1463. doi: 10.1016/j.hrthm.2010.06.037. [DOI] [PubMed] [Google Scholar]
- 10.Krieger N, Chen JT, Waterman PD, Soobader MJ, Subramanian SV, Carson R. Geocoding and monitoring of US socioeconomic inequalities in mortality and cancer incidence: does the choice of area-based measure and geographic level matter?: the Public Health Disparities Geocoding Project. Am J Epidemiol. 2002;156:471–482. doi: 10.1093/aje/kwf068. [DOI] [PubMed] [Google Scholar]
- 11.Rubin DB. The design versus the analysis of observational studies for causal effects: parallels with the design of randomized trials. Stat Med. 2007;26:20–36. doi: 10.1002/sim.2739. [DOI] [PubMed] [Google Scholar]
- 12.Breiman L. Bagging predictors. Machine Learning. 1996;26:123–140. [Google Scholar]
- 13.Ahmed A, Husain A, Love TE, Gambassi G, Dell'Italia LJ, Francis GS, Gheorghiade M, Allman RM, Meleth S, Bourge RC. Heart failure, chronic diuretic use, and increase in mortality and hospitalization: an observational study using propensity score methods. Eur Heart J. 2006;27:1431–1439. doi: 10.1093/eurheartj/ehi890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blackstone EH. Comparing apples and oranges. J Thorac Cardiovasc Surg. 2002;123:8–15. doi: 10.1067/mtc.2002.120329. [DOI] [PubMed] [Google Scholar]
- 15.Ong KL, Cheung BM, Man YB, Lau CP, Lam KS. Prevalence, awareness, treatment, and control of hypertension among United States adults 1999-2004. Hypertension. 2007;49:69–75. doi: 10.1161/01.HYP.0000252676.46043.18. [DOI] [PubMed] [Google Scholar]
- 16.Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N, Hailpern SM, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O'Donnell C, Roger V, Sorlie P, Steinberger J, Thom T, Wilson M, Hong Y American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics--2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117:e25–146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 17.Meschia JF, Merrill P, Soliman EZ, Howard VJ, Barrett KM, Zakai NA, Kleindorfer D, Safford M, Howard G. Racial Disparities in Awareness and Treatment of Atrial Fibrillation. The REasons for Geographic and Racial Differences in Stroke (REGARDS) study. Stroke. 2010;414:581–7. doi: 10.1161/STROKEAHA.109.573907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singh R, Cohen SN, Krupp R, Abedi AG. Racial differences in ischemic cerebrovascular disease. J Stroke Cerebrovasc Dis. 1998;7:352–357. doi: 10.1016/s1052-3057(98)80054-2. [DOI] [PubMed] [Google Scholar]
- 19.Brugada R, Tapscott T, Czernuszewicz GZ, Marian AJ, Iglesias A, Mont L, Brugada J, Girona J, Domingo A, Bachinski LL, Roberts R. Identification of a genetic locus for familial atrial fibrillation. N Engl J Med. 1997;336:905–911. doi: 10.1056/NEJM199703273361302. [DOI] [PubMed] [Google Scholar]
- 20.Chen YH, Xu SJ, Bendahhou S, Wang XL, Wang Y, Xu WY, Jin HW, Sun H, Su XY, Zhuang QN, Yang YQ, Li YB, Liu Y, Xu HJ, Li XF, Ma N, Mou CP, Chen Z, Barhanin J, Huang W. KCNQ1 gain-of-function mutation in familial atrial fibrillation. Science. 2003;299:251–254. doi: 10.1126/science.1077771. [DOI] [PubMed] [Google Scholar]
- 21.Ellinor PT, Lunetta KL, Glazer NL, Pfeufer A, Alonso A, Chung MK, Sinner MF, de Bakker PI, Mueller M, Lubitz SA, Fox E, Darbar D, Smith NL, Smith JD, Schnabel RB, Soliman EZ, Rice KM, Van Wagoner DR, Beckmann BM, van Noord C, Wang K, Ehret GB, Rotter JI, Hazen SL, Steinbeck G, Smith AV, Launer LJ, Harris TB, Makino S, Nelis M, Milan DJ, Perz S, Esko T, Kottgen A, Moebus S, Newton-Cheh C, Li M, Mohlenkamp S, Wang TJ, Kao WH, Vasan RS, Nothen MM, MacRae CA, Stricker BH, Hofman A, Uitterlinden AG, Levy D, Boerwinkle E, Metspalu A, Topol EJ, Chakravarti A, Gudnason V, Psaty BM, Roden DM, Meitinger T, Wichmann HE, Witteman JC, Barnard J, Arking DE, Benjamin EJ, Heckbert SR, Kaab S. Common variants in KCNN3 are associated with lone atrial fibrillation. Nat Genet. 2010;42:240–244. doi: 10.1038/ng.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benjamin EJ, Rice KM, Arking DE, Pfeufer A, van Noord C, Smith AV, Schnabel RB, Bis JC, Boerwinkle E, Sinner MF, Dehghan A, Lubitz SA, D'Agostino RBS, Lumley T, Ehret GB, Heeringa J, Aspelund T, Newton-Cheh C, Larson MG, Marciante KD, Soliman EZ, Rivadeneira F, Wang TJ, Eiriksdottir G, Levy D, Psaty BM, Li M, Chamberlain AM, Hofman A, Vasan RS, Harris TB, Rotter JI, Kao WH, Agarwal SK, Stricker BH, Wang K, Launer LJ, Smith NL, Chakravarti A, Uitterlinden AG, Wolf PA, Sotoodehnia N, Kottgen A, van Duijn CM, Meitinger T, Mueller M, Perz S, Steinbeck G, Wichmann HE, Lunetta KL, Heckbert SR, Gudnason V, Alonso A, Kaab S, Ellinor PT, Witteman JC. Variants in ZFHX3 are associated with atrial fibrillation in individuals of European ancestry. Nat Genet. 2009;41:879–881. doi: 10.1038/ng.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gudbjartsson DF, Arnar DO, Helgadottir A, Gretarsdottir S, Holm H, Sigurdsson A, Jonasdottir A, Baker A, Thorleifsson G, Kristjansson K, Palsson A, Blondal T, Sulem P, Backman VM, Hardarson GA, Palsdottir E, Helgason A, Sigurjonsdottir R, Sverrisson JT, Kostulas K, Ng MC, Baum L, So WY, Wong KS, Chan JC, Furie KL, Greenberg SM, Sale M, Kelly P, MacRae CA, Smith EE, Rosand J, Hillert J, Ma RC, Ellinor PT, Thorgeirsson G, Gulcher JR, Kong A, Thorsteinsdottir U, Stefansson K. Variants conferring risk of atrial fibrillation on chromosome 4q25. Nature. 2007;448:353–357. doi: 10.1038/nature06007. [DOI] [PubMed] [Google Scholar]
- 24.Body SC, Collard CD, Shernan SK, Fox AA, Liu KY, Ritchie MD, Perry TE, Muehlschlegel JD, Aranki S, Donahue BS, Pretorius M, Estrada JC, Ellinor PT, Newton-Cheh C, Seidman CE, Seidman JG, Herman DS, Lichtner P, Meitinger T, Pfeufer A, Kaab S, Brown NJ, Roden DM, Darbar D. Variation in the 4q25 chromosomal locus predicts atrial fibrillation after coronary artery bypass graft surgery. Circ Cardiovasc Genet. 2009;2:499–506. doi: 10.1161/CIRCGENETICS.109.849075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marcus GM, Alonso A, Peralta CA, Lettre G, Vittinghoff E, Lubitz SA, Fox ER, Levitzky YS, Mehra R, Kerr KF, Deo R, Sotoodehnia N, Akylbekova M, Ellinor PT, Paltoo DN, Soliman EZ, Benjamin EJ, Heckbert SR for the Candidate-Gene Association Resource (CARe) Study. European Ancestry as a Risk Factor for Atrial Fibrillation in African Americans. Circulation. 2010;122:2009–2015. doi: 10.1161/CIRCULATIONAHA.110.958306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Osranek M, Fatema K, Qaddoura F, Al-Saileek A, Barnes ME, Bailey KR, Gersh BJ, Tsang TS, Zehr KJ, Seward JB. Left atrial volume predicts the risk of atrial fibrillation after cardiac surgery: a prospective study. J Am Coll Cardiol. 2006;48:779–786. doi: 10.1016/j.jacc.2006.03.054. [DOI] [PubMed] [Google Scholar]
- 27.Tanabe K, Yamaguchi K, Tani T, Yagi T, Katayama M, Tamita K, Kinoshita M, Kaji S, Yamamuro A, Morioka S, Okada Y, Kihara Y. Left atrial volume: predictor of atrial fibrillation in patients with degenerative mitral regurgitation. J Heart Valve Dis. 2007;16:8–12. [PubMed] [Google Scholar]
- 28.Marcus GM, Olgin JE, Whooley M, Vittinghoff E, Stone KL, Mehra R, Hulley SB, Schiller NB. Racial differences in atrial fibrillation prevalence and left atrial size. Am J Med. 2010;123:375.e1–375.e7. doi: 10.1016/j.amjmed.2009.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sanders P, Morton JB, Davidson NC, Spence SJ, Vohra JK, Sparks PB, Kalman JM. Electrical remodeling of the atria in congestive heart failure: electrophysiological and electroanatomic mapping in humans. Circulation. 2003;108:1461–1468. doi: 10.1161/01.CIR.0000090688.49283.67. [DOI] [PubMed] [Google Scholar]
- 30.Faggiano P, D'Aloia A, Zanelli E, Gualeni A, Musatti P, Giordano A. Contribution of left atrial pressure and dimension to signal-averaged P-wave duration in patients with chronic congestive heart failure. Am J Cardiol. 1997;79:219–222. doi: 10.1016/s0002-9149(96)00720-5. [DOI] [PubMed] [Google Scholar]
- 31.Prasongsukarn K, Abel JG, Jamieson WR, Cheung A, Russell JA, Walley KR, Lichtenstein SV. The effects of steroids on the occurrence of postoperative atrial fibrillation after coronary artery bypass grafting surgery: a prospective randomized trial. J Thorac Cardiovasc Surg. 2005;130:93–98. doi: 10.1016/j.jtcvs.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 32.Patti G, Chello M, Candura D, Pasceri V, D'Ambrosio A, Covino E, Di Sciascio G. Randomized trial of atorvastatin for reduction of postoperative atrial fibrillation in patients undergoing cardiac surgery: results of the ARMYDA-3 (Atorvastatin for Reduction of MYocardial Dysrhythmia After cardiac surgery) study. Circulation. 2006;114:1455–1461. doi: 10.1161/CIRCULATIONAHA.106.621763. [DOI] [PubMed] [Google Scholar]
- 33.Allessie MA, Boyden PA, Camm AJ, Kleber AG, Lab MJ, Legato MJ, Rosen MR, Schwartz PJ, Spooner PM, Van Wagoner DR, Waldo AL. Pathophysiology and prevention of atrial fibrillation. Circulation. 2001;103:769–777. doi: 10.1161/01.cir.103.5.769. [DOI] [PubMed] [Google Scholar]
- 34.Gaborit N, Steenman M, Lamirault G, Le Meur N, Le Bouter S, Lande G, Leger J, Charpentier F, Christ T, Dobrev D, Escande D, Nattel S, Demolombe S. Human atrial ion channel and transporter subunit gene-expression remodeling associated with valvular heart disease and atrial fibrillation. Circulation. 2005;112:471–481. doi: 10.1161/CIRCULATIONAHA.104.506857. [DOI] [PubMed] [Google Scholar]
- 35.Koch CG, Li L, Van Wagoner DR, Duncan AI, Gillinov AM, Blackstone EH. Red cell transfusion is associated with an increased risk for postoperative atrial fibrillation. Ann Thorac Surg. 2006;82:1747–1756. doi: 10.1016/j.athoracsur.2006.05.045. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


