Abstract
We describe here the use of inorganic manganese based particles as convertible MRI agents. As has been demonstrated with iron oxide particles, manganese oxide and manganese carbonate particles can be internalized within phagocytotic cells, being subsequently shuttled to endosomes and/or lysosomes. As intact particles, only susceptibility induced MRI contrast is exhibited, most often see as dark contrast in susceptibility weighted images. Modulation of MRI contrast is accomplished by the selective degradation of these particles within the endosomal and lysosomal compartments of cells. Upon particle deconstruction in the endosomes and lysosomes, the dissolved Mn2+ acts as a T1 agent, eliciting bright contrast in T1 weighted images. This modulation of MRI contrast is demonstrated both in vitro in cells in culture, and also in vivo, in rat brain. These particles are the potential building blocks for an entire class of new environmentally responsive MRI contrast agents.
Keywords: MRI, manganese, cells, contrast agents, brain
INTRODUCTION
Many strategies have been devised for using MRI to sense molecular and cellular changes. These efforts largely rely either directly on the expression of enzymes or indirectly by their actions. These enzymes can be inducible, either chemically or biologically, or can be constitutively expressed. For instance, induced expression of beta-galactosidase in cells loaded with EGadMe results in cleavage of the sugar moiety off the top of EGadMe, exposing the gadolinium atom (1). This increases the relaxivity of the agent, resulting in bright contrast in T1 weighted images. Similarly, the induced expression of tyrosinase, resulting in the production of melanin in cells, results in enhanced relaxivity due to increased intracellular metals absorption (2, 3). A third example is constitutively expressed proteins which affect NMR properties, most useful for cell tracking experiments. Two examples of this are ferritin expression (4), which by way of dark, T2- or T2*-weighted contrast, is intended to report on cellular location, and engineered poly-lysine expression (5), which by way of chemical exchange saturation transfer effect, also reports on the location of cells.
MRI contrast agents act by influencing the bulk properties of the water interacting with or surrounding the agent. Most nano- or microparticle formulations affect T2 and T2* by altering the local magnetic field in their vicinity. Other agents, usually small manganese or gadolinium based molecules, in addition to possessing T2 and T2* effects, act as T1 agents, as the unpaired electrons on these agents catalyze the reestablishment of longitudinal magnetization of nearby water protons. MR imaging in the presence of particulate agents usually creates dark contrast for the T2 and/or T2* affecting agents and bright contrast for the T1 agents. In many cases, however, these strategies have taken advantage only of half of the available dynamic range in the image, that is, introducing bright or dark contrast on top of tissue signal intensity. Figure 1 pictorially demonstrates this. Figure 1A depicts the activation of a gadolinium based chelate (1), which, using T1 weighted MRI, creates bright contrast in an image, where before there was tissue signal intensity. Conversely, Figure 1B illustrates the accumulation of iron oxide nanoparticles in tissue, which, using appropriate pulse sequences, renders dark contrast, where there was previously tissue signal intensity. The MRI contrast agent we are reporting on here is shown in Figure 1C, and has the potential to take advantage of the full dynamic range in an image. In this case, a T2 or T2* contrast agent is initially present which creates dark contrast. Cellular changes, such as gene expression, induce alterations in the particles which converts the contrast agent into a T1 contrast agent, eliciting bright contrast.
Figure 1.
Illustration of various molecular and cellular MRI contrast phenomena. A) Bright contrast elicited from an activatable gadolinium based contrast agent, B) dark contrast elicited from the accumulation of targeted iron oxide nanoparticles at a tumor site, C) initial dark contrast from manganese based nanoparticle converts to bright contrast upon dissolution.
Here, we demonstrate the use of inorganic manganese based particles as convertible MRI contrast agents. The mechanism of this action is controlled dissolution. It has been observed that upon internalization of nano- and microparticles for cellular MRI, particles are exclusively located within endosomes and/or lysosomes (6, 7). These are cellular compartments which degrade foreign materials, due to the abundance of proteolytic enzymes and chelating agents, and an acidic environment. Indeed, studies have demonstrated that dextran coated iron oxide nanoparticles dissolve within these cellular compartments within hours, resulting in the release of free iron (8). We have purposefully applied this concept to insoluble, inorganic manganese based particles. Manganese oxides (MnO, MnO2, Mn3O4) and manganese carbonate (MnCO3) are insoluble at neutral pH and can be formulated into nano- or microparticles. As intact particles, all have magnetic susceptibilities which cause dark contrast using T2*-weighted MRI (9). Upon internalization by cells and localization within endosomes and/or lysosomes, the particles dissolve, releasing manganese 2+ ions, which are strong T1 MRI contrast agents. Thus, the dissolution of these particles converts dark MRI contrast to bright.
MATERIALS AND METHODS
Molecular dissolution
50 millimoles MnO2 was placed in 500 ml of each of three vigorously stirring solutions; 20 mM sodium citrate, pH 5.0; 150 mM phosphate buffered saline, pH 7.4; and DMEM F-12 growth medium, pH 7.4. In acidic environments, MnO2 dissolves to form the redox stable Mn2+, even though its oxidation state in the compound is +4. 1.5 ml samples were removed with a 3 ml syringe at the following time points, 0, 15, 30, 45, 60, 90, 150 minutes, and sterile filtered through 0.22 micron syringe filters to remove any floating particulates. Samples were prepared for MRI in glass tubes and sealed with wax film. To calculate the r1 molar relaxivity of MnCl2 and MnO2 at 4.0 Tesla, tubes containing various concentrations of MnCl2 (0, 2, 10, 20, 50, 100 micromolar) and MnO2 (0, .5, 1, 2 and 5 millimolar) were prepared in deionized water. T1 maps of each set of samples were calculated at 4.0 Tesla using a standard saturation recovery scheme employing spin echo readouts. Thirty repetition times were collected logarithmically spaced, ranging from 20 ms to 15 sec. Region of interests were measured from the images for each tube at each time point. Linearization of the Bloch equations relating T1 relaxation time as a function of repetition time allowed plotting natural log (Signal Intensity/Equilibrium Signal Intensity) versus TR−1 to retrieve T1. T2 maps were acquired for the MnCl2 sample set as well as for a sample set containing the same concentrations of MnCO3. For this, a multi-echo spin echo sequence was used with parameters of: 16 echo times, echo spacing = 9 ms, repetition time = 10 seconds. From these maps, r2 (transverse molar relaxivity) was calculated for each compound.
In vitro, intracellular molecular dissolution
325 mesh MnCO3 and Mn3O4 were manually ground using a mortar and pestle, then used without size sorting. Under electron microscopy, particles were in the size range of 200 nanometers to 5 microns. To investigate the ability of cells to endocytose particles and then dissolve them, MnCO3 in PBS was added to confluent culture dishes of mouse embryonic fibroblasts (~ 5 million cells) at 1650, 165, 33 and 8.25 µg in 10 ml growth medium, and allowed to incubate 24 hours. Controls received only PBS. Previous studies have demonstrated the ability of fibroblasts to endocytose micron size magnetic particles (7, 10). To test whether particles could dissolve in growth medium alone, 33 µg of particles were incubated in either growth medium only or PBS only (no cells). Cells were then washed to remove free particles, trypsinized and centrifuged in eppendorf tubes. Cell tubes underwent rapid T1 mapping at 11.7 T using a multi-slice Look-Locker imaging sequence (11). In plane resolution was 200 microns, with 1 mm slice thickness.
In vivo, intracellular molecular dissolution and manganese tract tracing
All animal procedures were carried out in accordance with the approval of the National Institutes of Neurological Disorders and Stroke Animal Use and Care Committee. To investigate the ability of the dissolved manganese from the particles to trace neural pathways, either MnCO3 (6 animals) or Mn3O4 nanoparticles (5 animals) were injected directly into the right somatosensory cortex (S1) of six week old anesthetized rats at a concentration of 660 µg in 2 µl PBS. Previous reports have demonstrated the ability of brain cells to endocytose free particles, in vivo (12). Immediately after injections, T1 maps were acquired of the entire rat brain, in vivo, using a multi-slice Look-Locker imaging sequence with 100 micron in plane resolution, 1 mm slice thickness. Rats were then revived and returned to the animal facility. At day 10, T1 maps were again acquired of the entire rat brain, in vivo, using the identical multi-slice Look-Locker imaging sequence. T1 maps were calculated in Matlab.
RESULTS
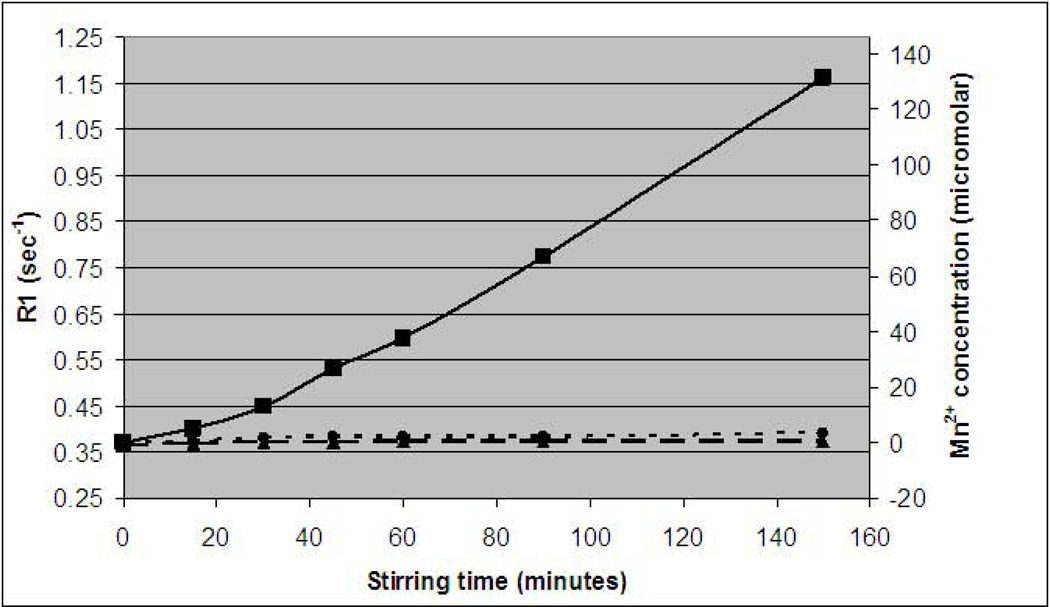
We attempted to dissolve MnO2 in three different solutions, relevant to intracellular and lysosomal/endosomal environments. MRI was used to determine the amount of dissolved Mn2+ ion by way of its ability to shorten T1 (increase R1 relaxation rate) of water. As can be seen in Figure 2, a substantial increase in water R1 relaxation rate was observed for the MnO2 incubated in 20 mM citrate, pH 5.0, with no observable effect from the MnO2 in either PBS, pH 7.4 or growth medium, pH 7.4. The 20 mM citrate, pH 5.0 was used to simulate the endosome/lysosomes chemical environment, typically pH 4.5–6.0, as has been previously reported (8). Indeed, while free Mn2+ ion concentrations in the two samples at neutral pH were near zero, after 150 minutes of stirring, Mn2+ concentrations in the pH 5.0 sample had risen to over 130 micromolar. These measurements were made using the measured r1 molar relaxivity of MnCl2 which was 5.8 mM−1 s−1. The measured r1 molar relaxivity of MnO2 was 0.07 mM−1 s−1. This low r1 is in accordance with previously measured r1 for inorganic manganese based nanoparticles (13).
Figure 2.
Dissolution of MnO2 particles in media as a function of time. R1 relaxation rate (1/T1), and calculated free Mn2+ ion concentration for MnO2 stirred in (solid line) 20 mM sodium citrate, pH 5.0; (dashed line) 150 mM phosphate buffered saline, pH 7.4; and (dotted line) DMEM F-12 growth medium, pH 7.4. Only at pH 5.0 does the particle dissolve, detected as an increase in R1 relaxation rate as Mn2+ is generated. Free Mn2+ was calculated from the r1 molar relaxivity obtained for MnCl2, which was 5.8 mM−1 sec−1.
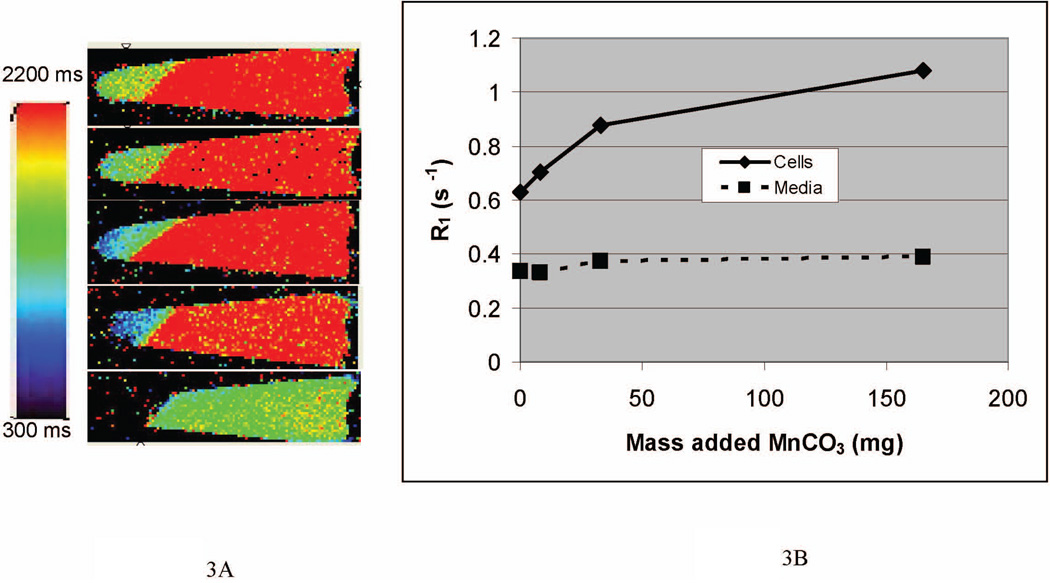
To test the ability of cells to dissolve inorganic manganese particles and release Mn2+ ions, cells were labeled in culture with various amounts of 200 nanometers to 5 microns MnCO3 particles. We have previously demonstrated the ability of cells to endocytose particles as large 5.8 microns (7). After 24 hours incubation, cells containing manganese particles could be observed by light microscopy. MRI investigations showed that after 24 hours incubation with particles, cells began to dissolve some of the particles. This was observed as a decrease in the T1 of the cells due to free Mn2+. Figure 3A shows T1 maps of cells labeled with various quantities of MnCO3 particles for 24 hours. The T1 of cells without manganese particle incubation was 1595 ms, with the T1 of the saline at 2980 ms. Addition of 8.25, 33 and 165 µg of particles to the cells caused reductions in the T1 of the cells to 1420, 1140 and 925 milliseconds. The addition of 1650 µg of particles resulted in undetectable signal intensity, likely due to T2* effects from either the remainder of the intact particles or excessive Mn2+, which can also give T2* effects at high concentration. The T1 of the saline bathing the cells also decreased, likely due to some cell death during centrifugation of the cells, releasing Mn2+ into the saline. Additionally, it can be seen that cells pelleted inhomogeneously, with cells at the bottom of the tube having shorter T1 than cells above. This was likely due to heterogeneous labeling of cells, causing more heavily labeled cells to sink faster during centrifugation. Accordingly, increased Mn2+ would be created and retained in these cells, and hence the lower T1 measured.
Figure 3.
A) T1 maps of cells labeled with various quantities of MnCO3 particles for 24 hours. T1 of labeled cells decreased in a dose dependent manner. The T1 of the saline bathing the cells also decreased, likely due to some cell death during centrifugation of the cells, releasing Mn2+ into the saline. B) Plotting R1 relaxation rate versus amount of MnCO3 shows nearly 75% increase in R1 relaxation rate for 165 microgram labeling, with an 11% increase for the bathing saline.
Figure 3B converts these T1 values to R1 relaxation rates (1/ T1), which depends linearly on concentration. Here it can be observed that R1 increases for the samples incubated with 8.25, 33 and 165 µg of particles increased 12%, 40% and 72%, respectively, with minimal increases in the R1 relaxation rate of the saline bathing the cells. Control experiments, where particles were incubated in only growth medium or PBS resulted in no change in T1, indicating that no dissolution had occurred.
Next, to ensure that the T1 effect seen by the dissolution of inorganic manganese particles was due to the release of Mn2+, MnCO3 and Mn3O4 particles were injected directly into the primary somatosensory cortex (S1) of adult rats. We have previously demonstrated the ability for neural cells to endocytose large particles directly in situ (12). The hypothesis was that cells would endocytose particles, with particles trafficking to lysosomes, whereupon cells would dissolve them to form Mn2+. This Mn2+ would be free to tract trace down active neural pathways, eliciting T1 contrast, as is standard experimental protocol for neural tract tracing employing manganese enhanced MRI (14). Figure 4 shows T1 maps of a slice from the injection location from two animals, one each for MnCO3 and Mn3O4 particle injections, immediately after injection and 10 days after injection. Clearly visible are the dark contrast areas resulting from T2* effects from the presence of manganese particles in the injection sites. Importantly, in each animal, the right thalamus had significantly decreased T1 ten days post-injection. Indeed, in 8 of 11 rats injected with either MnCO3 or Mn3O4 particles, marked T1 decrease was measured in the ipsilateral thalamus to the injection site ten days post-injection. The average decrease in T1 among these animals was 155 ms, from 1147 +/− 37 to 992 +/− 66 ms, corresponding to an increase in R1 relaxation rate of 15%. This was statistically significant, p<.001, as revealed by paired student t-test. In the three animals where T1 decrease was not observed in the thalamus, dark injection sites were not observed either, indicating failed injections. Furthermore, for the animal shown in Figure 5, not only is there T1 shortening in the ipsilateral thalamus, but additionally, the secondary somatosensory cortex displayed a decrease in T1 from 1390 to 1250 ms, corresponding to an increase in R1 relaxation rate of 11%. Thalamus and secondary somatosensory cortex are known anterograde targets of S1.
Figure 4.
T1 maps of two rat brains injected with either MnCO3 (top row) or Mn3O4 (bottom row) both immediately after injection of particles and ten days post injection. In both animals, substantial T1 decreases can be observed in the thalamus (arrows) ipsilateral to the injection sites.
Figure 5.
In vivo convertible manganese MRI. T1 maps of the injection site immediately after injection with MnCO3 (left) and 10 days post injection (right). Numbers in the table are T1 in milliseconds for relevant regions of the brain from this animal. This animal displayed ipsilateral T1 decrease not only in the thalamus (arrow), but also in the secondary somatosensory cortex (circle), corresponding to a 11% increase in R1 relaxation rate.
DISCUSSION
There is interest in creating switchable or activatable MRI contrast agents that respond to changes in cell physiology. Here we describe the use of inorganic manganese oxides and carbonate as convertible MRI contrast agents, that is, agents that can be converted from low r1, high r2* agents, to high r1, low r2* agents. Inorganic manganese oxides and carbonates are virtually insoluble at neutral, cytosolic pH. As an example, the Ksp (solubility product constant) of MnCO3 at pH 7.0 is ~ 2 ×10−11. This means that at equilibrium, the Mn2+ concentration is maximally less than 5 micromolar; nearly undetectable by MRI (11). Indeed, for the insoluble manganese particle MnO2, the measured r1 was less than 0.1 mM−1 s−1. As metal oxides and carbonates are Arrhenius bases, their dissolution can be accelerated by the presence of excess protons, i.e. acidic solutions. Thus, as insoluble nanoparticles, the inorganic manganese particles have low r1 and exhibit high r2*, however, their incorporation into low pH environments inside cells causes release of Mn2+ with concomitant increase in r1 and a decrease in r2*.
We have demonstrated that cells can endocytose inorganic manganese based particles and can dissolve them to form ionic Mn2+. Solubilization only occurred upon internalization of particles into low pH compartments of the cell, such as endosomes and lysosomes. The resultant Mn2+ species had high r1 and caused cells to turn bright on T1 weighted imaging sequences after 24 hour incubation with the particles. Additionally, the decomposition of the nanoparticle decreases the susceptibility induced r2*. A similar gadolinium based contrast agent that relies on low pH has been reported (15).
We also demonstrated that the converted manganese could be transported by neurons in the rat brain in tract tracing experiments. Ten days after injection of manganese particles into S1 of rats, substantial T1 decreases were observed in the thalamus and in one case, secondary somatosensory cortex. Thalamus and secondary somatosensory cortex are known anterograde targets of S1. In all animals, the area of dark contrast in S1 was decreased ten days later, further demonstrating the decomposition of the original, susceptibility generating nanoparticles. It is unlikely that particles dissolved outside of cells in CSF, as in vitro experiments failed to demonstrate particles dissolving in either growth medium or PBS following 24 hour incubation. An important result is that the manganese enhanced effect was robust even ten days after administration. Most manganese tract tracing experiments lose signal after only a few days (16) which demonstrates the potential of having a slow release form of manganese for track tracing experiments.
A major issue with manganese based contrast agents is toxicity. Indeed, Aoki, et al, observed significant cell death of lymphocytes labeled with MnCl2 at concentrations above 1.0 mM (17). Advances in particle coating and composition can improve bioavailability, cellular targeting and toxicity. These avenues are currently being pursued.
CONCLUSION
We describe here the use of inorganic manganese based particles as convertible MRI agents. As intact constructs, these particles exhibit only susceptibility induced MRI contrast, most often see as dark contrast in susceptibility weighted images. Following particle deconstruction, the dissolved Mn2+ acts as a T1 agent, eliciting bright contrast in T1 weighted images. Modulation of MRI contrast is accomplished by the selective degradation of these particles within the endosomal and lysosomal compartments of cells. These particles are the potential building blocks for an entire class of new environmentally responsive MRI contrast agents.
ACKNOWLEDGENTS
This research was supported, in part, by the NINDS Intramural Research Program of NIH. This work was also supported, in part, by a National Institute of Neurological Disorders and Stroke P30 grant (NS-52519). We thank Dr. Kai –Hsiang Chuang for assistance with the Look Locker T1 imaging. The NINDS EM facility is acknowledged for their assistance with particle EM. We also thank Dr. Mildred Cohn for valuable comments on this manuscript.
References
- 1.Louie AY, Huber MM, Ahrens ET, Rothbacher U, Moats R, Jacobs RE, Fraser SE, Meade TJ. In vivo visualization of gene expression using magnetic resonance imaging. Nat Biotechnol. 2000;18(3):321–325. doi: 10.1038/73780. [DOI] [PubMed] [Google Scholar]
- 2.Weissleder R, Simonova M, Bogdanova A, Bredow S, Enochs WS, Bogdanov A., Jr. MR imaging and scintigraphy of gene expression through melanin induction. Radiology. 1997;204(2):425–429. doi: 10.1148/radiology.204.2.9240530. [DOI] [PubMed] [Google Scholar]
- 3.Enochs WS, Petherick P, Bogdanova A, Mohr U, Weissleder R. Paramagnetic metal scavenging by melanin: MR imaging. Radiology. 1997;204(2):417–423. doi: 10.1148/radiology.204.2.9240529. [DOI] [PubMed] [Google Scholar]
- 4.Cohen B, Ziv K, Plaks V, Israely T, Kalchenko V, Harmelin A, Benjamin LE, Neeman M. MRI detection of transcriptional regulation of gene expression in transgenic mice. Nat Med. 2007;13(4):498–503. doi: 10.1038/nm1497. [DOI] [PubMed] [Google Scholar]
- 5.Gilad AA, McMahon MT, Walczak P, Winnard PT, Jr., Raman V, van Laarhoven HW, Skoglund CM, Bulte JW, van Zijl PC. Artificial reporter gene providing MRI contrast based on proton exchange. Nat Biotechnol. 2007;25(2):217–219. doi: 10.1038/nbt1277. [DOI] [PubMed] [Google Scholar]
- 6.Hinds KA, Hill JM, Shapiro EM, Laukkanen MO, Silva AC, Combs CA, Varney TR, Balaban RS, Koretsky AP, Dunbar CE. Highly efficient endosomal labeling of progenitor and stem cells with large magnetic particles allows magnetic resonance imaging of single cells. Blood. 2003;102(3):867–872. doi: 10.1182/blood-2002-12-3669. [DOI] [PubMed] [Google Scholar]
- 7.Shapiro EM, Skrtic S, Koretsky AP. Sizing it up: cellular MRI using micron-sized iron oxide particles. Magn Reson Med. 2005;53(2):329–338. doi: 10.1002/mrm.20342. [DOI] [PubMed] [Google Scholar]
- 8.Arbab AS, Wilson LB, Ashari P, Jordan EK, Lewis BK, Frank JA. A model of lysosomal metabolism of dextran coated superparamagnetic iron oxide (SPIO) nanoparticles: implications for cellular magnetic resonance imaging. NMR Biomed. 2005;18(6):383–389. doi: 10.1002/nbm.970. [DOI] [PubMed] [Google Scholar]
- 9.Wisner ER, Merisko-Liversidge E, Kellar K, Katzberg RW, Karpinski PH, Amparo EG, Drake C, Griffey SM, Brock JM. Preclinical evaluation of manganese carbonate particles for magnetic resonance imaging of the liver. Acad Radiol. 1995;2(2):140–147. doi: 10.1016/S1076-6332(05)80149-7. [DOI] [PubMed] [Google Scholar]
- 10.Shapiro EM, Skrtic S, Sharer K, Hill JM, Dunbar CE, Koretsky AP. MRI detection of single particles for cellular imaging. Proc Natl Acad Sci U S A. 2004;101(30):10901–10906. doi: 10.1073/pnas.0403918101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chuang KH, Koretsky A. Improved neuronal tract tracing using manganese enhanced magnetic resonance imaging with fast T(1) mapping. Magn Reson Med. 2006;55(3):604–611. doi: 10.1002/mrm.20797. [DOI] [PubMed] [Google Scholar]
- 12.Shapiro EM, Gonzalez-Perez O, Garcia-Verdugo JM, Alvarez-Buylla A, Koretsky AP. Magnetic resonance imaging of the migration of neuronal precursors generated in the adult rodent brain. Neuroimage. 2006;32(3):1150–1157. doi: 10.1016/j.neuroimage.2006.04.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Na HB, Lee JH, An K, Park YI, Park M, Lee IS, Nam DH, Kim ST, Kim SH, Kim SW, Lim KH, Kim KS, Kim SO, Hyeon T. Development of a T1 contrast agent for magnetic resonance imaging using MnO nanoparticles. Angew Chem Int Ed Engl. 2007;46(28):5397–5401. doi: 10.1002/anie.200604775. [DOI] [PubMed] [Google Scholar]
- 14.Pautler RG, Silva AC, Koretsky AP. In vivo neuronal tract tracing using manganese-enhanced magnetic resonance imaging. Magn Reson Med. 1998;40(5):740–748. doi: 10.1002/mrm.1910400515. [DOI] [PubMed] [Google Scholar]
- 15.Himmelreich U, Aime S, Hieronymus T, Justicia C, Uggeri F, Zenke M, Hoehn M. A responsive MRI contrast agent to monitor functional cell status. Neuroimage. 2006;32(3):1142–1149. doi: 10.1016/j.neuroimage.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 16.Lee JH, Silva AC, Merkle H, Koretsky AP. Manganese-enhanced magnetic resonance imaging of mouse brain after systemic administration of MnCl2: dose-dependent and temporal evolution of T1 contrast. Magn Reson Med. 2005;53(3):640–648. doi: 10.1002/mrm.20368. [DOI] [PubMed] [Google Scholar]
- 17.Aoki I, Takahashi Y, Chuang KH, Silva AC, Igarashi T, Tanaka C, Childs RW, Koretsky AP. Cell labeling for magnetic resonance imaging with the T1 agent manganese chloride. NMR Biomed. 2006;19(1):50–59. doi: 10.1002/nbm.1000. [DOI] [PubMed] [Google Scholar]